Abstract
Objective
To determine whether use of a primary pull-through would result in equivalent perioperative and long-term complications compared with the two-stage approach.
Summary Background Data
During the past decade, the authors have advanced the use of a primary pull-through for Hirschsprung disease in the newborn, and preliminary results have suggested excellent outcomes.
Methods
From May 1989 through September 1999, 78 infants underwent a primary endorectal pull-through (ERPT) procedure at four pediatric surgical sites. Data were collected from medical records and a parental telephone interview (if the child was older than 3 years) to assess stooling patterns. A similar group of patients treated in a two-stage fashion served as a historical control.
Results
Mean age at the time of ERPT was 17.8 days of life. Comparing primary ERPT with a two-stage approach showed a trend toward a higher incidence of enterocolitis in the primary ERPT group compared with those with a two-stage approach (42.0% vs. 22.0%). Other complications were either lower in the primary ERPT group or similar, including rate of soiling and development of a bowel obstruction. Median number of stools per day was two at a mean follow-up of 4.1 ± 2.5 years, with 83% having three or fewer stools per day.
Conclusions
Performance of a primary ERPT for Hirschsprung disease in the newborn is an excellent option. Results were comparable to those of the two-stage procedure. The greater incidence of enterocolitis appears to be due to a lower threshold in diagnosing enterocolitis in more recent years.
The Soave or endorectal pull-through was introduced by Franco Soave at the Institute G. Gaslini in 1955. 1 Use of this procedure has been conventionally approached with the placement of a decompressing colostomy once the diagnosis is made. This is followed by a definitive pull-through procedure once the child’s intestine is decompressed and he or she reaches approximately 10 kg body weight. The use of a primary endorectal pull-through (ERPT) in the management of patients with Hirschsprung disease represents a significant change from the classic approach to the treatment of this disease. The first successful modern report of a primary pull-through for Hirschsprung disease came from So et al in 1980. 2 Subsequently, because of the simplified nature of this approach and the potential for cost savings, our group and others have reported on this procedure and have shown it to be a safe option. 3–7
One major objection to performing a primary ERPT in a neonate is the concern that delicate structures such as the muscular sphincters may be injured. Clearly, the most important outcome parameter to evaluate is the child’s overall stooling pattern. Because of the relatively short period of time during which the primary ERPT procedure has become widely performed, few data are available regarding long-term outcomes. Further, there is considerable variability regarding the age at which surgeons have performed these one-stage procedures, ranging from the first week of life to several years of age. This makes the interpretation of outcomes difficult.
The purpose of this study was to evaluate the immediate and long-term results of a primary pull-through in the newborn period, performed at four major pediatric surgical sites using the same surgical technique. We hypothesized that the use of a primary pull-through would result in equivalent perioperative and long-term complication rates compared with the two-stage approach while reducing the number of surgical procedures performed and thereby reducing exposure to such complications.
METHODS
Study Design
The study was a multicenter, retrospective review of patient charts and a telephone survey. From May 1989 through September 1999, 78 neonates underwent ERPT at four pediatric surgical sites: the C.S. Mott Children’s Hospital at the University of Michigan Health Systems; M.S. Hershey Medical Center, Hershey, Pennsylvania; Children’s Hospital of Los Angeles, Los Angeles, California; and Spectrum Health Center, Grand Rapids, Michigan. All surgeons performing the procedure were taught the technique by the senior author (A.G.C.). Data were collected from medical records. In addition, parents of patients who were older than 3 years were interviewed on the phone for an assessment of stooling patterns.
All patients were diagnosed with Hirschsprung disease and underwent ERPT in the first 2 months of life. Patients undergoing ERPT at an older age were excluded. Data were recorded on an identical data sheet and abstracted by one person at each site. Before this abstraction, the study sheets and the selected patients were reviewed to ensure uniformity in how the data would be abstracted and the types of patients selected. Data included age at presentation, symptoms, modes of diagnosis, length of aganglionosis, and associated anomalies. Early and late complications, including whether the patient required hospital admission or medication, were recorded. The patient/family survey (for patients older than 3 years) was based on a previous survey performed to assess stooling problems after a pull-through procedure for Hirschsprung disease. 8 The stooling record examined the frequency of stooling and the presence of incontinence as well as constipation. The stooling pattern was then rated as poor to excellent (Table 1). Enterocolitis was clinically graded using a previously established system from mild (grade 1) to severe (grade 3). 9 Hirschsprung-associated enterocolitis (HAEC) included patients with abdominal distention, fever, and explosive diarrhea. More severe symptoms included patients with hemodynamic instability.
Table 1. PARENT TELEPHONE INTERVIEW AND STOOLING GRADING SHEET
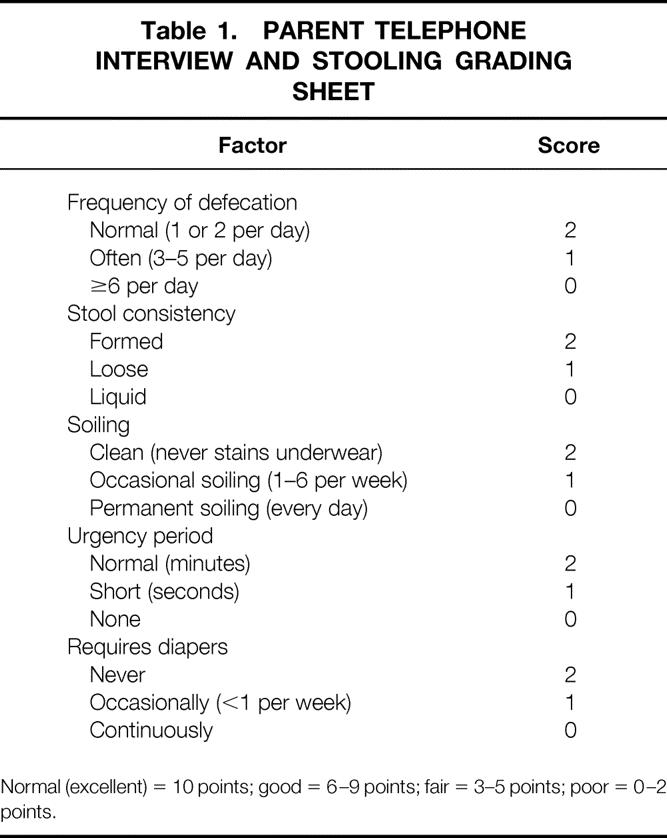
Normal (excellent) = 10 points; good = 6–9 points; fair = 3–5 points; poor = 0–2 points.
The study was approved by the hospital’s institutional review board, and all patient records were kept confidential.
Statistical Analysis
Our primary outcome measure was the incidence of HAEC; secondary outcome measures were the incidence of early and late postoperative complications, and stooling patterns. Included in these latter complications were stricture rate, leakage rate, overall death and complication rates, and incidence of soiling and constipation. Results are expressed as mean ± SD. The unpaired t test, chi-square, and logistic and linear regression were used for statistical analysis, using SPSS 9.0.0 (SPSS Science, Chicago, IL). A similar group of patients treated in a two-stage fashion served as an historical control. For this control group, we used a previously constructed database of 131 children treated at the C.S. Mott Children’s Hospital during the past 15 years who underwent a two-stage approach. Data were complete in 103 of these patients for an accurate assessment of patient outcomes. There is a potential for bias in the selection of some of these patients for a two-stage approach (i.e., potentially a sicker or older group of patients). However, most of these patients underwent a two-stage approach either because it was more commonly performed during that period, or they were referred late from an outside hospital and had a markedly dilated colon that did not permit the performance of a primary pull-through.
Surgical Technique
The surgical approach has been previously described and consists of the following basic principles. 10 The newborn undergoes serial rectal washouts, and digital dilatations of the rectum are performed the day before surgery. The last of the rectal irrigations has 1% neomycin added to it. Broad-spectrum intravenous antibiotics are given before the beginning of surgery. In general, a hockey-stick incision is made in the left lower quadrant, and the endorectal dissection was carried out from inside the abdominal cavity. However, during the past 2 years, nine patients have undergone a primary pull-through using a perineal approach with or without laparoscopic assistance. 11 Ganglionic bowel is mobilized proximally and transected at the transition level. The endorectal dissection is then started approximately 2 cm below the peritoneal reflection. The dissection is continued distally down to 0.5 cm above the dentate line in the newborn. The submucosal/mucosal cuff is everted out of the rectum and opened anteriorly. The ganglionic bowel is brought down through the muscular cuff, and the anastomosis is performed outside the anal cavity. The top of the muscular cuff is then attached to the pull-through bowel to prevent the intestine from prolapsing. Before discharge, a cotton-tipped applicator is inserted into the anus to break up any adhesions between the opposite sides of the anastomosis. At 3 weeks after surgery, gentle rectal dilatations are performed with either a #6 or #7 Hegar dilator.
With increasing experience with the primary pull-through, we have grown to appreciate the indications and contraindications. Indications include a healthy infant who is diagnosed with Hirschsprung disease in the newborn period. The most common contraindication is a delay in the diagnosis, with a resultant dilation of the more proximal colon. This dilation is best seen with a contrast enema study, which is highly recommended in most patients. Additional contraindications include an infant who has significant enterocolitis or associated medical conditions that might complicate a prolonged surgical case, such as congenital heart disease. Although some have viewed total colonic aganglionosis as a contraindication, we have successfully cared for these patients with a primary pull-through.
RESULTS
Demographics
Eighty children were initially identified at the four study sites: University of Michigan, n = 41; Hershey Medical Center, n = 13; Grand Rapids, Michigan, n = 13; and West Covina, California, n = 13. Two of these patients were diagnosed and underwent their primary pull-through beyond 2 months of age and were excluded, leaving 78 patients for analysis. Mean birthweight was 3.4 ± 0.5 kg. Mean gestational age was 38.5 ± 1.8 weeks (range 32–42), and nine infants were less than 37 weeks’ gestation. There were 57 (71%) boys. Racial distribution was white, 71%; black, 14%; Hispanic, 14%, and Asian, 1%. The level of aganglionosis was rectum, n = 7 (9%); rectosigmoid, n = 29 (37%); sigmoid, n = 22 (28%); descending colon, n = 7 (9%); ascending colon, n = 3 (4%); and total colonic, n = 8 (10%). Mean age at diagnosis was 13.5 ± 13.1 days. The mean age at ERPT was 17.8 ± 14.0 days (range 2–67).
Trisomy 21 was found in 11 patients (13.8%). Other associated anomalies were found in 17 patients (21%) and included predominately cardiac anomalies in 13.8% of all patients. Presenting symptoms included abdominal distention (91%), emesis (70.5%), diarrhea (9%), and lethargy (6%). Meconium was passed within 24 hours in 13%, between 24 and 48 hours in 41%, and greater than 48 hours in 35%; this information was unavailable in the remaining 12% of patients. A contrast enema was diagnostic in 61 of the 67 patients who underwent the study (91%). Retention of barium (overnight) was seen in only 46% of patients; however, in many of these patients, the information was missing or a repeat film was not obtained. Confirmation of the diagnosis of Hirschsprung disease was made by a rectal biopsy in all patients.
The demographics of the control group of children who underwent a two-stage procedure showed several important similarities and differences. Sex distribution (69% boys) and the incidence of associated anomalies (31%) were similar to the primary ERPT group. Length of aganglionosis was also similar to the primary ERPT group (rectum, 30%; rectosigmoid, 44%; descending colon, 6%; transverse colon, 10%, total colonic, 8%; unknown, 1%). Racial distribution was also similar (86% white, 8% black, 2% Asian). However, the mean age for the control group at diagnosis was 8.8 ± 16.6 months. Also, because all patients had a colostomy placed, the mean age at pull-through was considerably later than in the primary group, at 19.6 ± 23.9 months. Finally, considerably more patients in the two-stage group had preoperative enterocolitis (22.7% vs. 5% in the primary ERPT group).
No statistical differences in basic patient demographics were noted among the study sites, including age at pull-through (P = .57), extent of aganglionosis (P = .21), or incidence of early (P = .82) or late (P = .38) complications. However, the incidence of HAEC was greater at one center (see below).
Surgical Course and Hospital Stay
Intraoperative complications occurred in five infants: tension at the anastomosis in two and a poor blood supply to the pull-through in three. No major anesthetic complications were noted. Two patients had a protective colostomy placed at the time of their primary pull-through, and both of these colostomies were taken down at 2 months of age without incident. Oral diet was started at a mean of 1.8 ± 0.4 days, and full feedings were achieved at a mean of 3.5 ± 1.4 days.
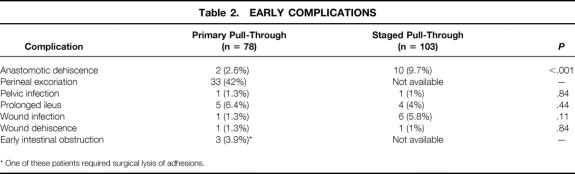
Early Complications
Early complications (within 3 months of ERPT) are listed in Table 2. Five patients (6%) had a prolonged ileus (greater than 4 days) that delayed their discharge from the hospital; however, none required additional surgery. Perineal excoriation was found in 33 patients (42%); all could be managed with local control.
Table 2. EARLY COMPLICATIONS
* One of these patients required surgical lysis of adhesions.
Two patients were taken back to surgery. The first child had previously undergone exploration on two occasions at an outside hospital for a potential bowel obstruction without the diagnosis of Hirschsprung disease. He was subsequently transferred to the C.S. Mott Children’s Hospital, where the correct diagnosis was made. He had an uneventful pull-through; however, he was taken back to surgery on the third postoperative day for necrotizing fasciitis. At reexploration, no evidence of an anastomotic disruption or peritoneal contamination was found. Despite debridement of the abdominal wall, the child died on the fourth postoperative day. In the second child, a small bowel obstruction developed 3 months after the pull-through, and the child underwent an uneventful small bowel enterolysis.
A comparison of early complications between the primary and two-stage groups showed little difference. The rate of anastomotic dehiscence was statistically greater in the two-stage group, even though the procedure was performed in much older children (mean age at ERPT 19.8 ± 23.8 months). A higher rate of wound infections was also noted in the two-stage group, although the difference was not significant.
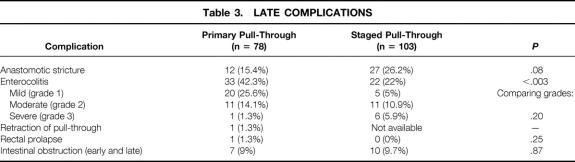
Late Complications
Late complications are listed in Table 3. A mild retraction of the pull-through segment developed in one child; it was successfully managed with simple dilations. Anastomotic narrowing or strictures developed in 12 patients (15%). In general, these strictures were mild; 11 of them required only serial dilations with Hegar dilators during clinic visits, and one child required a rectal dilation under anesthesia. No child with a stricture required a surgical stricturoplasty, and no child required a redo pull-through. There was no correlation between stricture formation and tension at the anastomosis (P = .54), poor blood supply (P = .45), or the level of aganglionosis (P = .15). In addition, smaller children were not at greater risk for stricture formation (P = .17). In one child, a mild rectal prolapse developed and was corrected with a local surgical procedure. Including the child with an early bowel obstruction, a postoperative bowel obstruction developed in a total of seven patients (9%). One infant with a postoperative bowel obstruction had a volvulus of his small bowel after an ERPT for total colonic Hirschsprung disease and lost approximately a third of his small intestine. The infant required total parenteral nutrition for more than 3 years but is now completely on enteral nutrition only. Another patient with long segment disease had progressive difficulty in stooling after her ERPT despite repeated rectal biopsies showing normal ganglion cells and nerves. She subsequently underwent both an anal sphincterotomy and sphincterectomy, with transient improvement after each procedure. Because of persistent stooling difficulties, she had a loop colostomy placed 4 years after her pull-through. This ostomy was subsequently closed after a repeat anal myomyectomy, and she is now having normal stooling after her last surgery.
Table 3. LATE COMPLICATIONS
Comparison of late complications with patients undergoing a two-stage approach (see Table 3) showed few differences between the groups except for HAEC rates.
Enterocolitis
Four patients (5%) had a preoperative diagnosis of HAEC. All of them were admitted and treated with antibiotics, with a subsequent diagnosis of Hirschsprung disease and a pull-through once the enterocolitis had resolved. In general, these were fairly mild cases, because those with severe HAEC generally underwent a staged procedure with an initial colostomy. Enterocolitis after the pull-through was noted in 33 patients (42%). The clinical grade of enterocolitis was grade 1, 20 patients; grade 2, 11 patients; and grade 3, 1 patient. A significantly greater incidence of HAEC was noted in children undergoing a primary ERPT versus those undergoing a two-stage approach (see Table 3). It was our impression that this increased incidence may have been due to a higher degree of attention in making the diagnosis of HAEC during this latter time period. This appears to be confirmed by examining a breakdown of the clinical grade of enterocolitis. There was a much greater incidence of low-grade HAEC in the primary group compared with the two-stage group (Table 3).
There was a high correlation (P < .002) between the development of HAEC and the performance of the pull-through at one institution (C.S. Mott Children’s Hospital). To determine whether the rates of other complications differed between study sites, a cross-tabulation of other early and late complications was performed. This showed no correlation between any of the study sites and the development of intraoperative complications (P = .12), early complications (P = .82), or late complications, including anastomotic stricture (P = .15), pull-through retraction (P = .81), or soiling (P = .57).
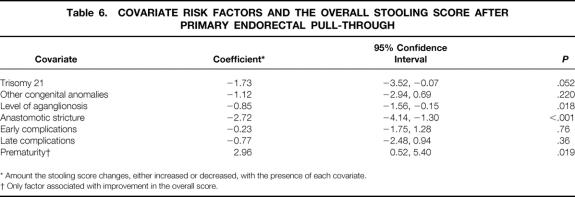
Logistic regression analysis of covariates that may have contributed to the development of HAEC after pull-through was performed (Table 4). This analysis showed a higher incidence of HAEC in patients with an anastomotic stricture (P = .004). An almost significant difference was seen in patients diagnosed with perioperative malnutrition (P = .058; malnutrition was defined by subjective global assessment). If length of aganglionosis was analyzed as a separate risk factor, there was a slight correlation (odds ratio = 4.44, P = .04) between longer length (ascending colon and more proximally) and development of HAEC. Importantly, however, by modeling a number of covariates, no significance was found between longer segment aganglionosis (P = .122) and the development of HAEC after pull-through. Also, no correlation was noted with the development of HAEC and trisomy 21 (P = .232), nor those with a history of HAEC before pull-through (P = .23).
Table 4. COVARIATE RISK FACTORS FOR ENTEROCOLITIS
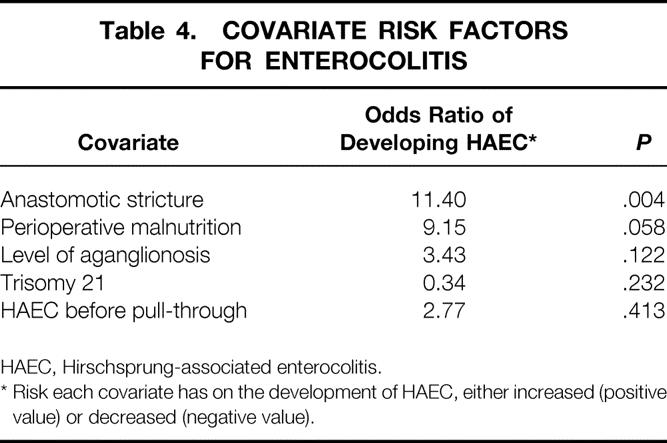
HAEC, Hirschsprung-associated enterocolitis.
* Risk each covariate has on the development of HAEC, either increased (positive value) or decreased (negative value).
Stooling Patterns
Fifty-four of the patients were older than 3 years, and their parents took part in a telephone interview about stooling patterns. Stool frequency consisted of 33 children with one or two bowel movements per 24 hours, 10 with three per 24 hours, 4 with four per 24 hours, and 5 with greater than four per 24 hours. Stool was formed in 21, loose in 31, and liquid-like in 2. Constipation was the most frequently noted problem: 22 (28%) patients had some degree of constipation. Symptoms of constipation were noted weekly in 4 children and monthly in 11. The remaining patients with constipation had symptoms infrequently. Laxatives were used in 15 patients, or 19% of the series; however, the average use in these patients was six times per month, and many of these patients used only one or two doses per month. After surgery, several patients required rectal washouts; however, use of rectal washouts for passage of stool more than 3 months after the pull-through was required in only two children.
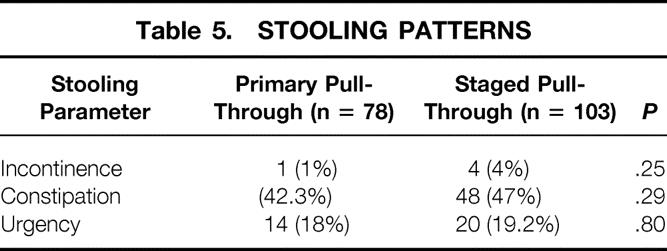
Soiling was noted in only two patients, one of whom had long segment aganglionosis (ascending colon and more proximal). Urgency with stooling was noted in 14 patients (18%). Intermittent use of diapers was required in 21 children. Table 5 shows there was no significant differences in stooling patterns between patients undergoing a primary ERPT and those with a two-stage approach.
Table 5. STOOLING PATTERNS
Using our modified scoring system for grading stool function (Table 6), 5 patients had completely normal stooling, 29 had a good stooling pattern, 17 had a fair stooling pattern, and 3 had poor stooling function. The median age at which continence was achieved was 34 months, and 92% achieved continence by 48 months. As with constipation, stool frequency declined with age. The mean age in years of patients who had one bowel movement per day was 5.4 ± 2.2, whereas the mean age of children having four or more bowel movements per day was 2.6 ± 2.4. Using linear regression analysis, this approached significance (P = .10; relative risk of a decline in stool frequency with age, −0.32; 95% confidence interval, −0.70, 0.07).
Table 6. COVARIATE RISK FACTORS AND THE OVERALL STOOLING SCORE AFTER PRIMARY ENDORECTAL PULL-THROUGH
* Amount the stooling score changes, either increased or decreased, with the presence of each covariate.
† Only factor associated with improvement in the overall score.
Linear regression analysis was performed to correlate a low stooling score with potential risk factors. Covariates associated with a poor stooling score were increasing length of aganglionosis (P = .018) and a history of an anastomotic stricture (P < .001). There was a slight correlation of a poor stooling score with trisomy 21 (P = .052). Factors such as intraoperative complications (P = .25), early complications (P = .76), and late complications (P = .36) did not correlate with soiling. A correlation was noted between prematurity and an improved stooling score (P = .02).
DISCUSSION
This report represents the largest series of children undergoing ERPT in the newborn period. Our results indicate that the overall complication rate and long-term stooling patterns were not significantly different from those of patients undergoing a staged approach. The goals of treating a child with Hirschsprung disease should be to attain anorectal function that is as near to normal as possible. The best approach is to bring ganglionic bowel down to a point just above the dentate line. Conventionally, this has been performed with a staged approach consisting of a leveling colostomy followed by a subsequent pull-through with closure of the colostomy (two-stage approach).
Use of a primary pull-through has been controversial. Although Swenson was the first to perform a primary pull-through, he was discouraged by a high rate of anastomotic leakage. Surprisingly, however, the rate of leakage in their patients undergoing the procedure at 4 months or younger (2/28) was not significantly different (P = .57, as calculated from their published data) than that of those undergoing a staged procedure (10/215). 12 Many of the deaths and complications in Swenson’s series were due to the perioperative management of these infants, and this care has dramatically improved during the past several decades. Thus, it may have been premature at that time to discount the use of a primary pull-through.
So et al 2 described their experience with infants undergoing a primary pull-through in 1980. Since that report, several other groups have reported their experience with this technique. 5,13–16 Results have varied; the differences may be due to the fact that the timing of these procedures ranged from the first week of life to several years of age. 13,17 In some cases the pull-through was protected by a colostomy, but in most cases it was not. So’s group recently updated their initial report. 18 It describes 84 patients, but half of these are older children (more than 1 year old). The description of their results is limited, but the continence rate is low (81.5%). We are unsure whether this is due to a limited follow-up or poorer results. No patient in So et al’s series developed enterocolitis; this may reflect a difference in the definition of, and criteria for diagnosing, HAEC rather than an actual difference between the two series.
Aside from the one child who developed necrotizing fasciitis, early complications were uncommon in our patients. Late complications were also mild. The most common complication was an anastomotic stricture. Although seen in 17% of patients, it was mild in most, and none of these patients required reoperation. The development of a stricture was unrelated to many covariates that we anticipated might have an association, including the child’s size (prematurity) and poor blood supply or tension at the anastomosis. Further, the early development of anastomotic dehiscence and the late development of anastomotic stricture were both statistically higher in our two-stage group. Complications in other series have been reported with variable consistency, thereby making comparisons difficult. Similar to the results of our study, Skarsgard et al 16 found no difference in complication rates between patients who underwent surgery before or after 30 days of life.
The rate of enterocolitis varies widely in published reports, from a high of 42% in our series to a low of 1.4% in the group reported by Nmadu. 5 Although we find this increased incidence troublesome, our overall impression is that the rate of clinically severe HAEC is not appreciably different. There was a clear difference in the rate of this diagnosis at one hospital (C.S. Mott Children’s Hospital) compared with the other three study sites. Our impression is that there is a very low threshold for making the diagnosis of HAEC at this one hospital compared with the others. Although this difference may relate to the surgical technique, the clinical grade of enterocolitis in our patients was low (only one had a clinical grade of 3), suggesting that the difference may relate to the surgical group’s definition of enterocolitis itself. Also, the fact that other perioperative and long-term complication rates were not significantly different between the study sites suggests that this is most likely the case. If one looks at the incidence of enterocolitis reported without including the C.S. Mott Children’s Hospital component, the incidence declines to 26%, which certainly falls within the range reported in many series of staged pull-throughs, 19–21 including our own previous series (22%). 9 It is also possible that the younger age at which these patients are undergoing ERPT may predispose them to develop enterocolitis, potentially from a less mature intestinal immune system. Also, the small caliber of the anastomosis may lead to a partial narrowing, which might predispose to a higher incidence of enterocolitis. Perhaps the only way this could be completely addressed would be to implement a prospective, randomized study. Because of the increasing trend toward the use of a primary pull-through, such a randomized trial may offer considerable insight into the safety of such an approach.
Because of the recent trend toward approaching a primary ERPT by a purely perineal approach, the incidence of HAEC was also examined in this group of nine patients. The incidence in the perineal group was 55% (mean of 0.9 ± 0.4 episodes per patient) and was not significantly different than the transabdominal group (P = .08, using a chi-square analysis).
Logistic regression analysis of our data was interesting in that many of the previously identified risk factors for HAEC were not noted in this series. 19 Specifically, no correlation was noted between the development of HAEC and trisomy 21, long segment aganglionosis, or HAEC before the pull-through. However, patients in whom an anastomotic stricture developed and those with malnutrition were at a higher risk for developing HAEC. Although the connection between these covariates and HAEC is unknown, one could speculate that in patients with anastomotic strictures, the relative obstruction may have contributed to the development of bacterial overgrowth and HAEC. In fact, in a recent review of HAEC by Hackam et al, 18 a similar association of postoperative stricture and HAEC was also noted. Because patients with malnutrition may have a depressed immune system, patients with malnutrition may be unable to manifest an appropriate immune response against their enteric flora. 23
Stooling patterns have been poorly characterized in the late follow-up of most of the reported series of Hirschsprung disease. This is primarily due to the short follow-up times reported in many of these series. Although several authors of primary pull-through series claim that their patients are continent, 24,25 this must be viewed with caution, because follow-up in many of these patients was no longer than 2 or 3 years. A previous evaluation of stooling patterns in patients after ERPT clearly showed a return to normal of stooling frequency over time. 8
Constipation was seen in 28% of our patients. Most symptoms were mild and readily treated with intermittent use of laxatives. This rate of constipation is similar to that seen in other reviews of primary ERPT. 5 Continence rates were good in our patients. Evaluation was limited to children older than 3 years to ensure that these rates were accurate. Poor stooling scores were noted in only three children, and two of them had total colonic aganglionosis.
In conclusion, a primary ERPT in the neonatal period should be viewed as an acceptable procedure in the management of the infant with Hirschsprung disease. The approach should be restricted to infants who are stable and show no signs of enterocolitis at the time of the pull-through procedure. The risk factors for the development of HAEC and those affecting stooling scores should identify potential high-risk patients and may help surgeons avoid or anticipate future complications of this disease.
Discussion
Dr. James A. O’Neill, Jr. (Nashville, Tennessee): This manuscript details a 10-year experience with 78 infants who had primary endorectal pull-through procedures at four different institutions, although half of them came from the University of Michigan Children’s Hospital.
Now, while primary pull-throughs for Hirschsprung’s disease have been performed for many years, beginning with Dr. Swenson of this organization, the earlier efforts were all in older children, and this was done very selectively because of the attendant morbidity encountered at that time. The reason primary pull-throughs were not done was that there was higher mortality and morbidity. But over time, with improvements in anesthetic management and other aspects of care, two-stage pull-through procedures have been performed at an earlier and earlier time. So this revisiting of primary pull-through is understandable, particularly in the infant, and it has gradually been undertaken by most pediatric surgical centers, but there is no question this is the largest series.
If you look at the results of two-stage operations over the years—and they have been well tabulated—the results have been excellent. So the benchmark figures are good ones for your comparison. With the exception of postoperative enterocolitis, complications, results, and everything else are essentially identical to two-stage operations. This is excellent considering the small size of your patients. So overall the results with up to 10 years of follow-up are such that you certainly deserve everyone’s congratulations for a pacesetting report.
I would like to pose a few questions to clarify a few things for us. First, the manuscript indicates that this series of primary pull-throughs is not consecutive but that patients with severe enterocolitis preoperatively were treated by staged procedures. Would you share with us the current indications you use for primary pull-through in infants?
Secondly, can you really be sure that patients from four different sites were being selected for operation with exactly the same criteria and that they were evaluated in exactly the same way?
Third, this is not a randomized study and the comparison is with historical controls, but no details are given as to whether the controls were age-matched and whether everything was the same when the historical group was treated, except for that overlap period, as at the present time. Would you tell us about that? Wouldn’t the validity of this procedure be best evaluated in a randomized fashion?
Finally, the incidence of postoperative enterocolitis in this series is of concern, as you have pointed out. Previously, your group at Michigan has reported a 17% to 20% incidence of postoperative enterocolitis with two-stage procedures, and you have gotten that up to 26% with perhaps stricter criteria. Presumably you have been using similar criteria all along, so the incidence of enterocolitis at 42% is certainly something which bears closer investigation. Do you have any other explanations for this? For example, the way you treat—it is identical to the way that Clostridium difficile is treated. Were any of your patients surveyed for this?
Presenter Dr. Daniel H. Teitelbaum (Ann Arbor, Michigan): Your first question related to the fact that this wasn’t a consecutive series and how did we overall go about selecting which patients might be best for a primary pull-through as opposed to a staged approach. I think that we still remain very conservative about which patients we decide to perform a primary pull-through on. As you well know, many of these patients are quite small in size and often quite debilitated at the time when they present to us.
I think our threshold for performing this procedure would have to be those patients that present fairly early in life. Once we get beyond 3 or 4 months of age, the colon has become so dilated in nature that to safely perform an anastomosis into a relatively small anal diameter would really put the patient at very high risk for leakage.
The other contraindication is those patients that present with enterocolitis. We did have one death in our series, and that was a child who developed a necrotizing fasciitis. I do have to believe that that was due to perhaps being overzealous in performing a primary pull-through in a fairly small child.
In order to examine adequate numbers, we did utilize four different sites. We were very careful about the sites we selected. First, each of the sites had surgeons that were taught the procedure by the senior author, Dr. Arnold Coran. This allowed for a very high degree of uniformity in the way the procedure was performed. Second, before we performed any of our analyses, we went back and looked at the demographic data from each of the particular sites and found that they were extremely well matched. There was no difference in the age of the patients, the age at the time of the pull-through, or the level of the aganglionosis in each of the sites.
The controls are another story. Those were historically based as the bulk of our patients who are now undergoing primarily pull-throughs. And you are right, the best way to do this would be to perform a randomized series. I think the problem is that the shift in the United States is really going towards a primary pull-through and it will be difficult in most places to present a particular option of a two-stage pull-through when one might do just as well with a single-stage pull-through.
Although we did find a higher incidence of enterocolitis in our primary pull-through procedures, I truly think that those patients who suffered from severe enterocolitis were not significantly different; it was more likely the manner in which we declared a patient having enterocolitis. The other three sites, aside from Ann Arbor, had a 26% incidence of enterocolitis, which was identical to our two-stage pull-through procedure group. It can be a very subjective matter of whether somebody has enterocolitis or is having abdominal distension or increased diarrhea from another cause. There was not a higher incidence of Clostridium difficile in our study.
Dr. Robert J. Touloukian (New Haven, Connecticut): I concur with Dr. Teitelbaum and Dr. O’Neill that performing primary pull-through is a trend that is occurring in all children’s hospitals by qualified pediatric surgeons who have experience in treating Hirschsprung’s disease. However, the risk of enterocolitis in these patients is of serious concern to us and needs to be studied very carefully, and I am grateful to Dr. Teitelbaum and his colleagues for having done this study. However, we must recall that there are certain safeguards inherent in the two-stage operation.
First and most important is confirmation of the pathology at the colostomy. This allows the surgeon to review the final pathology and be absolutely certain that normal ganglion cells are present and there is no evidence of neuronal intestinal dysplasia, which indeed increases the risk of postoperative enterocolitis.
Second, patients who have a two-stage operation generally can be monitored for adequate weight gain as well as adequate decompression of the proximal colon in the ganglionic area, so that the pull-through is done electively and at a time when the patient’s condition is optimal.
Third, the anal reconstruction is done, of course, at a slightly older age, which reduces the risk of anal stenosis.
I have two questions: One, have you correlated the pathology from the four different institutions to be certain that you have adequate ganglion cells at the pull-through site and there is no increased incidence of neuronal intestinal dysplasia?
Number two: How have you studied these patients preoperatively to be certain that their colon is adequately decompressed prior to their pull-through procedure, either by plain film radiography or by determination that they are in an edema program that has been successful?
Number three: Do you routinely calibrate or dilate the patient postoperatively to reduce the risk of anal stricture?
Dr. Teitelbaum: To answer your first question, I do think that there is a high degree of anxiety when one performs a primary pull-through. One has to be sure that these are performed at a site where a pediatric pathologist is readily available, or at least a pathologist who is highly capable of telling what true ganglion cells are and whether there are hypertrophied nerves suggestive of a transition zone. Despite our operating at a multitude of different sites in Michigan, we routinely send our patients down to the University of Michigan when we do a pull-through to make sure that we have a pediatric pathologist looking at our pathology specimens.
We did not read all the specimens at our study site. However, there have been no misreadings in this cadre of 78 patients, although I would be remiss in saying that we haven’t had misreadings done in other patients who have had a two-stage procedure performed.
I routinely get a barium enema on all of my patients because I want to assess the degree of colonic dilatation, and it also gives me a road map of where the site of aganglionosis ends before I begin my pull-through procedure. Additionally, adopting Dr. Langer’s more recent perineal approach (J Pediatr Surg 1999;34:148–52), I like having the road map of knowing whether this is a left-sided colonic disease, that can be approached primarily from a pure peritoneal approach, as opposed to requiring a laparotomy or laparoscope.
Finally, I calibrate all of my patients postoperatively on a fairly routine basis. And following what was published by Dr. Marty and Dr. Johnson a few years ago, I have been very aggressive in having many of my patients undergo rectal decompression with washouts for the first few months after their pull-through. I think this leads to a significant decrease in the number of enterocolitic complications.
Footnotes
Correspondence: Daniel H. Teitelbaum, MD, Section of Pediatric Surgery, University of Michigan Medical Center and C.S. Mott Children’s Hospital, F3970, Box 0245, Ann Arbor, MI 48109.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail: dttlbm@umich.edu
Accepted for publication April 2000.
References
- 1.Soave F. Hirschsprung’s disease: a new surgical technique. Arch Dis Child 1964; 39: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.So HB, Schwartz DL, Becker JM, et al. Endorectal “pull-through” without preliminary colostomy in neonates with Hirschsprung’s disease. J Pediatr Surg 1980; 15: 470–471. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi A. One-stage neonatal reconstruction without stoma for Hirschsprung’s disease. Sem Pediatr Surg 1998; 7: 170—173. [DOI] [PubMed] [Google Scholar]
- 4.Georgeson KE, Cohen RD, Hebra A, et al. Primary laparoscopic-assisted endorectal colon pull-through for Hirschsprung’s disease: a new gold standard. Ann Surg 1999; 229:678–6782; discussion 682–683. [DOI] [PMC free article] [PubMed]
- 5.Nmadu PT. Endorectal pull-through and primary anastomosis for Hirschsprung’s disease. Br J Surg 1994; 81: 462–464. [DOI] [PubMed] [Google Scholar]
- 6.Pierro A, Fasoli L, Kiely E. Staged pull-through for rectosigmoid Hirschsprung’s disease is not safer than primary pull-through. J Pediatr Surg 1997; 32: 505–509. [DOI] [PubMed] [Google Scholar]
- 7.Cilley RE, Statter MB, Hirschl RB, Coran AG. Definitive treatment of Hirschsprung’s disease in the newborn with a one-stage procedure. Surgery 1994; 115: 551–556. [PubMed] [Google Scholar]
- 8.Teitelbaum D, Drongowski R, Chamberlain J, Coran A. Long-term stooling patterns in infants undergoing a primary endorectal pull-through (ERPT) for Hirschsprung’s disease. J Pediatr Surg 1997; 32: 1049–1053. [DOI] [PubMed] [Google Scholar]
- 9.Elhalaby EA, Coran AG, Blane CE, et al. Enterocolitis associated with Hirschsprung’s disease: a clinical-radiological characterization based on 168 patients. J Pediatr Surg 1995; 30: 76–83. [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum DH, Coran AG. Primary pull-through in the newborn. Sem Pediatr Surg 1998; 7: 103–107. [DOI] [PubMed] [Google Scholar]
- 11.Langer JC, Minkes RK, Mazziotti MV, et al. Transanal one-stage Soave procedure for infants with Hirschsprung’s disease. J Pediatr Surg 1999; 34: 148–152. [DOI] [PubMed] [Google Scholar]
- 12.Carcassonne M, Morisson-Lacombe G, Letourneau JN. Primary corrective operation without decompression in infants less than three months of age with Hirschsprung’s disease. J Pediatr Surg 1982; 17: 241–243. [DOI] [PubMed] [Google Scholar]
- 13.Kücükaydin M, Okur H, Turan C, et al. Swenson’s operation for neonatal Hirschsprung’s disease. Eur J Surg 1993; 159: 487–489. [PubMed] [Google Scholar]
- 14.Samuel M, Freeman N. Primary modified Duhamel operation for Hirschsprung’s disease in infants. Pediatr Surg Int 1994; 9: 61–63. [Google Scholar]
- 15.Skarsgard E, Superina R, Shandling B, Wesson D. Initial experience with one-stage endorectal pull-through procedures for Hirschsprung’s disease. Pediatr Surg Int 1996; 11: 480–482. [DOI] [PubMed] [Google Scholar]
- 16.Skarsgard D, Superina R, Shandling B, Wesson D. Initial experience with one-stage endorectal pull-through procedures for Hirschsprung’s disease. Pediatr Surg Int 1996; 11: 480–482. [DOI] [PubMed] [Google Scholar]
- 17.So HB, Becker JM, Schwartz DL, Kutin ND. Eighteen years’ experience with neonatal Hirschsprung’s disease treated by endorectal pull-through without colostomy. J Pediatr Surg 1998; 33: 673–675. [DOI] [PubMed] [Google Scholar]
- 18.Hackam DJ, Filler RM, Pearl RH. Enterocolitis after the surgical treatment of Hirschsprung’s disease: risk factors and financial impact. J Pediatr Surg 1998; 33: 830–833. [DOI] [PubMed] [Google Scholar]
- 19.Teitelbaum DH, Qualman SJ, Caniano DA. Hirschsprung’s disease. Identification of risk factors for enterocolitis. Ann Surg 1988; 207: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rescorla FJ, Morrison AM, Engles D, et al. Hirschsprung’s disease. Evaluation of mortality and long-term function in 260 cases. Arch Surg 1992; 127: 934–942. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda K, Goto S. Diagnosis and treatment of Hirschsprung’s disease in Japan. An analysis of 1628 patients. Ann Surg 1984; 199: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marty TL, Matlak ME, Hendrickson M, et al. Unexpected death from enterocolitis after surgery for Hirschsprung’s disease. Pediatrics 1995; 96 (1 Pt 1):118–121. [PubMed] [Google Scholar]
- 23.Marty TL, Seo T, Sullivan JJ, et al. Rectal irrigations for the prevention of postoperative enterocolitis in Hirschsprung’s disease. J Pediatr Surg 1995; 30: 652–654. [DOI] [PubMed] [Google Scholar]
- 24.Cass D. Aganglionosis: associated anomalies. J Paediatr Child Health 1990; 26: 351–354. [DOI] [PubMed] [Google Scholar]
- 25.Carcassonne M, Guys JM, Morrison-Lacombe G, Kreitmann B. Management of Hirschsprung’s disease: curative surgery before 3 months of age. J Pediatr Surg 1989; 24: 1032–1034. [DOI] [PubMed] [Google Scholar]