Abstract
Objective
To determine the incidence of tracheal stenosis, voice and breathing changes, and stomal complications after percutaneous dilatational tracheostomy (PDT).
Methods
From December 1992 through June 1999, 420 critically ill patients underwent 422 PDTs. There were 340 (81%) long-term survivors, 100 (29%) of whom were interviewed and offered further evaluation by fiberoptic laryngotracheoscopy (FOL) and tracheal computed tomography (CT). Tracheal stenosis was defined as more than 10% tracheal narrowing on transaxial sections or coronal and sagittal reconstruction views. Forty-eight patients agreed to CT evaluation; 38 patients also underwent FOL. CT and FOL evaluations occurred at 30 ± 25 (mean ± standard deviation) months after PDT.
Results
Twenty-seven (27%) patients reported voice changes and 2 (2%) reported persistent severe hoarseness. Vocal cord abnormalities occurred in 4/38 (11%) patients, laryngeal granuloma in 1 (3%) patient, focal tracheal mucosal erythema in 2 (5%) patients, and severe tracheomalacia/stenosis in 1 (2.6%) patient. CT identified mild (11–25%) stenosis in 10 (21%) asymptomatic patients, moderate (26–50%) stenosis in 4 (8.3%) patients, 2 who were symptomatic, and severe (>50%) stenosis in 1 (2%) symptomatic patient. Ten patients (10%) reported persistent respiratory problems after tracheal decannulation, but only four agreed to be studied. Two patients had moderate stenosis, and one had severe stenosis. One patient’s CT scan was normal. No long-term stomal complications were identified or reported.
Conclusions
Subjective voice changes and tracheal abnormalities are common after endotracheal intubation followed by PDT. Long-term follow-up of critically ill patients identified a 31% rate of more than 10% tracheal stenosis after PDT. Symptomatic stenosis manifested by subjective respiratory symptoms after decannulation was found in 3 of 48 (6%) patients.
Tracheostomy is one of the most common elective surgical procedures performed on critically ill patients. Percutaneous dilatational tracheostomy (PDT) is now considered a safe and cost-effective alternative to standard surgical tracheostomy. The technique of PDT that is most frequently used was introduced by Ciaglia et al 1 in 1985. Many investigators have since published their experience with this technique and have concluded that the procedure is safe and cost-effective. 2–9 The perioperative complications of PDT are well described 10,11 and are considered less than for open surgical tracheostomy. 10,11
Late complications, particularly tracheal stenosis, are more difficult to quantify because many patients undergoing PDT are critically ill and may die or be discharged from the hospital before decannulation. In patients who survive their critical illness or injury, access to long-term follow-up is often difficult because of the complexity of follow-up at tertiary referral centers, persistent medical problems, or a reluctance to return for evaluation by survivors who are asymptomatic. Recent studies to determine the incidence of late complications from PDT have used a variety of methods, including questionnaires, 12–15 tracheal roentgenograms, 16 tracheal tomograms, 17 magnetic resonance imaging, 18 laryngotracheoscopy, 12,13 and various pulmonary function tests. 12,15 The purpose of our study was to determine the incidence of tracheal stenosis and other late complications of PDT in a population of critically ill patients who survived their initial hospital stay and were successfully decannulated. A high-resolution computed tomography (CT) technique used to identify bronchial stenosis in lung transplant recipients 19–22 was used as a noninvasive method of evaluation to identify all degrees of tracheal stenosis.
METHODS
All procedures performed during this follow-up study were performed in accordance with the ethical standards required by the Investigational Review Board of East Texas Medical Center. Informed consent was obtained from all patients who agreed to have fiberoptic laryngoscopy and tracheal CT. From December 1992 through June 1999, 422 PDTs were performed on 420 critically ill medical, surgical, cardiac, and trauma patients. The authors’ technique for performing PDT has been reported previously, along with the early perioperative complication rate. 7 All PDTs were performed by one of six board-certified general surgeons using intratracheal bronchoscopic visual guidance. Endotracheal and tracheostomy cuff inflation pressures were measured at least every 8 hours and maintained at 20 to 25 cm H2O by a certified respiratory therapist.
Based on an earlier report of 54 patients evaluated for tracheal stenosis after PDT, 17 it was determined that 100 patients evaluated for long-term symptoms and complications would allow for estimation of the tracheal stenosis rate with a 10% absolute error rate. This would allow for a 95% confidence interval if the rate of tracheal stenosis was determined to be 25% to 45%.
Information provided in the medical and billing records of all patients who survived their initial hospital stay was used to locate patients for follow-up. Multiple attempts were made to contact these patients by telephone. One hundred patients were eventually contacted and agreed to answer questions about voice changes, respiratory problems since decannulation, and cosmetic considerations in relation to their tracheal scars. All 100 patients regardless of symptoms, were then offered evaluation by fiberoptic laryngotracheoscopy (FOL) and tracheal CT. At the time of CT, photographs of the tracheostomy stoma sites were also made.
All tracheal CT scans were performed with a General Electric CT Scanner (HiSpeed CT/i, GE, Milwaukee, WI) with standard reconstruction software. The protocol for CT scanning required 3-mm transaxial collimation beginning at the level of the hyoid bone and extending to the carina. A 20-cm field of view was used with standard interpolation and algorithm. Pitch was set at 1.0 to 1.5 with a 1.5-mm overlap. Coronal and sagittal reconstructions were performed at 1.5 mm using shaded surface reconstruction. The degree of tracheal stenosis was determined by measuring the greatest percentage of narrowing in the transaxial plane and corroborating these findings with coronal and sagittal tracheal reconstructions. Tracheal stenosis was defined as greater than 10% reduction in the transaxial tracheal diameter. If coronal or sagittal reconstructions indicated a stenosis greater than that found on transaxial views, the larger percentage stenosis was recorded. Under topical anesthesia with 4% lidocaine, a fiberoptic laryngoscope (Jet-Med Inc., St. Louis, MO) was passed nasally to evaluate the vocal cords and the posterior pharyngeal and subglottic areas. Any abnormalities identified by FOL were recorded. Interpretation of CT results and all FOL procedures were performed by two of the authors (K.S., M.S.) who had not been involved in the original PDT procedures and had no knowledge of the patients’ clinical symptoms.
Data were collected on all 100 patients regardless of whether they agreed to further evaluation by CT and FOL. Data points included gender, age at the time of tracheostomy, date of tracheostomy, date of removal of the tracheostomy cannula, total orotracheal intubation time before tracheostomy, and any patient-perceived changes in voice or breathing patterns. Long-term stomal complications were also recorded, and patients were asked about possible considerations of cosmetic stomal site revision. The 100 patients in the study group were compared with patients who had undergone PDT but were not available for follow-up to ensure that patients who entered the study were representative of the entire group for age, gender, orotracheal intubation time, and perioperative complications. The possibility of selection bias in the study group of 100 patients was also evaluated by comparing all data points between the patients who agreed to and those who did not agree to tracheal CT and FOL. Patients with greater than 10% tracheal stenosis were compared with patients with no CT evidence of significant stenosis to determine differences in patient characteristics or perioperative complications that might predispose to tracheal stenosis. Other risk factors that may influence the incidence and severity of tracheal stenosis include chronic underlying pulmonary disease, infectious tracheobronchitis and other pulmonary infections, steroid use, and traumatic or field intubations. Patients with more than 10% tracheal stenosis by CT were compared with patients with no CT evidence of significant tracheal stenosis to determine any differences in these risk factors.
All data collection and analyses were performed using Data Desk 6.0 Statistical Analysis Program (Data Description, Inc., Ithaca, NY). Significant differences among groups were determined using the Fisher exact test for categorical data and the F-test analysis of variance for continuous data. Unless otherwise specified, all data were reported as mean ± standard deviation. P < .05 was considered statistically significant.
RESULTS
The perioperative complications in 420 patients who underwent 422 PDTs are summarized in Table 1. The overall death rate for PDT was 0.2% (1/422). The one death occurred in an elderly patient with end-stage chronic obstructive pulmonary disease and congestive heart failure who developed bilateral pneumothoraces during the procedure. The surgeon believed that increased transpulmonary pressures created by introducing the bronchoscope through a 7.5-mm endotracheal tube may have caused the pneumothoraces, because the tracheal dilation and tracheostomy tube placement was not difficult and was performed with no apparent technical complications.
Table 1. PERIOPERATIVE COMPLICATIONS
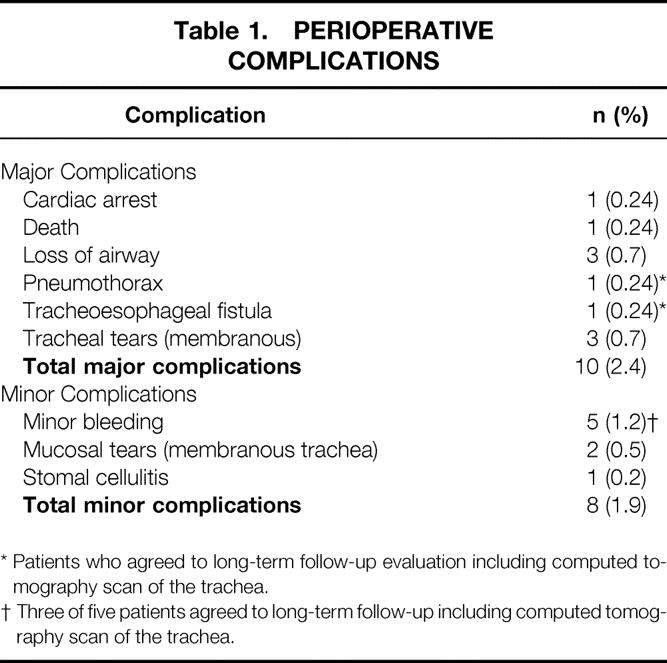
* Patients who agreed to long-term follow-up evaluation including computed tomography scan of the trachea.
† Three of five patients agreed to long-term follow-up including computed tomography scan of the trachea.
Forty-two patients died during their initial hospital stay. Three hundred seventy-eight patients (90%) survived their initial hospital stay and were potentially evaluable. Information provided by telephone revealed that 38 patients had died since discharge from the hospital. No deaths were attributed to PDT. Thirty-two patients were located but could not participate in the study. Long-term follow-up occurred at 30 ± 23 months (median 26 months, range 1–82 months) for the 100 patients who agreed to be interviewed. This study group was significantly younger at the time of tracheostomy compared with the 320 patients who were not available for long-term follow-up (Table 2). No differences were identified between these two groups in terms of gender, intubation time before tracheostomy, or perioperative complications.
Table 2. COMPARISON OF STUDY GROUP WITH PATIENTS NOT INTERVIEWED
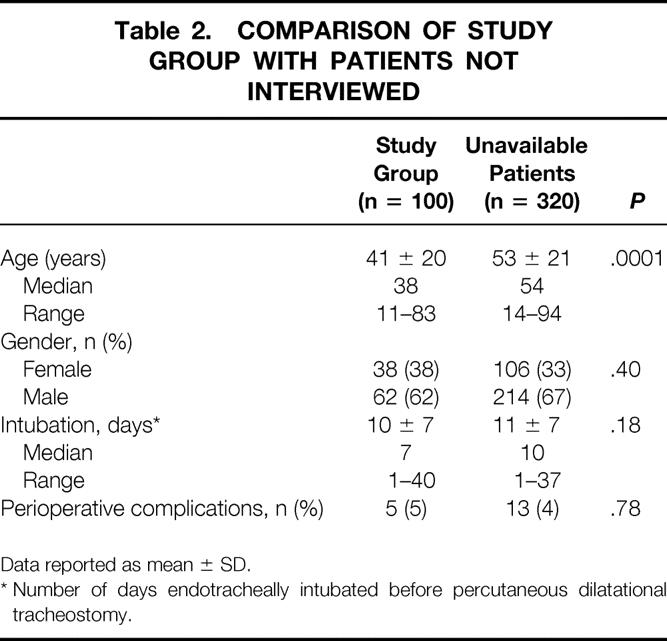
Data reported as mean ± SD.
* Number of days endotracheally intubated before percutaneous dilatational tracheostomy.
Follow-up for the 48 patients who agreed to CT and FOL occurred at 30 ± 25 months (median 21 months, range 3–80 months). A comparison of the 48 patients who agreed to CT and FOL with 52 patients who were interviewed but refused further testing is provided in Table 3. With the exception of the hospital day that tracheostomy was performed and total length of hospital stay, these two groups were similar for all variables studied. Five of the 18 patients who had perioperative complications (Table 1) were identified for long-term follow-up, and all 5 patients agreed to undergo CT and FOL.
Table 3. COMPARISON OF PATIENTS WHO UNDERWENT TESTING WITH THOSE WHO DID NOT
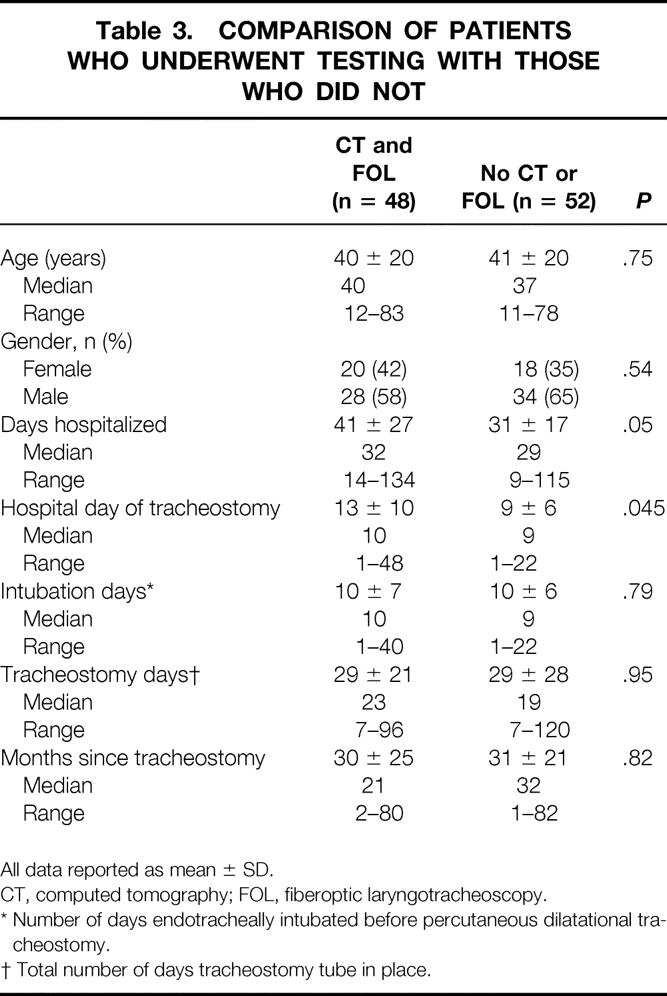
All data reported as mean ± SD.
CT, computed tomography; FOL, fiberoptic laryngotracheoscopy.
* Number of days endotracheally intubated before percutaneous dilatational tracheostomy.
† Total number of days tracheostomy tube in place.
Tracheal CT identified more than 10% tracheal stenosis in 15 of 48 (31%) patients. Twenty-five (52%) patients had normal CT scans (Fig. 1) and eight (17%) patients had insignificant (≤10%) narrowing. One (2%) patient reported persistent respiratory problems after tracheal decannulation despite a completely normal CT and FOL. Mild (11–25%) stenosis was identified in 10 (21%) asymptomatic patients. Moderate (26–50%) stenosis was identified in four (8.3%) patients, two of whom reported persistent respiratory problems after decannulation (Figs. 2 through 5), and severe (>50%) stenosis occurred in one (2%) patient (Fig. 6). With the exception of one patient with severe (>50%) diffuse tracheal stenosis and tracheomalacia (Fig. 6), all of the stenoses occurred at the stomal level.
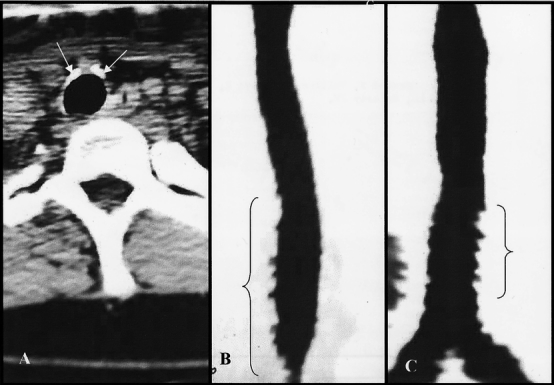
Figure 1. Computed tomography scan of a normal trachea after percutaneous dilatational tracheostomy. (A) Axial view. Arrows indicate perichondrial hypertrophy from tracheostomy with no luminal narrowing. (B, C) Sagittal and coronal reconstruction views. Indentations at bracketed areas are normal pulsatile artifacts from the adjacent great vessels.
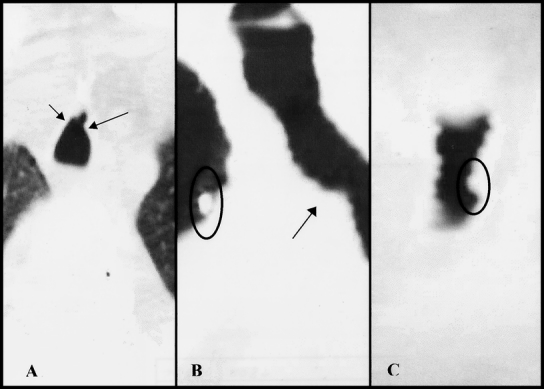
Figure 2. Patient with 30% tracheal stenosis after percutaneous dilatational tracheostomy. (A) Axial view. Arrows indicate areas of anterior stenosis. (B) Sagittal view with arrow indicating area of stenosis. Circle indicates marker used to identify stoma site. (C) Coronal view with circle indicating area of stenosis.
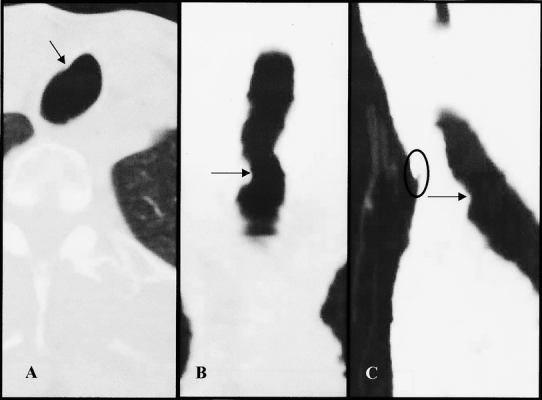
Figure 3. Patient with 35% tracheal stenosis. Arrows indicate areas of stenosis. Circle denotes stomal site with marker. (A) Axial view. (B) Coronal view. (C) Sagittal view.
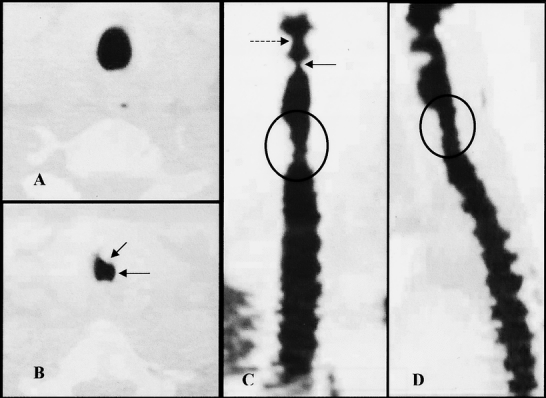
Figure 4. Patient with 45% tracheal stenosis. (A) Axial view of normal trachea just above the stoma level. (B) Axial view at stoma level. Arrows denote areas of anterior and lateral stenosis. (C) Coronal view with circled area of stenosis. Dotted arrow denotes false vocal cords. Solid arrow indicates true vocal cords. (D) Sagittal view with circle denoting area of stenosis.
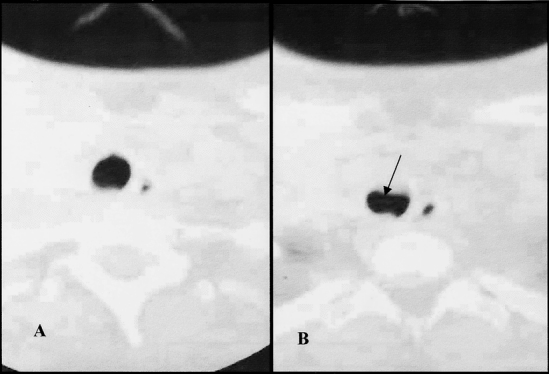
Figure 5. Patient with 33% tracheal stenosis. (A) Axial view 6 mm above area of stenosis. (B) Axial view of beginning of stenotic area. Arrow indicates area of anterior stenosis.
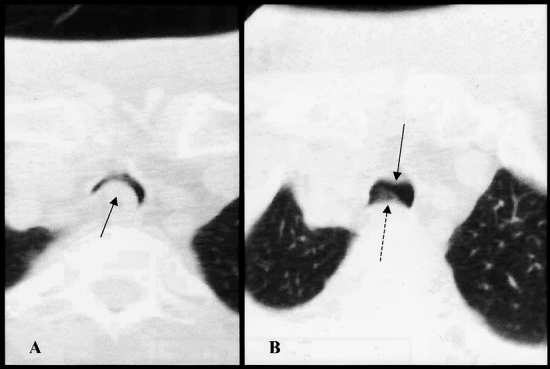
Figure 6. Patient with severe stenosis and tracheomalacia after percutaneous dilatational tracheostomy. (A) Axial view. Arrow indicates area of severe tracheomalacia with bulging of the posterior membranous tracheal wall and esophagus causing 90% obstruction of the tracheal lumen. (B) Axial view 9 mm cephalad to A. Solid arrow indicates anterior tracheal scarring and stenosis. Dotted arrow denotes posterior tracheomalacia.
The patient with severe stenosis was interviewed further and was minimally symptomatic. This patient’s PDT was complicated by development of a tracheoesophageal fistula that healed spontaneously during her initial hospital stay. Her stenosis was 4 cm long, beginning at the stoma and extending to 3 cm above the carina (Fig. 6). Because of the severity of her stenosis, pulmonary function studies were performed and revealed a forced vital capacity of 57% of predicted and an FEV1 of 45% of predicted (0.92 L). The contour of the patient’s flow volume loop was significantly diminished, with a flattened expiratory flow limb but a relatively normal inspiratory flow limb. Fiberoptic tracheoscopy identified anterior movement of the membranous trachea with forced exhalation indicative of tracheomalacia. Despite these findings, the patient reported only mild dyspnea on exertion.
No differences in age, gender, days intubated before tracheostomy, days of tracheal cannulation, or perioperative complications were identified when patients with CT evidence of tracheal stenosis were compared with patients with no CT evidence of tracheal stenosis (Table 4). Other potential risk factors for tracheal stenosis are compared in Table 5.
Table 4. COMPARISON OF PATIENTS WITH OR WITHOUT EVIDENCE OF STENOSIS
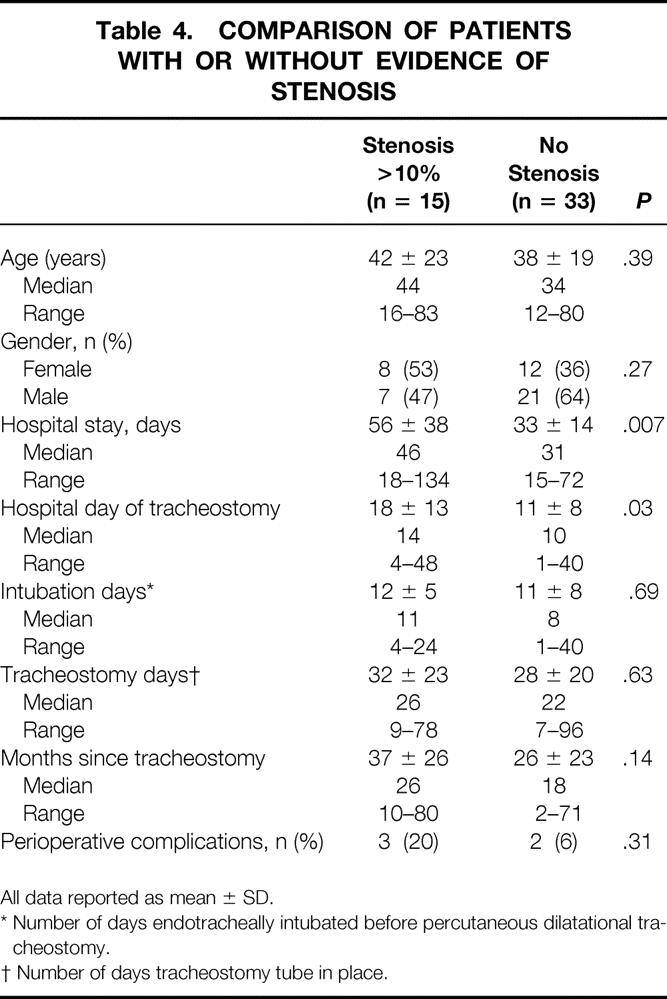
All data reported as mean ± SD.
* Number of days endotracheally intubated before percutaneous dilatational tracheostomy.
† Number of days tracheostomy tube in place.
Table 5. RISK FACTORS FOR TRACHEAL STENOSIS
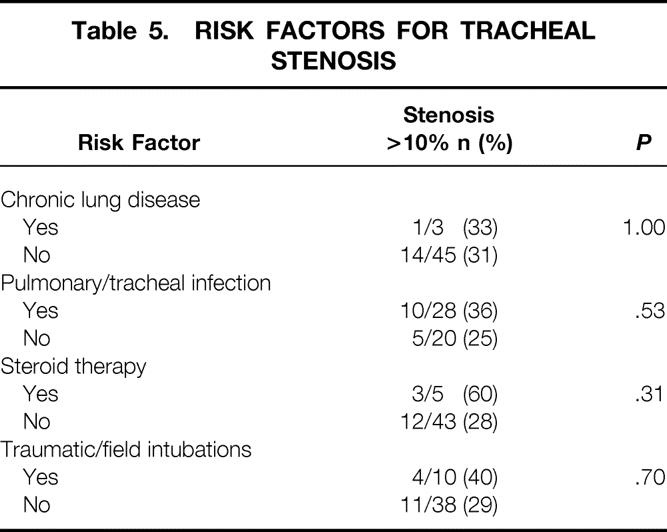
Thirty-eight of the 48 patients who had CT agreed to further evaluation by FOL. Thirty (79%) patients had no abnormalities. Eight (21%) patients had abnormal findings: one (2.6%) had laryngeal granuloma, four (11%) had vocal cord abnormalities, two (5%) had mild focal edema and erythema of the larynx, and one (2.6%) had severe tracheomalacia and tracheal stenosis (Fig. 6).
Ten of 100 (10%) patients reported persistent respiratory problems after tracheal decannulation. Four of these patients agreed to have a tracheal CT performed. The degrees of stenoses were 20%, 30%, and more than 50%. The fourth patient had a completely normal tracheal CT scan. Subjective voice changes were reported in 27 (27%) patients. Two (2%) patients reported major voice changes manifested by persistent hoarseness. There were no reported long-term stomal problems, although 15 (15%) patients were considering cosmetic scar revision.
DISCUSSION
In critically ill patients, PDT is rapidly becoming the procedure of choice for long-term airway control. Its advantages include the relative ease of performing the procedure at the bedside, the documented cost savings, and the low perioperative complication rate. 6,23–26 These reported advantages have led to an increase in the number of tracheostomies being performed in critically ill patients. A recent study by Simpson et al 27 showed that the number of critically ill patients at their facility undergoing tracheostomy had increased from 8.5% to 16.8% of all critically ill patients since the advent of PDT. Tracheostomy was also being performed significantly earlier, at a median of 4 versus 8 days of mechanical ventilation. 27
Although the early complications of PDT are well documented and are reported to compare favorably with the complication rates associated with standard open tracheostomy, 1–11,24–26 there is less information concerning late complications, particularly tracheal stenosis. Indeed, although thousands of PDTs have been performed in the United States and Europe, our literature review identified only a few studies with long-term follow-up and relatively few patients who have undergone objective studies to identify tracheal stenosis. 12–18,26 Several different tests have been used to identify tracheal stenosis after PDT. These include plain tracheal roentgenograms, 16 tracheal tomograms, 17 pulmonary function tests, 12,15 laryngotracheoscopy, 12,13 and magnetic resonance imaging. 18 We chose a high-resolution tracheal CT technique previously used to identify bronchial stenosis and other central airway problems after lung transplantation. 19–22 We believed that this noninvasive, high-resolution technique would provide the most accurate information.
In 1992, Ciaglia and Graniero 6 were the first to report any long-term follow-up for patients who had PDT. Their follow-up was based primarily on clinical assessment, but many of the patients were reported to have had pulmonary function tests and laryngotracheal roentgenograms. They initially reported no long-term complications, but in a later report of 254 patients, tracheal stenosis had developed in two. 23 The actual number of patients who were available for follow-up in these studies was not given.
Hill et al 24 evaluated 214 of 356 patients after PDT who were decannulated. They identified eight (3.7%) patients in whom symptomatic tracheal stenosis developed.
Cobean et al 25 evaluated 28 of 35 (80%) patients who were available for long-term follow-up at 7.5 ± 4.8 months (range 1–18). Late postoperative complications were reported in 2 (7%) patients. One patient had rest stridor 1 month after decannulation and was found to have subglottic stenosis, which required surgical correction. A second patient reported a hoarse voice but no difficulty breathing, and no further examination was performed. Details of how the remaining 26 patients were evaluated were not given. Others have also reported no significant tracheal lesions when patients were evaluated by clinical assessment and FOL. 26
Few studies have used objective testing to determine structural changes in the trachea after PDT in both symptomatic and asymptomatic patients. Most investigators have focused on patients with symptoms of airway compromise. To paraphrase Dr. Ciaglia, patients who have had PDT may develop a minor degree of pathologic change and stenosis that would cause no symptoms or signs of airway difficulty even during exercise; these anatomical abnormalities should therefore be considered of no practical importance. 6 Although this conclusion may be valid in patients during normal daily activities, asymptomatic tracheal stenosis may have profound clinical significance in patients during vigorous exercise or with associated upper airway edema from infectious processes. Tracheal stenosis may also become an important factor if patients require tracheal intubation at a later date for emergency airway control or elective surgery. 28 Knowledge of the true incidence of tracheal stenosis after PDT is also important because patients often do not become symptomatic until stenosis greater than 75% of the tracheal diameter occurs or the actual diameter is reduced to 5 mm. 29,30
Several studies have reported an overall incidence of tracheal stenosis after PDT of 0% to 10%. 10,12–15,18 These studies rely primarily on clinical symptoms leading to some form of objective testing in a few patients. Such patient selection may not be representative of the overall population.
VanHearn et al 17 attempted follow-up on 80 patients who were successfully decannulated after PDT. Fourteen patients died before follow-up, leaving 66 patients available for evaluation at 3 to 39 months (mean 16 months) after decannulation. Voice changes, most of which were considered minor by the patients, were identified in 13 of 61 (21%) patients. Fifty-four of 66 (82%) patients were evaluated by tracheal tomography, and 14 (26%) patients were found to have more than 10% stenosis. Eleven (20%) patients had tracheal stenosis of 10% to 25%, with 25% to 50% tracheal stenosis being found in two (4%) patients. Severe stenosis, defined as 50% to 75%, was found in one (2%) patient. The authors found a correlation between the level of experience of the person performing the tracheostomy and the risk for developing tracheal stenosis after the procedure.
Walz et al 16 identified 141 patients who were successfully decannulated of 326 patients who had PDT at seven intensive care units in Germany. One hundred six patients were evaluated with coronal and sagittal plane tracheal radiographs. Tracheal stenosis of 10% or greater was identified in 46 (44%) patients. Follow-up was performed at 9.9 ± 5.6 months (range 6–33) after decannulation. Median time of tracheal cannulation was 24 days (range 4–292), and mean duration of endotracheal intubation before tracheostomy was 4 days (range 0–31). In contrast to historical reports in patients after open tracheostomy, 29,30 all of the tracheal stenoses occurred in the area of the tracheostomy stoma, with no stenoses identified at the level of the cuff or the cannula tip. In the Walz et al study, 16 the degree of stenosis was 11% to 25% in 19 (18%) patients, 26% to 50% in 23 (22%) patients, and more than 50% in 4 (3.7%) patients. Only one patient was symptomatic from tracheal stenosis.
The present study using CT identified an overall incidence of tracheal stenosis of 31%. Only 3 of 15 (20%) patients with tracheal stenosis were symptomatic, giving an overall rate of symptomatic tracheal stenosis of 6%. This incidence is similar to that identified by vanHearn et al 17 and Walz et al. 16 The present study is unique in that all 422 tracheostomies were performed by a small group of general surgeons experienced in the technique. In addition, all of the PDTs were performed with bronchoscopic guidance and pretracheal blunt dissection to identify the tracheal rings before tracheal needle puncture. These techniques are now also recommended by Ciaglia and colleagues. 23 We believe that the routine use of these techniques contributed to the low perioperative complication and death rates (Table 1).
Despite the low perioperative complication rate and other perceived benefits of PDT, long-term follow-up of both symptomatic and asymptomatic patients indicates that the incidence of tracheal stenosis is significant. 16,17 Rates of moderate to severe stenosis range from 6%17 to 26%. 16 We failed to identify any risk factors associated with the development of tracheal stenosis other than the finding that patients in whom tracheal stenosis developed had a significantly longer hospital stay and underwent tracheostomy later in their hospital course (Tables 4 and 5). We also failed to determine whether the subjective voice changes, vocal cord abnormalities, and perceived respiratory problems were secondary to endotracheal intubation, PDT, a combination of both procedures, or residual pulmonary dysfunction as a result of chronic pulmonary changes. Further studies are needed to determine whether early PDT versus prolonged endotracheal intubation has any effect on the long-term complications identified in this study.
There is little debate concerning the safety and efficacy of PDT. Perioperative complications are low when compared with open tracheostomy. 5,31–33 However, our results and those of others 16,17 indicate that there is a quantifiable risk of long-term complications, including tracheal stenosis. Surgeons and patients should be made aware of these late complications, despite the observation that most patients with moderate to severe stenosis are asymptomatic.
Footnotes
Correspondence: Scott Norwood, MD, FACS, 1020 East Idel St., Tyler, TX 75701.
Presented at the 65th International Scientific Assembly of the American College of Chest Physicians, November 3, 1999, Chicago, Illinois.
E-mail: ettrauma@ballistic.com
Accepted for publication December 21, 1999.
References
- 1.Ciaglia P, Firshing R, Syniec C. Elective percutaneous dilatational tracheostomy: a simple bedside procedure: preliminary report. Chest 1985; 87: 715–719. [DOI] [PubMed] [Google Scholar]
- 2.Paul A, Marelli D, Chiu RCJ, et al. Percutaneous endoscopic tracheostomy. Ann Thorac Surg 1989; 47: 314–315. [DOI] [PubMed] [Google Scholar]
- 3.Friedman Y, Mayer AD. Bedside percutaneous tracheostomy in critically ill patients. Chest 1993; 104: 532–535. [DOI] [PubMed] [Google Scholar]
- 4.Marelli D, Paul A, Manolidis S, et al. Endoscopic guided percutaneous tracheostomy: early results of a consecutive trial. J Trauma 1990; 30: 433–435. [PubMed] [Google Scholar]
- 5.Hazard P, Jones C, Benitone J. Comparative clinical trial of standard operative tracheostomy with percutaneous tracheostomy. Crit Care Med 1991; 19: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 6.Ciaglia P, Graniero KD. Percutaneous dilatational tracheostomy; results and long-term follow-up. Chest 1992; 101: 464–467. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez L, Norwood S, Roettger R, et al. Bedside percutaneous tracheostomy with bronchoscopic guidance in critically ill patients. Arch Surg 1996; 131: 129–132. [DOI] [PubMed] [Google Scholar]
- 8.Toursarkissian B, Zweng TN, Kearney PA, et al. Percutaneous dilatational tracheostomy: report of 141 cases. Ann Thorac Surg 1994; 57: 862–867. [DOI] [PubMed] [Google Scholar]
- 9.Winkler WB, Karnik R, Seelman O, et al. Bedside percutaneous dilational tracheostomy with endoscopic guidance: experience with 71 ICU patients. Intensive Care Med 1994; 20: 476–479. [DOI] [PubMed] [Google Scholar]
- 10.Moe KS, Schmid S, Stoecki SJ, Weymuller EA. Percutaneous tracheostomy: a comprehensive evaluation. Ann Otol Rhinol Laryngol 1999; 108: 384–391. [DOI] [PubMed] [Google Scholar]
- 11.Trottier SJ, Hazard PB, Sakabu SA, et al. Posterior tracheal wall perforation during percutaneous dilatational tracheostomy. An investigation into its mechanism and prevention. Chest 1999; 115: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 12.Leonard RC, Lewis RH, Singh B, van Heerden PV. Late outcome from percutaneous tracheostomy using the Portex kit. Chest 1999; 115: 1070–1075. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbower TJ, Morris JA, Eddy VA, Ries WR. The long-term complications of percutaneous dilatational tracheostomy. Am Surg 1998; 64: 82–87. [PubMed] [Google Scholar]
- 14.Wagner F, Nasseri R, Laucke U, Hetzer R. Percutaneous dilatational tracheostomy: results and long-term outcome of critically ill patients after cardiac surgery. Thorac Cardiovasc Surg 1998; 46: 352–356. [DOI] [PubMed] [Google Scholar]
- 15.Law RC, Carney AS, Manara AR. Long-term outcome after percutaneous dilational tracheostomy. Anaesthesia 1997; 52: 51–56. [DOI] [PubMed] [Google Scholar]
- 16.Walz MK, Peitgen K, Thürauf N, et al. Percutaneous dilatational tracheostomy—early results and long-term outcome of 326 critically ill patients. Intensive Care Med 1998; 24: 685–690. [DOI] [PubMed] [Google Scholar]
- 17.vanHearn LWE, Goei R, dePloeg I, et al. Late complications of percutaneous dilatational tracheostomy. Chest 1996; 110: 1572–1576. [DOI] [PubMed] [Google Scholar]
- 18.Callanan V, Gillmore K, Field S, Beaumont A. The use of magnetic resonance imaging to assess tracheal stenosis after percutaneous dilatational tracheostomy. J Laryngol Otol 1997; 111: 953–957. [DOI] [PubMed] [Google Scholar]
- 19.Quint LE, Whyte RI, Kazerooni EA, et al. Stenosis of the central airways: evaluation by using helical CT with multiplanar reconstructions. Radiology 1995; 194: 871–877. [DOI] [PubMed] [Google Scholar]
- 20.Kauczor HU, Wolcke B, Fischer B, et al. Three-dimensional helical CT of the tracheobronchial tree. AJR 1996; 167: 419–424. [DOI] [PubMed] [Google Scholar]
- 21.Locicero J, Costello P, Campos CT, et al. Spiral CT with multiplanar and three-dimensional reconstructions accurately predicts tracheobronchial pathology. Ann Thorac Surg 1996; 62: 811–817. [DOI] [PubMed] [Google Scholar]
- 22.McAdams HP, Palmer SM, Erasmus JJ, et al. Bronchial anastomotic complications in lung transplant recipients: virtual bronchoscopy for non-invasive assessment. Radiology 1998; 209: 689–695. [DOI] [PubMed] [Google Scholar]
- 23.Marx WA, Ciaglia P, Graniero KD. Some important details in the technique of percutaneous dilatational tracheostomy via the modified Seldinger technique. Chest 1996; 110: 762–766. [DOI] [PubMed] [Google Scholar]
- 24.Hill BB, Zweng TN, Maley RH, et al. Percutaneous dilatational tracheostomy: report of 356 cases. J Trauma 1996; 40: 238–244. [DOI] [PubMed] [Google Scholar]
- 25.Cobean R, Beals M, Moss C, Bredenberg CE. Percutaneous dilatational tracheostomy. A safe, cost-effective bedside procedure. Arch Surg 1996; 131: 265–271. [DOI] [PubMed] [Google Scholar]
- 26.Fischler MP, Kuhn M, Cantieni R, et al. Late outcome of percutaneous dilatational tracheostomy in intensive care patients. Intensive Care Med 1995; 21: 475–481. [DOI] [PubMed] [Google Scholar]
- 27.Simpson TP, Day CJE, Jewks CF, Manara AR. The impact of percutaneous tracheostomy on intensive care unit practice and training. Anaesthesia 1999; 54: 179–197. [DOI] [PubMed] [Google Scholar]
- 28.Carney AS. Letter to the editor. J Laryngol Otol 1998; 112: 559. [Google Scholar]
- 29.Weber AL, Grillo HC. Tracheal stenosis. An analysis of 151 cases. Radiol Clin North Am 1978; 16: 291–308. [PubMed] [Google Scholar]
- 30.Heffner JE, Miller S, Sahn SA. Tracheostomy in the intensive care unit. Part 2: complications. Chest 1986; 90: 430–436. [DOI] [PubMed] [Google Scholar]
- 31.Griggs WM, Myburg JA, Worthley LIG. A prospective comparison of a percutaneous tracheostomy technique with standard surgical tracheostomy. Intensive Care Med 1991; 17: 261–263. [DOI] [PubMed] [Google Scholar]
- 32.Crofts SL, Alzeer A, McGuire GP, et al. A comparison of percutaneous and operative tracheostomies in intensive care patients. Can J Anaesth 1995; 42: 775–779. [DOI] [PubMed] [Google Scholar]
- 33.Friedman Y, Fildes J, Mizock B, et al. Comparison of percutaneous and surgical tracheostomies. Chest 1996; 110: 480–485. [DOI] [PubMed] [Google Scholar]


