Abstract
Objective
To determine the factors that influence patient survival after in vivo split liver transplantation (SLT).
Summary Background Data
Split liver transplantation is effective in expanding the donor pool, and its use reduces the number of deaths in patients awaiting orthotopic liver transplantation. Early SLTs were associated with poor outcomes, and acceptance of the technique has been slow. A better understanding of the factors that influence patient and graft survival would be useful in widening the application of SLT.
Methods
During a 3.5-year period, 55 right and 55 left lateral in vivo split grafts were transplanted in 102 pediatric and adult recipients. The authors’ in vivo split technique has been previously described. Median follow-up was 14.5 months. Recipient, donor, and surgical variables were analyzed for their effect on patient survival after SLT.
Results
Overall survival rates of patients who received an SLT were not significantly different from those of patients who received whole organ transplants. Survival of left lateral segment recipients, at median follow-up time, was 76% versus 80% in patients receiving a trisegment. Fifty of 102 patients (49%) were high-risk urgent recipients (United Network for Organ Sharing [UNOS] status 1 and 2A) and 52 (51%) were nonurgent recipients (UNOS status 2B, 3). High-risk recipients had a survival rate significantly lower than that of nonurgent recipients. By univariate comparison, two variables—UNOS status and number of transplants per patient—were significantly associated with an increased risk of death. Preoperative recipient mechanical ventilation, preoperative prothrombin time, donor sodium level, donor length of hospital stay, and warm ischemia time approached significance. The type of graft (right vs. left) did not reduce the survival rate after transplantation. Multivariate logistic regression analysis identified UNOS status and length of donor hospital stay as independent predictors of survival.
Conclusions
Patient survival of in vivo SLT is not significantly different from that of whole-organ orthotopic liver transplantation. The variables affecting outcome of in vivo SLT are similar to those in whole-organ transplantation. in vivo SLT should be widely applied to expand a severely depleted donor pool.
Orthotopic liver transplantation (OLT) has become a well-established modality for treatment of previously fatal liver disease. The advent of new immunosuppressive agents and refinement of surgical techniques have accounted for remarkable progress in the years since the first OLT was performed in 1963. 1,2 The past decade has also witnessed an exponential increase in the number of patients awaiting liver transplantation. Currently, it is estimated that more than 14,500 patients are listed for liver transplantation, with only 4,500 cadaveric livers available annually. With more than 4 million Americans affected with hepatitis C, 25% to 35% of whom will develop cirrhosis, the transplant community is anticipating an epidemic of patients requiring transplantation. Such an increasing discrepancy between donor supply and demand has resulted in an increasing death rate for adult and pediatric patients awaiting OLT. In addition, in the past 5 years, there has been an increase in the number of livers transplanted into critically ill patients, with a higher rate of complications and death after OLT.
To maximize donor organ use in children and adults, four procedures have evolved from the fundamental principle that a portion of the liver with a suitable vascular pedicle, bile duct, and venous drainage, along with sufficient functional hepatocyte mass, can sustain hepatic function in a patient as well as a whole organ. Reduced-size liver transplantation 3–9 was the wellspring for this effort, followed by adult-to-pediatric living-related transplantation, 10,11 cadaveric split liver transplantation (SLT), 12–32 and more recently adult-to-adult living donor transplantation. 33
Pichlmayr et al 12 in 1988 reported the first clinical attempt at an ex vivo SLT. One year later, Bismuth et al 13 described two patients with fulminant hepatic failure, each receiving a split graft. Broelsch et al 15 reported the first series of 30 SLTs in 21 children and 5 adults. In this early experience, patient survival was inferior to that reported in series of cadaveric whole-liver transplants. 28 Despite skepticism as to the lasting role of SLT, several European centers, faced with increasing waiting list death rates because of donor scarcity, pursued the split liver option. The results of a collective experience of 50 donor livers, providing 100 grafts during a 5-year period, from the European Split Liver Registry, demonstrated no significant difference from the results of conventional whole-organ orthotopic liver transplantation during the same period. 16 Such results renewed interest in SLT, as evidenced by more recent series of ex vivo SLT. 14,19,24 Despite marked improvements of outcome in these series, high-risk patients appeared to have a worse outcome than nonurgent recipients.
A modification of the ex vivo splitting technique is in vivo splitting, which involves an extension of the techniques for living-related donor liver procurement that is completed in the heart-beating cadaver donor. At UCLA, we first attempted in vivo SLT in 1992. Our first experience was not favorable, but after establishing a living-related liver program and accruing experience in 30 cases, we resumed the in vivo split liver program in 1996. In that same year, Rogiers et al 30 reported their initial experience with split grafts, which demonstrated superior results compared with ex vivo split liver techniques.
There have been only a few published reports of in vivo SLT 29–35 because the procedure has been performed only during the past 4 years. Initial results from these few series, which involved a limited number of patients, suggested an improvement over the ex vivo experience, with higher patient and graft survival rates and a lower incidence of complications. However, larger series of in vivo SLT are required to confirm these results. Further, a better understanding of the factors that influence the outcome of SLT is critical for the successful application of SLT to expand a severely depleted cadaveric donor pool.
This report examined the outcome of in vivo SLT and analyzed the factors affecting patient survival in a large number of recipients.
METHODS
Patients and Cadaveric Donors
From July 1996 to December 1999, 58 cadaveric livers were split in vivo to generate 58 trisegmental and 58 left lateral segment grafts. One cadaveric liver was reduced in vivo to a left lobe for transplantation into a pediatric patient. Another liver was split in vivo into right and left lobes that were transplanted into two adult recipients. Sixteen trisegmental grafts and one left lateral segment were shared with local centers. Five left lateral segments and two right trisegments were not used for transplantation because of prolonged cold ischemia times. Two right trisegmental transplants that were performed in other centers were excluded because of the absence of complete records to allow statistical analysis. Thus, a total of 110 split cadaveric liver grafts were included in this study. For the purpose of analysis, the split right lobe and the two left lobes were included with the right trisegmental and the left lateral segmental grafts, respectively.
Retrospective analysis of the patient records was performed. Survival rates of patients who received an SLT were compared with those of a contemporary cohort of 628 adult and pediatric patients who underwent whole-liver transplantation during the same period.
Candidates for OLT were assigned, according to their medical condition, to one of the following United Network for Organ Sharing (UNOS) categories:
Status 1: patients in intensive care with expected survival less than 7 days
Status 2: continuous inpatient
Status 3: at home, but requires medical attention.
In 1997 UNOS status 1 classification for adult recipients was modified into UNOS status 1 (patients with fulminant hepatic failure, acute Wilson disease, primary nonfunction and hepatic artery thrombosis after OLT) and UNOS status 2A (patients with chronic liver disease in intensive care). Previous UNOS status 2 was changed to 2B. Thus, urgent recipients were UNOS status 1 and 2A; nonurgent recipients were UNOS status 2, 2B, and 3.
In Vivo Splitting Technique
Livers from cadaveric donors that were considered suitable for splitting by the procuring surgical team were split in vivo as previously described. 28,31 In brief, the left lateral segment was mobilized and the left hepatic artery was isolated after identification of the arterial branch to segment 4. The left branch of the portal vein was isolated after ligation of all branches to the caudate lobe, followed by isolation of the left hepatic vein. Portal vein branches to segment 4 were ligated and divided to the right of the umbilical fissure. The liver parenchyma was divided between the left lateral segment and segment 4 using electrocautery. The left hepatic bile duct and hilar plate were divided sharply. At this stage of dissection, the two isolated hepatic grafts, which included a right trisegmental (segments 1, 4, 5, 6, 7, and 8) and a left lateral segmental (segment 2 and 3) allograft, were perfused with University of Wisconsin solution after cannulation of the inferior mesenteric vein and the infrarenal aorta. The left hepatic artery, the left branch of the portal vein, and the left hepatic vein were divided, and each graft was packaged separately.
In one donor where the liver was reduced in vivo to a left lobe (segments 1, 2, 3, and 4), a formal right hepatectomy was performed in which the liver parenchyma was divided to the right of segment 4. The donor vena cava, celiac axis, portal vein, and common bile duct were preserved with the left lobe. In the donor liver that was split into a right and a left lobe, the liver parenchyma was divided to the right of segment 4. The right lobe was separated by dividing the right hepatic vein, the right hepatic artery, and the left bile duct above the biliary bifurcation. The donor vena cava, celiac axis, and portal vein were preserved with the left lobe; the common bile duct was preserved with the right lobe.
Recipient Procedure
The right trisegmental allograft was prepared on the bench in a manner identical to a whole cadaveric graft, with preservation of the entire length of the celiac axis, portal vein, bile duct, and vena cava. The right trisegmental allograft was implanted in the same manner as a whole organ. 31 Biliary reconstruction was performed using a choledochocholedochostomy over a T-tube or by means of a Roux-en-Y hepaticojejunostomy with an external or internal stent.
The left lateral segmental allograft was transplanted in a fashion similar to the adult-to-pediatric living-related transplant procedure, in which the recipient vena cava is preserved during the initial phase of recipient hepatectomy. The liver allograft was implanted in a piggyback fashion in which the venous outflow tract of the donor graft was anastomosed to the confluence of the recipient hepatic veins. Biliary reconstruction was completed by a Roux-en-Y hepaticojejunostomy with an internal stent.
The immunosuppression regimen consisted of dual tacrolimus-based immunosuppression including tacrolimus and steroids. 34,36
Statistical Analysis
Survival analysis was performed using the Kaplan-Meier method. Group survival curves were compared using the log-rank test for nonparametric data. For univariate survival analysis, continuous variables that may affect patient survival were dichotomized at the median value. In addition, all variables were analyzed for independent significance using multivariate logistic regression analysis. P < .05 was considered significant.
RESULTS
Donor Characteristics
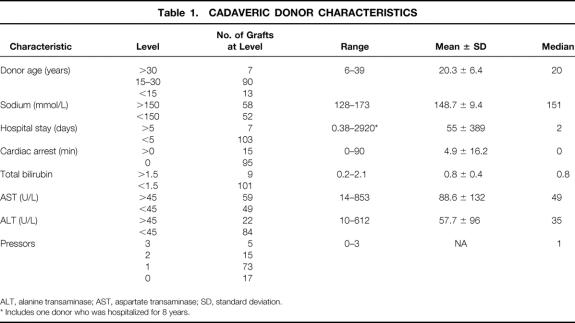
All cadaveric livers from donors younger than 40 years of age were considered for in vivo splitting. As shown in Table 1, the median donor age was 20 years and the median sodium level was 151 mmol/L. Donors with a history of cardiac arrest or hospital stays of more than 5 days were considered for split liver harvests in urgent situations. Fifteen split liver grafts were obtained from nine donors with a history of cardiac arrest that ranged from 3 to 90 (37.7 ± 28.8) minutes. Five grafts originated from three donors with prolonged hospital stays ranging from 6 to 8 (7 ± 1) days. Two grafts were obtained from one donor with a hospital stay of 8 years. Most donors required one pressor to provide hemodynamic stability. Only 17 grafts were obtained from donors without pressor support. Fifteen grafts were harvested from donors receiving two pressors, and five grafts were obtained from donors receiving three pressors. Donor pressors included dopamine 2 to 20 (7.9 ± 4.9) μg/kg/min, epinephrine 0.1 to 1.4 (0.83 ± 0.67) μg/min, phenylephrine 3.3 to 50 (21.8 ± 21.5) μg/min, or norepinephrine 4 to 15 (8 ± 6) μg/min.
Table 1. CADAVERIC DONOR CHARACTERISTICS
ALT, alanine transaminase; AST, aspartate transaminase; SD, standard deviation.
* Includes one donor who was hospitalized for 8 years.
This study included 110 (54 right trisegments, 53 left lateral segments, 1 right lobe, 2 left lobes) split cadaveric livers that were used for transplantation. Forty-seven right trisegments, one left lobe, one right lobe, and one left lateral segment were transplanted in adult recipients. Pediatric patients received 52 left lateral segments, 7 right trisegments, and 1 left lobe.
Recipient Characteristics
One hundred two adult and pediatric (younger than 16 years) patients received 110 SLTs. Recipient age was 0.25 to 73 years (mean 24.8 ± 25). Recipient weight was 4 to 113 kg (mean 38.9 ± 31.2). Ninety-five patients received one split graft only. Six patients received two split grafts, and one pediatric patient received three left lateral segment transplants. End-stage liver disease caused by biliary atresia was the most common cause of transplantation in pediatric patients; hepatitis C and fulminant liver failure were the leading causes of transplantation in the adult population (Table 2.
Table 2. RECIPIENT DIAGNOSIS
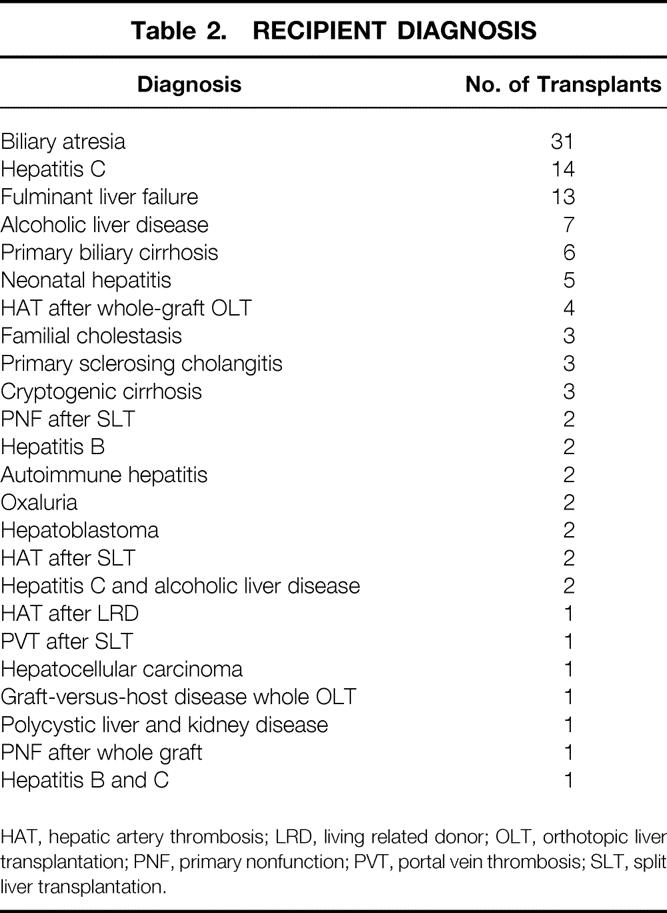
HAT, hepatic artery thrombosis; LRD, living related donor; OLT, orthotopic liver transplantation; PNF, primary nonfunction; PVT, portal vein thrombosis; SLT, split liver transplantation.
At the time of OLT, 50 of 102 (49%) recipients were UNOS status 1 or 2A and required urgent transplantation. Fifty-two recipients (51%) were nonurgent (UNOS status 2, 2B, and 3). Preoperative total serum bilirubin was 0.2 to 37.5 mg/dL (mean 9.8 ± 9.4), prothrombin time was 11 to 100 seconds (mean 17.5 ± 14.6), and serum creatinine was 0.1 to 6.6 mg/dL (mean 0.95 ± 0.9). Serum aspartate and alanine transaminases (AST and ALT) were 11 to 17,556 U/L (1,037 ± 2,460) and 9 to 6,170 U/L (664 ± 1,240), respectively. At the time of OLT, cold ischemia time was 1.3 to 10.2 hours (mean 5.6 ± 2, median 5.4). Median warm ischemia was 35.5 minutes (mean 41 ± 19, range 17–103).
Patient Survival
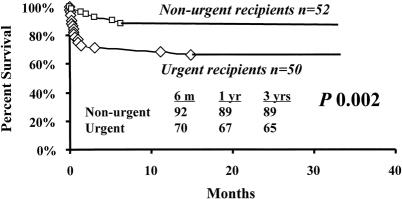
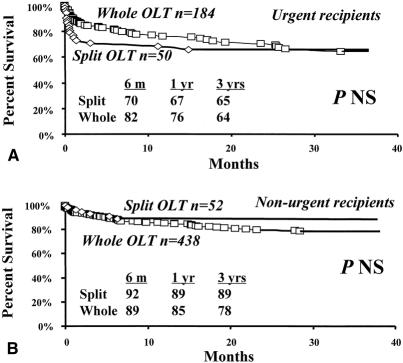
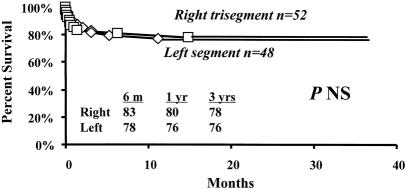
Kaplan-Meier patient survival estimates for all patients who received SLT are shown in Figure 1. Median follow-up was 14.5 months (range 0–38.7). Overall survival rates of patients undergoing SLT at 6 months and 1 and 3 years were 81%, 79%, and 77%, respectively. A matched control cohort of 628 adult and pediatric patients who received a whole-organ transplant had corresponding survival rates of 86%, 82%, and 74% (median follow-up time 16.3 months, range 0–41.9; see Fig. 1). There was no significant difference in survival between patients who received a SLT and patients who received whole organs. However, among patients who received an SLT, corresponding survival rates for nonurgent recipients were 92%, 89%, and 89% (Fig. 2). Corresponding survival rates for urgent recipients were 70%, 67%, and 65%. Survival for urgent recipients was significantly lower than for nonurgent recipients (P = .002; see Fig. 2). Figure 3A demonstrates that there was no significant difference in survival for urgent recipients who received an SLT compared with urgent recipients receiving a whole graft (70%, 67%, and 65% vs. 82%, 76%, and 64%). Similarly, there was no significant difference in survival for nonurgent recipients who received either a split or a whole-organ graft (92%, 89%, and 89% vs. 89%, 85%, and 78%; see Fig. 3B). Trisegment recipients exhibited overall survival rates of 83%, 80%, and 78%, which was not significantly different from the survival rates of left segment recipients (78%, 76%, and 76%;Fig. 4).
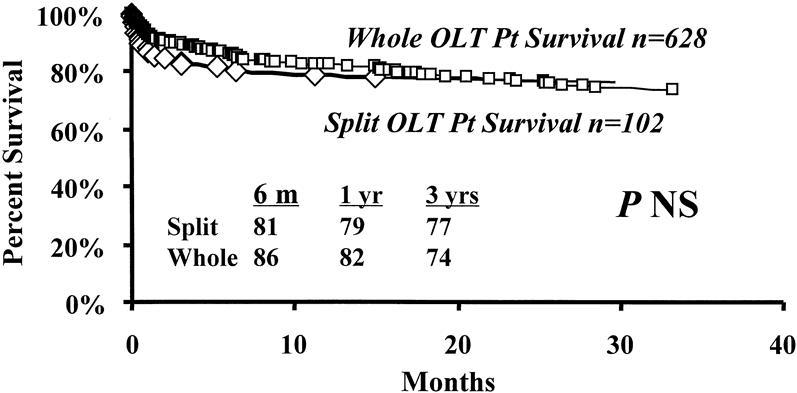
Figure 1. Kaplan-Meier patient survival curves. Overall patient survival after split liver transplantation (♦) compared with a contemporary cohort of patients who received a whole-liver graft (▪).
Figure 2. Kaplan-Meier survival estimates of urgent (♦) versus nonurgent (▪) recipients of split liver transplantation.
Figure 3. Patient survival estimates according to United Network for Organ Sharing (UNOS) status before transplantation. (A) Survival of urgent (UNOS status 1 and 2A) patients after split liver (♦) versus whole-organ (▪) transplantation. (B) Survival of nonurgent (UNOS status 2, 2B, and 3) recipients after split liver (♦) versus whole-organ (▪) transplantation.
Figure 4. Patient survival after a right (▪) or left (♦) segmental graft.
Causes of Death After SLT
Ten children and 13 adults died after SLT (Table 3). Eighteen deaths occurred in 50 urgent recipients (36%). In contrast, only five deaths occurred in 52 nonurgent recipients. Sepsis was the leading cause of death in urgent recipients, resulting in 7 of the 18 deaths (41%). Graft failure was the underlying cause of death in only four urgent patients. Three of five deaths in nonurgent recipients were caused by graft failure. Only three late deaths occurred after SLT (161, 341, and 453 days) and were caused by multiple organ failure in one and sepsis in two patients. Twenty of 23 deaths (87%) occurred within the first 100 days after surgery. Because splitting of the liver may have contributed to recipient deaths within 100 days of transplantation, factors that may influence patient survival during this period were subjected to further analysis.
Table 3. CAUSES OF PATIENT DEATHS AFTER IN VIVO SLT
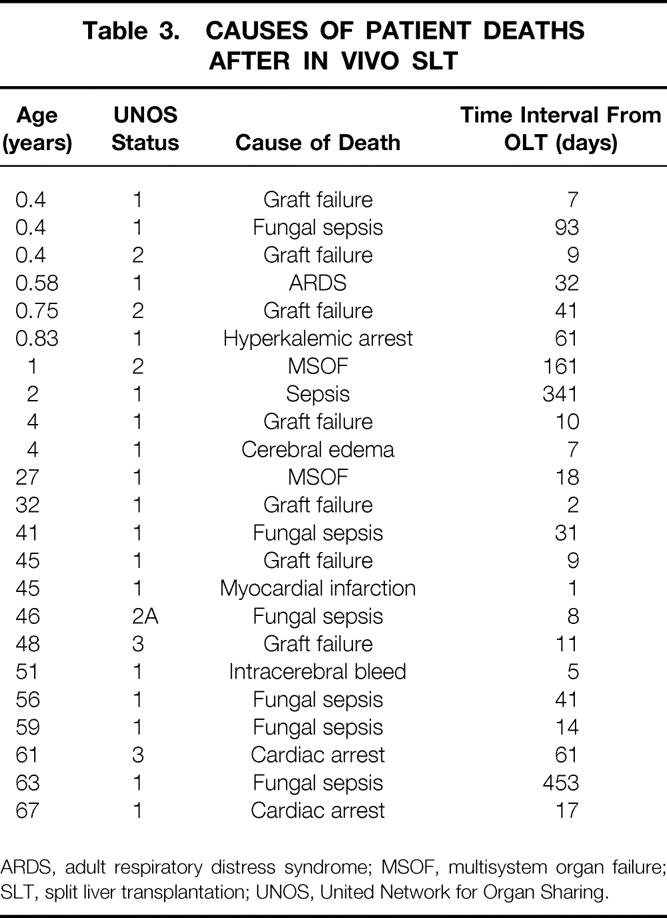
ARDS, adult respiratory distress syndrome; MSOF, multisystem organ failure; SLT, split liver transplantation; UNOS, United Network for Organ Sharing.
Predictors of Patient Survival After SLT
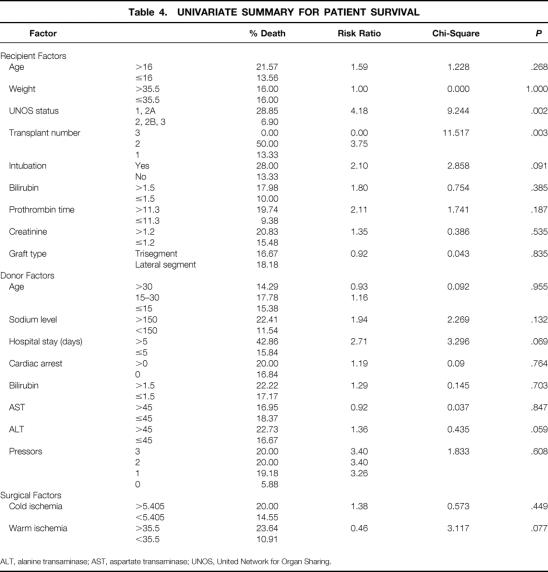
Preoperative recipient, donor, and surgical variables were studied for their impact on survival in the first 100 days after SLT. As shown in Table 4, the recipient variables were age, weight, UNOS status before transplantation, number of transplants per patient, pretransplant mechanical ventilation, preoperative bilirubin, prothrombin time, serum creatinine, and split liver graft type. The donor variables were age, sodium level, length of hospital stay before procurement, history of cardiac arrest, donor serum bilirubin, AST, ALT, and number of donor pressors. The two surgical variables were cold and warm ischemia times.
Table 4. UNIVARIATE SUMMARY FOR PATIENT SURVIVAL
ALT, alanine transaminase; AST, aspartate transaminase; UNOS, United Network for Organ Sharing.
On univariate comparison, two variables were significantly associated with patient survival after SLT (see Table 4): UNOS status of the patient (urgent vs. nonurgent;P = .002) and number of transplants per patient (two vs. one, P = .003). Preoperative recipient mechanical ventilation (P = .091), preoperative prothrombin time more than 11.3 (P = .187), donor sodium level more than 150 mmol/L (P = .132), donor hospital stay more than 5 days (P = .069), and warm ischemia time more than 35.5 minutes (P = .077) all approached statistical significance. Neither the type of graft used (right trisegment vs. left lateral segment, P = .835) nor the age of the recipient (older than 16 years vs. younger than 16;P = .268) influenced patient survival after SLT.
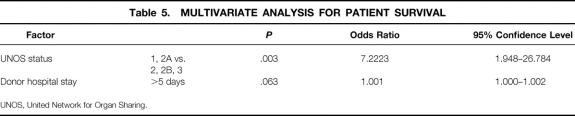
All variables were subjected to multivariate logistic regression analysis (Table 5). Of these, one recipient and one donor variable were independent predictors of survival: UNOS status of the recipient (urgent vs. nonurgent, P = .003) and donor hospital stay more than 5 days (P = .063), with relative risk factors of 7.2 and 1, respectively.
Table 5. MULTIVARIATE ANALYSIS FOR PATIENT SURVIVAL
UNOS, United Network for Organ Sharing.
DISCUSSION
This study represents the largest report to date of in vivo SLT. Transplantation of 102 recipients with 110 SLTs during 3.5 years achieved overall patient survival rates of 79%, 78%, and 78% at 1, 2, and 3 years, respectively. The survival rate of nonurgent recipients was significantly higher than that of urgent high-risk patients. Comparison with a contemporary cohort of 628 patients who underwent a whole-organ transplant during the same period revealed no survival advantages for whole-organ transplantation compared with SLT. Further, patients receiving right-sided split grafts had an equivalent survival rate to those receiving left-sided grafts.
The first series of in vivo SLT in 1996 by Rogiers et al, 30 which included 14 transplantations from seven splitting procedures, demonstrated a 6-month patient survival rate of 92%. Three of 14 (21.4%) recipients were urgent patients. Recently, Reyes et al 35 reported an overall 1-year survival rate of 96% in 29 patients undergoing in vivo SLT. Eight of 29 (27.5%) recipients were high-risk patients. Similarly, our early experience at UCLA, reported by Goss et al, 31 demonstrated an overall patient survival rate of 92% at 6 months and 1 year. This series included 26 patients who underwent 28 in vivo SLTs. Ten of 26 (38.4%) were urgent high-risk recipients. Such initial reports are characterized by a limited number of patients and short follow-up periods. During the past 4 years, we have extensively used the in vivo split procedure to maximize our cadaveric pool for both adult and pediatric patients. Although our overall patient survival rates of 81%, 79%, and 77% at 6 months and 1 and 3 years, respectively, are lower than previous reports using this procedure, the current study included a high number of high-risk patients: at the time of transplantation, 49% were high-risk (UNOS status 1 and 2A) recipients. When patients were stratified according to UNOS status, survival of nonurgent recipients was superior to that of high-risk recipients (92%, 89%, and 89% vs. 70%, 67%, and 65% at 6 months and 1 and 3 years, respectively;P = .002). Among urgent high-risk recipients, survival of patients who received a SLT was not significantly different from those who received a whole organ (70%, 67%, and 65% vs. 82%, 76%, and 64% at 6 months and 1 and 3 years, respectively). Similarly, survival of nonurgent recipients was similar whether a split or a whole organ was used (92%, 89%, and 89% vs. 89%, 85%, and 78% at 6 months and 1 and 3 years, respectively). Thus, the high percentage of urgent recipients may have affected the overall survival of patients undergoing SLT. Nevertheless, patient survival rates after SLT were equivalent to those of whole-organ transplantation.
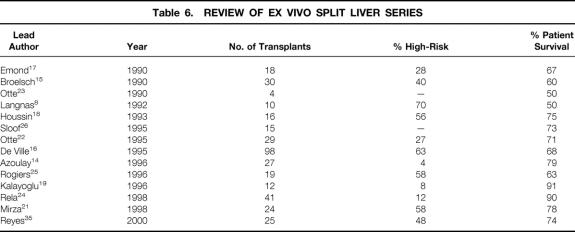
The adverse effect of high-risk recipients on patient survival is clearly demonstrated in the ex vivo split experience (Table 6). A collective experience of 50 donor livers, providing 100 grafts during a 5-year period, was reported from the European Split Liver Registry by de Ville. 16 In this report, patient 6-month survival rates were stratified according to the patient’s pretransplant status, which included elective nonurgent versus urgent high-risk recipients. In the former situation, the patient survival rate was 88.9% for children and 80% for adults. In the urgent setting, patient survival rates were 61.3% for children and 67.7% for adults. These results were compared with the European Liver Transplant Registry of conventional OLT performed during the same period and showed no significant difference. In fact, the survival rate for elective adult recipients receiving an SLT was higher than the rate for those who received a whole graft (88.9% vs. 80.3%). More recent reports by Azoulay et al, 14 Rogiers et al, 25 Kalayoglu et al, 19 Rela et al, 24 Mirza et al, 21 and Reyes et al 35 confirm poor survival of high-risk recipients undergoing ex vivo SLT. Since 1996, as shown in Table 6, some series have demonstrated 1-year patient survival rates of 91% and 90%. 19,24 However, only 8% and 12% of patients were high-risk. Other series that exhibited patient survival rates of 63%, 78%, and 74% included a high percentage of urgent high-risk patients (58%, 58%, and 48%, respectively). 25,21,35 Thus, despite marked improvements of outcome in these series, the use of ex vivo SLT in high-risk patients is associated with a worse outcome than in nonurgent recipients. Initial reports on in vivo SLT suggested that the in vivo procedure may offer patient survival advantages over the ex vivo technique in urgent recipients. 30,31,35 The current study, in addition to recent ex vivo reports, 14,19,21,24,25,35 suggest that both techniques are applicable to high-risk recipients; patient survival rates are lower, but are nevertheless equivalent to those of whole-organ transplantation. However, the in vivo technique, compared with the ex vivo procedure, produces a lower rate of biliary complications 28,31 and avoids excessive blood loss from the cut surface of the liver on reperfusion. 29 Further, mandatory back-table manipulation for ex vivo splitting is associated with graft rewarming, increased susceptibility to hepatic ischemia/reperfusion hepatic injury, and prolonged cold ischemia time. 28 Such deleterious effects are avoided with the in vivo procedure.
Table 6. REVIEW OF EX VIVO SPLIT LIVER SERIES
The death rate for urgent recipients in the current study was 36% for high-risk recipients, compared with only 9.6% in nonurgent patients. Twenty of 23 deaths (87%) occurred in the first 100 days after transplantation. Eighteen of the 23 deaths were in urgent recipients (78%). Sepsis, but not graft failure, was the leading cause of death in these patients (7/18, 41%). Fungal infections were the most common cause of sepsis, occurring in six of seven recipients who died of sepsis. In addition, sepsis was the cause of death in two of three patients who died more than 100 days after transplantation. Such results are similar to those reported with whole-organ transplantation. In patients who underwent OLT for hepatitis C virus at our institution, sepsis was the cause of death in 37.8% of patients who died after whole-organ OLT. 36 Similarly, 26% of deaths in patients undergoing transplantation for primary sclerosing cholangitis were related to sepsis. 37 A high incidence of death from sepsis in the late postoperative period has also been reported after whole-organ transplantation. 38,39
Univariate analysis of recipient, donor, and surgical factors identified two significant variables that reduce patient survival after SLT: recipient UNOS status 1 and 2A and need for retransplantation (two vs. one transplants). Preoperative mechanical ventilation and elevated prothrombin time were two other recipient variables that approached significance. All such variables, particularly an advanced UNOS status, have been identified by multiple other studies as predictors of poor patient survival after whole-organ OLT. 40–46 Age group, number of transplants, and UNOS status were identified as independent risk factors for patient death in a study that evaluated 250 patients who underwent retransplantation at UCLA. 41 Other variables that approached significance on univariate analysis in the current study included donor sodium level more than 150 mmol/L, donor length of hospital stay of more than 5 days, and surgical warm ischemia time of more than 35.5 minutes. A large study that analyzed 340 OLTs demonstrated that a donor plasma sodium level of more than 155 mmol/L and ABO incompatibility were independent predictors for patient and graft survival. 45 The deleterious effect of prolonged warm ischemia time on the outcome of transplantation is well established. 46,47 Similarly, a prolonged donor hospital stay has been clearly demonstrated to reduce graft and patient survival after whole-organ grafts. 46,48,49 In addition, multivariate analysis in our study identified a donor hospital stay of more than 5 days and UNOS status of recipients as independent predictors of survival after SLT. In contrast to other published reports, our study did not demonstrate that the age of the recipient 41,50 or the type of graft used (right vs. left) reduced patient survival. 4,14 It therefore appears that factors affecting patient survival after SLT are similar to those previously identified for whole-organ transplantation.
Despite the intuitive appeal of SLT as an innovative approach to the scarcity of liver donors, poor initial results with reduced-size and ex vivo split grafts have resulted in slow acceptance of the procedure. In recent years, national debate on organ allocation has underscored the acute need for a renewed interest in SLT. The current data clearly indicate that the outcome of in vivo SLT is equivalent to that achieved by whole-organ transplantation. We have therefore pursued an aggressive policy to split suitable livers into right trisegmental and left lateral grafts to benefit both the adult and pediatric populations. 28 We are pursuing the option of a right and a left lobe split that would benefit two appropriately size-matched adult recipients. We have also developed institutional donor, surgical, and recipient criteria for SLT. Hemodynamically stable cadaveric donors younger than 45 years are considered for the split procedure if the liver is deemed suitable by the harvesting team. We avoid using livers from donors with sodium levels more than 155 mmol/L, with hospital stays of more than 5 days, or with a history of cardiac arrest, or who are receiving more than one pressor, except in desperate circumstances. Cold ischemia time is ideally kept to less than 6 hours, and transplantation of split grafts with cold ischemia times of more than 10 hours should be avoided. Warm ischemia time should not exceed 45 minutes. With the current organ allocation system that favors urgent recipients, it is practically impossible to avoid transplantation of split organs in UNOS status 1 or 2A recipients. Moreover, splitting a liver graft for an urgent recipient is likely to benefit another nonurgent patient. Such insight is validated by our current results that demonstrate equal survival rates of high-risk patients, whether they received a split or a whole-organ graft.
In summary, in vivo SLT can be performed in nonurgent recipients with excellent results. Despite a lower patient survival rate in high-risk recipients, SLT can be applied to urgent patients with results that are equivalent to those expected with whole organs. Independent variables reducing patient survival after SLT include a donor hospital stay of more than 5 days and high-risk urgent patients. Other factors that may affect patient survival include retransplantation, preoperative mechanical ventilation, elevated prothrombin time, donor sodium level and prolonged warm ischemia. SLT should be applied to expand the donor organ pool and benefit a larger number of patients.
Acknowledgments
The authors thank M. Farrell-Ross, MS, for her expert statistical analyses of the data and N. Feduska for his efforts in organ procurement.
Discussion
Dr. Christoph Broelsch (Essen, Germany): This remarkable paper and the surgical achievement definitely sets a new standard by the UCLA group in performing liver transplantation. It is clearly shown that it is not the type of transplant that the patient receives, but clearly the time when the transplant is needed and the graft that should be available.
It was exactly 10 years ago when we first presented a series of 30 split and 30 recipients from my Chicago group, with an outcome of about 15%, inferior to the standard results, and a biliary tract complication rate of about 30%, which was unacceptable and prompted a reluctance of the acceptance of the procedure. As causes for the worse results, there were a few primary nonfunctioning grafts and there were circulation problems with the segment 4 for anatomical reasons. For several years then, splitting has been reluctantly applied, while living-related transplants, particularly for children, by performing left lateral segmentectomies, had obtained excellent results!
Simultaneously, because of the need for organs, cadaveric splits as well as increasing living-donor hemihepatectomies were performed by several centers. The first series was reported last year at the ASA Meeting by Fan from Hong Kong, the background there being that no cadaveric organs were available. Here, as well as in Europe, we do have cadaveric donors, and many in the field concur with you that the split approach should be more widely applied.
The way you describe it, it looks really simple and well organized, like young donors, even on pressures or even with cardiac arrest, are the ones that should be used while many donor hospitals agree on letting you perform those procedures with little burden on their resources. It apparently worked in all the cases you described. But in reality, you only did this about 60 times in 31/2 years while you did almost 700 other transplants. My first question, therefore, is: How many times did you really try to set up this splitting and fail for some reason? Is it worth the effort to set up a system, at least in your region, and what is the potential?
The second question: How would you then apply to a more nation-wide set-up as we have proposed in the transplant area but are facing tremendous physician resistance?
The limitations for the procedure you described are clearly that in your splitting system, excluding lobar in situ or ex vivo splitting, you need a child and an adult as a pair. But there are not as many children as there are potential liver segments, including the ones deriving from the living-related procedures and you are bypassing the real problem, which is two adults needing a part of a split. This conflict, I believe, can only be solved by using both the living-related hemihepatectomy procedure as well as the cadaveric split procedures. Do you see both procedures supplementing each other? Or do you see a growing competition, or even one procedure replacing the other?
The last small point is your definition of “in vivo split.” I would rather like to refer to an “in situ procurement,” as we suggested. The “in vivo” procedure, I believe, cannot be performed on a cadaveric brain-dead donor. By definition, it is an “in situ split,” because there is no more “vita” or “vivere” in a deceased donor.
Presenter Dr. Ronald W. Busuttil (Los Angeles, California): Your first two questions basically want to address the issue of the logistics of in situ or in vivo split liver transplantation. Clearly, it can be a logistical nightmare.
When we first started the procedure, we tried very carefully to discuss it with the other members of the team within our region, and we took a significant period of time to make them understand that this was something that we should be trying and that we would do everything we could not to impact on the procurement of the hearts, the pancreata, et cetera. In fact, the system that we have now is that we say it is going to take us 11/2 hours and no more to do the in situ split liver transplant. If we can’t do it, we stop at that point, perfuse the organs, and then complete the split on the back table. But most of the time, it can be done.
One of the most important issues is: Does this impact the viability of other grafts? We have looked at that, and in fact there has been no deleterious effect on hearts, pancreas, or kidneys when the in situ liver procedure has been done.
With regard to a national system, UNOS has now instituted a voluntary plan allowing the sharing of split organs. This has not been actually put into place yet, and I think we are going to have to see how this works.
We think that these organs, as was indicated in the presentation by Dr. Ghobrial, really have to come from very good donors. Ischemia time has to be kept to a minimum. And I am concerned about sending these organs across the country, although we have shared them with other centers with fairly good results.
The third question was: What role does the in situ split have vis-a-vis the LRD in adults? I do think they that are complementary. As you know, the adult LRD program in this country has only been rolling over the last 12 to 18 months. About 200 cases have been done. The chief concern is the issue of donor safety. Of the 200 cases that have been done, there has been at least a 10% complication rate in the donor. So I think we have to proceed with that cautiously. I think if we have a procedure that avoids any risk to the donor, probably it would be the preferential procedure.
Dr. Goran B. Klintmalm (Dallas, Texas): One lesson learned from the UCLA group is that these excellent results do not come cheaply nor freely. The data helps us determine how to select donors and recipients and how to execute the split liver transplantation. The results presented to us today show us what can be achieved when performing large volumes of these surgeries. In experienced hands, the result of the transplantation of a split liver graft for the recipient of either the right or left segment is equal to the results of full-size organ grafting. I have four questions to ask the authors.
Number one, when evaluating donors or potential split liver organ donation, there is constantly a discussion of how perfect the donor needs to be. Several extremely important views are shared by the authors in the manuscript. The use of pressors in the donors is okay, preferably not more than one, but two or even three is permissible if good blood pressure is maintained. Even a history of cardiac arrest in the donor does not preclude organ donation. However, what caught my attention was the amount of transaminase increase that you accepted. You had donors with AST as high as 853 and ALT as high as 612. My question is: How do these enzyme elevations go into your judgment on the suitability of split liver organ transplantation?
Number two, I assume that you define warm ischemia time as the time from when the graft is taken out of the ice until reperfusion occurs. The importance of warm ischemia time was well recognized in the old days of kidney transplantation. This is something not widely discussed in the liver transplantation, and has virtually disappeared over the past years from the debate in kidney transplantation as well. We have recently seen a couple of publications where the importance of warm ischemia time has reemerged, and it is also mentioned in your paper. My question is: How important do you think warm ischemia time is and how much do we need to reemphasize the importance of getting the livers in quickly to minimize preservation injuries?
Number three: Retransplantation is being brought about because of the poor results. In your previous report of retransplantation at UCLA, you reported a 55% survival for 1 year and 47% for 5 years beginning at the time of the second transplant. My question is: In a time of extreme need, where primary transplant outcomes are running better than 85%, should patients in need of a second transplant receive a lower priority?
Number four: Dr. Ghobrial and Dr. Busuttil, I understand that you aggressively tried to do a split in every suitable donor. However, it is unclear to me what your policy is on how to allocate the split liver grafts that result from these procedures. The question is: Are you truly looking to split liver donors only when you have an urgent patient, since half of your patients were urgent recipients? Or do you split every graft that you think you can get away with and then allocate the grafts to your recipients according to regular UNOS standards? In this process, do you ask for permission of the right lobe recipient to resect the left lateral segment for a pediatric recipient?
In conclusion, this presentation by the UCLA group has provided us with the fundamental guidelines on how to proceed with split liver transplantation. It will help guide policy not only on the local program level, but also on the national level.
Dr. Busuttil: Dr. Klintmalm, your first question referred to how good does the donor have to be—as good as it possibly can be. I think the best results are going to be obtained with a pristine donor. Our policy now is to go out and look at every donor with the intention to split. I think one of the most important parameters is how the liver looks at the time that you see the liver at the time of procurement.
The issue about the transaminase elevation is important. I am not sure that was not a recipient transaminase. In any event, we look at the transaminases, and obviously we look at the trend more than any absolute number.
Regarding warm ischemia, with ex vivo split liver, you split the liver on a back bench, there is rewarming that occurs when you fix the liver. I think you have to minimize warm ischemia as much as possible, and that is what we do with any type of transplant that we perform.
Regarding the philosophical issue of whether we should do a retransplantation on these patients, I think this is a personal philosophy. It is very difficult, as I think I have stated before in front of this organization, to abandon a patient who has a failed graft and not retransplant them. But I think in this time of crucial donor shortage, we have to be somewhat circumspect in that and exercise judgment not only for our own patients but for all the other patients on the list. I think, yes, if I have a patient who has a PNF or an hepatic artery thrombosis, I do indeed think those patients need to be retransplanted. If they are out 2 weeks with sepsis, dialysis, and ventilator dependence, obviously you are not going to want to retransplant those patients.
Your last question is very, very important, and that is the issue of to whom you offer split liver transplants. I think to do this you have to fully inform your patients, and basically what we have now developed is a two-tier list of recipients. We inform the patients at the time of evaluation that we have a split liver program, and tell them they may accept a split liver or they may not. We only do it in those patients who have accepted it, and they can back out at any time, just like they can for adult living-related transplantation. And we will do it in elective patients, obviously, put in the right lobe, if the patient has accepted it early on.
Dr. Mark B. Adams (Milwaukee, Wisconsin): Dr. Busuttil, can you tell me why you think there is a significant advantage to the in vivo versus the bench if the liver is kept cold during the bench work?
Dr. Busuttil: First of all, when you do the ex vivo technique it takes about 21/2 hours to cut the liver on the bench, there is rewarming, and I think that there is an increased incidence of graft dysfunction. This has been shown in all of the series reported. The overall incidence of biliary complications in the ex vivo technique is approximately 20%, whereas in the in situ technique the biliary complication rate is about 2% to 3%.
Secondly, the bleeding you see in the ex vivo is higher than it is than in vivo, because in the in vivo, you achieve hemostasis during the time of procurement. So complications are less with the in situ, and the results with urgent patients in which the ex situ has been performed are not comparable to what we see with the in situ.
Dr. Andreas G. Tzakis (Miami, Florida): Dr. Busuttil, would you please define any exclusion criteria for donors for a split that you might have, and also any size considerations? I notice that one of your recipients was 113 kg. Are there any age limitations?
Dr. Busuttil: We showed one exclusion criteria, being in the hospital for greater than 5 days. I think that the ultimate exclusion criteria is when you go and look at the liver, if the liver doesn’t look good, you don’t split it.
Your other question was size considerations. I think you can basically put a triseg in anybody that you would consider putting a whole organ graft in. Again, that is a judgment issue of whether you think you have got sufficient hepatocyte mass.
As we get more experienced, the age is going up. I remember Dr. Starzl once saying that he would never take a donor over 55—until he reached 55 years of age. So as our experience is increasing, we are going up. We have had now a donor who was, I think, about 54 years of age that we have split. But again, younger and better is better.
Footnotes
Correspondence: Ronald W. Busuttil, MD, PhD, Dumont-UCLA Transplant Center, UCLA School of Medicine, 10833 LeConte Ave., 77–132 CHS, Los Angeles, CA 90095.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail: rbusutti@mednet.ucla.edu
Accepted for publication April 2000.
References
- 1.Starzl TE, Marchiaro TL, Von Kaulla K, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963; 117: 659–676. [PMC free article] [PubMed] [Google Scholar]
- 2.NIH Consensus Development Conference Statement. Liver transplantation. Hepatology 1984; 4: 1075. [PubMed] [Google Scholar]
- 3.Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery 1984; 95: 367–370. [PubMed] [Google Scholar]
- 4.Broelsch CE, Emond JC, Thistlethwaite JR, et al. Liver transplantation, including the concept of reduced-size liver transplants in children. Ann Surg 1988; 208: 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broelsch CE, Emond JC, Whitington PF, et al. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts and living related segmental transplants. Ann Surg 1990; 214: 368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emond JC, Whitington PF, Thistlethwaite JR, et al. Reduced-size orthotopic liver transplantation: use in the management of children with chronic liver disease. Transplantation 1989; 10: 867–872. [DOI] [PubMed] [Google Scholar]
- 7.Houssin D, Soubrane O, Boillot O, et al. Orthotopic liver transplantation with a reduced-size graft: an ideal compromise in pediatrics? Surgery 1992; 111: 532–542. [PubMed] [Google Scholar]
- 8.Langnas AN, Wagner CM, Inagaki M, et al. The results of reduced-size liver transplantation, including split livers, in patients with end-stage liver disease. Transplantation 1992; 53: 387–391. [DOI] [PubMed] [Google Scholar]
- 9.Otte JB, de Ville de Goyet J, Solak E, et al. Size reduction of the donor liver is a safe way to alleviate the shortage of size-matched organs in pediatric liver transplantation. Ann Surg 1990; 211: 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet 1988; 2: 497. [DOI] [PubMed] [Google Scholar]
- 11.Strong RW, Lynch SV, Ong TN, et al. Successful liver transplantation from a living donor to her son. N Engl J Med 1990; 322: 1505–1507. [DOI] [PubMed] [Google Scholar]
- 12.Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation einer spenderbeber auf zwei empfanger (splitting - transplantation): eine neue methode in der weiterentwicklung der lebersegment transplantation. Langenbecks Arch Chir 1988; 373: 127–130. [PubMed] [Google Scholar]
- 13.Bismuth H, Marino M, Castaing D. Emergency orthotopic liver transplantation in two patients using one donor. Br J Surg 1989; 76: 722–724. [DOI] [PubMed] [Google Scholar]
- 14.Azoulay D, Astarcioglu I, Bismuth H, et al. Split liver transplantation: the Paul Brousse Policy. Ann Surg 1996; 224: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broelsch CE, Emond JC, Whitington PF, et al. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts and living related segmental transplants. Ann Surg 1990; 214: 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Ville de Goyet J. Split liver transplantation in Europe, 1988–1993. Transplantation 1995; 59: 1371–1376. [DOI] [PubMed] [Google Scholar]
- 17.Emond JC, Whitington PF, Thistlethwaite JR, et al. Transplantation of two patients with one liver. Analysis of a preliminary experience with split-liver grafting. Ann Surg 1990; 212: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houssin D, Boillot O, Soubrane O, et al. Controlled liver splitting for transplantation in two recipients: technique, results and perspectives. Br J Surg 1993; 80: 75–80. [DOI] [PubMed] [Google Scholar]
- 19.Kalayoglu M, D’Alessandro AM, Knechtle JS, et al. Preliminary experience with split liver transplantation. J Am Coll Surg 1996; 182: 381–387. [PubMed] [Google Scholar]
- 20.Langnas AN, Wagner CM, Inagaki M, et al. The results of reduced-size liver transplantation, including split livers, in patients with end-stage liver disease. Transplantation 1992; 53: 387–391. [DOI] [PubMed] [Google Scholar]
- 21.Mirza DF, Achilleos O, Pirenne J, et al. Encouraging results of split-liver transplantation. Br J Surg 1998; 85: 494–497. [DOI] [PubMed] [Google Scholar]
- 22.Otte JB. Is it right to develop living related liver transplantation? Do reduced and split livers not suffice to cover the needs? Transpl Int 1995; 8: 69–73. [DOI] [PubMed] [Google Scholar]
- 23.Otte JB, de Ville de Goyet J, Alberti D, et al. The concept and technique of the split liver in clinical transplantation. Surgery 1990; 107: 605–612. [PubMed] [Google Scholar]
- 24.Rela M, Voregas V, Miniesan P, et al. Split liver transplantation: King’s College Hospital experience. Ann Surg 1998; 227: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogiers X, Malago M, Gawad KA, et al. One-year experience with extended application and modified techniques of split liver transplantation. Transplantation 1996; 61: 1059–1061. [DOI] [PubMed] [Google Scholar]
- 26.Sloof MJH. Reduced-size liver transplantation, split liver transplantation and living related transplantation in relation to donor organ shortage. Transpl Int 1995; 8: 65–68. [DOI] [PubMed] [Google Scholar]
- 27.Reyes J, Gerber D, Mazariegos GV, et al. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg 2000; 35: 283–290. [DOI] [PubMed] [Google Scholar]
- 28.Busuttil RW, Goss JA. Split liver transplantation. Ann Surg 1999; 229: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogiers X, Malago M, Habib N, et al. In situ splitting of the liver in heart-beating cadaveric organ donor for transplantation in two recipients. Transplantation 1995; 59: 1081–1083. [PubMed] [Google Scholar]
- 30.Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers: the ultimate expansion of the donor pool. Ann Surg 1996; 224: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goss JA, Yersiz H, Shackleton CR, et al. In situ splitting of the cadaveric liver for transplantation. Transplantation 1997; 64: 871–877. [DOI] [PubMed] [Google Scholar]
- 32.Ghobrial RM, Farmer DG, Yersiz H, et al. Split liver transplantation for expansion of the donor pool. Transplantation 1998; 67: S548. [Google Scholar]
- 33.Marcos A, Fisher R, Ham J, et al. Right lobe living donor liver transplantation. Transplantation 1999; 68: 798–803. [DOI] [PubMed] [Google Scholar]
- 34.Goss JA, Shackleton CR, McDiarmid SV. Long-term results of pediatric liver transplantation. An analysis of 569 transplants. Ann Surg 1998; 228: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes J, Gerber D, Mazariegos GV, et al. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg 2000; 35: 283–290. [DOI] [PubMed] [Google Scholar]
- 36.Ghobrial RM, Farmer DG, Baquerizo A, et al. Orthotopic liver transplantation for hepatitis C: outcome, effect of immunosuppression and causes of retransplantation during an eight-year single-center experience. Ann Surg 1999; 229: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graziadel IW, Wiesner RH, Marotta PJ, et al. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology 1999; 30: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 38.Asfar S, Metrakos P, Fryer J, et al. An analysis of late deaths after liver transplantation. Transplantation 1996; 61: 1377–1381. [DOI] [PubMed] [Google Scholar]
- 39.Ryckman FC, Alonso MH, Bucuvalas JC, Balistreri WF. Long-term survival after liver transplantation. J Pediatr Surg 1999; 34: 845–850. [DOI] [PubMed] [Google Scholar]
- 40.Wong T, Devlin J, Roland N, et al. Clinical characteristics affecting the outcome of liver retransplantation. Transplantation 1997; 64: 878–882. [DOI] [PubMed] [Google Scholar]
- 41.Markmann JF, Markowitz JS, Yersiz H, et al. Long-term survival after retransplantation of the liver. Ann Surg 1997; 226: 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doyle HR, Morelli F, McMichael J, et al. Hepatic retransplantation: an analysis of risk factors associated with outcome. Transplantation 1996; 61: 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baliga P, Merion RM, Turcotte JG, et al. Preoperative risk factor assessment in liver transplantation. Surgery 1992; 112: 704–710. [PubMed] [Google Scholar]
- 44.Spanier TB, Klein RD, Nasrawy SA, et al. Multiple organ failure after liver transplantation. Crit Care Med 1995; 23: 466–473. [DOI] [PubMed] [Google Scholar]
- 45.Figuras J, Busquets J, Grande L, et al. The deleterious effect of donor high plasma sodium and extended preservation in liver transplantation. A multivariate analysis. Transplantation 1996; 61: 410–413. [DOI] [PubMed] [Google Scholar]
- 46.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology 1994; 20: 829–838. [DOI] [PubMed] [Google Scholar]
- 47.Mimeault R, Grant D, Ghent C, Duff J, Wall W. Analysis of donor and recipient variables and early graft function after orthotopic liver transplantation. Transplant Proc 1989; 21: 3355. [PubMed] [Google Scholar]
- 48.Mor E, Klintmalm GB, Gonwa TA, et al. The use of marginal donors for liver transplantation. Transplant Proc 1992; 53: 383–386. [DOI] [PubMed] [Google Scholar]
- 49.Ploeg RJ, D’Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation: a multivariate analysis. Transplantation 1993; 55: 807–813. [DOI] [PubMed] [Google Scholar]
- 50.Cacciarelli TV, Esquivel CO, Moore DH, et al. Factors affecting survival after orthotopic liver transplantation in infants. Transplantation 1997; 64: 242–248. [DOI] [PubMed] [Google Scholar]