Abstract
Objective
To compare the safety and efficacy of intravenous (IV) ciprofloxacin plus IV metronidazole (CIP+MET) with that of IV piperacillin/tazobactam (PIP/TAZO) in adults with complicated intraabdominal infections, and to compare the efficacy of sequential IV-to-oral CIP+MET therapy with that of the IV CIP-only regimen.
Summary Background Data
Treatment of intraabdominal infections remains a challenge, mainly because of their polymicrobial etiology and attendant death and complications. Antimicrobial regimens using sequential IV-to-oral therapy may reduce the length of hospital stay.
Methods
In this multicenter, randomized, double-blind trial involving 459 patients, clinically improved IV-treated patients were switched to oral therapy after 48 hours. Overall clinical response was the primary efficacy measurement.
Results
A total of 282 patients (151 CIP+MET, 131 PIP/TAZO) were valid for efficacy. Of these patients, 64% CIP+MET and 57% PIP/TAZO patients were considered candidates for oral therapy. Patients had a mean APACHE II score of 9.6. The most common diagnoses were appendicitis (33%), other intraabdominal infection (29%), and abscess (25%). Overall clinical resolution rates were statistically superior for CIP+MET (74%) compared with PIP/TAZO (63%). Corresponding rates in the subgroup suitable for oral therapy were 85% for CIP+MET and 70% for PIP/TAZO. Postsurgical wound infection rates were significantly lower in CIP+MET (11%) versus PIP/TAZO patients (19%). Mean length of stay was 14 days for CIP+MET and 17 days for PIP/TAZO patients.
Conclusion
CIP+MET, initially administered IV and followed by CIP+MET oral therapy, was clinically more effective than IV PIP/TAZO for the treatment of patients with complicated intraabdominal infections.
The treatment of intraabdominal infection is challenging because of their polymicrobial nature 1–3 and accompanying death and complications. Treatment may be further complicated by bacteriologic resistance issues, such as the large number of β-lactamase-producing Enterobacteriaceae present in intraabdominal infections. For most episodes, antimicrobial regimens usually involve combination therapy, 4–11 although several monotherapy approaches have been studied. 5,6,12–15 Ciprofloxacin (CIP), a 1-cyclopropyl fluoroquinolone, has excellent activity against aerobic gram-negative facultative microbes commonly associated with intraabdominal infections 16,17 and also penetrates well into the peritoneal cavity. 18,19 In this study, CIP was used in combination with metronidazole (MET), which has proven efficacy in the treatment of intraabdominal infections. 13 One important advantage of CIP+MET combination therapy over conventional antibiotic regimens is that it permits patients to be switched from intravenous (IV) to oral therapy, thus maintaining the same antibiotic class and providing consistent susceptibility. Because of the excellent bioavailability of CIP (70%), 20 oral CIP 500 mg twice daily is bioequivalent to 400 mg of intravenous CIP q12h. 19 Treatment of serious abdominal infections with oral therapy can allow recovery at home, potentially lowering the risk of acquiring a nosocomial or IV catheter-related complication. Obviously, limiting the duration of hospital stay and also antimicrobial costs is desirable in the current healthcare environment.
The primary objective of this study was to compare the efficacy of IV CIP+MET with IV piperacillin/tazobactam (PIP/TAZO) for the treatment of complicated intraabdominal infections. A secondary goal was to compare the efficacy of IV CIP+MET alone with sequential IV-to-oral CIP+MET therapy. We hypothesized that CIP+MET would provide equivalent clinical resolution to the PIP/TAZO regimen.
METHODS
Study Design and Enrollment Criteria
This prospective, randomized, double-blind, multicenter clinical trial was conducted between September 1995 and May 1997. The protocol was approved by the institutional review board of each collaborating institution. Inpatients at least 18 years old with complicated intraabdominal infection requiring surgical intervention or percutaneous drainage in addition to parenteral antibiotics were eligible for entry into the study. Major reasons for exclusion from this trial included allergy, renal insufficiency, an indwelling peritoneal catheter, ascites with spontaneous bacterial peritonitis, abdominal infection secondary to acute pancreatitis, perforated peptic ulcer or traumatic upper gastrointestinal tract perforation of less than 24 hours’ duration, and lower gastrointestinal tract perforation of less than 12 hours’ duration. Patients were also excluded if their Acute Physiology and Chronic Health Evaluation (APACHE) II score was more than 30, if they were not expected to survive 48 hours, and if they had been given prior antibiotic therapy for this intraabdominal infection episode for 24 hours. Pregnant women or women who were breast-feeding were also excluded.
Randomization and Treatment Regimen
Once patients provided written informed consent, a pretherapy medical history was taken and a physical examination performed. Clinically relevant radiologic procedures were obtained, blood and urine samples were taken, and an APACHE II score was calculated. Specimens for culture and Gram stain were obtained during the surgical procedure. Patients with susceptible bacterial pathogens were assessed for microbiologic response. Susceptibility of isolated and identified aerobic and anaerobic organisms was determined using standard methods. 21,22
On enrollment, patients were stratified by the presence or absence of appendicitis. Patients were then randomized to receive either IV CIP (400 mg q12h) plus MET (500 mg q6h) or IV PIP/TAZO (3.375 g q6h). CIP was provided as Cipro IV or tablets (Bayer Corp., Pharmaceutical Division, West Haven, CT); MET was provided as an IV solution or as tablets (Lemmon Co., Kansas City, MO, or Rhone-Poulenc Rorer, Collegeville, PA); and PIP/TAZO ( Zosyn [United States], or Tazocin [Canada], Lederle Laboratories, Pearl River, NY) was provided as an IV solution. Patients were randomized to receive the study drug either before surgery or after postoperative confirmation of infection.
After 48 hours of IV therapy, the patient was switched to oral therapy if the investigator determined that the patient had a functioning gastrointestinal tract and had clinically improved. Patients receiving CIP+MET IV were given CIP+MET orally and an IV placebo infusion to maintain the blind. Patients not switched to an oral regimen continued to receive CIP+MET IV. Patients assigned to the PIP/TAZO IV therapy continued on this regimen if they were not considered candidates for oral therapy. However, if they were considered candidates for oral therapy, CIP+MET oral placebo was given to maintain the blind along with PIP/TAZO IV.
If antibiotics were still required at the time of hospital discharge, at the discretion of the physician, patients were prescribed antibiotics in an unmasked fashion. However, the overall treatment with antibiotics was not to exceed 14 days from the start of therapy.
Patients receiving at least one dose of a study drug were included in the intent-to-treat (safety) population. Safety of the study drug treatments was monitored by clinical and laboratory evaluation. Adverse events were classified subjectively by the investigator using his or her clinical expertise according to severity (mild, moderate, severe) and relation to the study drug (probable, possible, remote, none or not assessable).
Efficacy-Valid Criteria
To be considered for the efficacy-valid population, the following criteria were necessary: satisfied the aforementioned inclusion/exclusion criteria; study drug administered for at least 3 days for patients with appendicitis and 5 days for all other diagnoses, unless the patient failed to respond to treatment; and all predetermined clinical evaluations (if applicable) were completed. The microbiologically valid population included patients with a pretherapy intraabdominal pathogen. In addition, organisms had to have been isolated 24 hours before enrollment or within 48 hours of starting the study drug and had to be susceptible to both CIP and PIP/TAZO. Microbiologically valid patients could not have received concomitant antibacterial agents during the study or the posttherapy follow-up period, and all predetermined follow-up cultures had to be obtained, if clinically indicated. Patients were not excluded if they received a narrow-spectrum or nonsystemic antibiotic for an infection at a separate site.
Clinical Response
Patients were evaluated for clinical response by the investigator on the day therapy was switched from IV to oral therapy, on the day of hospital discharge (end of masked study drug therapy), and at the end of unmasked study drug therapy. Clinical responses were defined as follows:
Resolution: disappearance of signs and symptoms associated with an intraabdominal infection
Improvement: significant improvement in signs and symptoms
Failure: worsening or no significant change in the signs and symptoms
Indeterminate: patient died of another underlying condition, an inadequate surgical procedure, or no evaluation was completed.
A clinical evaluation was also completed at the 3- to 5-week posttherapy follow-up visit (1–3 weeks for appendicitis) and categorized as continued resolution (continued absence of signs and symptoms), relapse (return or worsening of signs and symptoms), or indeterminate (previous definitions not applicable).
The primary efficacy variable was defined as the proportion of efficacy-valid patients having an overall clinical response of resolution at both end of therapy and follow-up. The overall clinical response was defined as the worst evaluation obtained at either the end of therapy or the posttherapy follow-up visit. In addition, the response was considered an overall clinical failure if a wound infection, regardless of clinical response, developed at any time during therapy through the 3- to 5-week posttherapy follow-up visit.
Bacteriologic Response
The bacteriologic response for patients with a pretherapy intraabdominal infection was defined as follows:
Eradication: no organisms present
Presumed eradication: not possible to obtain material for culture from a patient who had responded clinically to therapy
Persistence: original causative organism still present
Presumed Persistence: not possible to obtain material for culture from a patient who had not responded clinically to therapy
Indeterminate: no bacterial evaluation possible, including organisms that were resistant to the study drug.
Superinfection was defined as the isolation of a new organism during the treatment period at a site other than the primary site of infection (excluding wound infection).
Using these definitions, bacteriologic success at the end of therapy was defined as eradication plus presumed eradication versus persistence. At posttherapy follow-up, bacteriologic success was defined as eradication versus eradication with relapse, and eradication versus eradication with reinfection.
Postsurgical Wound Infections
Investigators subjectively recorded postoperative wound infections based on their clinical experience as purulent, superficial cellulitis, or deep wound infection at the primary surgical sites. In addition, the type of wound closure used (i.e., primary [wound closed at time of surgery], delayed primary [attempt made to close wound 72–96 hours after surgery], or secondary [wound allowed to close on its own]) was also recorded.
Statistical Analysis
For demographic and baseline medical characteristics, a chi-square procedure was used to test for differences between groups for the categorical variables. For continuous variables, a one-way analysis of variance model, with a term for treatment, was used.
Two-sided 95% confidence intervals (CI) were constructed to detect differences in clinical and bacteriologic response rates between treatment groups. A Mantel-Haenszel weighting procedure was used, weighted by stratum.
With the sample size enrolled and the actual success rates of the study drugs, for the total efficacy-valid population the study had 90% power to detect equivalence and 65% power to detect equivalence in the IV-to-oral therapy subgroup (∝ = 0.025, one-sided). The maximal allowable difference between the success rates of the two treatment groups to determine equivalence was 20%.
RESULTS
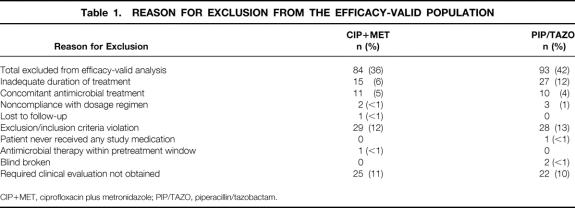
Four hundred fifty-nine patients were enrolled from 34 centers in the United States and Canada (235 CIP+MET IV, 224 PIP/TAZO IV). All patients (except one from the PIP/TAZO group who failed to receive study medication) were valid for safety. One hundred fifty-one (64%) patients who received CIP+MET and 131 patients who received PIP/TAZO (58%) were valid for efficacy. The primary reasons for the exclusion of the 177 patients were entry criteria violation and incomplete clinical evaluations (Table 1).
Table 1. REASON FOR EXCLUSION FROM THE EFFICACY-VALID POPULATION
CIP+MET, ciprofloxacin plus metronidazole; PIP/TAZO, piperacillin/tazobactam.
Premature discontinuation of study drug for any reason was similar in both groups: 34% for the PIP/TAZO group, 28% for the CIP+MET group (P = .120). Discontinuations resulting from adverse events (6% CIP+MET, 7% PIP/TAZO) and insufficient therapeutic effect (8% each group), the primary reasons for early study drug withdrawal, were similar between the two groups.
Demographic Information
For the efficacy-valid population, the two treatment groups were similar with respect to demographic (Table 2) and intraabdominal infection characteristics (Table 3). Appendicitis was the most common diagnosis. The APACHE II scores of the patients with appendicitis were lower, indicating less severe illness, than the scores for all patients together. The demographic and baseline infection characteristics for patients valid for safety were similar to those of the patients valid for efficacy, with no significant differences in any categories (data not shown). The proportions of patients with septicemia in the two treatment groups were equivalent, with 3% in the CIP+MET group and 5% in the PIP/TAZO group.
Table 2. DEMOGRAPHIC DATA FOR THE EFFICACY-VALID POPULATION
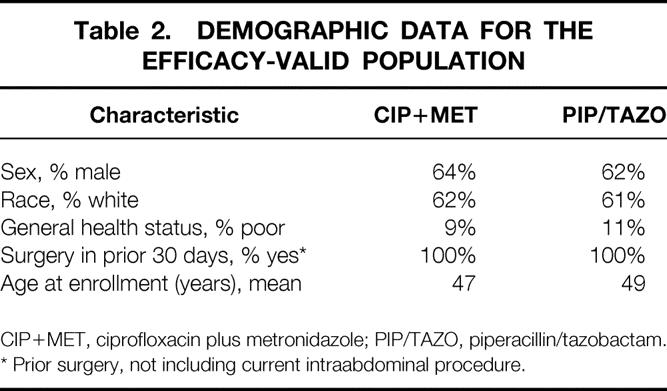
CIP+MET, ciprofloxacin plus metronidazole; PIP/TAZO, piperacillin/tazobactam.
* Prior surgery, not including current intraabdominal procedure.
Table 3. INTRAABDOMINAL INFECTION CHARACTERISTICS
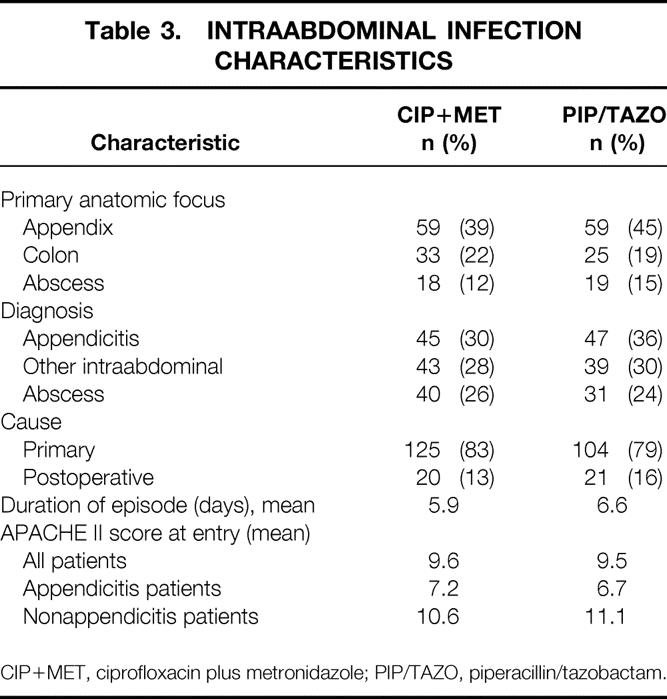
CIP+MET, ciprofloxacin plus metronidazole; PIP/TAZO, piperacillin/tazobactam.
Efficacy Results: Clinical Response
The overall clinical response of resolution was 74% in CIP+MET patients and 63% in PIP/TAZO patients (95% CI, 0.3–23.1%;P = .047) (Fig. 1). For the subgroup with appendicitis, 88% of CIP+MET patients demonstrated overall clinical resolution, compared with 64% of PIP/TAZO patients. Patients without appendicitis had similar overall clinical resolution rates.
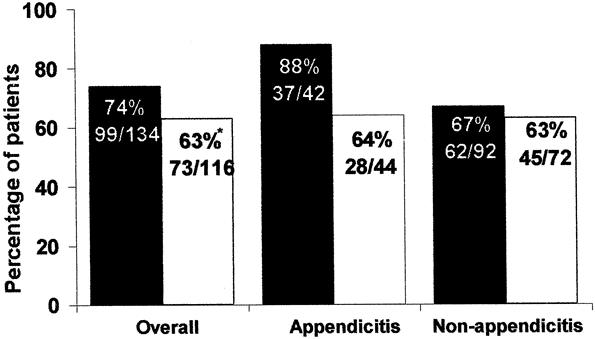
Figure 1. Clinical success rates for all patients valid for efficacy (number and percentage). *95% confidence interval, 0.3% to 23.1%. Black columns, ciprofloxacin plus metronidazole; white columns, piperacillin/tazobactam.
In the intent-to-treat analysis, an overall clinical response of resolution demonstrated the two regimens to be equivalent: 75% (132/176) for CIP+MET versus 69% (111/161) for PIP/TAZO (95% CI, −3.4–15.6%;P = .213).
End-of-Therapy Bacteriologic Response Efficacy Results
End-of-therapy bacteriologic eradication rates were similar between the groups: 77% for CIP+MET and 71% for PIP/TAZO (95% CI, −5.1–19.4%) (Fig. 2). For patients with appendicitis, bacteriologic eradication was achieved in 84% of CIP+MET patients versus 74% of PIP/TAZO patients. For the nonappendicitis stratum, bacteriologic success rates were 74% for CIP+MET versus 68% for PIP/TAZO.
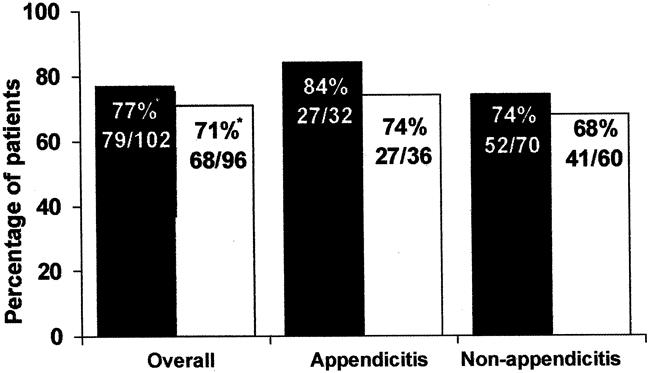
Figure 2. Bacterial eradication rates at end of therapy for patients valid for efficacy (number and percentage). *95% confidence interval, −5.1% to 19.4%. Black columns, ciprofloxacin plus metronidazole; white columns, piperacillin/tazobactam.
The end-of-therapy bacteriologic success rates for all patients valid for safety was similar to that for the efficacy-valid population: 107/138 (78%) CIP+MET, 100/131 (76%) PIP/TAZO.
Pretherapy Pathogens and Eradication Rates by Pathogen
The most common pathogen isolated before therapy was Escherichia coli (22% 92/421 CIP+MET, 23% 82/357 PIP/TAZO). Bacteroides fragilis was the next most commonly recovered pathogen (9% CIP+MET, 10% PIP/TAZO), followed by Streptococcus viridans (7% CIP+MET, 7% PIP/TAZO), Enterococcus spp (5% CIP+MET, 6% PIP/TAZO), Klebsiella pneumoniae (6% CIP+MET, 4% PIP/TAZO), and Pseudomonas aeruginosa (3% CIP+MET, 4% PIP/TAZO). The eradication rates for these organisms are shown in Table 4. Most patients (74%) had multiple causative organisms identified before therapy (mean 3.2 organisms).
Table 4. BACTERIOLOGIC ERADICATION RATES AT THE END OF THERAPY
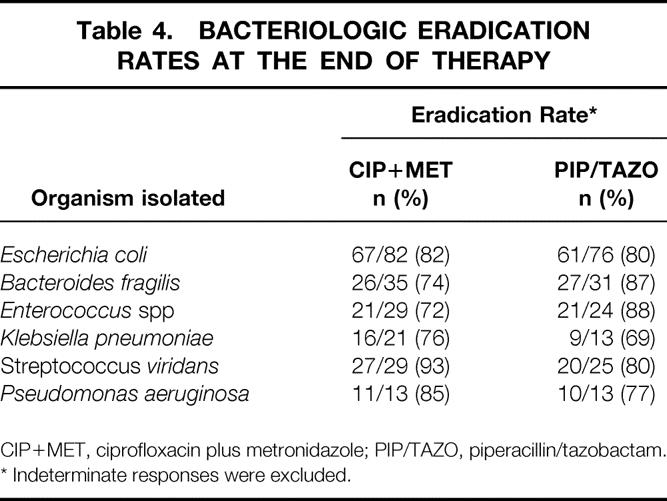
CIP+MET, ciprofloxacin plus metronidazole; PIP/TAZO, piperacillin/tazobactam.
* Indeterminate responses were excluded.
Superinfections and Reinfections
Of all efficacy-valid patients, nine CIP+MET patients had at least one superinfecting organism and four had at least one reinfecting organism at the intraabdominal site. The corresponding numbers for the PIP/TAZO group were eight and five. No specific organism was responsible for more than two reinfections, and only E. coli caused superinfections in more than two patients.
IV-to-Oral Switch: Clinical and Bacteriologic Success Rates
Efficacy-valid patients considered suitable for IV-to-oral switch therapy included 64% (96/151) CIP+MET patients and 57% (75/131) PIP/TAZO patients; 23 patients had indeterminate clinical responses. For the IV-to-oral subgroup, CIP+MET patients had a significantly higher overall clinical success rate of 85% (69/81) compared with 70% (28/39) for PIP/TAZO patients (95% CI, 1.6–28.7%;P = .028). In patients with appendicitis, the success rates were 89% (24/27) for CIP+MET patients and 68% (19/28) for PIP/TAZO patients. Patients without appendicitis had clinical success rates of 83% (45/54) for CIP+MET and 72% (28/39) for PIP/TAZO. Bacteriologic eradication rates for patients switched to oral therapy were 91% (53/58) for CIP+MET and 80% (43/54) for PIP/TAZO (95% CI, −1.3–24.9%). For the appendicitis subgroup, the CIP+MET group had similar bacteriologic eradication rates to the PIP/TAZO group (86% 18/21 vs. 79% 15/19). Corresponding rates for those without appendicitis were 95% (35/37) and 80% (28/35).
Wound Infections and Failures
In total, wound infections developed in 11% (16/151) of CIP+MET patients and 19% (25/131) of PIP/TAZO patients (P = .04). Of the 16 CIP+MET patients in whom a wound infection developed, 8/16 (50%) had a purulent infection compared with 18/25 (72%) of PIP/TAZO patients. Wound infections led to a longer hospital stay in 5/16 (31%) of the CIP+MET patients and 11/25 (41%) of the PIP/TAZO patients.
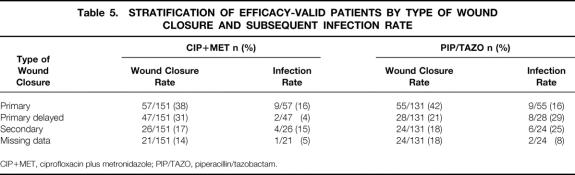
Rates of wound infection stratified by closure type are shown in Table 5. For the efficacy-valid population, primary wound closure (40%) was the most common type of wound closure used. No clear relation between infection rate and the timing of wound closure was seen.
Table 5. STRATIFICATION OF EFFICACY-VALID PATIENTS BY TYPE OF WOUND CLOSURE AND SUBSEQUENT INFECTION RATE
CIP+MET, ciprofloxacin plus metronidazole; PIP/TAZO, piperacillin/tazobactam.
Wound infection alone was responsible for four clinical failures (11%) in the CIP+MET group and five (12%) in the PIP/TAZO group. However, clinical failure plus a wound infection was present in 12/35 (34%) of the CIP+MET patients and 20/43 (47%) of the PIP/TAZO patients.
Length of Hospital Stay and Length of Therapy
For the efficacy-valid population, the mean length of hospital stay was 14 days for the CIP+MET group and 17 days for the PIP/TAZO group. This difference in length of stay was weighted toward patients with a long length of stay (>21 days): 15% (23/151) CIP+MET versus 21% (28/131) PIP/TAZO.
The mean total duration of treatment for efficacy-valid patients receiving only IV study drug was 9 days for the CIP+MET group and 10 days for the PIP/TAZO group. For those switched to oral therapy, the mean total duration of therapy was 10 days for CIP+MET and 11 days for PIP/TAZO. The corresponding mean length of oral therapy was 6 and 7 days.
Unmasked Study Drugs
Forty-six of 151 (30%) CIP+MET patients and 39 of 131 (30%) PIP/TAZO patients received antimicrobial agents during the unmasked phase of the study. Specifically, 34 of 46 (74%) CIP+MET patients and 25 of 39 (64%) PIP/TAZO patients received CIP (oral or IV). Further, 31 of 46 (67%) CIP+MET patients and 24 of 39 (62%) PIP/TAZO patients received MET (oral or IV). Amoxicillin/clavulanic acid was prescribed for 6 of 46 (13%) CIP+MET patients and 5 of 39 (13%) PIP/TAZO patients. For patients receiving unmasked drug, the mean total duration of therapy (masked plus unmasked) was 13 days in each treatment group. The mean length of unmasked therapy was 6 days for CIP+MET patients and 7 days for PIP/TAZO patients.
Clinical success rates were higher for patients treated with unmasked study drugs (85% CIP+MET vs. 72% PIP/TAZO). Of the patients who did not receive unmasked study drug, 70% of CIP+MET and 60% of PIP/TAZO patients achieved clinical success.
Adverse Events
Four hundred fifty-eight patients made up the safety population (235 CIP+MET, 223 PIP/TAZO). The most common drug-related events for CIP+MET were nausea (14%), diarrhea (7%), and vomiting (7%). For PIP/TAZO patients, nausea (17%), diarrhea (6%), and rash (5%) were reported most often.
Fourteen CIP+MET patients and 16 PIP/TAZO patients discontinued therapy or changed antibiotic treatment as a result of adverse events. Most of the events causing discontinuation in the CIP+MET group were related to injection site reaction or a digestive system event. Rash was the most common event leading to discontinuation in the PIP/TAZO group.
Of the 459 patients entered into this trial, 23 died (13 CIP+MET, 10 PIP/TAZO) within 30 days of the start of the study. Of the patients who died in the CIP+MET group, the investigator assigned the cause of death as accompanying disease in 11, and 1 each as bacterial infection plus accompanying disease and other. The numbers for the PIP/TAZO group were four from accompanying disease, two from bacterial infection plus accompanying disease, one from bacterial infection, and three “other.” Three efficacy-valid CIP+MET patients were considered to have clinical failure and died. Two efficacy-valid PIP/TAZO patients died: one was considered to have clinical failure, and the other had a clinical response of resolution. No deaths were caused by reinfection.
DISCUSSION
To our knowledge, this is the first clinical study comparing the safety and efficacy of CIP+MET with PIP/TAZO therapy for the treatment of complicated intraabdominal infections. In this large prospective study of more than 280 efficacy-valid patients, CIP+MET was found to be superior to PIP/TAZO in terms of overall clinical resolution. The serious nature of these infections was described by the mean APACHE II score for efficacy-valid patients of approximately 9.5, including two thirds of patients with a nonappendicitis diagnosis. A secondary finding was the lower postsurgical wound infection rate with CIP+MET compared with PIP/TAZO therapy. More than 60% of efficacy-valid patients could be switched to oral therapy. In this patient subset, overall clinical response after sequential CIP+MET therapy was also found to be superior to that of PIP/TAZO.
Solomkin et al 13 reported that IV CIP+MET, including an IV-to-oral subgroup, was at least as effective as imipenem/cilastatin for the treatment of complicated intraabdominal infections. Several previous studies have also shown PIP/TAZO to be an effective monotherapy for the treatment of intraabdominal infections when compared with either imipenem/cilastatin or gentamicin/clindamycin. 8,10,11 The current trial reconfirms the effectiveness of CIP+MET versus a well-studied contemporary standard monotherapy (PIP/TAZO).
Although our results demonstrated that CIP+MET was superior to PIP/TAZO in the treatment of intraabdominal infections, the overall clinical success rates were lower than anticipated (74% CIP+MET, 63% PIP/TAZO). These relatively low cure rates were most likely related to the fact that only one third of the patients had appendicitis. For appendicitis patients, the overall clinical resolution rate was higher (89% CIP+MET, 64% PIP/TAZO) than for the total efficacy-valid group. After a thorough review of the data, we could not identify any patient- or surgery-specific factors to explain the lower rates of success, especially for PIP/TAZO patients with appendicitis. However, any evaluation of success using subjective measures of success such as clinical judgment, even if predefined, is less precise than one using objective measures. The trial avoided any bias in this subjective assessment through a double-blind design.
As anticipated, patients who were able to be switched to oral medication had higher overall clinical resolution rates in both treatment groups (85% CIP+MET, 70% PIP/TAZO) compared with patients who could not be switched (56% CIP+MET, 53% PIP/TAZO). These findings are similar to the 86% overall clinical success rate found in the previous CIP+MET trial. 13 Sixty percent of patients enrolled in this trial were considered oral therapy candidates by the investigator after only 48 hours of initial IV therapy. Although strict criteria for IV-to-oral conversion were not established in this study, and the switch was not subject to randomization, it is worth noting the relatively large percentage of potential candidates for oral therapy, coupled with the high overall clinical resolution rate observed in the oral CIP+MET subgroup.
As hospital and administration costs rise, physicians are under increasing pressure to lower costs, often by reducing the length of hospital stay and treatment-related expenses. The overall length of stay for CIP+MET patients was 14 days versus 17 days for PIP/TAZO patients. The opportunity to discharge a patient early arises when patients are started on an IV antibiotic and are switched to oral therapy; this is possible with CIP+MET because both are available in IV and oral formulations. Although this study did not prospectively address cost-effectiveness, a reduction in length of stay in the CIP+MET group by 3 days could save money.
Wound infections are a costly sequela to intraabdominal surgery because they may extend the length of stay and increase antibiotic and other therapy costs. 23,24 Our data suggest that CIP+MET therapy provides high rates of clinical and bacteriologic success and may also reduce the incidence of wound infection (11% CIP+MET vs. 19% PIP/TAZO;P = .04). Because patients with wound infections were considered failures to respond in this study, the lower wound infection rates in the CIP+MET patients would contribute to the higher clinical success rates found in these patients. Further, the clinical response of failure plus a wound infection was seen in 8% of CIP+MET patients and 15% of PIP/TAZO patients.
The results of this study must be interpreted carefully, considering several limitations of the study design. The following scenarios may have affected the course of efficacy, sequelae, and the rate of adverse events:
At discharge, approximately one third of patients in each treatment group received an antibiotic in unmasked fashion, including CIP+MET.
Pretherapy antibiotics were received by approximately 90% of patients.
More CIP+MET patients had delayed primary closure compared with PIP/TAZO patients (31% vs. 21%, respectively), and delayed primary closure is thought to reduce the risk of wound infection.
Although nearly equal proportions of patients received CIP+MET as unmasked drug in both treatment groups, the influence of this treatment on overall clinical response cannot be fully ascertained. Nonetheless, the clinical success rates were higher for patients who received unmasked study drugs. The high use of pretherapy antibiotics could influence the efficacy outcomes and also the adverse event profile; however, the effect should be minimal because they were permitted for less than 24 hours.
In conclusion, the results of this study indicate that CIP+MET (IV only or IV-to-oral switch) is a safe and effective treatment for complicated intraabdominal infections. For overall clinical response, CIP+MET was found to be statistically superior to IV PIP/TAZO; bacteriologic response rates were equivalent between the two groups. Sequential (IV-to-oral) therapy with CIP+MET is an attractive alternative to IV PIP/TAZO for patients who show initial clinical improvement and who have a functioning gastrointestinal tract, although the frequent use of unmasked oral antibiotic therapy at hospital discharge in both study groups demonstrates that oral therapy can follow any initial IV antibiotic choice. Further, sequential therapy may reduce the costs of therapy by decreasing the length of hospital stay and potentially by minimizing complications associated with IV therapy.
Acknowledgments
The authors thank Blair E. Robertson, PhD, and Teresa Tartaglione, PharmD, for their critique and editorial contributions.
APPENDIX.
Investigators
The following were study participants:
Stephen Cohn, MD, Yale-New Haven Hospital, New Haven, CT; David Haas, MD, Vanderbilt University Medical Center, Nashville, TN; Samuel Wilson, MD, Irvine Medical Center, Orange, CA; Stephen Vogel, MD, University of Florida, Gainesville, FL; Frederic Chang, MD, St. Francis Regional Medical Center, Wichita, KS; Joseph Van De Water, MD, Mercer University School of Medicine, Medical Center of Central Georgia, Macon, GA; Pamela Lipsett, MD, Johns Hopkins Hospital, Baltimore, MD; Rodney Durham, MD, St. Louis University Medical Center, St. Louis, MO; Gary Garber, MD, Ottawa General Hospital, Ontario, Canada; Ori Rotstein, MD, Toronto General Hospital, Toronto, Canada; David Neal, MD, FACS, Tucson VA Medical Center, Tucson, AZ; Joseph Jemsek, MD, Medical Director Clinical Research, Charlotte, NC; William Cheadle, MD, University of Louisville Norton Hospital and University of Louisville, Louisville, KY; Larry Rumans, MD, IMID Medical Consultants, PA, Topeka, KS; Michael Salem, MD, George Washington University, Washington DC; Jeffrey Milsom, MD, Cleveland Clinic Foundation, Cleveland, OH; Philip Gordon, MD, FRCS, Jewish General Hospital, Montreal, Canada; Stuart Hamilton, MD, University of Alberta Hospital, Edmonton, Canada; Dean Gubler, MD, Naval Medicine Center San Diego, San Diego, CA; Timothy Buchman, MD, Washington University School of Medicine, St. Louis, MO; Carol Terregino, MD, Cooper Hospital, Camden, NJ; James Hebert, MD, Fletcher Allen Healthcare, Burlington, VT; Steven O’Marro, MD, St. John’s Hospital, Springfield, IL; Ronald Stewart, MD, University of Texas Hospital Health Science Center, San Antonio, TX; Michael Metzler, MD, University of Missouri School of Medicine, Columbia, MO; Mark Malangoni, MD, Metro Health Medical Center, Cleveland, OH; Donald Fry, MD, University of New Mexico, Albuquerque, NM; Richard Schwartz, MD, University of Kentucky, Lexington, KY; Albert Yellin, MD, Thomas Berne, MD, Peter Heseltine, MD, and Maria Appleman, MD, LAC & USC Medical Center, Los Angeles, CA; Peter Krumpe, MD, University of Nevada School of Medicine, Reno, NV; Richard Johnston, MD, Royal Alexandra Hospital, Edmonton, Alberta, Canada; and Richard Hall, MD, Victoria General Hospital, Halifax, Nova Scotia, Canada.
Footnotes
Correspondence: Stephen M. Cohn, MD, Dept. of Surgery, Division of Trauma/Surgical Critical Care (D-40), University of Miami School of Medicine, P.O. Box 01690, Miami, FL 33101.
Presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 24–27, 1998, San Diego, CA.
Supported by a research grant from Bayer Corp., Pharmaceutical Division, West Haven, CT.
Dr. Jungerwirth is currently with Procter & Gamble Pharmaceuticals, Cincinnati, OH.
E-mail: stephen.cohn@miami.edu
Accepted for publication September 24, 1999.
References
- 1.Gorbach S, Thadepalli H, Norsen J. Microorganisms in abdominal infections. In: Balowa A, DeHaan R, Dowall V, Guze L, eds. Anaerobic Bacteria: Role in Disease. Springfield, IL: Thomas; 1974: 399–407.
- 2.Swenson R, Lorber B, Michaelson T, Spaulding E. The bacteriology of intra-abdominal infections. Arch Surg 1974; 109: 398–399. [DOI] [PubMed] [Google Scholar]
- 3.McClean K, Sheehan G, Harding G. Intra-abdominal infection: a review. Clin Infect Dis 1994; 9: 100–106. [DOI] [PubMed] [Google Scholar]
- 4.Schentag J, Wels P, Reitberg D, et al. A randomized clinical trial of moxalactam alone versus tobramycin plus clindamycin in abdominal sepsis. Ann Surg 1983; 198: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson S. Results of a randomized multicenter trial of meropenem versus clindamycin/tobramycin for the treatment of intra-abdominal infections. Clin Infect Dis 1997; 24 (Suppl 2): S197–206. [DOI] [PubMed] [Google Scholar]
- 6.Solomkin J, Dellinger E, Christou N, Busuttil R. Results of a multicenter trial comparing imipenem/cilastatin to tobramycin/clindamycin for intraabdominal infections. Ann Surg 1990; 212: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackford A, Tally F, Reinhold R, et al. Prospective study comparing imipenem-cilastatin with clindamycin and gentamicin for the treatment of serious surgical infections. Arch Surg 1988; 123: 322–326. [DOI] [PubMed] [Google Scholar]
- 8.Vestweber K, Grundel E. Efficacy and safety of piperacillin-tazobactam in intra-abdominal infections. Eur J Surg 1994; 573 (Suppl): 57–60. [PubMed] [Google Scholar]
- 9.Niinikoski J, Havia T, Alhava E, et al. Piperacillin-tazobactam versus imipenem-cilastatin for treatment of intra-abdominal infections. Surg Gynecol Obstet 1993; 176: 255–261. [PubMed] [Google Scholar]
- 10.Brismar B, Malmborg A, Tunevall G, et al. Piperacillin-tazobactam versus imipenem-cilastatin for treatment of intra-abdominal infections. Antimicrob Agents Chemother 1992; 36: 2766–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polk H, Fink M, Laverdiere M, et al. Prospective randomized study of piperacillin/tazobactam therapy of surgically treated intra-abdominal infection. Am Surg 1993; 59: 598–605. [PubMed] [Google Scholar]
- 12.Condon R, Walker A, Sirinek K, et al. Meropenem versus tobramycin plus clindamycin for treatment of intra-abdominal infections: results of a prospective, randomized, double-blind clinical trial. Clin Infect Dis 1995; 21: 544–550. [DOI] [PubMed] [Google Scholar]
- 13.Solomkin J, Reinhart H, Dellinger E, et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intraabdominal infections. Ann Surg 1996; 223: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehtar S, Ball A. Intravenous Augmentin in bacteraemia and severe invasive polymicrobial sepsis. J Antimicrob Chemother 1985; 15: 765–771. [DOI] [PubMed] [Google Scholar]
- 15.Ball P, Watson T, Mehtar S. Amoxicillin and clavulanic acid in intra-abdominal and pelvic sepsis. J Antimicrob Chemother 1981; 7: 441–444. [DOI] [PubMed] [Google Scholar]
- 16.Strass C. Fluoroquinolone antibiotics: properties of the class and individual agents. Clin Ther 1992; 14: 348–375. [PubMed] [Google Scholar]
- 17.Fass R. In vitro activity of ciprofloxacin. Antimicrob Agents Chemother 1983; 24: 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergan T. Extravascular penetration of ciprofloxacin. A review. Diag Microbiol Infect Dis 1990; 13: 103–114. [DOI] [PubMed] [Google Scholar]
- 19.Rodvold K. Pharmacokinetics of IV ciprofloxacin. Infect Med 1991; 8 (Suppl C): 12–20. [Google Scholar]
- 20.Lettieri JT, Rogge MC, Kaiser L, et al. Pharmacokinetic profiles of ciprofloxacin after single intravenous and oral doses. Antimicrob Agents Chemother 1992; 36: 993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. Approved Standard M7–A3. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Villanova, PA: National Committee for Clinical Laboratory Standards; 1993.
- 22.National Committee for Clinical Laboratory Standards. Approved Standard M11–T2, Methods of susceptibility testing of anaerobic bacteria. Villanova, PA: National Committee for Clinical Laboratory Standards; 1988.
- 23.Zoutman D, McDonald S, Vethanayagan D. Total and attributable costs of surgical-wound infections at a Canadian tertiary-care center. Infect Control Hosp Epidemiol 1998; 19: 254–259. [DOI] [PubMed] [Google Scholar]
- 24.Olson M, Lee J. Continuous, 10-year wound infection surveillance. Arch Surg 1990; 125: 794–803. [DOI] [PubMed] [Google Scholar]