Abstract
Objective
To develop and validate a preoperative risk index for predicting postoperative respiratory failure (PRF).
Summary Background Data
Respiratory failure is an important postoperative complication.
Method
Based on a prospective cohort study, cases from 44 Veterans Affairs Medical Centers (n = 81,719) were used to develop the models. Cases from 132 Veterans Affairs Medical Centers (n = 99,390) were used as a validation sample. PRF was defined as mechanical ventilation for more than 48 hours after surgery or reintubation and mechanical ventilation after postoperative extubation. Ventilator-dependent, comatose, do not resuscitate, and female patients were excluded.
Results
PRF developed in 2,746 patients (3.4%). The respiratory failure risk index was developed from a simplified logistic regression model and included abdominal aortic aneurysm repair, thoracic surgery, neurosurgery, upper abdominal surgery, peripheral vascular surgery, neck surgery, emergency surgery, albumin level less than 30 g/L, blood urea nitrogen level more than 30 mg/dL, dependent functional status, chronic obstructive pulmonary disease, and age.
Conclusions
The respiratory failure risk index is a validated model for identifying patients at risk for developing PRF and may be useful for guiding perioperative respiratory care.
Postoperative pulmonary complications greatly contribute to the death and complication rates of surgery. It has been reported that 5% to 10% of all surgical patients and 9% to 40% of those undergoing abdominal surgery experience postoperative pulmonary complications. 1 In prior studies, the definition of what constitutes a postoperative pulmonary complication varied greatly. Atelectasis, 2,3 postoperative pneumonia, 2–4 acute respiratory distress syndrome, 4,5 and postoperative respiratory failure 6,7 have all been classified as postoperative pulmonary complications. Although the clinical significance of each of these complications varies, they are often grouped together in studies of risk factors for postoperative pulmonary complications. 8
Postoperative respiratory failure (PRF) is among the most serious of the postoperative pulmonary complications. It is most commonly defined as the inability to be extubated 48 hours after surgery, 6 although some investigators have used 5 days. 7 Among patients undergoing abdominal aortic aneurysm repair, the rate of PRF is 5% to 21%, depending on the type of aneurysm. 7 More importantly, the in-hospital death rate for patients with PRF is 40% to 42% versus 6% for those without PRF. 7
Prior studies have suggested that the risk factors for PRF include those that are patient-specific and those that are operation-specific. The patient-specific risk factors can be divided into those related to the patient’s general health status (e.g., age, 3 functional status, diabetes mellitus, cancer, alcohol use), pulmonary status (e.g., smoking, 3,4–6 chronic obstructive pulmonary disease [COPD], 9,10 increased body mass index, 3), neurologic status (e.g., impaired sensorium 3), cardiac status (e.g., myocardial infarction 6), and renal and fluid status (e.g., renal failure, 6, 7 blood transfusion 11). The operation-specific risk factors include the location of the incision in relation to the diaphragm, 3,7 emergent operation, and the type of anesthesia used (e.g., general vs. spinal).
The main objective of this study was to determine the preoperative predictors of PRF in a large multicenter observational cohort of subjects undergoing major noncardiac surgery. Specific goals included identifying predictors of PRF that are easily obtained and commonly accessible to care providers before surgery. Using these predictors, a risk assessment model and scoring system analogous to risk assessment models for postoperative cardiac complications 12–14 was developed and validated.
METHODS
Subjects were selected from the National Veterans Affairs Surgical Quality Improvement Program (NSQIP). A detailed description of the study methods has been published previously and is briefly summarized here. 15–17
Participating Hospitals
Phase I of the study included patients enrolled between Oct. 1, 1991, and Dec. 31, 1993, and was conducted at 44 Veterans Affairs Medical Centers (VAMCs) that were closely affiliated with university medical centers and offered both cardiac and noncardiac surgery. Phase II of the study included patients enrolled between Jan. 1, 1994, and Aug. 31, 1995, and was conducted at all 132 VAMCs that perform major surgery.
Selection of Subjects
All noncardiac operations performed under general, spinal, or epidural anesthesia were eligible for inclusion as index operations. Based on a review of the surgical death rates from the Veterans Affairs administrative discharge database for 1988 and 1989, selected operations with very low death rates such as dental procedures, central line insertions, dressing changes, and endoscopic procedures were excluded from the NSQIP. 18 Major transplantation procedures and cases entered into the study within the previous 30 days were also excluded. Because the data were obtained from veterans undergoing surgery in VAMCs, most of the patients were men. Female patients tended to be much younger and healthier than male patients and had better outcomes in the NSQIP. Specifically for this study, operations performed on patients who were female, ventilator-dependent, or comatose or had a do-not-resuscitate (DNR) order were excluded from analysis. All eligible operations were included at low-volume centers (<140 eligible operations per month). At high-volume centers (>140 eligible operations per month), the first 36 consecutive eligible operations were entered in each consecutive 8-day period, beginning with a different day each period. Only the first five transurethral resections of the prostate and tumors of the bladder performed in each 8-day cycle were included because of the large number of these procedures that were performed.
Data Collection
A surgical risk assessment nurse was assigned at each center to collect the data. These nurses completed in-depth training on the protocol and study definitions. Annual meetings and regular conference calls were conducted, and two traveling nurse coordinators performed site visits to maintain data reliability.
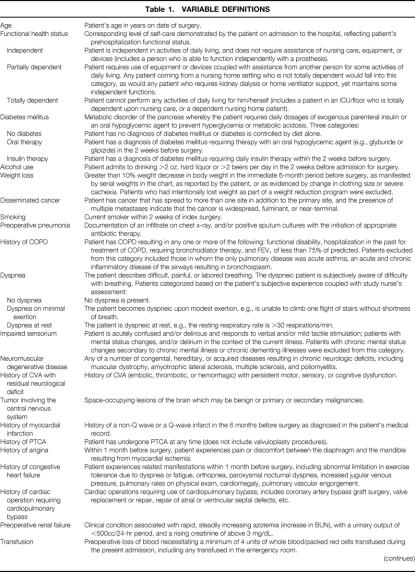
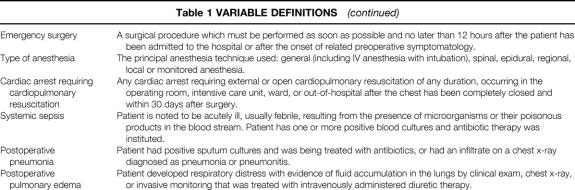
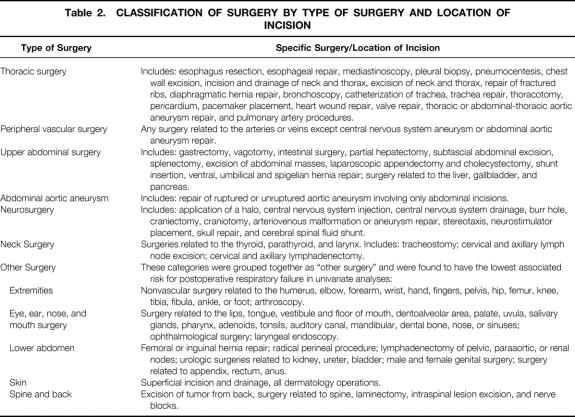
Generic preoperative, intraoperative, and postoperative variables were chosen on the basis of clinical relevance, reliability of data collection, and availability of data. Relevant variable definitions are given in Table 1. 18 Preoperative data were obtained by the study nurse from the medical chart or a surgical risk assessment profile that was completed by the surgical resident caring for the patient and was later verified by the study nurse from the medical chart. Preoperative laboratory values within 30 days of the index operation and closest to the time of operation were acquired automatically from the laboratory software in each center’s computer system. Intraoperative variables were collected from the surgical log and anesthesia record for the index operation. The index operation was defined as the first eligible operation performed on the patient. Other operations performed under the same anesthetic by the same or a different surgical team were also recorded. Specifically for this study, the type of surgery was primarily classified by the anatomical location of the surgical incision (Table 2).
Table 1. VARIABLE DEFINITIONS
Table 1A. VARIABLE DEFINITIONS (continued)
BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CVA, cerebral vascular accident; FEV1, forced expiratory volume in 1 second; PTCA, percutaneous transluminal coronary angioplasty.
Table 2. CLASSIFICATION OF SURGERY BY TYPE OF SURGERY AND LOCATION OF INCISION
Patients were followed for 30 days after surgery. Thirty-day surgical death was defined as death from any cause inside or outside the hospital within 30 days of surgery. Patients were defined as having respiratory failure if the total duration of ventilator-assisted respiration during the postoperative hospital stay exceeded 48 hours. Patients were defined as having unplanned intubation if they required placement of an endotracheal tube and mechanical or assisted ventilation because of respiratory or cardiac failure manifested by severe respiratory distress, hypoxia, hypercarbia, or respiratory acidosis subsequent to extubation after general anesthesia. For the analysis reported in this study, patients with respiratory failure or unplanned intubation were combined into the primary outcome variable of PRF.
The study nurse obtained outcomes information by chart review, interviews with care providers, reports from morbidity and mortality conferences, and communication with each patient on the 30th postoperative day by letter or by telephone. The nurse input all data into a surgical risk assessment module in each center’s computer system. A summary of the entire data record on each patient was forwarded to the chief of the surgical service 30 days after surgery for inspection. No later than 45 days after surgery, the case records were transmitted automatically to the statistical coordinating center for editing and analysis.
Missing Data
All data were greater than 99% complete, with the exception of the preoperative laboratory variables, the completeness of which depended on whether the specific laboratory tests had been ordered. The logistic regression models were run with missing laboratory values being assigned normal values (albumin = 40 g/L, blood urea nitrogen [BUN] = 10 mg/dL, creatinine = 1.0 mg/dL). This is based on the assumption that these relatively routine laboratory tests were not obtained in healthier patients, who would likely have normal values. The models were also analyzed using dummy variables to designate the group of patients with missing values. The β-coefficients for these dummy variables were not significantly different from the reference groups for each laboratory test. A regression procedure was also used to estimate missing values based on values of variables that were present, and the final models were evaluated using these generated values. 19 The β-coefficients of the models did not change significantly with any of these three methods, so the results reported are from the models run with missing values being assigned normal values.
Statistical Analysis
Data were analyzed using the SAS for UNIX software package (SAS, Cary, NC). Using patients from phase I, the univariate relationship between each variable and PRF was tested using a chi-square test for categorical variables and a Student t test for continuous variables. All variables were entered into a logistic regression model with PRF as the dependent variable. Continuous variables were categorized into ranges suggested by their relationship to PRF in univariate analyses. Dummy variables were created for each range, and all such dummy variables were used in the logistic regression models. Potential predictor variables that were not statistically significant at the P < .05 level were sequentially deleted from the base model until only predictor variables that were significant remained. All two-way interactions were analyzed, with none being statistically significant. The resulting model was designated model 1.
Model 1 was then used as the basis for developing a simplified model. Variables with multiple ranges in model 1 (e.g., albumin, BUN) were categorized into dichotomous variables. Starting with the variables that had the lowest odds ratios (OR), variables were sequentially eliminated from model 1. If the deletion of a variable resulted in a decrease in the c-index of less than 0.005, then that variable was excluded. Excluded variables were reintroduced into the model at various stages of model development to reassess their contribution to the explanatory power of the model. If the reintroduction of a previously deleted variable resulted in an increase in the c-index of less than 0.005, then that variable was excluded. The resulting model was designated model 2.
Development of Scoring System
Using the methodology of Le Gall et al, 20 point values were assigned to each predictor by multiplying the β-coefficients from model 2 by 10 and rounding off to the nearest integer. The point total for each patient was designated as the respiratory failure risk index and was used in a multiple logistic regression equation designed to convert the risk index to a probability of PRF. The risk index scores were highly skewed, so a shrinking power transformation, ln (respiratory failure risk index + 1), where ln indicates the natural logarithm, was incorporated into the model. 20
Model Performance
To assess the calibration of the models, formal goodness-of-fit (Hosmer-Lemeshow) tests 21 were performed on patients from both phase I (development set) and phase II (validation set). The Hosmer-Lemeshow test compares the expected and observed events in 10 equal subgroups defined by the deciles of predicted probabilities of PRF. The inclusion of the shrinking power transformation resulted in improved model calibration, reflected by a statistically nonsignificant goodness-of-fit statistic.
The c-index was used in the two datasets to evaluate discrimination. The c-index is the proportion of all possible pairs of patients with and without PRF for which the predicted probability of PRF in the patient with PRF is greater than that of the patient without PRF. 22 A c-index of 0.5 means no predictability; a c-index of 1.0 means perfect predictability.
RESULTS
Patient Characteristics and PRF Rates
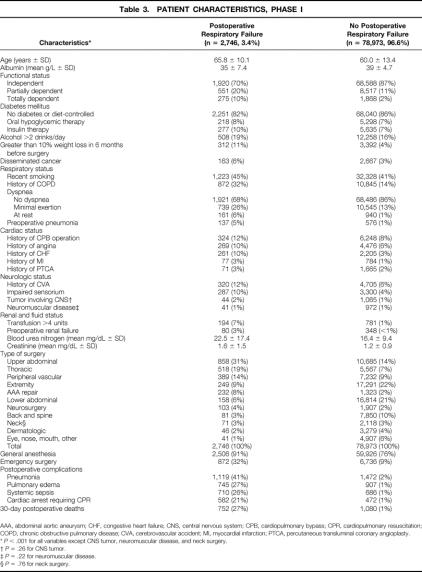
Of the 87,078 patients enrolled in phase I, 5,359 (6.2%) were excluded as noted above, leaving 81,719 for model development. Of the 107,241 patients enrolled in phase II, 7,851 (7.3%) were excluded, leaving 99,390 for model validation.
Table 3 displays the baseline characteristics of patients with and without PRF. There were 2,746 patients (3.4%) with PRF in phase I. Patients with PRF had a mean age of 65.8 ± 10.1 years versus 60.0 ± 13.4 years for those without PRF. In bivariate analysis, patients with PRF were significantly more likely to be classified as having dependent functional status and had lower baseline albumin levels. They were significantly more likely to report treatment for diabetes mellitus and to have a history of recent smoking, dyspnea, COPD, and preoperative pneumonia. Patients with PRF were significantly more likely to have characteristics related to cardiac, neurologic, and renal disease. Patients with PRF underwent significantly more upper abdominal, thoracic, peripheral vascular, abdominal aortic aneurysm repair, and neurosurgical operations than did patients without PRF. The most common postoperative complications associated with PRF included pneumonia, pulmonary edema, systemic sepsis, and cardiac arrest. Overall, the 30-day death rate was 27% in patients with PRF versus 1% in those without PRF.
Table 3. PATIENT CHARACTERISTICS, PHASE I
AAA, abdominal aortic aneurysm; CHF, congestive heart failure; CNS, central nervous system; CPB, cardiopulmonary bypass; CPR, cardiopulmonary resuscitation; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
*P < .001 for all variables except CNS tumor, neuromuscular disease, and neck surgery.
†P = .26 for CNS tumor.
‡P = .22 for neuromuscular disease.
§P = .76 for neck surgery.
Multivariable Analysis
It is possible that a significant proportion of the 1,080 patients who died and were classified in our analysis as not having PRF may have died within the first 48 hours after surgery while receiving mechanical ventilation. These early deaths represent potential cases of PRF that were unobserved (censored). To address this possibility, the number of deaths that occurred within 48 hours after surgery was determined. Of these 211 patients who died early, 39 had unplanned intubation and were included in our definition of PRF. The multivariable models were reanalyzed assuming that all the remaining 172 patients with early death would have developed PRF. The significant predictors and β-coefficients in these revised models were essentially identical to the original models; therefore, the results of the original models are presented.
Table 4 displays the results of the initial logistic regression model (model 1), which included all the significant preoperative predictors of PRF. Potential predictors that were excluded from model 1 included preoperative creatinine level, disseminated cancer, neuromuscular disease, central nervous system tumor, angina, myocardial infarction, angioplasty, and operation involving cardiopulmonary bypass. Significant predictors of PRF related to general health status included serum albumin level, age, dependent functional status, weight loss of greater than 10% in the 6 months before surgery, alcohol use, and insulin therapy for diabetes. The OR for developing PRF increased with decreasing serum albumin levels, especially with levels of less than 30 g/L. Increasing age was associated with increased odds of PRF, particularly for patients older than 60. Patients designated as having a totally dependent functional status had an OR of 2.2 (95% confidence interval [CI], 1.9–2.7) compared with those with an independent functional status; the OR was 1.5 (95% CI, 1.3–1.7) for those designated as having a partially dependent functional status.
Table 4. PREOPERATIVE PREDICTORS OF POSTOPERATIVE RESPIRATORY FAILURE (MODEL 1)
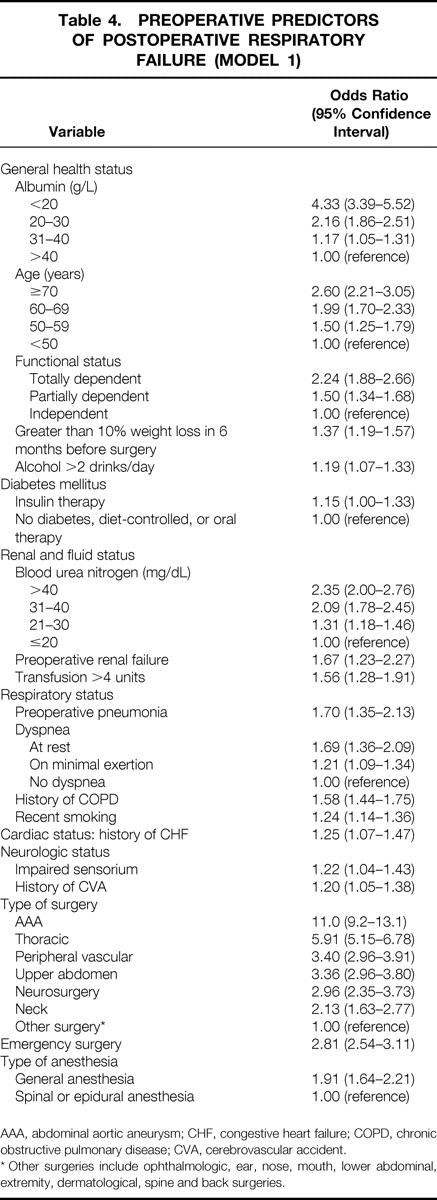
AAA, abdominal aortic aneurysm; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident.
* Other surgeries include ophthalmologic, ear, nose, mouth, lower abdominal, extremity, dermatological, spine and back surgeries.
Significant predictors related to renal and fluid status included BUN, preoperative renal failure, and preoperative transfusion of more than 4 units. Patients with a preoperative BUN of more than 40 mg/dL had an OR of 2.4 (95% CI, 2.0–2.8) compared with those with a BUN less than 20 mg/dL. Among the significant factors related to respiratory status, preoperative pneumonia had the highest OR, 1.7 (95% CI, 1.4–2.1). Among several potential predictors related to cardiac status, only a history of congestive heart failure was a significant predictor of PRF, with an OR of 1.3 (95% CI, 1.1–1.5). Impaired sensorium and a history of prior cerebrovascular accident with a residual neurologic deficit were the only factors related to neurologic status that were significant in multivariable analysis.
Operation-specific factors had the highest associated OR for developing PRF, particularly the type of surgery performed. The type of surgery performed was classified primarily by the location of the incision (Table 2). The reference group for type of surgery, designated as “other surgery,” included ophthalmologic, ear, nose, mouth, lower abdominal, extremity, dermatologic, spine, and back surgeries. These groups were chosen as the reference category because they had the lowest associated rates of PRF in univariate analysis. Patients undergoing surgery related to an abdominal aortic aneurysm had the greatest risk of developing PRF of any surgical category. Patients undergoing emergency surgery or surgery using general anesthesia were also found to have an increased risk of developing PRF.
Model 2 includes 11 predictors of PRF and is shown in Table 5. Patients undergoing abdominal aortic aneurysm surgery had an OR of 14.3 (95% CI, 12.0–16.9) compared with those undergoing “other surgery.” Patients undergoing thoracic surgery had an OR of 8.1 (95% CI, 7.2–9.3), and those undergoing neurosurgical, upper abdominal, or peripheral vascular surgery had an OR of 4.2 (95% CI, 3.8–4.7). Patients undergoing neck surgery had an OR of 3.1 (95% CI, 2.4–4.0), and patients undergoing emergency surgery had an OR of 3.1 (95% CI, 2.8–3.4). Patients with an albumin level less than 30 g/L had an OR of 2.5 (95% CI, 2.3–2.8), and patients with a BUN more than 30 mg/dL had an OR of 2.3 (95% CI, 2.0–2.6). The OR for patients with a partially or totally dependent functional status was 1.9 (95% CI, 1.7–2.1). For patients with a history of COPD, the OR was 1.8 (95% CI, 1.7–2.0). Compared with patients younger than 60, patients older than 70 had an OR of 1.9 (95% CI, 1.7–2.1), and patients 60 to 69 years old had an OR of 1.5 (95% CI, 1.4–1.7).
Table 5. PREOPERATIVE PREDICTORS OF POSTOPERATIVE RESPIRATORY FAILURE (MODEL 2)
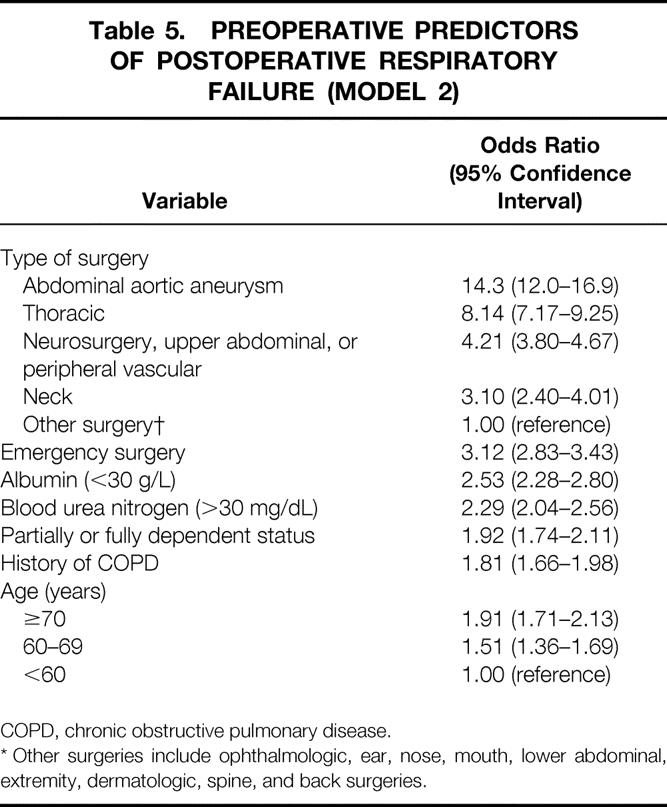
COPD, chronic obstructive pulmonary disease.
* Other surgeries include ophthalmologic, ear, nose, mouth, lower abdominal, extremity, dermatologic, spine, and back surgeries.
Model Calibration and Discrimination
Model 1 applied to patients from phase I resulted in a c-index of 0.846, indicating excellent discrimination. The Hosmer-Lemeshow goodness-of-fit statistic was 13.5 with 8 df (P > .095), indicating good fit of model 1 to the data. When model 1 was applied to patients from phase II, the c-index was essentially unchanged (c = 0.843) and the Hosmer-Lemeshow goodness-of-fit statistic was 8.16 with 8 df (P > .41), indicating that the model continued to have excellent discrimination and good fit in an independent dataset.
Model 2 had a slightly lower c-index than model 1 (c = 0.834). The Hosmer-Lemeshow goodness-of-fit statistic was 3.37 with 6 df (P > .76), indicating good fit of the model to the data. When model 2 was applied to phase II data, the c-index was essentially unchanged (c = 0.828) and the Hosmer-Lemeshow goodness-of-fit statistic was 0.97 with 6 df (P > .98), indicating that model 2 also had excellent discrimination and good fit in an independent dataset.
Respiratory Failure Risk Index
Point values were assigned to each predictor by multiplying the β-coefficients from model 2 by 10 and rounding off to the nearest integer. 20 Table 6 displays the point values assigned to each preoperative predictor used in calculating the respiratory failure risk index score. Based on the predicted probability associated with various scores, patients were categorized into five risk classes. Table 7 displays the number of phase I patients in each risk class, the associated predicted probability of PRF based on model 2, and the actual incidence of PRF in phase I and phase II patients.
Table 6. RESPIRATORY FAILURE RISK INDEX
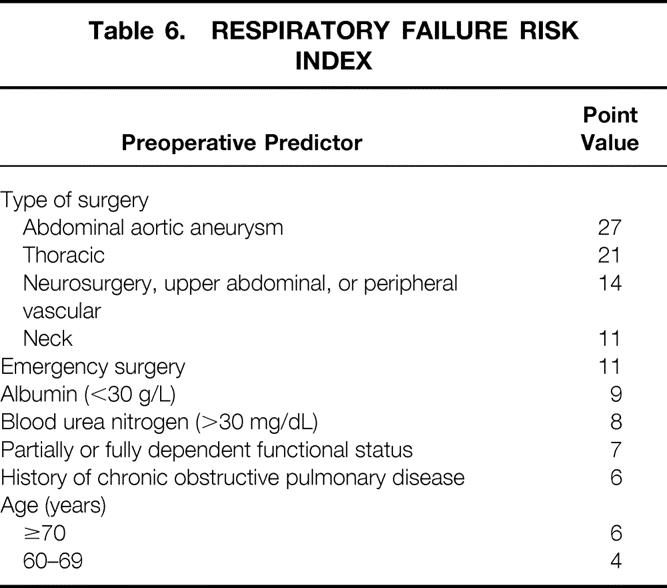
Table 7. RESPIRATORY FAILURE RISK INDEX SCORES FOR PHASE I AND PHASE II PATIENTS
PRF, postoperative respiratory failure.
* Number of phase I subjects in each risk class.
DISCUSSION
This study confirmed several preoperative predictors of PRF. Operation-specific risk factors, particularly the type of surgery, had the highest ORs. Prior studies have examined risk factors for PRF after high-risk operations, including abdominal aortic aneurysm repair, 6,7,9 thoracotomy, 2,10 colon resection, 3,5,10 and head and neck surgery. 4 All of these types of operations were associated with an increased risk of PRF in this study.
In some patients, the operation cannot be significantly modified, making operation-specific risk factors less reasonable targets for clinical intervention to decrease the rate of PRF. Prior studies consistently identified patient-specific risk factors similar to those in our respiratory failure risk index, such as age older than 60, 3,9,23 low albumin level, 5 renal insufficiency, 5–7 and prior history of COPD 9,10 or smoking. 4 The respiratory failure risk index points to several potentially treatable or correctable factors that may decrease the risk for PRF. Proper perioperative fluid management as reflected by a normal BUN, correction of possible malnutrition as reflected by a low albumin level, and performance of high-risk surgeries electively rather than waiting until an emergency surgery becomes necessary may all decrease the risk of PRF. Pulmonary rehabilitation, smoking cessation, and maximizing pharmacologic treatments to optimize the respiratory status of patients with COPD, asthma, and congestive heart failure may also decrease the risk for respiratory failure. Chumillas et al 24 found that preoperative and postoperative respiratory rehabilitation protected against postoperative pulmonary complications in moderate-risk and high-risk patients undergoing upper abdominal surgery.
The respiratory failure risk index provides a useful guide to evaluating preoperative risk for developing PRF. One major advantage of this study is that patient characteristics and outcomes were obtained prospectively with a level of clinical detail not found in administrative databases. Most prior studies examining risk factors for PRF depended on a retrospective review of administrative databases or medical chart review. 4,5,7,9,10 Missing data, inconsistencies in the use of variable definitions, and lack of clinical detail are some of the limitations encountered when using retrospectively obtained or administrative data. Another weakness of prior studies is the lack of validation of the models in independent datasets. 25,26 The NSQIP provided a unique opportunity to develop models on more than 80,000 patients and to validate the models on a separate set of more than 99,000 patients. The models developed in this study performed well in validation testing, maintaining almost all of their explanatory power.
The models developed in this study may be useful in future studies aimed at reducing PRF rates. In this study, we determined the preoperative risk factors for developing PRF. There are many intraoperative and postoperative factors that probably influence the risk of developing PRF as well, such as intraoperative ventilator and cardiovascular management, use of intravenous versus inhalation anesthetic maintenance, and use of intraoperative and early postoperative analgesia and sedation. Our models may be used to control for preoperative patient-specific and operation-specific risk factors in future studies designed to evaluate interventions in intraoperative and postoperative management. The models may also be used for case-mix adjustment in studies examining variation in PRF rates between hospitals.
The most common postoperative complications in phase I were postoperative pneumonia (3.6%), urinary tract infection (3.5%), and respiratory failure (3.4%). Notably, two of the top three postoperative complications were pulmonary complications. The 30-day death rate for patients with PRF was 27% versus 1% for patients without PRF. In contrast, cardiac arrest requiring cardiopulmonary resuscitation occurred in 1.5% of total patients; myocardial infarction occurred in only 0.7% of patients. The development and clinical use of preoperative cardiac risk assessment models 12–14 may have focused greater attention on maximizing cardiac status before surgery, as well as possible cancellation of surgery if the cardiac risk was deemed to be high. These results from the NSQIP raise concerns that similar attention needs to be given to postoperative pulmonary complications.
Some controversy exists over the best definition for PRF. PRF is most commonly defined as the inability to be extubated 48 hours after surgery, 6 although other investigators have used 5 days after surgery as their definition. 7 The definition of PRF used in this study was a combination of two separately measured outcomes from the NSQIP: unplanned intubation and inability to be extubated 48 hours after surgery. Thirty-seven percent of patients with PRF had the inability to be extubated, 29% had unplanned intubation, and 34% had both. For all three groups, the most commonly associated postoperative complications were pneumonia, pulmonary edema, systemic sepsis, and cardiac arrest. The 30-day death rate was 31% for reintubation patients and 23% for patients with the inability to be extubated. Initially, logistic regression models were developed for each of these groups separately. The significant predictors and β-coefficients in these initial models were essentially identical, and therefore these groups were combined.
There are several limitations to our study. Because veterans cared for at VAMCs have a high level of comorbid conditions, these models may not generalize to other, healthier populations. Another limitation was that women were excluded because of the caseload of the source hospitals. Patient-specific factors such as age and albumin level are likely to be relevant in women, but the associated ORs may be different. Future studies are needed to validate these models in other populations including women.
Another limitation was that other potentially important preoperative risk factors for PRF were not included in the NSQIP. During the planning stages of the NSQIP, a pilot study examining the feasibility of including pulmonary function test results revealed that it would be extremely difficult to obtain reliable information consistently across multiple institutions. The lack of pulmonary function test data did not allow assessment of the severity of underlying pulmonary diseases such as asthma, COPD, or restrictive lung disease. The definition of COPD used in the NSQIP was fairly broad and may have resulted in an underestimation of the risk associated with severe COPD. Body mass index, which has been shown to be associated with respiratory failure, was not recorded in the NSQIP. 3 Therefore, the respiratory failure risk index does not include an assessment of the importance of obesity or increased body mass index as risk factors for PRF.
Despite these limitations, the respiratory failure risk index may be helpful to clinicians and researchers in targeting perioperative testing and respiratory care to high-risk patients. Prior studies have been limited to patients undergoing specific types of operations 2–7,9,10 or patients with particular risk factors. 1,10 The respiratory failure risk index is unique in that it includes several patient-specific and operation-specific risk factors simultaneously, allowing for an accurate assessment of the preoperative risk of PRF associated with each individual risk factor. We found that the type of surgery performed has the highest associated risk for developing PRF and that the major patient-specific risk factors are related to general health status, renal and fluid status, and respiratory status. We hope that an increased awareness of the importance of postoperative pulmonary complications will develop through the clinical use of the respiratory failure risk index. We also hope that by using the models developed in this study, researchers will be able to evaluate future interventions aimed at reducing the rate of PRF.
APPENDIX
Chairperson’s Office: Shukri F. Khuri, MD (Chairperson); Jennifer Daley, MD (Co-Chairperson); Maureen Forbes, MS, RN, MPH (Health Services Researcher); Ellen M. Ciambriello (Program Assistant); Lynn-Marie Herlihy, BA (Program Assistant); Candace Savage (National Administrative Coordinator); Jeannette Spencer, RN, MS, CS (National Clinical Coordinator). Hines Center for Cooperative Studies in Health Services: John Demakis, MD, and William Henderson, PhD (Codirectors); James Gibbs, PhD (Health Services Researcher); Kwan Hur, MS (Biostatistician); Bharat Thakkar (Statistical Programmer); Robbin Denwood, RN, MSN, MBA (Data Coordinator); Sharon Urbanski (Statistical Assistant). Traveling Nurse Coordinators: Nancy Deegan, MSN, RN, San Antonio, TX; Jeannette Spencer, RN, MS, CS, Brockton/West Roxbury, MA; Debra Wilcox, MSN, RN, Denver, CO. VA Central Office: Galen Barbour, MD (AsCMD for Quality Management); Scott Beck, ME, CPQA (Special Assistant to AsCMD for Quality Management); Daniel Deykin, MD (Chief, Cooperative Studies Program, and Director, Health Services R&D); Gerald McDonald, MD (Deputy Chief of Surgery). Executive Committee: Shukri F. Khuri, MD (Chairperson), Brockton/West Roxbury, MA; Jennifer Daley, MD (Co-Chairperson), Brockton/West Roxbury, MA; J. Bradley Aust, MD, San Antonio, TX; Scott Beck, ME, CPQA, Washington DC; John Demakis, MD, Hines, IL; Peter J. Fabri, MD, Tampa, FL; James Gibbs, PhD, Hines, IL; Frederick Gover, MD, Denver, CO; Karl Hammermeister, MD, Denver, CO; William Henderson, PhD, Hines, IL; George L. Irvin III, MD, Miami, FL; Gerald McDonald, MD, Washington DC; Edward Passaro, Jr., MD, West Los Angeles, CA; Frank Scamman, MD, Director, National Anesthesia Service, Iowa City, IA; Jeannette Spencer, RN, MS, Brockton/West Roxbury, MA; John Stremple, MD, Pittsburgh, PA.
Expert Advisory Committee: Barbara McNeil, MD, PhD (Chairperson), Boston, MA; J. Bradley Aust, MD, San Antonio, TX; Paul Ebert, MD, Chicago, IL; Frank Harrell, PhD, Durham, NC; Lisa Iezzoni, MD, Boston, MA; John Mannick, MD, Boston, MA; L. Richard Smith, PhD, Durham, NC; J. William Thomas, PhD, Ann Arbor, MI.
Birmingham Information Systems Center: Alan Monoscky (Computer Specialist); Michael Montali (Senior Systems Analyst); Steve Musgrove (Computer Specialist). St. Louis Continuing Education Center: Donna Schoonover, RN, MSN, CS (Program Director). Durham Regional Medical Education Center: Linda Exner, MS, RN (Chief, Special Projects).
Current Participating VA Medical Centers, Chiefs of Surgery, and Surgical Clinical Nurse Reviewers. Albany, NY: Thomas K. Wu, MD; George A. Leamy, RN;Albuquerque, NM: Stuart Ford, MD; Tony L. Lantzer, BSN;Alexandria, LA: Hollis Reed, MD; Carol Rowe, BSN;Altoona, PA: Akbar M. Samii, MD; Phyllis Podrasky, RN;Amarillo, TX: Douglas Stephenson, DO; Sheree Keil, RN;Ann Arbor, MI: John F. Sweeney, MD; Linda S. Brooks, RN;Ashville, NC: Thomas J. Berger, MD; Marge Turcot, RN;Atlanta (Decatur), GA: Aaron S. Fink, MD; Renee Lawrence, RN;Augusta, GA: George I. Cue, MD; Connie Miller, RN;Baltimore, MD: Barbara Bass, MD; Nancy P. Specht, RN, BSN, MAS;Bay Pines, FL: Terry Wright, MD; Judith M. Girard, RN;Beckley, WV: Georges A. Hoche, MD; Pam Johnson;Big Spring, TX: Gaddum Reddy, MD; Jennan Swafford, RNC, MSN;Biloxi, MS: Larry Fontenelle, MD; Donna Wells, RN;Birmingham, AL: John J. Gleysteen, MD; Linda Helm-Little, RN;Boise, ID: Ernest C. Peterson, MD; Launa J. Nardella, BSN;Boston, MA: Willard Johnson, MD; Laura McDonald RN, BSN;Bronx, NY: A. James McElhinney, MD; Elias Enriquez, RN;Brooklyn, NY: Bimal C. Ghosh, MD, Wendy R. Trimboli, BSN, MA, CPHQ;Buffalo, NY: Irineo Gutierrez, MD; Mary Ann Blake, RN, MSN;Castle Point, NY: A. James McElhinney, MD; Barbara Powers, RN;Charleston, SC: John Allison, MD; Stephen E. Johnston, RN, MSN, CNA;Cheyenne, WY: D. Michael Kilpatrick, MD; Nina J. Pike, RN, BSN;Chicago (Lakeside), IL: Robert V. Rege, MD; Denise Ostrowski, RN;Chicago (Westside), IL: Donald K. Wood, MD; Carbena Daniels, RN;Cincinnati, OH: Robert A. Bower, MD; Elaine Hardin, RN;Clarksburg, WV: Juanito V. Chua, MD; Lisa R. Michael, RN;Cleveland, OH: John Raaf, MD; Mary Ann Bobulsky RN, BSN;Columbia, MO: Debra Koivunen, MD; Barbara Von Thun, RN, MSN;Columbia, SC: John Jeffrey Brown, MD; Joanne K. Ogg, RN, MN, CS;Dallas, TX: Richard H. Turnage, MD; Bernice Willis, RN;Danville, IL: Jin Kim, MD; David Lohnes, RN, BSN;Dayton, OH: Samuel A. Aderonojo, MD; Shirley Ribak, RN, MSN;Denver, CO: Frederick L. Grover, MD; Donna LoSaso, RN;Des Moines, IA: David Sidney, MD; Cathy S. Sandle, RN, BSN;Detroit, MI: Robert Kozol, MD; Barbara L. Bieke, RN, MSN;Dublin, GA: Noel Nellis, MD; Teresa Fagan, RN;Durham, NC: Theodore Pappas, MD; C. Jean Hanchey, RN;East Orange, NJ: Frank E. Gump, MD; Anna T. Detschel, RN;Erie, PA: Prabhu Negi, MD; Denise Albertson, RN;Fargo, ND: Mark O. Jensen, MD; Priscilla K. Stroh, RN;Fayetteville, AR: Pat O’Donnell, MD; Carol Wolgamott, RN;Fayetteville, NC: Arthur McGuire, MD; Nancy Albaladejo, RN, MSA, CNA;Fort Harrison, MT: Michael Agee, MD; Edna L. Clausen, BSN;Fort Meade, SD: Mark F. Blum, MD; Teresa Gabeline, RN;Fort Wayne, IN: Sun Guo, MD;Fresno, CA: Gregory Wille, MD; Elena J. Eaton, RN, BSN;Gainesville, FL: Timothy Flynn, MD; Linda D. Carter, RN;Grand Island, NE: Danitsu Hirar, MD; Cynthia E. Hansen, RN;Grand Junction, CO: Earl Howells, MD; Karen L. Rogers, RN, BA;Hampton, VA: Ali Farpour, MD; Sol F. Aquinaldo, RN, BSN;Hines, IL: Charles H. Andrus, MD, FACS; Kristine L. Johnson, RN, BSN, BA, BS;Hot Springs, SD: Mark F. Blum, MD; Marcia Bishop, RRA;Houston, TX: James W. Jones, MD; Barbara J. Anderson, LPN; Clara Kistner, RN;Huntington, WV: Timothy Canterbury, MD; Rena K. Black, RN;Indianapolis, IN: Dolores Cikirt, MD; Connie Adams, RN;Iowa City, IA: Barcellos Winston, MD; Isabelle A. Olson, RN;Iron Mountain, MI: Robert L. Alexander, MD; Terri M. Danielson, RN, ADN;Jackson, MS: Kenneth Simon, MD; Shiela Ann Buck, RN, BSN;Kansas City, MO: Mary McAnew, MD, and Betty Drees, MD; Becky Ganaban, RN, Berta Graves, RN;Kerrville, TX: Mauro Gangai, MD; Theresa Rangel, BS, MA, EdPsy;Lake City, FL: Juan R. Baralat, MD; Charlotte L. Lintz, RN, MSN;Leavenworth, KS: Chris C. Haller, MD; Mary Lee Driscoll, RN, BSN, CNOR;Lebanon, PA: Peter Mucha, Jr., MD; Vicki L. Leibich, LPN;Lexington, KY: Thomas Schwarcz, MD; Rose Mary Collins, RN;Lincoln, NE: Danitsu Hirar, MD; Judy L. Sanne, RN;Little Rock, AR: Nicholas P. Lang, MD; Richard D. Bloesch, RN;Loma Linda, CA: Gerrold Longerbeam, MD; Gillian Gomulka, RN;Long Beach, CA: Edward A. Stemmer, MD; Delores Whalen, RN;Louisville, KY: Richard Neal Garrison, MD; Ruth A. Meadows, RN;Madison, WI: James Starling, MD; Kathy Gruber, RN;Manchester, NH: Willard Johnson, MD; Patricia M. Stevens, RN, BSN;Marion, IL: Rama Iyengar, MD; Jane A. Hale, RN;Martinez, CA: Pauline Velez, MD; Marytess Baula, RN;Martinsburg, WV: C. R. Kamath, MD; Jeffrey B. Spoon, PA, BS;Memphis, TN: Eugene Mangiante, MD; Anita L. Garrison, RN;Miami, FL: A. J. Furst, MD; Nancy Box, RN;Milwaukee, WI: Charles Aprahamian, MD; Christine A. Tyler, RN, BSN;Minneapolis, MN: Donald G. McQuarrie, MD; Jane Bonawitz-Conlin, RN;Montgomery, AL: Eddie Warren, MD; Jeulia E. Hendrick, RN, BSN, MS;Mountain Home, TN: David Walters, MD; Joyce F. Hamm, RN, BSN;Murfreesboro, TN: Rudolph Comberbatch, MD, FACS, Margaret Cantrell, RN;Muskogee, OK: Glenn Lytle, MD; Terry Maycher, RN;Nashville, TN: Walter H. Merrill, MD; Jeanette B. Pujol, BSN, RNC;New Orleans, LA: Paul R. Hastings, MD, FACS; Donna M. Gray, RNC, CCRN;New York, NY: Alex C. Solowey, MD; Jacqueline H. Parker, RN, BSN;North Chicago, IL: B. F. Kepley, MD; Kathleen Mega, BSN;Northport, NY: Eugene P. Mohan, MD; Sheila Dahl, RN, MSN;Oklahoma City, OK: Donald R. Carter, MD; Rouchelle Osborn, RN, MS;Omaha, NE: Thomas Lynch, MD; Sharon M. Tighe, RN;Palo Alto, CA: Thomas Burdon, MD; Jacie Epperson, RN, BSN;Philadelphia, PA: Steven Raper, MD; Miriam S. Moskowitz, RN, MSN;Phoenix, AZ: Gerald Schmitz, MD, FACS; Seaton West, RN;Pittsburgh, PA: John Stremple, MD; Susan Layne, RN;Portland, OR: Cliff Deveney, MD; Elizabeth McCollum, RN;Providence, RI: Michael P. Vezeridis, MD; Carol Maynard, RN;Reno, NV: Ralph DePalma, MD; Jerylann E. Gale, RN;Richmond, VA: Hunter Holmes McGuire, Jr., MD; Gail Laub, RN;Roseburg, OR: Norman Marshall, MD; Dennis Morehouse, RN;Saginaw, MI: Isa Salti, MD;St. Louis, MO: Frank E. Johnson, MD; Mary Louise Smith, RN, MSN;Salem, VA: Wayne H. Wilson, MD; Rebecca P. Evans, RN, BSN;Salisbury, NC: Barbara Temeck, MD; Lisa H. Noonan, RN, BSN, CPHQ;Salt Lake City, UT: Leigh Neumayer, MD; Sandy McMaster, RN;San Antonio, TX: O. LaWayne Miller, MD; Linda M. Porazzi, RN, MSN, CNS;San Diego, CA: Nicholas A. Halasz, MD; Gail P. Maxwell, RN, BA;San Francisco, CA: Jeffrey Norton, MD; Rita J. Sears, RN, MS;San Juan, PR: Ernesto Rive-Mora, MD; Saribelle Reyes-Frau, RN, BSN;Seattle, WA: Richard Bell, MD; Julie K. Kieras, RN, BSN;Sepulveda, CA: Howard Reber, MD; Betty Wright, RN;Shreveport, LA: James Evans, MD; Lillian Thornhill, RN;Sioux Falls, SD: John Ryan, MD; Becky Poss, RN;Spokane, WA: Meredith Richmond, MD; Shelly Sumner, RHIP, CPIM;Syracuse, NY: Michael Sobel, MD; John E. LeBeau, RN;Tacoma, WA: Richard Bell, MD; Julie K. Kieras, RN, BSN;Tampa, FL: Peter J. Fabri, MD; Kathryn S. Bowns, RN;Temple, TX: P. Pandya, MD; Carolyn Broussard, RN;Togus, ME: Martyn Vickers, MD; Patricia A. Wotton, RN;Topeka, KS: C. N. Radhakrishna, MD; Allen Zander, RN;Tucson, AZ: Martin L. Dresner, MD; Christopher R. Brown, RN, BSN;Tuskegee, AL: Eddie Warren, MD; Joice Promisee, RN, BSN;Washington, DC: John Harmon, MD; Deborah T. Fleming, RN, BSN;West Haven, CT: Barbara Kinder, MD; Kathy Maher-Cleary, RN;West Los Angeles, CA: Edward Livingston, MD; Marilyn DeGroot, RN;West Palm Beach, FL: James Schell, MD; Rosa Caraballo, RN;West Roxbury, MA: Shukri Khuri, MD; Jeannette Spencer, RN, MS, CS;White River Junction, VT: Martha McDaniel, MD; Lisa Ryder, RN;Wichita, KS: Joseph K. Robertson, MD; Stephanie Lentz, RN;Wilkes-Barre, PA: Feroz Sheikh, MD; Beth A. Chaken, RN, MSN;Wilmington, DE: Claude Lieber, MD; Evie Logue, RN.
Footnotes
Correspondence: Ahsan M. Arozullah, MD, MPH, University of Illinois at Chicago, Section of General Internal Medicine (M/C 787), 840 S. Wood St., Chicago, IL 60612.
The National Veterans Administration Surgical Quality Improvement Program was funded by the Office of Patient Care Services and the Health Services Research and Development Service of the Department of Veterans Affairs.
Dr. Arozullah was an Ambulatory Care Fellow of the Department of Veterans Affairs during this project. Dr. Daley is a Senior Research Associate in the Career Development Award Program of the Veterans Affairs Health Services Research and Development Service.
E-mail: arozulla@uic.edu
Accepted for publication February 2, 2000.
References
- 1.Wong DH, Weber EC, Schell MJ, et al. Factors associated with postoperative pulmonary complications in patients with severe chronic obstructive pulmonary disease. Anesth Analg 1995; 80: 276–284. [DOI] [PubMed] [Google Scholar]
- 2.Dales RE, Dionne G, Leech JA, Lunau M, Schweitzer I. Preoperative prediction of pulmonary complications following thoracic surgery. Chest 1993; 104: 155–159. [DOI] [PubMed] [Google Scholar]
- 3.Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest 1997; 111: 564–571. [DOI] [PubMed] [Google Scholar]
- 4.McCulloch TM, Jensen NF, Girod DA, Tsue TT, Weymuller EA. Risk factors for pulmonary complications in the postoperative head and neck surgery patient. Head and Neck 1997; 19: 372–377. [DOI] [PubMed] [Google Scholar]
- 5.Ondrula DP, Nelson RL, Prasad ML, Coyle BW, Abcarian H. Multifactorial index of preoperative risk factors in colon resections. Dis Colon Rectum 1992; 35: 117–122. [DOI] [PubMed] [Google Scholar]
- 6.Svensson LG, Hess KR, Coselli JS, Safi HJ, Crawford ES. A prospective study of respiratory failure after high-risk surgery on the thoracoabdominal aorta. J Vasc Surg 1991; 14: 271–282. [PubMed] [Google Scholar]
- 7.Money SR, Rice K, Crockett D, et al. Risk of respiratory failure after repair of thoracoabdominal aortic aneurysms. Am J Surg 1994; 168: 152–155. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell CK, Smoger SH, Pfeifer MP, et al. Multivariate analysis of factors associated with postoperative pulmonary complications following general elective surgery. Arch Surg 1998; 133: 194—198. [DOI] [PubMed] [Google Scholar]
- 9.Calligaro KD, Azurin DJ, Dougherty MJ, et al. Pulmonary risk factors of elective abdominal aortic surgery. J Vasc Surg 1993; 18: 914–921. [PubMed] [Google Scholar]
- 10.Kroenke K, Lawrence VA, Theroux JF, Tuley MR, Hilsenbeck S. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest 1993; 104: 1445–1451. [DOI] [PubMed] [Google Scholar]
- 11.Ebert JP, Grimes B, Niemann KMW. Respiratory failure secondary to homologous blood transfusion. Anesthesiology 1985; 63: 104–106. [DOI] [PubMed] [Google Scholar]
- 12.Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 1977; 297: 845–850. [DOI] [PubMed] [Google Scholar]
- 13.Palda VA, Detsky AS. Perioperative assessment and management of risk from coronary artery disease. Ann Intern Med 1997; 127: 313–328. [DOI] [PubMed] [Google Scholar]
- 14.Eagle KA, Brundage BH, Chaitman BR, et al. Guidelines for perioperative cardiovascular evaluation for noncardiac surgery: an abridged version of the report of the American College of Cardiology/American Heart Association task force on practice guidelines. Mayo Clin Proc 1997; 72: 524–531. [DOI] [PubMed] [Google Scholar]
- 15.Khuri SF, Daley J, Henderson W, et al, for the National Veterans Administration Surgical Risk Study. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg 1995; 180: 519–531. [PubMed] [Google Scholar]
- 16.Khuri SF, Daley J, Henderson W, et al, for the National Veterans Administration Surgical Risk Study. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg 1997; 185: 315–327. [PubMed] [Google Scholar]
- 17.Daley J, Khuri SF, Henderson W, et al, for the National Veterans Administration Surgical Risk Study. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg 1997; 185: 328–340. [PubMed] [Google Scholar]
- 18.National Veterans Affairs Surgical Risk Adjustment Study, Non-Cardiac. Data Managers’ Terms, Definitions, Revised Sept. 5, 1991.
- 19.Roberts JS, Capalbo GM. A SAS macro for estimating missing values in multivariate data. In: Proceedings of the 12th Annual SAS User Group International Conference, Feb. 8–11, 1987, Dallas, TX; 1987: 939–941.
- 20.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270: 2957–2963. [DOI] [PubMed] [Google Scholar]
- 21.Lemeshow S, Hosmer DW. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiology 1982; 115: 92–106. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143: 29–36. [DOI] [PubMed] [Google Scholar]
- 23.Hall JC, Tarala RA, Hall JL, et al. A multivariate analysis of the risk of pulmonary complications after laparotomy. Chest 1991; 99: 923–927. [DOI] [PubMed] [Google Scholar]
- 24.Chumillas S, Ponce JL, Delgado F, Viciano V, Mateu M. Prevention of postoperative pulmonary complications through respiratory rehabilitation: a controlled clinical study. Arch Phys Med Rehabil 1998; 79: 5–9. [DOI] [PubMed] [Google Scholar]
- 25.Brooks-Brunn JA. Validation of a predictive model for postoperative pulmonary complications. Heart Lung 1998; 27: 151–158. [DOI] [PubMed] [Google Scholar]
- 26.Melendez JA, Carlon VA. Cardiopulmonary risk index does not predict complications after thoracic surgery. Chest 1998; 114: 69–75. [DOI] [PubMed] [Google Scholar]