Abstract
Objective
To examine the impact of laparoscopic nephrectomy and recipient education on the proportion of kidney recipients who could identify a potential live donor, and on the live donor (LD) transplantation rate.
Summary Background Data
Laparoscopic donor nephrectomy (LDN) results in less postoperative surgical pain, a shorter hospital stay, and quicker recovery than the standard open donor nephrectomy (ODN). The authors hypothesized that the availability of this less invasive surgical technique would enhance the willingness of family and friends to donate.
Methods
The study population consisted of 3,298 end-stage renal disease patients referred for kidney transplant evaluation between November 1991 and February 2000, divided into three groups. The first group received no formal LD education and had only ODN available. The second group received formal education about the LD process and had only ODN available. The third group had both formal LD education and LDN available. Records were examined to determine what proportion of each group had any potential donors tissue-typed, and the rate at which they received an LD transplant.
Results
Before LDN availability and formal LD education, only 35.1% of referrals found a potential donor, and only 12.2% received an LD transplant within 3 years. Institution of a formal education program increased the volunteer rate to 39.0%, and 16.5% received an LD transplant. When LDN became available, 50% of patients were able to find at least one potential donor, and within 3 years 24.7% received an LD transplant. Regression analysis indicated that availability of LDN was independently associated with a 1.9 relative risk of receiving an LD transplant. Kaplan-Meier death-censored 1- and 3-year graft survival rates for ODN transplants were 95.8% and 90.6%, versus 97.5% and 94.8% for LDN.
Conclusions
The availability of LDN and an LD family education program has doubled the live donor transplantation rate, and outcomes remain excellent.
Live donor (LD) kidney transplantation is the ideal solution to end-stage renal disease (ESRD). Compared with dialysis, kidney transplantation offers an improved quality of life, a reduced death rate, and much lower costs to ESRD reimbursement systems. 1–3 Compared to cadaver kidney transplantation, LD transplants offer substantially superior graft function and survival. 4 Unfortunately, LD transplantation remains an underused form of renal replacement therapy. In 1997, there were 300,000 patients with ESRD in the United States, but only 3,844 (1.3% of the ESRD population, and 1.7% of the 220,000 patients receiving dialysis) received an LD transplant. 5,6 Certain prominent subgroups of the ESRD population are less likely to receive an LD transplant. Among the 105,000 black patients with ESRD (29.5% of the ESRD population), only 525 (0.5%) received an LD transplant in 1997. Worse still were the 170,000 ESRD patients older than 64 (47.6% of the ESRD population): only 144 (0.09%) received an LD transplant that year.
Although transplant physicians understand the advantages of LD transplantation, the decision to proceed is often made by persons who are not transplant professionals, including patients, their potential donors, referring physicians, and third-party payers. Therefore, efforts to improve the use of LD transplantation must address the concerns of these communities and must include efforts to educate them about the relative merits of the various forms of renal replacement therapy. To fill the void of information that exists in most ESRD recipients and their families when they arrive for their first evaluation, we organized a formal family education program in 1994. This program resulted in a small but significant improvement in both LD volunteer rates and transplant rates. 7
One poorly quantified barrier to LD transplantation is the concern among both potential donors and recipients about the pain and personal inconvenience associated with the live kidney donation. To address this concern, we introduced laparoscopic donor nephrectomy (LDN) into our clinical practice in 1996. The concept of LDN was originally described in a porcine model by Gill et al. 8 The first clinically successful LDN was performed by Kavoussi and Ratner in 1995. 9 Compared with open donor nephrectomy (ODN), LDN has been shown to reduce significantly the donor’s postoperative analgesic requirements, length of hospital stay, and length of convalescence. 10 Most importantly, the late results of LD transplants using the LDN technique are equivalent to those performed with ODN. 11 The current study was conducted to determine whether availability of this minimally invasive surgery for removal of donor kidneys altered the likelihood that a live donor could be identified.
METHODS
Study Population
The University of Maryland transplant program was completely restructured in 1991. The population of patients for this study consists of the 3,298 potential kidney recipients referred to the program for transplant evaluation from the time of this restructuring in 1991 through February 2000. The study compares three groups in that population, divided according to the time period when patients had their initial evaluation by the transplant team. The first time period extended from 1991 until a formal recipient family education program was integrated into the evaluation process in October 1994 (group 1). The second time period extended from October 1994 until introduction of LDN into the practice in March 1996 (group 2). The third period, with both education and LDN available, extended from March 1996 until February 2000 (group 3).
The kidney recipient evaluation process has been described. 12 Patients with serious contraindications to transplantation at the time of referral, and who were never considered to be potential candidates and were never registered on our waiting list, were excluded from this analysis. Between 1991 and 1998, persons with insulin-dependent diabetes who also met the criteria for simultaneous pancreas transplantation 13 were encouraged to consider LD kidney transplantation with subsequent cadaver pancreas-after-kidney transplantation. After May 1998, these patients were encouraged to consider simultaneous cadaver pancreas and LD kidney transplantation. 14
Formal Family Education Program
From 1991 until 1994, potential recipients and their families were not being fully informed of the relative consequences of deciding on LD versus cadaver kidney transplantation. It was believed that consistency was desirable and would be best achieved if the program were conducted by two or three trained nurse coordinators who were familiar with the LD process and the literature regarding the risk to the donor. Therefore, during the last quarter of 1994, a formal LD family education program was designed. An 8.5-minute educational videotape was produced in 1995 and was subsequently shown to all potential recipients and family members and friends who accompanied them to the transplant office.
Discussions by the coordinators, supplemented by the videotape, emphasize the LD experience with respect to the donor’s preoperative testing, surgical procedure, possible short- and long-term complications, and the expected loss of time from work and the financial impact on the donor. The video gives actual life experiences from the donor’s point of view and included donors from a variety of ethnic backgrounds. The coordinators also routinely compare cadaver and LD kidney transplantation in terms of expected waiting times, graft function, and survival times. The effects of the biologic relationship between the donor and recipient, including the effect of tissue type compatibility, are described.
Donor Nephrectomy
Between 1991 and March 1996, donor nephrectomy was performed by the standard open technique, using a flank incision. In March 1996, the laparoscopic technique 9,10 was introduced, and it has been used since then almost exclusively. In 1998, the technique was modified to minimize the chance of stripping the periureteral vascular tissue, and this resulted in a reduction in the ureteral complication rate. 15 In the early experience, a periumbilical incision was used for extraction of the kidney, but more recently the donor surgeons have preferred the Pfannenstiel bikini-type incision for cosmetic reasons.
From March 1996 onward, all potential kidney recipients and their families were informed of the availability of LDN, as well as its risks and benefits. After the advent of LDN, another video presentation was developed that describes the LDN perioperative experience in layman’s language. This video is shown to all kidney referral patients and their family members during the transplant evaluation.
Postoperative Management
An accelerated-discharge clinical pathway was instituted in October 1996. The goal is safe discharge from the hospital after LD transplantation on postoperative day 2. 16
Postoperative immunosuppression with antilymphocyte antibody was used for 5 to 14 days for recipients of HLA-mismatched kidneys until July 1996, after which time it was given only to those with delayed graft function or those thought to be at high risk for early rejection. Cyclosporine microemulsion (Neoral, Novartis, Summit, NJ) was used as the initial oral maintenance immunosuppressant until it was replaced in November 1997 by tacrolimus (Prograf, Fujisawa USA, Deerfield, IL). Cyclosporine and tacrolimus 12-hour trough levels were measured daily during the hospital stay, then twice weekly for the first month, then weekly for 2 months, then monthly. Initial target levels for cyclosporine of 300 to 350 ng/mL were tapered by 1 year to 200 to 250 ng/mL. Initial target levels for tacrolimus of 15 to 20 ng/mL were tapered by 1 year to 5 to 10 ng/mL. Mycophenolate mofetil (CellCept, Roche Pharmaceuticals, Nutley, NJ) was also given to all patients in the study, except where prohibited by a research protocol, at a dose of 1 g twice daily; black patients taking cyclosporine were dosed at 1.5 g twice daily. All patients received oral prednisone, which was tapered from 2 mg/kg/day after surgery to 0.3 mg/kg/day within 2 weeks of transplantation, then to 10 mg/day by 6 months. Immunosuppressant drugs and doses were adjusted as needed to minimize medication side effects.
Unexplained renal dysfunction prompted ultrasound-guided percutaneous biopsies, which, together with an assessment of the clinical course, were used to determine treatment of rejection. Three bolus doses of methylprednisolone, followed by a 2-week prednisone taper, were used to treat mild cases of acute rejection. Moderate to severe cases of acute rejection, and mild cases unresponsive to corticosteroids, were treated with 7 to 14 days of OKT3, Atgam, or thymoglobulin (SangStat Medical Corp., Menlo Park, CA).
Data Collection and Analysis
Kidney transplant referrals were identified by searching the Division of Transplantation database. Corresponding potential living donors were identified by analyzing immunogenetics laboratory records to determine the number of persons per referral who had submitted blood samples to determine their candidacy for donation. Lists of donor–recipient pairs were then compiled. Clinical data on referred patients were obtained from electronic and paper medical records.
Statistical comparisons of groups of discrete data were made with a t test (normally distributed data, expressed as the mean ± SEM) or the Mann-Whitney rank-sum test (skewed data, expressed as the median). Proportions were compared with the chi-square test. The Kaplan-Meier product limit method was used to calculate transplantation rates and survival rates, which were compared with the log-rank test. The association of covariates with the time-dependent outcome variable, LD transplantation, was calculated with the Cox proportional hazards model. The model was developed by forward variable selection using the likelihood-ratio test. Significance for all tests was P < .05. Statistics were calculated with SPSS Graduate Pack 8.0 for Windows (SPSS Inc., Chicago, IL).
RESULTS
The annual rate of referrals for kidney transplant evaluation increased from 285 patients in 1992 to 608 patients in 1999. There were 764 patients in group 1, 559 patients in group 2, and 1,975 patients in group 3. The referrals were 60.6% male and 40.6% black. The mean age of the population was 48.5 ± 0.2 years (range 5–83); 32.6% were older than 55 at the time of their initial transplant evaluation.
The distribution of the familial relationships of potential LDs to their corresponding recipients for the entire study period was as follows: 32% sibling, 27% child, 23% unrelated, 11% parent, and 7% distantly related. The distribution of potential LD relationships for the period before introduction of LDN (38% sibling, 21% child, 26% unrelated, 12% parent, and 3% distantly related) was significantly different (P < .0001, chi-square) from the distribution after the LDN program began (28% sibling, 24% child, 29% unrelated, 9% parent, and 10% distant related), with increases in the percentage of both unrelated and distantly related potential donors.
As shown in Figure 1, before the LD education program began, 35.1% of patients had at least one potential donor tissue-typed, compared with 40.7% in the period after it began (P < .05, chi-square). There was a further increase to 49.7% of patients with at least one potential donor after introduction of LDN (P < .001). Among the white registrants, 39.0% of those in group 1, 43.1% in group 2, and 54.1% in group 3 had at least one potential donor; the corresponding figures for black registrants were 29.9%, 37.5%, and 43.3%. Within each race, the difference in the volunteer rate between group 1 and group 3 was significant (P < .0001 for both races). However, the incremental rise in the donation rate after LDN was added to the program (group 2 vs. group 3) was only significant for white patients (white, P = .008; black, P = .14).
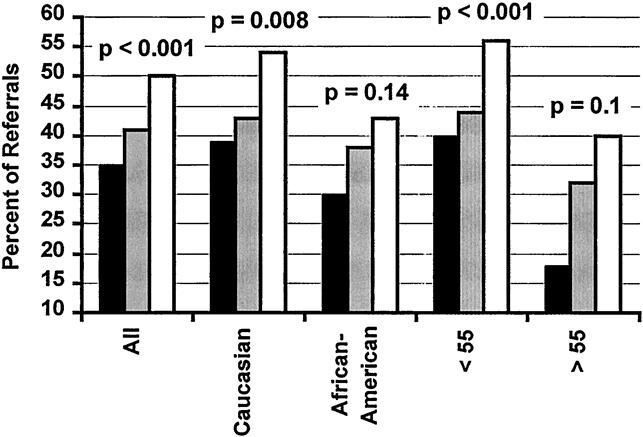
Figure 1. Percentage of kidney transplant referrals who had at least one potential live kidney donor tissue-typed. Black bar, group 1 (open donor nephrectomy, no formal family education program); gray bar, group 2 (open donor nephrectomy with formal family education program); white bar, group 3 (laparoscopic donor nephrectomy with formal family education program). P values refer to a chi-square comparison of groups 2 and 3.
Among the younger registrants (those 55 or younger at the time of evaluation), 40.0% of those in group 1, 44.2% in group 2, and 55.6% in group 3 had at least one potential donor. The corresponding figures for the older registrants (those older than 55) were 18.2%, 32.3%, and 39.8%. Within each age group, the difference in the volunteer rate between group 1 and group 3 was significant (P < .0001 for both). However, the incremental rise in the donation rate after LDN was added to the program was significant only for the younger group of patients (younger, P = .0001; older, P = .10). As expected from these data, there was a large difference in volunteer rates between young, white referrals and older, black referrals (31.0% vs. 53.9%, P < .0001).
In the patients who had at least one potential donor, the average number of persons tissue-typed per patient was 1.8 ± 0.7 for group 1, 1.7 ± 0.7 for group 2, and 1.8 ± 0.5 for group 3 (P = NS, Mann-Whitney).
Among those who received transplants at the time of this analysis, the median time from the initial visit to the transplant office to the LD transplant was 154 days, versus 454 days for the cadaver transplants (P < .0001, Mann-Whitney). The rate of LD kidney transplantation was higher (P = .08) for group 2 than for group 1 (Fig. 2); the rate was significantly higher (P < .0001) for group 3 versus group 2. The Kaplan-Meier curves in Figure 2 indicate that at 3 years, the estimated percentage of patients who receive an LD transplant was 12.2% for group 1, 16.3% for group 2, and 24.7% for group 3.
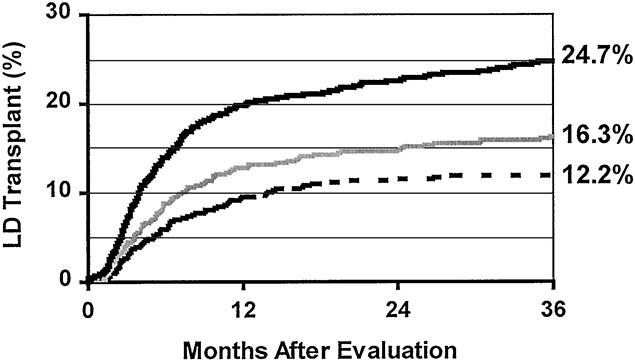
Figure 2. Kaplan-Meier rates of living donor transplantation among all kidney registrants. The lowest rate was for group 1 patients (dashed line). The transplant rate was higher after introduction of the formal family education program (gray line, group 2;P = .08 vs. group 1, log-rank test). The rate again increased significantly with the addition of laparoscopic donor nephrectomy (black line, group 3;P < .0001 vs. group 2).
A further analysis was conducted to determine whether the observed increase in the rate of LD transplantation in the LDN era was due to increased interest in donation in the local population, or rather to an influx of patients from remote parts of the country who came to the program specifically for access to the LDN technique. Patient were classified as local or remote according to their state of residence. Local patients were those from Maryland, Pennsylvania, Delaware, Virginia, West Virginia, New Jersey, and the District of Columbia. Remote referrals were those from the remaining states. Before initiation of the LDN program, 1,323 patients were evaluated, 1,293 (98%) local and 30 (2%) remote. After the LDN program began, 1,975 patients were evaluated, 1,826 (92%) local and 149 (8%) remote (P = .001 vs. remote patients in the period before LDN, chi-square). Considering only remote patients, 5 of the 30 patients (17%) evaluated before the LDN program began received a transplant compared with 60 of the 149 patients (40%) evaluated afterward (P = .025). This indicates that a large portion of the increase in remote referrals is due to patients specifically seeking LDN expertise. When the LD transplantation rates were calculated among only Maryland local patients, in group 3 the transplantation rate was still significantly higher than in group 2 (P = .0005, log-rank).
To determine whether certain characteristics of the referral population were associated with the LD transplantation rate, a Cox regression analysis was performed using time to LD transplantation as the dependent variable (Table 1). Patient records were examined to determine the values of covariates that might potentially affect the LD transplant rate, including the referred patient’s sex, race, age, marital status, educational level, employment status, number of living first-degree family members, and availability of the LDN technique. Values of these covariates were then used to construct a statistical model. The model indicated that sex, marital status, and educational level were not associated with rates of LD transplantation that were significantly different from average. Black referral race and age older than 55 at the time of evaluation were associated with significantly lower-than-average rates of LD transplantation (relative risks of 0.6 for each). In contrast, recipients who were employed, those educated about the availability of the LDN technique, and those with more than four living first-degree family members received LD transplants at a significantly higher rate than average (relative risks of 2.1, 1.9, and 1.3, respectively). The single factor under the direct control of the transplant center (making LDN available and informing patients of such) was associated with a highly significant (nearly twofold) increase in LD transplantation rates.
Table 1. ASSOCIATIONS BETWEEN RECIPIENT CHARACTERISTICS AND TIME TO LD TRANSPLANTATION
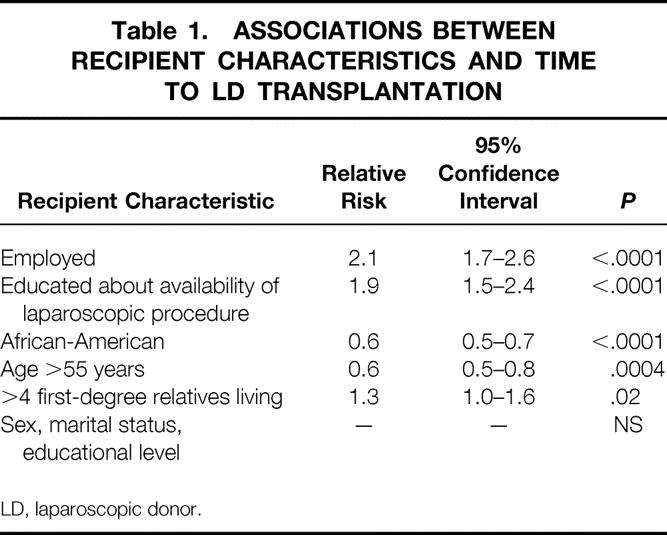
LD, laparoscopic donor.
Patient and Graft Survival
The 1- and 3-year patient survival rates for the 620 LD transplants performed in these patients were 97.1% and 95.0%. The corresponding 1- and 3-year graft survival rates were 94.3% and 88.5%. The patient survival rate was no different for 168 patients transplanted with kidneys procured with ODN versus the 452 procured with the LDN technique (ODN, 99.4% and 97.5%; LDN, 96.1% and 93.9%;P = .06). Similarly, the 1- and 3-year graft survival rate was no different for ODN kidney recipients versus the LDN group (ODN, 95.2% and 88.3%; LDN, 93.9% and 89.6%;P = .93). Several of the graft losses were due to patient death with a functioning graft. Considering only the 452 transplants performed in the LDN group, there were 21 patient deaths between 0 to 24 months after the transplant (average 7.3 ± 1.6 months). The causes of death and the time after the transplant were six sudden deaths at home (0.5, 1.4, 3.1, 4.2, 11.2, and 19.0 months), five cases of sepsis (1.7, 3.6, 7.9, 14.2, and 15.6 months), three cardiac arrests during the hospital stay for the transplant, three cases of posttransplantation lymphoproliferative disorder (at 4.8, 5.0 and 8.4 months), one case of pulmonary embolism (at 4.8 months), one motor vehicle accident (at 2.1 months), one case of liver failure (at 20.5 months), and one stroke (at 23.9 months). Because death with function is a cause of graft failure that indicates a disease process in the recipient rather than a consequence of the donation technique, the 3-year death-censored graft survival rates for the ODN and LDN kidney transplants were calculated (Fig. 3). Death-censored 1- and 3-year graft survival rates were again no different for ODN kidney referrals versus the LDN group, and they were slightly higher for the LDN group (ODN, 95.8% and 90.6%; LDN, 97.5% and 94.8%;P = .17). These data indicate that the LDN technique had no demonstrable adverse effect on graft survival compared with ODN.
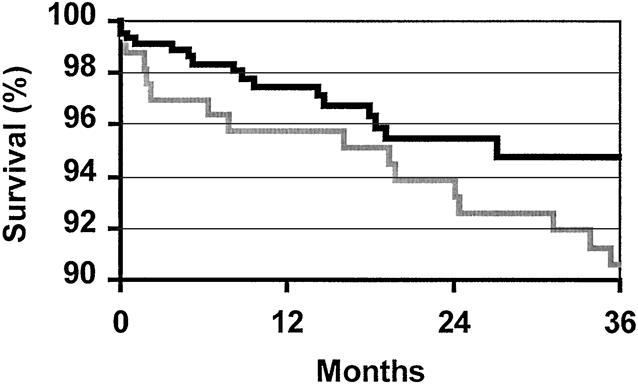
Figure 3. Three-year Kaplan-Meier death-censored graft survival rates for open donor nephrectomy (gray line, n = 168, 90.6%) and laparoscopic donor nephrectomy (black line, n = 452, 94.8%) living donor kidney transplants. Graft survival rates between the two groups were not significantly different (P = .17).
The 1- and 3-year patient survival rates for the 1,163 cadaver kidney transplants in these patients were 93.2% and 86.6%. These rates were significantly lower than in the 620 LD kidney recipients (P < .0001). The cadaver 1- and 3-year graft survival rates of 84.5% and 76.1% were also significantly lower than those for the LD kidney transplants (P < .0001). A comparison of 3-year graft survival curves for the 417 white and 194 black patients in the series who received LD transplants, and the 716 white and 443 black patients who received cadaver kidneys was performed. The 3-year graft survival rate for LD transplants was 89.1% in white patients versus 87.3% in black patients (P = .38). These rates were significantly better than the cadaver kidney 3-year graft survival rates in the corresponding subgroups—76.9% in whites (P < .0001) and 74.2% in blacks (P = .007).
A comparison was made between the 499 recipients 55 years of age or younger and the 121 recipients older than 55 who received LD transplants. The 3-year graft survival rate for LD transplants was 91.3% in the younger patients versus 84.5% in the older group (P = .02). This statistically significant 6.8% difference was predominantly due to differences in patient death with function (3-year patient survival rates were 97.1% for the younger patients vs. 86.0% for the older ones, P < .0001). When graft survival was calculated with deaths censored, there was no statistical difference between the younger and older recipients (3-year death-censored graft survival rates were 92.3% for the younger patients vs. 95.7% for the older ones, P = .86).
DISCUSSION
Our results show that the availability of LDN is associated with significant increases in both LD volunteer rates and transplant rates. The percentage of patients who had at least one potential donor tissue-typed was 41% before the LDN program was begun and 50% afterward. Among those referred for transplantation and followed up for 3 years after the initial evaluation, the LD transplant rate was 25% among patients apprised of LDN during their initial visit versus only 16% among those evaluated before the LDN program began.
The combined effect of family education and the availability of LDN was particularly dramatic. Before either of these program changes were made, the percentage of recipients who had one or more persons tissue-typed was only 35%, and the 3-year transplant rate was only 12%. The first two cohorts of patients studied here were originally discussed in a publication that described the benefits of the education program alone. 7 This prior work showed that an LD education program improves volunteer rates, an effect that was especially pronounced in the subgroups of patients with the lowest rates (black and elderly recipients). The current study showed that both an educational initiative and LDN are independently useful adjuncts to an LD transplant program.
Some of the increase in LD transplantation during the LDN era was attributable to gravitation of patients to the program from remote states. However, when only local referrals were considered, the transplant rate was still significantly higher among potential recipients who were first informed about the availability of LDN at the time of evaluation compared with those evaluated before the LDN program began. Before the LDN program, only 2% of recipients came from outside the local area, versus 7% afterward. The high rate of LD transplantation (40%) among remote referrals evaluated during the LDN era indicates that many of them came specifically for LDN expertise. The fact that donors and recipients are willing to travel thousands of miles for access to LDN speaks for the tremendous appeal that the procedure offers to some patients.
Although regression analysis in the current study indicated that black recipient race was associated with a significantly lower LD transplant rate than average, the combined program changes described here significantly improved the black volunteer rate from 30% to 43%. We believe it is very important to encourage black families to consider LD transplantation, because the benefits of the procedure to this community are especially prominent. Nationally, black recipients listed for a cadaver kidney transplant in 1996 waited a median of 1,313 days for a kidney, compared with only 681 days for whites. 6 The median wait for an LD transplant in the current study of 154 days is therefore only 12% of the time that a black patient might expect to wait for a cadaver organ. Frequently, an LD transplant can be set up within 4 to 6 weeks of the initial evaluation. Further, LD transplantation is currently the best approach to improving graft survival rates among black recipients. The average life expectancy of a cadaver kidney transplant in a black recipient is only 6.2 years, compared with 11.1 years for whites. LD transplantation substantially improves black graft survival to nearly 9 years. 17
In this patient series, education and LDN doubled the volunteer rate among family members of older recipients, increasing from 18% to 40%. Elderly patients constitute a large and expanding subgroup of the ESRD population, but they are often systematically excluded from consideration as LD transplant recipients. This may be due to concerns by patients and physicians that elderly recipients represent a particularly high-risk group for transplantation. However, national data show that the 3-year LD kidney transplant graft survival rate of 79% among recipients older than 65 is within three percentage points of that for certain other high-risk groups, such as blacks (77%), retransplants (82%), and sensitized recipients (82%). It is therefore reasonable to offer LD transplantation to suitable elderly candidates, just as it is routinely offered to these other high-risk groups, rather than excluding them at an arbitrary age cutoff.
Increased rates of donation and transplantation resulting from the availability of LDN, in addition to its established beneficial effects on donor pain, hospital stay, and convalescence, would still not justify the use of LDN if the procedure jeopardized the outcome of the transplant. This does not appear to be the case. In the current series, the 3-year graft survival rate for kidneys procured with LDN (89.6%) was no different from that for ODN (88.3%, P = .93). When graft survival was calculated with deaths censored (a cause of graft failure unlikely to be related to the kidney procurement technique), the 3-year graft survival rate for LDN kidneys was 94.8%, again not statistically different from the death-censored ODN survival rate of 90.6% (P = .17). Prior studies have indicated that although the learning curve for this complex technique can be associated with slightly higher rates of delayed graft function and ureteral complications, long-term outcomes of LDN are the same or better than those with ODN. 11,15
The advantages of laparoscopic over open nephrectomy have been demonstrated for a variety of benign kidney diseases. 18 When the procedure is performed for benign disease, the patient derives the benefits from the technique during the postoperative period, but most often the availability of the technique per se does not influence the decision to proceed with surgery, which will be done one way or the other. With live kidney donation, however, the decision to proceed is more complex and is often more dependent on ill-defined psychological factors (e.g., the donor’s love for the recipient, or fear of hospitals and surgery) than on objective surgical indications. Because LDN directly addresses issues that are likely to pose substantial barriers to live kidney donation (the donor’s postoperative suffering and the amount of unreimbursed time off work), it is not surprising that the availability of the technique should exert a powerful positive influence on the decision to proceed.
In conclusion, we found that awareness by both donors and recipients that LDN hastens donor recovery and leads to excellent recipient results has significantly increased the chance that a live donor will be identified. This has resulted in significantly higher rates of LD transplantation, the optimal form of renal replacement therapy. Careful dissemination of this technique in the United States will lead to the increased use of LD transplantation for the treatment of ESRD.
Discussion
Dr. Clyde F. Barker (Philadelphia, Pennsylvania): Dr. Bartlett has chosen to approach his topic as scientifically as possible, but we have to recognize that it is not a randomized study. In the case of effectiveness of penicillin and the attractiveness of laparoscopic cholecystectomy, the difference from earlier treatments was so obvious that randomized studies were unnecessary. Whether laparoscopic donor nephrectomy is similar in requiring no randomized trial to demonstrate its superiority remains a question in my view. However, in one sense it is similar. I doubt that we will see any large randomized studies because (as in laparoscopic cholecystectomy) patients are driving us in the direction of doing donor nephrectomies laparoscopically. It certainly has happened at our own institution.
As Dr. Bartlett has pointed out, experience with this procedure was begun by his crosstown colleagues and has been further extended by him and his group, and rapidly adopted by others in this country. Patients are so enthusiastic about this method that it seems almost inevitable that this approach will soon be the usual one for living kidney donor operation everywhere.
But this question remains: Is this really as good in all-important respects as the time-honored method of open donor nephrectomy? Does it produce as good a result for the recipient? And is it as safe for the donor? During the last few years, kidney allograft survival has improved everywhere, perhaps at least in part due to availability of new immunosuppressive agents. Conceivably, the positive impact of better immunosuppressive drugs in his recent patients obscure a negative impact of complications or subtle damage to the kidney related to the laparoscopic nephrectomy. Would Dr. Bartlett’s graft survival in recent years have been even better if he had performed open donor nephrectomies instead? So I would like to ask him whether he has seen any serious complications in his donor group, any deaths, any near deaths, and does he know any of these at other institutions?
I would be interested in his thoughts are about the learning curve for this procedure. Should small centers that are doing only a few transplants per year be doing donor operations this way? Are there any advances in techniques such as using a hand-assist that would make it quicker or safer? And under what circumstances would he choose not to use this approach, but rather the conventional open donor nephrectomy?
Presenter Dr. Stephen T. Bartlett (Baltimore, Maryland): You suggested the possibility of a randomized study as an appropriate way to finally know the real difference between open donor nephrectomy and laparoscopic nephrectomy. I am aware that the University of Michigan team has instituted a randomized trial just as you have suggested. I do not know the details of the level of enrollment and specifically whether they are having difficulty getting patients to agree to enrollment. It certainly has been our impression that we would have difficulty enrolling patients in this kind of trial because the enthusiasm for the operation is so great. I believe that parallels the experience in the cholecystectomy era.
You also asked whether the operation is really as good as the open technique. I think that is not the case. We do not think we have had any technique-related graft losses in the past 460 cases. In a separate publication in the Journal of Urology, Dr. Stephen Jacobs from our group very carefully detailed the donor complications. He specifically addresses the question of early graft function and whether any graft loss could have eventuated from these complications. This has not been the case.
You asked specifically about some of the major complications we have experienced. There have been six out of the 460 cases. One case, for example, was a past-pointing error with the laparoscopic scissors; while dividing the ureter, the external iliac artery was divided as well. This was easily resolved by reanastomosis of the iliac artery appropriately, but the complication did require transfusion of 2 units of blood. We also had two cases where the stapler on the renal artery misfired and there was a very slight leaking at the renal artery take-off from the aorta. This was treated by direct suture, but in no way jeopardized the health of the donor or the quality of the kidney. There was one case requiring splenorrhaphy, and two cases requiring transfusion without reoperation. All of these major complications were in the first 60 cases.
You asked about some of the complications related to the ureter and some of the other factors that make the management of these cases better now than it was in the first 10, 20, and 30 cases we performed. One thing that became clear is that in the early series, renal artery spasm was not unusual. We now take care to volume-load the patient early in the procedure. There is data that pneumoperitoneum raises endothelin-1 levels and possibly reduces renal blood flow. To some degree, compensation with volume-loading of the potential donor can overcome this. Secondly, you can apply local papaverine and lidocaine to the surface of the renal artery to prevent spasm.
The issue of the ureteral necrosis simply resulted from the early technique of forceful tenting of the ureter to dissect it. This led to stripping of the ureteral artery and vein. The current technique involves division of the ureter and gonadal vein with a stapler and removal of all the intervening tissue. We now believe that the operation has been fine-tuned by our team, as well as many others, to a point now where it has achieved a very high level of safety.
Dr. James F. Burdick (Baltimore, Maryland): I have two questions. In the first place, when Ratner and Kavoussi originally devised this technique, of course, it was for this very reason, to improve the chances and decrease the disincentives that someone would be an organ donor. And we think at Hopkins that it really does that. We have the same impression. Establishing that point scientifically, of course, is complex.
The fraction of donor recipient pairs attracted from the outside you have addressed to some degree. I think we have also noted that people do tend to come from some distance for the perceived—we think real—advantages of the laparoscopic donor technique. Your fraction is 39% in the abstract in the booklet, and of course, the geography in the state of Maryland is partly responsible for that. But I wonder what the fraction is from out of state for cadaver donor recipients and if that also tends to confirm that the impact of referrals from the outside isn’t skewing your tendency to have an increased living donor fraction. This is particularly true since in the United States in general now there are many centers that have about a 50% fraction of living organ donations.
So as part of this question, if we reach the steady state that we would predict where everyone is using this technique in general, will it really have the impact that you are presuming over the same situation with the generally increased enthusiasm for organ donation in this country now as living donors without the technique?
The second question is related, and that is for cadaveric donors. Has this affected the degree of risk you are willing to accept for a patient with an extended cadaver donor rather than hoping that a living donor will come up? That is, are you decreasing the fraction of cadaver donors that you might have taken previously that have serious defects? Or is there no interchange between the two?
Dr. Bartlett: I think your first question got at the question of how much have we really scientifically assessed whether laparoscopic nephrectomy lowers the disincentive to donate. We have studied that question carefully. We have received a generous grant from the Roche Corporation for further study with focus groups, what potential and past donors consider in their decision to donate. I agree with you that the methodologic proof that this technique increases the rate of donors is quite difficult. Other methodologies really should be applied before the data that I am presenting you today is accepted without any kind of further scrutiny. Further scientific study is likely.
Your second question, I think, related to the number of cadaver transplant patients for which we utilize marginal donors in the Maryland area. As you well know, the Maryland area is one of the most disfavored regions in the United States in terms of waiting time for cadaveric kidney transplants. The state of Maryland falls at the most unfavorable end of a ten-fold difference in waiting time from region to region. Therefore, the two programs in Baltimore are required to use cadaveric transplants that are often of what we would refer to as marginal quality. I believe this all the more emphasizes why we need to highlight living kidney donation, to avoid unnecessary reliance on cadaveric kidneys that may have limited long-term function. Our data shows that the recipient should continue looking for a donor. The hazard analysis shows that while most donors are found soon after evaluation, others are identified after 2 to 3 years.
Dr. Mark B. Adams (Milwaukee, Wisconsin): We have noticed a similar increase in people who are willing to donate. But I guess that I have come to view this more as a hook than anything else. I think that everybody in transplantation has felt a deep responsibility for the safety of the donor, both short- and long-term. I think you have demonstrated, as other people have, that it is safe short-term. But obviously this procedure is transperitoneal and what will happen in the long-term in terms of, for example, bowel obstructions, I think is yet to be determined. I am curious if you have any information about that.
The second issue is that for the safety of the donor it is really primarily a left nephrectomy situation, and I think that most people feel uneasy at the present time with the current stapling technology to transect a short right renal vein with the risk that the staple line might not hold and the cava would fall open. I know you have done some right nephrectomies, so I would like to know your current view of this. I do think that it has significantly increased people’s willingness to donate, and as you have noted, that has increased access to renal transplantation.
Dr. Bartlett: As you described it, it is a hook for patients to come for evaluation and for donors to consider donating. I think there is no doubt that a patient, at least in the Baltimore area, would have to have recently descended from another planet to have not heard about the laparoscopic technique. I cannot say with certainty that our educational program and the availability of laparoscopy not influencing truly naive recipients prior to evaluation, and thus skewing the kind of patients evaluated in favor of those already disposed to a living donor transplant. That is why other methodologies may be needed to prove my point.
You asked about the use of the right kidney. Of the 460 we have done, 14 were right kidneys. They are more difficult. The right vein is very short, particularly after the staples are removed. I strongly encourage surgeons who use right kidneys to totally mobilize the recipient’s right iliac system, including division of the hypogastric veins, to make sure the anastomosis is performed as safely as possible. Given those precautions, the right kidney can be procured and transplanted quite safely.
Footnotes
Correspondence: Eugene J. Schweitzer, MD, 29 S. Greene St., #200, Baltimore, MD 21201.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
Supported by a grant from the Roche Laboratories, Inc.
Reprints will not be available.
E-mail: gschweitzer@umm.edu
Accepted for publication April 2000.
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 2.Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int 1996; 50: 235–242. [DOI] [PubMed] [Google Scholar]
- 3.Eggers PW. Effect of transplantation on the Medicare end stage renal disease program. N Engl J Med 1988; 318: 223–229. [DOI] [PubMed] [Google Scholar]
- 4.Cecka JM. Living donor transplants. In: Cecka MJ, Terasaki PI, eds. Clinical Transplants 1995. Los Angeles: UCLA Tissue Typing Laboratory; 1996: 363–377. [PubMed]
- 5.U.S. Renal Data System. USRDS 1999 Annual Data Report. Bethesda, MD: The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, April 1999.
- 6.1999 Annual Report of the U.S. Scientific Registry for Transplant Recipients and the Organ Procurement and Transplantation Network. Transplant Data: 1989–1998. Rockville, MD: U.S. Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation; UNOS, Richmond, VA.
- 7.Schweitzer EJ, Yoon S, Hart J, et al. Increased living donor volunteer rates with a formal recipient family education program. Am J Kidney Dis 1997; 29: 739–745. [DOI] [PubMed] [Google Scholar]
- 8.Gill IS, Carbone JM, Clayman RV, et al. Laparoscopic live-donor nephrectomy. J Endourol 1994; 8: 143–148. [DOI] [PubMed] [Google Scholar]
- 9.Ratner LE, Ciseck LJ, Moore RG, Cigarroa FG, Kaufman HS, Kavoussi LR. Laparoscopic live donor nephrectomy. Transplantation 1995; 60: 1047–1049. [PubMed] [Google Scholar]
- 10.Flowers JL, Jacobs S, Cho E, et al. Comparison of open and laparoscopic live donor nephrectomy. Ann Surg 1997; 226: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira JM, Cangro CB, Fink JC, et al. A comparison of recipient renal outcomes with laparoscopic versus open live donor nephrectomy. Transplantation 1999; 67: 722–728. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett ST, Farney AC, Jarrell BE, et al. Kidney transplantation at the University of Maryland. In: Cecka MJ, Terasaki PI, eds. Clinical Transplants 1998. Los Angeles: UCLA Tissue Typing Laboratory; 1999: 177–185. [PubMed]
- 13.Brayman KL, Najarian JS, Sutherland DER. Transplantation of the pancreas. In: Cameron JL, ed. Current Surgical Therapy, 4th ed. St. Louis: Mosby-Year Book; 1992.
- 14.Farney AC, Cho E, Schweitzer EJ, et al. Simultaneous cadaver pancreas living donor kidney transplantation (SPLK): a new approach for the type 1 diabetic uremic patient. Ann Surg 2000 (in press). [DOI] [PMC free article] [PubMed]
- 15.Philosophe B, Kuo PC, Schweitzer EJ, et al. Laparoscopic versus open donor nephrectomy. Comparing ureteral complications in the recipients and improving the laparoscopic technique. Transplantation 1999; 68: 497–502. [DOI] [PubMed] [Google Scholar]
- 16.Schweitzer EJ, Wiland A, Evans D, et al. The shrinking renal replacement therapy “break-even” point. Transplantation 1998; 66: 1702–1708. [DOI] [PubMed] [Google Scholar]
- 17.Cecka JM. The UNOS scientific renal transplant registry. In: Cecka JM, Terasaki PI, eds. Clinical Transplants 1998. Los Angeles: UCLA Tissue Typing Laboratory; 1999: 1–15.
- 18.Kerbl K, Clayman RV, McDougall EM, et al. Transperitoneal nephrectomy for benign disease of the kidney: a comparison of laparoscopic and open surgical techniques. Urology 1994; 43: 607. [DOI] [PubMed] [Google Scholar]


