Abstract
Objective
To determine the treatment efficacy, safety, local tumor control, and complications related to radiofrequency ablation (RFA) in patients with cirrhosis and unresectable hepatocellular carcinoma (HCC).
Summary Background Data
Most patients with HCC are not candidates for resection because of tumor size, location, or hepatic dysfunction related to cirrhosis. RFA is a technique that permits in situ destruction of tumors by means of local tissue heating.
Methods
One hundred ten patients with cirrhosis and HCC (Child class A, 50; B, 31; C, 29) were treated during a prospective study using RFA. Patients were treated with RFA using an open laparotomy, laparoscopic, or percutaneous approach with ultrasound guidance to place the RF needle electrode into the hepatic tumors. All patients were followed up at regular intervals to detect treatment-related complications or recurrence of disease.
Results
All 110 patients were followed up for at least 12 months after RFA (median follow-up 19 months). Percutaneous or intraoperative RFA was performed in 76 (69%) and 34 patients (31%), respectively. A total of 149 discrete HCC tumor nodules were treated with RFA. The median diameter of tumors treated percutaneously (2.8 cm) was smaller than that of lesions treated during laparotomy (4.6 cm). Local tumor recurrence at the RFA site developed in four patients (3.6%); recurrent HCC subsequently developed in other areas of the liver in all four. New liver tumors or extrahepatic metastases developed in 50 patients (45.5%), but 56 patients (50.9%) had no evidence of recurrence. There were no treatment-related deaths, but complications developed in 14 patients (12.7%) after RFA.
Conclusions
In patients with cirrhosis and HCC, RFA produces effective local control of disease in a significant proportion of patients and can be performed safely with minimal complications.
Hepatocellular carcinoma (HCC) is a common cause of death in patients with cirrhosis. Cirrhosis develops as a result of chronic liver injury secondary to extrinsic environmental factors in the vast majority of affected individuals. Hepatitis B or C virus infection, aflatoxin B1 or other mycotoxin ingestion, and prolonged ethanol abuse are the major causes of cirrhosis. 1 The worldwide population at risk for developing HCC because of one or more of these etiologic factors numbers in the tens of millions: in the United States and Italy, for example, there are an estimated 7.5 million persons with chronic hepatitis C virus infection. 2,3
In most patients with HCC, treatment options are limited by the liver dysfunction caused by chronic inflammation and cirrhosis. Although complete surgical resection of HCC offers the best chance of long-term survival, cirrhosis may limit the amount of parenchymal resection that will be tolerated and increases the risk of postoperative liver failure and death. 4–6 Systemic or regional chemotherapy is at best palliative in a small subset of patients (although rarely patients have sufficient tumor downstaging to convert an unresectable to a resectable lesion), but at the cost of significant side effects and a reduced quality of life. 1 In most patients with cirrhosis and HCC confined to the liver, resection is not safe, and local tumor-ablation therapies are considered as alternative treatment options.
Radiofrequency ablation (RFA) is a thermal treatment technique designed to produce localized tumor destruction by heating tumor tissue to temperatures that exceed 50°C. When tumor cells are heated to temperatures above 45° to 50°C for more than 3 minutes, intracellular protein denaturation and melting of lipid bilayers results in direct tumor cell death. 7–10 RFA uses alternating current passed across needle electrode arrays placed directly into the tumor. Ionic stimulation induced by the alternating current in tissue surrounding the electrode array produces gradual frictional heating, and the tissue temperatures rise to 80° to 110°C, which results in coagulative necrosis of the tissue in proximity to the electrode. The basic principle is similar to that of the surgical electrocautery units used to achieve intraoperative hemostasis.
We performed this prospective study using RFA to treat HCC in patients with cirrhosis to determine treatment efficacy, safety, local tumor control, patterns of failure, and treatment-related complications.
METHODS
All patients were enrolled in a prospective, ongoing protocol, approved by the institutional review boards at the University of Texas M.D. Anderson Cancer Center and the G. Pascale National Cancer Institute, evaluating RFA of malignant liver tumors. A detailed verbal and written description of the procedure was provided to all patients, and informed written consent was obtained before treatment. All patients were deemed to have unresectable hepatic disease based on tumor multifocality, tumor proximity to major vascular structures precluding a margin-negative resection, or the presence of severe cirrhosis with functional hepatic reserve inadequate to tolerate the necessary hepatic resection. Patients were considered for RFA even if they had tumor abutting a major portal or hepatic vein branch or the inferior vena cava, but they were excluded if tumor involved the main right or left bile duct (or both) because of the likelihood of destruction of the major bile ducts by RFA. All patients in this series had a core biopsy of the liver documenting cirrhosis.
Baseline evaluation included a history and physical examination; serum laboratory tests consisting of a complete blood count, platelets, coagulation profile, hepatitis B and C virus serology, renal panel, electrolytes, albumin, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, alkaline phosphatase, total bilirubin, and serum alpha-fetoprotein (AFP); computed tomography (CT) or magnetic resonance imaging (MRI) scan of the abdomen and pelvis; and a chest radiograph. Patients were excluded from RFA if their platelet count was less than 40,000/μL or if the prothrombin time was prolonged more than 1.5 times above normal. However, if platelet or fresh-frozen plasma transfusions corrected the abnormal laboratory values to meet these criteria, the patient received treatment. The same battery of serum blood tests was obtained 7 days after RFA, then again 1 month after treatment. At 1 month and then every 3 months up to 3 years after treatment, a CT or MRI scan of the abdomen, a chest radiograph, and the same serum laboratory tests were obtained. Based on the clinical history, physical examination, and serum laboratory findings, the clinical severity of the patient’s cirrhosis was scored using the Child-Turcotte-Pugh system. 11 All patients had histologic confirmation of HCC from prior intraoperative biopsy or from CT- or ultrasound-guided percutaneous fine-needle aspiration.
Patients with small cancers that were easily imaged on transabdominal ultrasonography, particularly those with cirrhosis too severe to tolerate a celiotomy, were considered for percutaneous ultrasound-guided RFA. A biopsy guide attached to the transabdominal ultrasound probe was used to assist RFA needle electrode placement for percutaneous treatment; the ultrasound biopsy guide was not used for laparoscopic or intraoperative treatment.
All patients undergoing percutaneous RFA were treated in the operating room and received intravenous anesthesia consisting of a propofol infusion (3–6 mg/kg/hr) in combination with a remifentanil infusion (0.05–0.075 μg/kg/min). The propofol and remifentanil infusion rates were adjusted based on the patient’s respiratory rate and analgesic request. All patients breathed spontaneously an enriched oxygen mixture at 35% to 50%. When the RFA treatment was completed, the propofol and remifentanil infusions were stopped and the patient was allowed to recover.
All other patients, primarily those with larger tumors, tumors abutting major intrahepatic blood vessels, or tumors near the liver capsule where percutaneous treatment could produce thermal injury to an adjacent organ, were treated during a laparoscopic or open surgical procedure under general anesthesia. Intraoperative or laparoscopic ultrasonography was used to place the RF needle into the lesions to be treated in these patients.
Patients were treated using the RF 2000 generator system (RadioTherapeutics Corp., Mountain View, CA). The RF 2000 system consists of a generator that supplies up to 100 W of power, a LeVeen monopolar array needle electrode (3.5-cm maximum array diameter), and two indifferent dispersive electrode pads applied to the patient’s skin (much like the grounding pads used for hemostatic electrocautery during surgical procedures). The LeVeen needle electrode is a 15-gauge, 15- to 25-cm-long insulated cannula that contains 10 individual hook-shaped electrode arms that are deployed in situ after ultrasound-guided placement of the needle electrode into the liver tumor. For tumors less than 2.5 cm in diameter, the multiple array is deployed into the center of the tumor. For larger lesions, the array is first deployed at the most posterior interface (ultrasonographically) between tumor and normal liver parenchyma; it is subsequently withdrawn and redeployed at 2.0- to 2.5-cm intervals in the tumor (Fig. 1). Optimal positioning of the electrode permits complete destruction of tumor and at least a 1-cm zone of normal liver parenchyma.
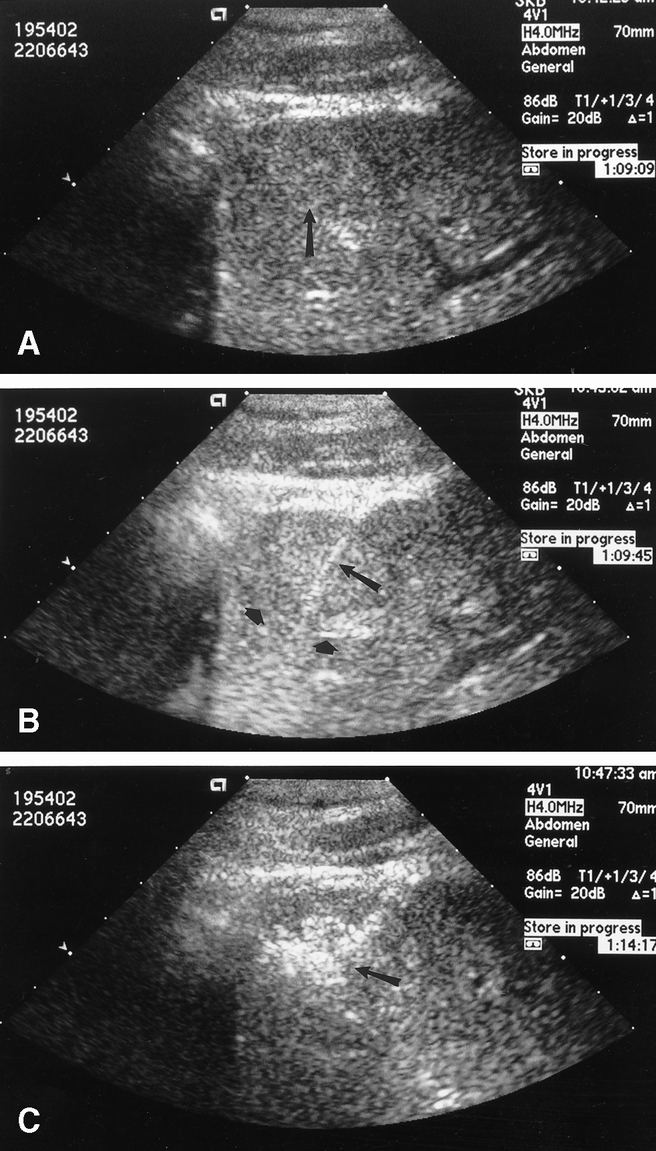
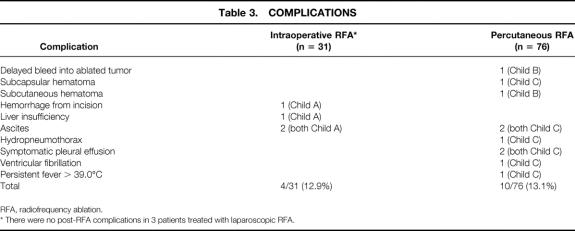
Figure 1. (A) Transabdominal ultrasonography demonstrating a solitary hepatocellular carcinoma (arrow). (B) The radiofrequency ablation needle (long arrow) has been placed and the multiple array opened (short arrows) at the deep interface between tumor and hepatic parenchyma. (C) The area of tumor and liver treated with radiofrequency ablation becomes hyperechoic on ultrasound (arrow). The multiple array is subsequently retracted back into the needle electrode sheath, the needle is pulled back approximately 2 cm, and then the array is redeployed to complete the radiofrequency ablation treatment of the tumor and a rim of surrounding hepatic parenchyma.
After deployment of the multiple array, the initial power is applied at 50 W and then increased in 10-W increments at 1, 2, 3, and 4 minutes to a maximum power of 90 W. Treatment continues until power roll-off occurs, indicating a precipitous drop in power output as tissue impedance increases markedly from coagulative necrosis. After a 20-second pause, power is reapplied at 75% of the maximum power achieved until power roll-off again occurs. Thus, each tumor or area within a large tumor is treated with a two-phase application of RF power before retracting the multiple array and repositioning or removing the needle electrode.
All patients were followed up to detect acute or chronic complications related to the RFA treatment. Patients treated with percutaneous RFA underwent a CT or MRI scan 1 week after treatment to assess for evidence of viable, incompletely treated tumor. If any areas suspicious for viable tumor were detected, the patient was immediately retreated with percutaneous RFA of the area. Abdominal CT or MRI scans, chest radiographs, and serum AFP tests were performed every 3 months to detect evidence of residual or recurrent tumor in the RFA lesions and to monitor for the development of new hepatic or extrahepatic metastatic disease.
The statistical analysis of differences between results was determined using a signed rank test and analysis of variance (Statview 4.5, Abacus Concepts, Inc., Overland Park, KS). Significance was determined at the 95% confidence interval.
RESULTS
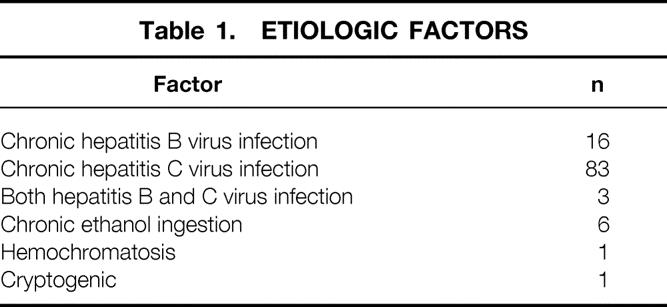
Only patients who had been followed up for at least 12 months after RFA of HCC were included in this report. All 110 patients met this criterion; none were lost to follow-up. The median follow-up in this group was 19 months. There were 71 men and 39 women, with a median age of 59 years (range 33–81). The risk factors for cirrhosis are listed in Table 1. The Child-Turcotte-Pugh class at the time of RFA treatment was A in 50 patients (45.4%), B in 31 (28.2%), and C in 29 (26.4%).
Table 1. ETIOLOGIC FACTORS
RFA was used to destroy a total of 149 discrete, ultrasonographically detectable HCC tumor nodules in the 110 patients. RFA was used to treat one tumor in 85 patients (77.3%), two tumors in 14 patients (12.7%), three tumors in 8 patients (7.3%), and four tumors in 3 patients (2.7%). Percutaneous RFA was performed in 76 patients (69.1%), 31 patients (28.2%) were treated during an open celiotomy, and 3 patients (2.7%) were treated with a laparoscopic approach. There was no difference in the age or sex distribution of patients treated with an intraoperative versus a percutaneous approach. Of the 34 patients treated during an open or laparoscopic procedure, 33 had Child class A disease and one had class B. In contrast, most patients with clinically severe cirrhosis were treated with a percutaneous approach (class A, 17 patients; class B, 30 patients; class C, 29 patients;P < .01 vs. intraoperative approach).
None of the 34 patients treated with an intraoperative or laparoscopic approach had evidence of viable residual tumor requiring retreatment on the first postoperative CT or MRI scan. However, 6 of the 76 patients (7.9%) treated with a percutaneous approach had evidence of incompletely treated tumor on the first post-RFA imaging study, necessitating immediate retreatment with percutaneous RFA of the residual viable tumor areas.
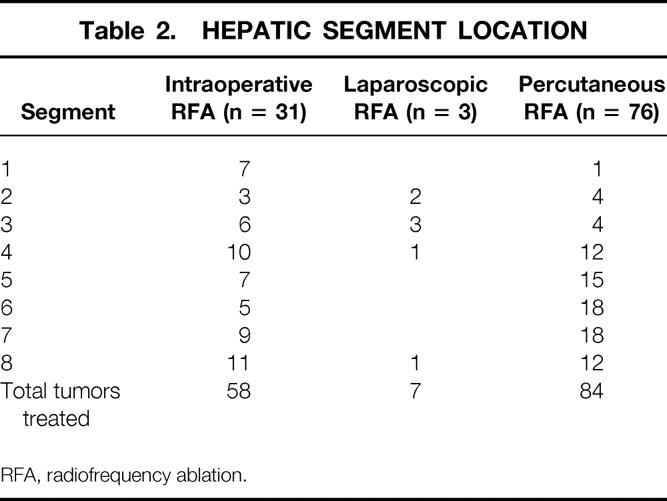
The tumors treated percutaneously were smaller than those treated during laparotomy or laparoscopy (2.8 ± 0.8 cm vs. 4.6 ± 1.7 cm, respectively;P < .01). The HCC tumor nodules treated with RFA were found in all segments of the liver (Table 2). 12
Table 2. HEPATIC SEGMENT LOCATION
RFA, radiofrequency ablation.
Serum AFP values were elevated (>5.0 ng/mL) in 79 patients (71.8%) at the time of diagnosis of HCC. Serum AFP levels were remeasured 1 month after RFA treatment: AFP values after RFA in all 110 patients (4.5 ± 0.4 ng/mL) were significantly lower than the AFP measurements before RFA treatment (118.4 ± 24.2 ng/mL, P < .01). The posttreatment serum AFP values in the 79 patients with elevated serum AFP levels before RFA were also significantly lower than pre-RFA values (6.1 ± 0.5 ng/mL vs. 194.4 ± 42.9 ng/mL, respectively;P < .01). Serum AFP values did not return to less than 5.0 ng/mL in 26 of the 79 patients (32.9%) whose AFP levels were elevated before RFA; these 26 had AFP values after RFA that ranged from 5.2 to 20.0 ng/mL.
There were no deaths during or in the 90 days after RFA in these 110 patients. Treatment-related complications developed in 14 patients (12.7%, Table 3). Treatment-related complications arose in 4 of the 50 patients with Child class A disease (8%), 2 of the 31 class B patients (6.5%), and 8 of the 29 class C patients (27.6%). The four patients in whom ascites developed after RFA treatment were treated successfully with diuretic therapy. The patient with a hydropneumothorax and the two with symptomatic pleural effusions required drainage of the affected hemithorax for only 2 to 4 days. Ventricular fibrillation developed at the conclusion of a percutaneous RFA treatment in one patient; this resolved after a single electrical discharge using low-voltage transthoracic cardioversion paddles. Subsequent evaluation showed no evidence of myocardial ischemia or injury. A single patient had persistent high fevers for 10 days after RFA; two separate aspirations of the treated lesion showed no bacterial, fungal, or acid-fast organism growth, and the fever resolved spontaneously. Upper abdominal pain developed in one patient 4 days after percutaneous RFA; this patient was found to have bled into the ablated tumor and was treated with transfusion of two units of packed red blood cells and transarterial embolization of the right hepatic artery. A single subcapsular and a single subcutaneous hematoma were noted and were associated with local pain for 1 week after percutaneous RFA, but neither patient required blood transfusion, and symptoms resolved within 1 week. A single patient had persistent bleeding from the subcostal wound after an intraoperative RFA; it was successfully treated with reoperation and suture ligation of several bleeding points in the subcutaneous tissue. Transient jaundice developed in one patient with a serum total bilirubin level elevated to 8.5 mg/dL and an elevation of the prothrombin time to 1.6 times the normal value. The patient received two units of fresh-frozen plasma intravenously, and the serum bilirubin level and prothrombin time returned to normal within 2 weeks of the RFA treatment. No other patients had clinically significant coagulopathies or thrombocytopenia requiring transfusion.
Table 3. COMPLICATIONS
RFA, radiofrequency ablation.
* There were no post-RFA complications in 3 patients treated with laparoscopic RFA.
No patient had evidence of thermal injury to adjacent structures or organs (diaphragm, kidney, stomach, duodenum, or colon), nor were there any biliary fistulas or bile collections. All of the patients treated percutaneously or laparoscopically were discharged from the hospital 1 day after RFA treatment. However, 9 of the 10 patients who had complications after percutaneous RFA were readmitted to the hospital briefly for management of their complications (the patient with ventricular fibrillation did not require readmission). The average hospital stay for patients treated with intraoperative RFA was 4 days (range 2–7).
In 4 of the 110 patients (3.6%), HCC recurred at the periphery of lesions treated with RFA, but HCC recurred in only 4 of the 149 tumors treated (2.7%). All four local recurrences developed in tumors more than 4.0 cm in diameter (range 4.0–7.5)—one treated during open laparotomy, one treated laparoscopically, and two treated percutaneously. All local recurrences were evident within 6 months after RFA. There were no local recurrences in any of the six tumors that required two RFA sessions because of incomplete tumor necrosis after the first percutaneous RFA treatment. There were no central recurrences of tumor; the recurrence at the periphery of the ablated tumor must be considered to be incomplete ablation of the HCC. All four of the patients in whom a local recurrence developed also had evidence of metastases at other sites.
At a median follow-up of 19 months, HCC recurred in 54 patients (49.1%). The site of first recurrence was local in 4 patients, other sites in the liver in 37 patients, pulmonary metastases in 9 patients, bone metastases in 2 patients, peritoneal metastases in 1 patient, and adrenal metastases in 1 patient. In 14 of the 37 patients (37.8%) in whom a new solitary site of recurrence developed in the liver, the new tumor was treated with percutaneous RFA. The median follow-up after the second RFA treatment in these 14 patients was only 6 months, but 12 of the 14 had no evidence of new hepatic or extrahepatic metastases. Recurrent HCC developed in 37 of the 85 patients (43.5%) who underwent RFA of a solitary HCC, in 8 of the 14 (57.1%) with RFA of two tumors, in 6 of the 8 (75%) with RFA of three tumors, and in all 3 of the patients with RFA of four tumors. Of the 54 patients in whom recurrent HCC developed after RFA, 53 were diagnosed within 12 months of the initial RFA treatment; a single patient was diagnosed with new lung metastases 15 months after RFA.
The survival and disease status of all 110 patients are known. Twenty-eight patients (25.4%, 14 treated percutaneously, 14 treated surgically) died as a result of recurrent HCC, 26 patients (25.5%, 16 treated percutaneously, 10 treated surgically) were alive but had developed recurrent HCC, 53 patients (48.2%, 43 treated percutaneously, 10 treated surgically) were alive with no evidence of hepatic or extrahepatic recurrence of HCC, and 3 patients (2.7%, all treated percutaneously) died of causes not related to HCC (myocardial infarction, hepatic failure, bleeding gastroesophageal varices). The 14 patients in whom a solitary hepatic recurrence of HCC developed and was retreated with RFA are included in the 26 patients considered alive with recurrent disease. As noted above, 12 of these 14 patients had no radiographic evidence of new metastases but are included in the group of patients who had recurrence after the initial RFA treatment. Twelve of these 14 patients were alive as of this writing with no evidence of recurrent HCC. Thus, at the time of this report, 65 patients (59.1%) were alive without radiographic evidence of recurrent HCC. Twenty-four of the 26 patients (92.3%) whose serum AFP values did not return to normal after the initial RFA treatment are included in the 54 patients who were subsequently diagnosed with recurrent HCC.
DISCUSSION
The use of heat to treat human neoplasms is not a recent development. The first recorded descriptions of therapeutic thermal tumor ablation come from the Edwin Smith and Ebers surgical papyri, describing the use of cautery with heated implements or oils to destroy tumors. 13 The practices described in these papyri date to the 2nd Egyptian Dynasty and the physician Imhotep approximately 5,000 years ago. 13 The intentional application of an electrical current to produce thermal ablation of tumors can be traced to the early 20th century. In 1909, Pozzi coined the term “fulguration” in a description of curative treatment of superficial skin cancers with a high-frequency Oudin spark. 14 Doyen subsequently applied a groundplate to patients to improve electrofulguration of tumors. 15 Electrocautery devices are now routinely used by surgeons to achieve hemostasis during surgical procedures.
Simple, straight-needle electrodes for RFA of human tumors are used infrequently because of the small (usually <1.0 cm in diameter) zones of necrosis produced around the needle. The geometry of the RF current pathway around the ablation electrode creates a relatively uniform zone of radiant/conductive heat in the first few millimeters of electrode–tissue interface. The conductive heat emitted from the tissue radiates out from the electrode, and if the tissue impedance is low, a dynamic expanding sphere of ablated tissue is created. If the tissue is heated and desiccated too rapidly, a high-impedance tissue coagulum forms around the needle electrode and markedly reduces further propagation of heat into the tissue surrounding the electrode. When the tissue is heated gradually, the final size of the zone of heat-ablated tissue is proportional to the square of the RF current, also known as the RF power density. The RF power/current delivered using a monopolar electrode with an indifferent dispersive grounding pad decreases in proportion to the square of the distance from the electrode. Thus, the tissue temperature falls rapidly with increasing distance from the electrode, resulting in temperatures high enough to produce coagulative necrosis of tissue within only 5 to 15 mm of the needle electrode.
Novel RF needle electrode designs have been developed with multiple-array hook electrodes that are deployed from the needle tip into the tumor. 16–18 The insulated 14- to 18-gauge needle electrode shaft is placed into the tumor with the multiple-hook array retracted. Using real-time ultrasonographic guidance, the array is deployed from the needle tip into the tumor. The multiple-array hook electrodes are available in maximum diameters of 2.0 to 5.0 cm. In contrast to the small areas of tumor tissue ablation created by simple needle electrodes, multiple-array electrodes, such as the LeVeen electrode we used, can produce zones of coagulative necrosis 2.0 to 6.0 cm in diameter.
The incidence of HCC in Western countries is relatively low compared with other solid cancers, but worldwide HCC is one of the most common human malignancies, with approximately 1 million new cases diagnosed annually. 1 The geographic variation in the incidence of HCC is largely related to the endemic or hyperendemic incidence of hepatitis B or C virus infection in corresponding regions. 19–24 Programs that screen high-risk viral hepatitis patients for HCC usually use serum AFP and transabdominal ultrasonography in an attempt to detect early-stage, treatable HCC. Unfortunately, even aggressive screening programs diagnose resectable HCC in less than half of the patients. 25 Thus, we are investigating the use of RFA to treat patients with cirrhosis and unresectable HCC based on tumor location, multifocality, or severity of cirrhosis.
Other local therapies have been used to treat HCC. Direct image-guided injection of absolute ethanol has been used extensively around the world. Percutaneous ethanol injection (PEI) is usually performed under transabdominal ultrasonographic guidance, with the tumor injected with 5 to 10 mL of ethanol twice a week. The volume of ethanol required to ablate the tumor is estimated based on the diameter of the HCC. For tumors less than 2 cm, three to five injection sessions are required; five to eight sessions are necessary for tumors 2 to 3 cm in diameter. 26 A study of 207 HCC patients with tumors less than 5 cm in diameter treated with PEI was performed in Italy. 27 The HCC was solitary in 162 patients and multiple in 45. In the 162 patients with solitary tumors, the 1-year survival rate after PEI was 90% and the 3-year survival rate was 63%. In contrast, the 3-year survival rate in patients with multiple tumors injected with ethanol was only 31%. Patient compliance with PEI has been a problem because of the multiple injections required and the pain associated with the treatment, but serious complications such as intraperitoneal hemorrhage, hepatic insufficiency, bile duct necrosis or biliary fistula, hepatic infarction, and hypotension occur in less than 5% of patients. 28 Local recurrence rates have been reported infrequently in most studies of PEI. Most reports mention that local recurrence is common in tumors greater than 5 cm in diameter and recommend that PEI not be used to treat such large HCCs. 26–28 However, a recent report of PEI in tumors less than 3 cm in diameter found a local recurrence rate of 38%. 29 After a 3-year follow-up of all patients, HCC had recurred locally or at other intrahepatic sites in 81% of the patients. 29 Because PEI requires multiple treatment sessions and was associated with a high local recurrence rate, the authors recommended that PEI be considered only for tumors less than 1.5 cm in diameter and that all other patients with small HCCs be treated with resection or other definitive, one-treatment ablation techniques, such as RFA.
Hepatic cryotherapy has been used during the past two decades to treat patients with unresectable primary and metastatic liver tumors. Most information on local tumor recurrence and complications after cryotherapy comes from patients treated for colorectal cancer liver metastases. In these patients, the rate of local recurrence in the cryoablated tumor has been reported to range from 2.5% to 44%. 30 In a collective review of the reported experience with hepatic cryotherapy, the overall treatment-related death rate was only 1.6%, but the overall complication rate was almost 50%. 30 Complications after cryoablation of hepatic tumors included cracking of the iceball, hemorrhage, coagulopathy, thrombocytopenia, myoglobinuria, acute renal failure, symptomatic pleural effusion, biloma/biliary fistula, abscess, and intraabdominal infection. The largest published series using cryotherapy to treat HCC in 235 patients reported that there were no treatment-related deaths, but complications and local recurrence in the cryoablated tumors was not reported. 31 This study is also difficult to interpret because cryotherapy alone was used in only 78 patients (33.2%); most of the patients were treated with cryotherapy plus hepatic artery ligation, transarterial chemoembolization, hepatic artery infusion chemotherapy, or resection of the frozen tumor. Our group has abandoned the use of cryotherapy to treat primary or metastatic liver tumors based on our finding of a significantly higher local tumor recurrence rate with cryoablation compared with RFA (13.6% vs. 2.2%, P < .01) and a much higher complication rate after cryoablation (40.7% vs. 3.3%, P < .001). 32
Heat ablation of liver tumors can also be performed using microwave coagulation therapy or laser-induced thermotherapy. 33–35 Like RFA, these procedures can be performed during an open laparotomy, with laparoscopic or thoracoscopic guidance, or percutaneously. The effectiveness of these treatments is currently limited by the small zones of necrosis achieved with the rapid heating and desiccation of the tissue around the microwave or laser probes. Multiple insertions of the probes are required to treat tumors more than 1 cm in diameter with microwave coagulation therapy, or more than 2 cm in diameter with laser-induced thermal ablation. 33–35 Currently, the treatment complexity of placing multiple intratumoral probes and the cost for these microwave or laser systems (at least 10 times higher than RF generators and needle electrodes) limits the clinical utility of these alternative thermal ablation techniques.
Another treatment that is often considered in patients with cirrhosis and HCC, particularly those with solitary, small tumors, is orthotopic liver transplantation (OLT). The risk of cancer recurrence in patients undergoing liver transplantation for HCC is high in patients with tumors greater than 5 cm in diameter, vascular invasion by tumor, poorly differentiated tumors, multicentric tumors, or extrahepatic disease. 36 Many transplant centers will not consider liver transplantation in HCC patients with any of these negative prognostic factors. In patients with small (<5 cm in diameter), solitary, encapsulated HCC who undergo OLT, the 5-year overall survival rate is almost identical to that for patients undergoing liver transplantation for nonmalignant liver disease. 37 Thus, OLT is a valid consideration in this highly selected group of HCC patients. OLT should not be considered instead of resection if patients have a resectable HCC and adequate functional hepatic reserve to tolerate the resection. Studies comparing the outcome of patients who underwent resection versus liver transplantation for HCC have demonstrated similar survival and recurrence rates. 37–39 Thus, OLT should be considered for patients with three or fewer HCC tumors that are small and are deemed unresectable based on tumor location or severity of cirrhosis. Unfortunately, the length of time patients must spend waiting for a suitable donor organ has limited the effectiveness of OLT. Some HCC patients on transplant waiting lists drop out because of tumor progression and the development of negative prognostic findings during a prolonged period waiting for a donor organ. Some transplant surgeons are using percutaneous or laparoscopic RFA to treat HCCs detected in patients with cirrhosis on the transplant waiting list in an attempt to attain local control of tumor and prevent progression (Robert Goldstein, MD, personal communication, April 2000).
We found a relatively low treatment-related complication rate after RFA because of careful treatment planning. We have avoided thermal injury to adjacent structures such as the diaphragm or bowel by using an open or laparoscopic approach to retract adjacent structures away from tumors near the liver capsule. Careful review of pretreatment imaging is important for patients treated percutaneously to avoid thermal injury to adjacent structures. Proper monitoring and state-of-the-art anesthesia care are also key factors to enhance the rate of successful percutaneous RFA treatment. Local recurrence rates after percutaneous RFA are higher in studies performed by interventional radiologists, suggesting the importance of appropriate oncologic surgical evaluation and management of these patients. 40
An advantage of percutaneous RFA is the short hospital stay required after treatment. In properly selected patients, it should be possible to perform percutaneous RFA on an outpatient, day surgery basis.
One important disadvantage to RFA in general is the inability to determine accurately the exact area that has been coagulated using real-time ultrasonographic monitoring. Unlike the easily defined margin of the cryoablation iceball seen ultrasonographically, there is no distinct demarcation between RF-ablated and viable tissue. This is not an issue if the HCC is small (<2.5 cm in diameter) and will be destroyed by a single placement of the multiple-array electrode and application of an RF treatment cycle. It is a problem when multiple overlapping zones of tumor ablation must be performed to ensure destruction of the tumor and a surrounding rim of hepatic parenchyma. The thermally ablated tissue becomes hyperechogenic on ultrasound, making it impossible to distinguish the deep margin of the ablation zone. Thus, when multiple placements of the RF needle electrode are needed, the deep junction of the tumor and hepatic parenchyma should be treated first by opening the multiple array at that interface between tumor and liver. The needle is then progressively pulled back more superficially into the tumor at approximately 2.0-cm intervals to create overlapping zones of coagulative necrosis (see Fig. 1).
Intraoperative or laparoscopic ultrasonography provides better resolution of the tumor and RFA treatment compared with transabdominal ultrasonography for percutaneous treatment, which provides one probable explanation for the finding of no incomplete ablations in the 65 HCCs treated during laparotomy or laparoscopy compared with the 7.1% (6/84) incidence of incomplete RFA in the HCCs treated percutaneously. Incomplete tumor destruction has been reported in up to 18% of liver cancers treated percutaneously with RFA, underscoring both the importance of careful treatment planning to overlap the zones of necrosis and the need for early post-RFA CT or MRI to detect incompletely treated tumor after percutaneous RFA. 40 Our local recurrence rate of 3.6% after RFA of HCC is similar to local recurrence rates of 6% to 12% reported in recent surgical RFA series. 17,18 Currently, we are evaluating color flow Doppler ultrasonography with and without intravenously administered ultrasound contrast (Levovist, Schering Plough, Kenilworth, NJ) to determine whether this technique will improve our accuracy in monitoring the zone of coagulative necrosis induced by RFA, thus reducing the incidence of incomplete treatment and local recurrence.
Resection, PEI, cryoablation, RFA, and other techniques of thermal ablation are treatments designed to achieve local control of malignant hepatic tumors. Resection or local destruction of tumor can produce long-term disease-free and overall survival in a subset of patients but cannot overcome the tumor biology in patients who already have micrometastatic disease at the time of their initial therapy. Even though we had a local failure rate of only 3.6%, recurrent HCC developed almost half the patients (49.1%) after RFA. Just as a second liver resection can be performed in a few patients, it was possible to perform a second RFA treatment for a solitary liver recurrence of HCC in 14 of our 110 patients (12.7%). In the patients who had an elevated serum AFP level before RFA treatment, AFP was a useful marker both for recurrence of cancer and for the presence of subclinical, untreated disease. HCC recurred in more than 90% of the patients whose serum AFP value did not return to normal after RFA. Unfortunately, serum AFP levels are not elevated in all HCC patients, particularly in those with small tumors. Serum AFP values are normal in 40% of patients with HCC less than 2 cm in diameter and in 27.5% of those with tumors 2 to 5 cm in diameter. 41 Serum AFP levels were not elevated in 28.2% of our patients, but in the patients who did have elevated levels, the test was useful to assess for recurrence of HCC.
We believe RFA is a safe and effective local treatment option in patients with cirrhosis and unresectable small HCC. Using current RFA devices, it is difficult to destroy reliably tumors greater than 5 to 6 cm in diameter, but new equipment to produce larger zones of ablation is being investigated. We are performing a randomized, prospective trial comparing RFA with PEI in patients with HCC less than 4.0 cm in diameter to determine local control and overall survival rates. Clearly, RFA must be considered as no more than an effective technique to produce local control in most liver cancer patients, with the possibility of producing long-term, disease-free survival in some of these patients. To improve the overall outcome and survival in HCC patients, we must continue to search for aggressive multimodal treatment programs to eradicate micrometastatic disease that can be combined with treatments such as RFA to destroy macroscopic liver tumors.
Discussion
Dr. John M. Daly (New York, New York): Hepatocellular carcinoma is the most common malignancy worldwide. In this country particularly, the occurrence of hepatitis C should continue to lead to an increasing incidence of primary liver tumors. Because of poor responses to medical therapy, new approaches are clearly needed.
As Dr. Curley and his colleagues have noted, frequently the size, location, the number of tumor nodules and the underlying liver function preclude operative resection of hepatocellular cancers. Local ablative treatments therefore do have a role. I would like to emphasize several points from his presentation and ask several questions.
Percutaneous approaches were used in 69% of patients and laparoscopic approaches were used in only 3%. Now, many investigators have noted additional lesions when disease is staged by laparoscopy, perhaps as many as 15% to 30% of patients. In your own series, incomplete ablation was more common with the percutaneous approach, 7% versus zero, compared with those that either had open or laparoscopic approaches. Does this not suggest that laparoscopy should be more liberally applied in your methodology?
Would CT scan be a better method to observe needle placement and tumor destruction if the percutaneous method is used? New liver lesions were seen in 37 patients during your follow-up time of at least a year. Were they more common in the patients who were treated percutaneously than those who were treated using either the open or laparoscopic approach?
As expected, complications are more common in Child class C patients. If these patients have two or more lesions, because of their complication rate and because of the frequency of local regional recurrence, could you use a predictive model in which you would say some of these patients are not candidates for this particular approach?
Finally, as you pointed out, this is a local ablative technique. What do we have on the horizon that might diminish new liver lesions in these patients, most of whom, as you suggested, are infected with hepatitis B or C? Can we use radiofrequency ablation as a bridge to transplantation? Does regional therapy have a role?
Presenter Dr. Steven A. Curley (Houston, Texas): In answer to your first question regarding increasing the role of laparoscopy, my feeling on that is a definite yes, we are now using laparoscopically guided RF ablation more often. In the experience presented today, we had only three patients treated laparoscopically; subsequently we have treated another 14 or 15 patients laparoscopically. These more recently treated patients are not included in this report because I don’t have 12-month follow-up data on them.
Interestingly enough, we have found in this small, anecdotal group treated laparoscopically that we diagnosed one or two small satellite nodules using laparoscopic ultrasonography in four patients that were not detected on preoperative imaging. Clearly, I think laparoscopic ultrasonography may have a role in detecting small hepatic tumors not seen on preoperative imaging studies. It is also possible to detect extrahepatic disease in some patients using a laparoscopic approach. So, if the patient will tolerate a laparoscopic procedure, many of the patients we treated percutaneously in the series reported today are now treated with a laparoscopic approach.
Regarding your question about CT-directed radiofrequency ablation, ultrasound is a readily available and inexpensive technique. We have not used CT guidance for radiofrequency ablation procedures. There are a number of radiologists around the country who are using CT-directed RFA of liver tumors. CT guidance is more time- and labor-intensive. The other problem with CT-directed radiofrequency ablation is that it can be difficult to perform general or monitored-sedation anesthesia in patients treated in the CT radiology suite. Some significant complications have been reported in patients undergoing treatment in a CT suite without adequate anesthesia. I will reemphasize that even our patients treated percutaneously are under the care of an anesthesiologist during treatment in the operating room.
In terms of liver recurrence, we did not note a difference in the incidence of recurrence comparing those patients treated percutaneously versus those treated during open laparotomy. Probably it is because many of the patients we treated during a laparotomy had larger tumors or multifocal disease, so there is a definite selection bias of patients with a higher risk to recur in the laparotomy group. Because of these factors, there was not a significant difference in recurrence of disease between the two groups.
You asked about predicting complications; most of our patients were Child class B or C cirrhotics, and we assumed that those patients with more clinically severe cirrhosis would have a higher complication rate after radiofrequency ablation. This was not true for class B cirrhotics compared to class A, but class C cirrhotics did have a higher complication rate. There were several Child class C cirrhotics who did not initially fit our criteria for RFA, meaning that they were thrombocytopenic or had a significant coagulopathy. We corrected these abnormalities with transfusion of appropriate blood products. We thought that this latter subset of patients would have a higher complication rate. They did not. At this time, I don’t believe we can adequately assess the risk of complications following RFA based on only Child class criteria in cirrhotic patients.
The use of radiofrequency ablation prior to orthotopic liver transplantation is being done. I happen to know that the transplant group from Dallas is going to report a paper this year describing the use of radiofrequency ablation in patients on the transplant waiting list. Obviously, a concern in patients with a small hepatocellular cancer is drop-off of the list because of progression of malignant disease if they are waiting a long time for a donor organ. So there is interest in using radiofrequency ablation prior to orthotopic liver transplantation. I think this will continue to be the case because I have had several patients who are on transplant lists sent to me specifically for radiofrequency ablation treatment of hepatocellular carcinoma, after which they maintain active status on the waiting list.
Dr. Douglas Fraker (Philadelphia, Pennsylvania): This type of prospective phase 2 clinical trial with 100% follow-up of treated patients is a remarkable achievement and provides very accurate and important information regarding performance of a new technique such as radiofrequency ablation.
I have several questions and comments for Dr. Curley. You stated that you treat or intend to treat a rim of normal liver, approximately 1 cm, around each tumor nodule to get a complete ablation. In my experience using the same technique, when you do that you create a larger lesion that is persistent and the radiologists often interpret this as tumor progression because you have a larger-size mass lesion on follow-up scan. What is the most optimal way to follow these patients and how accurate is it in terms of determining local recurrence? And is this more of problem in patients with colorectal metastases in which the lesions are in general more hypovascular compared to the more vascular hepatomas?
Second, the average size of the lesion treated by your open technique was 4.6 cm, meaning that if you treat with a rim of normal tissue around that, it is a 6-cm ablation area. Using a 3.5-cm thermal probe, if you do the math, the volume of sphere related to the radius tube, that is approximately 6 to 8 overlapping thermal lesions. For your large lesions in the range of 7 cm, it is over 20. You stated that you now have a cut-off of 5 or 6 cm. What number of thermal lesions did you use to treat these lesions in the open technique and what is your operating time in terms of ablation?
Third, what proportion of hepatoma patients referred to M.D. Anderson for this treatment are eligible for your protocol? How many patients are eliminated because of the overall tumor volume or proximity to major bile duct structures?
Finally, if these results of a less than 4% local recurrence rate with a follow-up of >1 year with complications of 13% and no mortalities are reproducible, should this become the standard technique? You stated that you are initiating a randomized trial comparing an ablation to ethanol injection. If you were asked to design the next clinical trial for multimodality treatment of hepatoma and cirrhotic patients, what would your suggestions be?
Dr. Curley: As you know very well, one of the mistakes you can make when you initiate a program using radiofrequency ablation is to fail to involve your diagnostic radiologists. I learned this lesson the hard way when I first started performing radiofrequency ablation and got several anxious calls from our radiologists asking what I had done to these patients, the radiologists noting a “large black hole” in the liver where there was once tumor. I have subsequently involved the radiologists in the design and evaluation of our radiofrequency ablation protocols, and they are publishing several pieces of work describing the CT or MRI scan differences between local recurrence and the hyperemic early inflammatory response around the RFA lesion seen in some patients. It is important to involve the diagnostic radiologists and let them know that this is a treatment you are planning to perform, and that you expect a postablation radiographic lesion that is larger than the original tumor.
Regarding your comments about treating larger tumors, you are absolutely right—treating a large tumor volume with the currently available devices takes a long time and you must carefully create overlapping zones of necrosis. In general, the larger the tumor, the longer the RFA treatment time. For example, we treated one lesion that was 10 cm in diameter. It took 2 hours 55 minutes to complete the ablation. The patient’s core body temperature rose to 40.2°C because of the large volume of tissue treated with thermal ablation.
I will no longer treat very large tumors with radiofrequency ablation because of the long treatment time and the increased incidence of local recurrence. The current feeling in our group is that until improvements in RF technology and equipment become available that will allow us to treat larger tumors, we use 5- to 6-cm maximum tumor diameter as our cut-off for both primary and metastatic tumors. Again, this is because of the long treatment time, the higher local recurrence rate, and the difficulty in confirming complete destruction of the tumor with real-time ultrasonographic monitoring, even though we carefully plan the treatment with overlapping zones of ablation. My personal belief is that currently, RFA should be applied very selectively, if at all, to treat tumors larger than 6 cm.
You asked the question of how many patients referred to M.D. Anderson with hepatocellular cancer are candidates for RFA. Unfortunately in this country, we do not have good hepatocellular cancer screening programs for high-risk individuals, such as patients with chronic hepatitis B or C virus infection, and thus, less than 25% of the patients I see with HCC are candidates for this type of treatment. The majority of the HCC patients we see at M.D. Anderson have tumors that are too large, are multifocal, or have metastases to extrahepatic sites at presentation. We do have a large group from Italy and southern Europe diagnosed with small hepatocellular cancers during an active screening program for patients chronically infected with hepatitis B or C virus. In Italy, we are diagnosing a larger proportion of patients with 2- or 3-cm diameter solitary hepatocellular cancers; these patients are often ideal candidates for RFA.
The last question you asked regards the role of radiofrequency ablation in a prospective clinical trial. I think an important trial would be a multiarm study including all of the local liver tumor ablation techniques, primarily cryoablation, radiofrequency ablation, and percutaneous ethanol injection. My personal opinion is that radiofrequency ablation will be superior to ethanol injection. Ethanol injection, as you know, requires multiple treatments, patient compliance is not always great because of the pain associated with treatment, and you cannot guarantee good diffusion of ethanol throughout the tumor. There is a recent paper from Japan that reports an almost 40% local recurrence rate after treatment of hepatocellular cancers <3cm in diameter with ethanol injection. RFA usually requires a single treatment session. I must say, however, that I am doubtful that such a multiarm trial will be performed.
Dr. Goran B. Klintmalm (Dallas, Texas): At Baylor we began thermal ablation protocols in 1998. Like you, we have very encouraging results in cirrhotic as well as in noncirrhotic patients with primary and metastatic lesions. Dr. Robert Goldstein of my team has spearheaded this work, as well as other members of the Baylor Liver and Pancreas Disease Center. He has treated 40 lesions in 26 patients. What is unique about our experience is that we have done transplants in seven of these patients after their ablation, and the experience has been studied with H&E as well as NADH stains. The mean time from the ablation to the transplant was 222 days and as long as 535.
In the pathology of the explants of these seven patients, only two patients showed any evidence of viable tumor cells. One who had a 5-cm lesion had only 5% viable tumor mass at the edge rim of it, probably related to the probe size that you allude to. The second patient had a complete ablation of the tumor but a microscopic focus distant to the primary site.
I have three questions. The first concerns the imaging of cirrhotic patients. There continues to be a debate whether CT scans versus gadolinium MR should be used. In our institution, we use gadolinium MR exclusively to evaluate and follow these patients. What is your experience with CT versus MR in the management of cirrhotic patients, particularly postablation?
My second question addresses the available technology. RITA has recently introduced a 5-cm probe, and we are one of the sites that has this available to us. Our experience with this has dramatically changed our ability to treat large lesions. Have you had a chance to use these 5-cm probes? And what effect do you think these large ablation probes would have in the management of the patients?
Lastly, there is clearly a learning curve in the use of this technology. Do you have any recommendations on how to introduce this technology and educate surgeons so we continue to have the successful outcomes as you and Dr. Goldstein have demonstrated?
Dr. Curley: In answer to your first question about CT versus MR, our radiologists are currently doing a study evaluating the accuracy of MR versus CT to follow patients after RFA. We do a three-phase helical protocol CT on our patients before and after RFA treatment.
I am familiar with the new RITA device, and I have also used the other devices on the market, including some new experimental electrodes and generators from RadioTherapeutics, Inc., that are designed to create 5- to 6-cm zones of necrosis. We must continue to study these new tools and evaluate them critically to determine reduction in treatment time and effective treatment of larger tumors. As I mentioned to Dr. Fraker, currently I am not willing to treat tumors larger than 6 cm in diameter with RFA because of the long treatment times and increased incidence of local recurrence. With improvements in the technology, perhaps we will again expand our indications to tackle larger tumors.
Finally, I think there is a key point regarding the introduction of any new technology, somewhat analogous to the early introduction of laparoscopic surgical procedures, where there was a steep learning curve. I believe we must initiate concerted educational efforts to teach the principles, tactics, indications, and limitations of RFA. Currently, we are involved in developing educational programs that will include both didactic and hands-on experience for individuals interested in learning RFA techniques. I think that advancing these types of educational programs, by working through the American College of Surgeons and through societies like this, will allow us to put together courses and materials to educate surgeons in the technique.
Dr. Henry A. Pitt (Milwaukee, Wisconsin): Dr. Curley, you have interpreted your data as saying that half of your patients had developed new tumors within a year and that half of those did not have return of their tumor markers to normal within the interim period. I would interpret your data as suggesting that most of those patients actually had their tumors at the beginning of the treatment period and that they were not detected. As others have suggested, we just don’t have a good way of detecting these small lesions in cirrhotic livers.
At the Medical College of Wisconsin, we have some data that suggest that, compared to any form of preoperative detection, intraoperative palpation, inspection, and ultrasound remain the gold standard. This interpretation raises the question of whether we really should be managing any of these patients with percutaneous techniques when we are missing perhaps half of the lesions in half of the patients.
Dr. Curley: Your point is well taken. This goes along with the point made by Dr. Daly, and as I mentioned, we are now switching over to a laparoscopic approach in a number of HCC patients with class A or B cirrhosis. With laparoscopy, the surgeon doesn’t have the advantage of palpation. Frankly, in my personal experience with cirrhotic livers, I often don’t know if I am feeling tumor versus an area of macronodular regeneration, so hepatic palpation may not be as helpful in cirrhotic livers.
Clearly, the laparoscopic ultrasound or the open intraoperative ultrasound provides superior resolution compared to transcutaneous ultrasound. There are a couple of new radiographic technologies coming down the line that we want to study, including handheld MR probes and some of the new power ultrasound units that will give us 3-D imaging capabilities. Whether these new imaging modalities will be useful, I don’t yet know.
I agree with you regarding multifocal disease in many of the patients and I suspect that a number of the patients who developed “new” intrahepatic disease probably had small satellite nodules at the time of the initial RFA treatment. As you know, these chronic hepatitis B- and C-virus–infected patients are at high risk to have multifocal disease. We are not always able to visualize these small, subclinical tumors using transcutaneous ultrasonography, CT, or MRI scans before performing a percutaneous RFA treatment.
Footnotes
Correspondence: Steven A. Curley, MD, FACS, University of Texas M.D. Anderson Cancer Center, Dept. of Surgical Oncology, Box 106, 1515 Holcombe Blvd., Houston, TX 77030
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail: scurley@mdanderson.org
Accepted for publication April 2000.
References
- 1.Watkins KT, Curley SA. Liver and bile ducts. In: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, eds. Clinical Oncology, 2nd ed. New York: Churchill Livingstone; 2000: 1681–1748.
- 2.Williams I. Epidemiology of hepatitis C in the United States. Am J Med 1999; 107: 2S–9S. [DOI] [PubMed] [Google Scholar]
- 3.Guadagnino V, Stroffolini T, Rapicetta M, et al. Prevalence, risk factors, and genotype distribution of hepatitis C virus infection in the general population: a community-based survey in southern Italy. Hepatology 1997; 26: 1006–1011. [DOI] [PubMed] [Google Scholar]
- 4.Fan S-T, Ng IOL, Poon RTP, et al. Hepatectomy for hepatocellular carcinoma. The surgeon’s role in long-term survival. Arch Surg 1999; 134: 1124–1130. [DOI] [PubMed] [Google Scholar]
- 5.Nagasue N. Liver resection for hepatocellular carcinoma: indications, techniques, complications, and prognostic factors. J Hepatobil Pancr Surg 1998; 5: 7–13. [DOI] [PubMed] [Google Scholar]
- 6.Mazziotti A, Grazi GL, Cavallari A. Surgical treatment of hepatocellular carcinoma in cirrhosis: a Western experience. Hepato-Gastroenterology 1998; 45: 1281–1287. [PubMed] [Google Scholar]
- 7.Rossi S, Fornari F, Paties C, Buscarini L. Thermal lesions induced by 480-KHz localized current field in guinea pig and in pig livers. Tumori 1990; 76: 54–57. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez H, van Sonnenberg E, D’agostine H, et al. Percutaneous tissue ablation by radiofrequency thermal energy as a preliminary to tumor ablation. Minim Invasive Ther 1993; 2: 299–305. [Google Scholar]
- 9.McGahan JP, Browning PD, Brock JM, et al. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990; 25: 267–270. [DOI] [PubMed] [Google Scholar]
- 10.Lounsberry W, Goldschmidt V, Linke C. The early histologic changes following electrocoagulation. Gastrointest Endosc 1995; 41: 68–70. [DOI] [PubMed] [Google Scholar]
- 11.Child CG, Turcotte JG. Surgery in portal hypertension. Major Problems in Clinical Surgery: The Liver and Portal Hypertension. Philadelphia: WB Saunders; 1964: 1. [PubMed]
- 12.Couinaud C. Le foie. Etudes anatomiques et chirurgicales. Vol. 1. Paris: Masson; 1957.
- 13.Breasted JH. The Edwin Smith Surgical Papyrus. Chicago: Chicago University Press; 1930: 54.
- 14.Kelly HA, Ward GE. Electrosurgery. Philadelphia: WB Saunders; 1932: 1–9.
- 15.Goldwyn RM. Bovie: the man and the machine. Ann Plast Surg 1979; 2: 135–153. [PubMed] [Google Scholar]
- 16.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies. Ann Surg 1999; 230: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilchik AJ, Rose DM, Allegra DP, et al. Radiofrequency ablation: a minimally invasive technique with multiple applications. Cancer J Sci Am 1999; 5: 356–361. [PubMed] [Google Scholar]
- 18.Siperstein A, Garland A, Engle K, et al. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Ann Surg Oncol 2000; 7: 106–113. [DOI] [PubMed] [Google Scholar]
- 19.Beasley RP, Hwang LY. Epidemiology of hepatocellular carcinoma. In: Vyas GN, Dienstag JL, Hoofnagle JH, eds. Viral Hepatitis and Liver Disease. Ft. Lauderdale, FL: Grune & Stratton; 1984: 209–231.
- 20.Beasley RP, Hwang LY, Lin CC, et al. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22,707 men in Taiwan. Lancet 1981; 2: 1129–1133. [DOI] [PubMed] [Google Scholar]
- 21.Seeff LB. Natural history of hepatitis C. Hepatology 1997; 26: 21S–28S. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Hiyama T, Tsukuma H, et al. Cumulative risk of hepatocellular carcinoma in hepatitis C virus carriers: statistical estimations from cross-sectional data. Jpn J Cancer 1994; 85: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda K, Saitoh S, Koida I, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology 1993; 18: 47–53. [PubMed] [Google Scholar]
- 24.Benvegnu L, Fattovich G, Noventa F, et al. Concurrent B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. A prospective study. Cancer 1994; 74: 2242–2244. [DOI] [PubMed] [Google Scholar]
- 25.Izzo F, Cremona F, Ruffolo F, et al. Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis. Ann Surg 1998; 227: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 1997; 12: S319–S328. [DOI] [PubMed] [Google Scholar]
- 27.Livraghi T, Bolondi L, Lazzaroni S, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer 1992; 69: 925–929. [DOI] [PubMed] [Google Scholar]
- 28.Fujimoto T. The experimental and clinical studies of percutaneous ethanol injection therapy (PEIT) under ultrasonography for small hepatocellular carcinoma. Acta Hepatol Jpn 1988; 29: 52–56. [Google Scholar]
- 29.Hasegawa S, Yamasaki N, Hiwaki T, et al. Factors that predict intrahepatic recurrence of hepatocellular carcinoma in 81 patients initially treated by percutaneous ethanol injection. Cancer 1999; 86: 1682–1690. [DOI] [PubMed] [Google Scholar]
- 30.Seifert JK, Junginger T, Morris DL. A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb 1998; 43: 141–154. [PubMed] [Google Scholar]
- 31.Zhou XD, Tang ZY. Cryotherapy for primary liver cancer. Semin Surg Oncol 1998; 14: 171–174. [DOI] [PubMed] [Google Scholar]
- 32.Pearson AS, Izzo F, Fleming RY, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg 1999; 178: 592–599. [DOI] [PubMed] [Google Scholar]
- 33.Seki T, Wakabayashi M, Nakagawa T, et al. Percutaneous microwave coagulation therapy for solitary metastatic liver tumors from colorectal cancer: a pilot clinical study. Am J Gastroenterol 1999; 94: 322–327. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita Y, Sakai T, Maekawa T, et al. Thoracoscopic transdiaphragmatic microwave coagulation therapy for a liver tumor. Surg Endosc 1998; 12: 1254–1258. [DOI] [PubMed] [Google Scholar]
- 35.Vogl TJ, Mack MG, Roggan A, et al. Internally cooled power laser for MR-guided interstitial laser-induced thermotherapy of liver lesions: initial clinical results. Radiology 1998; 209: 381–385. [DOI] [PubMed] [Google Scholar]
- 36.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg 1998; 228: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mor E, Kaspa RT, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med 1998; 129: 643–653. [DOI] [PubMed] [Google Scholar]
- 38.Otto G, Heuschen U, Hofmann WJ, et al. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg 1998; 227: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30: 1434–1440. [DOI] [PubMed] [Google Scholar]
- 40.Rhim H, Dodd GD 3rd. Radiofrequency thermal ablation of liver tumors. J Clin Ultrasound 1999; 5:221–229. [DOI] [PubMed]
- 41.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer 1989; 64: 1700–1707. [DOI] [PubMed] [Google Scholar]