Abstract
Objective
To evaluate the endpoints of complications (specifically pancreatic fistula and total complications) and death in patients undergoing pancreaticoduodenectomy.
Summary Background Data
Four randomized, placebo-controlled, multicenter trials from Europe have evaluated prophylactic octreotide (the long-acting synthetic analog of native somatostatin) in patients undergoing pancreatic resection. Each trial reported significant decreases in overall complication rates, and two of the four reported significantly lowered rates of pancreatic fistula in patients receiving prophylactic octreotide. However, none of these four trials studied only pancreaticoduodenal resections, and all trials had high pancreatic fistula rates (>19%) in the placebo group. A fifth randomized trial from the United States evaluated the use of prophylactic octreotide in patients undergoing pancreaticoduodenectomy and found no benefit to the use of octreotide. Prophylactic use of octreotide adds more than $75 to the daily hospital charge in the United States. In calendar year 1996, 288 patients received octreotide on the surgical service at the authors’ institution, for total billed charges of $74,652.
Methods
Between February 1998 and February 2000, 383 patients were recruited into this study on the basis of preoperative anticipation of pancreaticoduodenal resection. Patients who gave consent were randomized to saline control versus octreotide 250 μg subcutaneously every 8 hours for 7 days, to start 1 to 2 hours before surgery. The primary postoperative endpoints were pancreatic fistula, total complications, death, and length of hospital stay.
Results
Two hundred eleven patients underwent pancreaticoduodenectomy with pancreatic-enteric anastomosis, received appropriate saline/octreotide doses, and were available for endpoint analysis. The two groups were comparable with respect to demographics (54% male, median age 66 years), type of pancreaticoduodenal resection (60% pylorus-preserving), type of pancreatic-enteric anastomosis (87% end-to-side pancreaticojejunostomy), and pathologic diagnosis. The pancreatic fistula rates were 9% in the control group and 11% in the octreotide group. The overall complication rates were 34% in the control group and 40% in the octreotide group; the in-hospital death rates were 0% versus 1%, respectively. The median postoperative length of hospital stay was 9 days in both groups.
Conclusions
These data demonstrate that the prophylactic use of perioperative octreotide does not reduce the incidence of pancreatic fistula or total complications after pancreaticoduodenectomy. Prophylactic octreotide use in this setting should be eliminated, at a considerable cost savings.
Pancreaticoduodenectomy is a commonly performed surgical procedure at many institutions, being used for various malignant and benign diseases of the pancreas and periampullary region. In many centers the perioperative death rate for pancreaticoduodenectomy is now less than 5%, 1–5 with the leading causes of postoperative death hemorrhage, cardiac events, and sepsis. 6
In contrast to this low death rate, the incidence of major postoperative complications after pancreaticoduodenectomy approaches 40% to 50%. 1–6 In most series, the three most common complications are early delayed gastric emptying, wound infection, and pancreatic fistula (resulting from a pancreatic-enteric anastomotic leak). In the setting of pancreaticoduodenal resection, pancreatic fistula is variably defined as the persistent drainage of 50 mL or more of amylase-rich fluid on or after postoperative day 3 through 10. The incidence of pancreatic anastomotic leak varies from 5% to 25% in most series.
Because pancreatic fistula has been identified as such a common problem after pancreaticoduodenectomy, numerous techniques for managing the pancreatic remnant (body and tail of the pancreas) have been studied. 7 These techniques have included suture ligation of the pancreatic duct without enteric drainage, 8 pancreatic ductal occlusion with polymers, 9,10 and various modifications of the pancreaticojejunal anastomosis (e.g., end-to-end vs. end-to-side, invagination vs. duct-to-mucosa, isolated Roux-en-Y limb). 11–13 Pancreatic drainage to the stomach has also been evaluated by many groups, 14,15 with the only prospective randomized trial finding similar pancreatic fistula rates (11–12%) when comparing pancreaticogastrostomy and pancreaticojejunostomy. 16
A pharmacologic approach to reduce the rate of pancreatic fistula has been proposed that involves perioperative inhibition of pancreatic exocrine secretion. This concept originated in 1979, using a continuous perioperative infusion of the native tetradecapeptide somatostatin at a dosage of 250 μg/hour in patients undergoing pancreaticoduodenectomy, with the authors reporting a reduced complication rate. 17 Octreotide is a synthetic octapeptide analog of native somatostatin, which is more potent and has a longer half-life. 18,19
To date, four randomized, controlled, double-blind, multiinstitution studies from Europe have evaluated subcutaneous octreotide as prophylaxis against complications in patients undergoing elective pancreatic resection. 20–23 Each of these four trials reported that octreotide (at a dosage of 100 μg three times a day) significantly reduced the overall complication rate. Two of the four trials reported that octreotide was associated with a significantly lower incidence of pancreatic fistula. However, these trials are not without criticism. 24 All included many types of pancreatic resections (e.g., pancreaticoduodenectomy, distal pancreatectomy, enucleations), the pancreatic fistula rates in the control groups were high (≥19%), and in none of the trials was there a significant decrease in the overall death rate. Nonetheless, these four European trials have influenced the practice of many surgeons, who now routinely administer octreotide to patients undergoing elective pancreatic resection. 25,26 Also, a recent meta-analysis has used these four European octreotide trials to conclude that the use of octreotide is a cost-effective strategy in patients undergoing elective pancreatic resection. 27
In contrast to the published studies supporting the use of octreotide, Lowy et al 28 have reported data in direct opposition to the European multicenter trials and the meta-analysis results. This prospective, randomized trial from the M.D. Anderson Cancer Center evaluated 110 patients undergoing only pancreaticoduodenectomy. The rates of clinical pancreatic fistula and perioperative complications were 6% and 25% in the control group and 12% and 30% in the octreotide group. This study showed no benefit to the use of octreotide.
The current prospective, randomized, double-blinded, placebo-controlled, single-institution trial was designed to evaluate the primary endpoints of complications (specifically pancreatic fistula and total complications) and death in patients undergoing pancreaticoduodenectomy. A secondary analysis was performed to evaluate cost issues regarding the use of octreotide.
METHODS
This study was approved by the Joint Committee on Clinical Investigation of the Johns Hopkins University School of Medicine. Patients were recruited into the study before surgery on the basis of anticipated elective pancreaticoduodenal resection, and appropriate informed consent was obtained. Between February 1998 and February 2000, 383 patients were recruited into this study in anticipation of pancreaticoduodenectomy.
Randomization, Exclusions, and Study Drugs
Enrolled patients (n = 383) were randomized before surgery to either the octreotide or the control group by means of a randomly generated number pattern. The octreotide and control saline placebo were prepared in the Investigational Drug Pharmacy and were identical in appearance, volume, and labeling (labeled as “octreotide study drug”), thereby masking the nursing staff, physicians, and patients to their contents. Patients received the octreotide study drug subcutaneously before surgery (within 2 hours of the start of surgery) and every 8 hours after surgery for 7 days, in a volume of 0.25 mL. Patients in the octreotide group (n = 104) received 250 μg octreotide per dose for a total of 22 perioperative doses. Patients in the control group (n = 107) similarly received 22 perioperative doses of saline.
After enrollment and randomization, patients were excluded from the study for the following reasons: patient did not undergo pancreaticoduodenectomy (n = 118), patient underwent total pancreatectomy (n = 14), or patient did not receive at least a 5-day course of octreotide study drug (n = 40). After these exclusions, 211 patients remained in the cohort for outcome analyses.
Surgical Techniques
Patients underwent pancreaticoduodenal resection as a partial pancreatectomy, with either pylorus preservation or distal gastrectomy, as described previously in detail. 1,16 Vagotomy, tube gastrostomy, and feeding jejunostomy were not used. All pancreatic anastomoses were hand-sewn in two layers after mobilizing the pancreatic remnant for 2 to 4 cm. Silk (3–0) was used for the outer layer and polyglactin (3–0) for the inner layer. Pancreaticojejunostomy was performed in either end-to-side or end-to-end fashion at the surgeon’s discretion, as previously described. 29 Pancreaticogastrostomy was used in only two patients, as previously described. 16
At the conclusion of the pancreaticoduodenal reconstruction, one or two 3/16" or 1/4" round silicone closed-suction drains (Relia Vac, Davol, Cranston, RI) were introduced through separate left-sided abdominal stab incisions and placed in the vicinity of the pancreatic-enteric anastomosis.
Postoperative Management
All patients received histamine H2-receptor antagonists during the postoperative hospital stay as prophylaxis against stress and marginal ulceration. Most patients received erythromycin lactobionate (200 mg intravenously every 6 hours from postoperative day 2 to discharge) as prophylaxis against early delayed gastric emptying. 30
Surgically placed drains in the vicinity of the pancreatic anastomosis were left undisturbed, with their outputs recorded daily for at least 4 postoperative days. Aliquots of the drainage were sent for amylase determination between postoperative days 3 and 7. In the absence of a pancreatic fistula (defined below), the drains were removed. In the presence of a pancreatic fistula, management was left to the discretion of the primary surgeon. Because the presence of a pancreatic fistula was considered a primary endpoint, if a pancreatic fistula was observed the primary surgeon could elect to stop the octreotide study drug and begin octreotide therapy.
Data Collection
Data were collected prospectively on all patients and included history, details of the surgical procedure, a surgeon questionnaire (type of resection performed, pancreatic texture [soft, intermediate, hard], and number of drains), pathologic analysis of the resected specimen, and clinical information regarding the postoperative course and complications. Follow-up was complete through February 2000. Data collection was performed by study nurses who were not aware of each patient’s group allocation (octreotide or control).
Study Endpoints
The primary study endpoints were pancreatic fistula, complications, and death. Pancreatic fistula was defined as drainage of greater than 50 mL amylase-rich fluid (more than threefold elevation above upper limit of normal in serum) per day through the surgically placed drains on or after postoperative day 10, or pancreatic anastomotic disruption demonstrated radiographically. Other complications were defined in standard fashion, as previously described. 6,16,30
A secondary study endpoint concerned the cost of octreotide and the potential cost savings associated with the cessation of its use.
Statistical Analyses
The study design predicted the number of patients necessary for statistical validity (one-sided). This was based on the premise of improving the pancreatic fistula rate from 15% to 5%, with alpha set at 0.05 and beta set at 0.2, yielding a power of 80%. One hundred twenty-nine patients were calculated to be required in each arm of the study, for a total study population of 258 patients. The study was reviewed annually by an informal Data Safety Monitoring Board, assessing for adverse events and endpoints. At the first annual review (n = 77), the pancreatic fistula rate was 15.8% in the octreotide group and 10.3% in the control group (P = NS). At the second annual review (n = 164), the pancreatic fistula rate was 13% in the octreotide group and 9% in the control group (P = NS). These data failed to reveal a benefit with the prophylactic use of octreotide. After careful evaluation of the entire study and additional subgroup analyses, it was determined that octreotide therapy had no benefit in any subgroup, and the study was terminated.
Comparability of the octreotide and control groups was verified with the Student t test and chi-square statistics. Results are reported as mean ± standard error of the mean. Significance was accepted at the 5% level. Multivariate comparisons were performed using a stepwise regression of the variables found to be significant on univariate analysis.
Cost Calculations
Data regarding the cost of supporting this study, as well as the daily pharmacy charges for octreotide, were provided by the Investigational Drug Pharmacy.
RESULTS
Patient Population
The study population consisted of 211 patients, 104 in the octreotide group and 107 in the control group (Table 1). The mean patient age was 64 ± 0.9 years and was similar in the two groups. There were no differences between the groups in terms of gender, race, or multiple preoperative factors.
Table 1. PATIENT CHARACTERISTICS AND PREOPERATIVE FACTORS
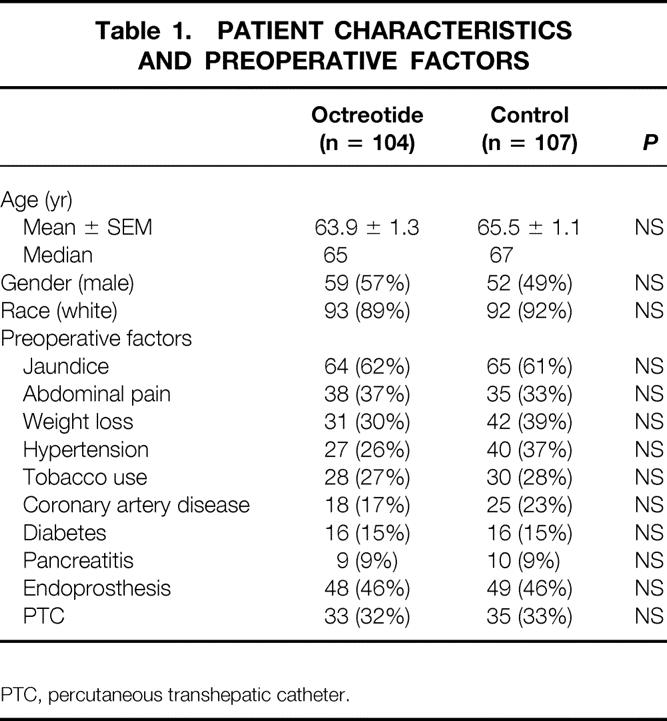
PTC, percutaneous transhepatic catheter.
No significant differences between the groups were observed in terms of multiple intraoperative parameters (Table 2). Most resections involved pylorus preservation, and most pancreatic-enteric anastomoses were performed as an end-to-side pancreaticojejunostomy. Radical or extended pancreaticoduodenectomy was performed in 17% of patients. The texture of the pancreas at the neck transection site was judged by the surgeon to be soft in 37% of patients, intermediate in 36% of patients, and hard in 27% of patients.
Table 2. INTRAOPERATIVE PARAMETERS
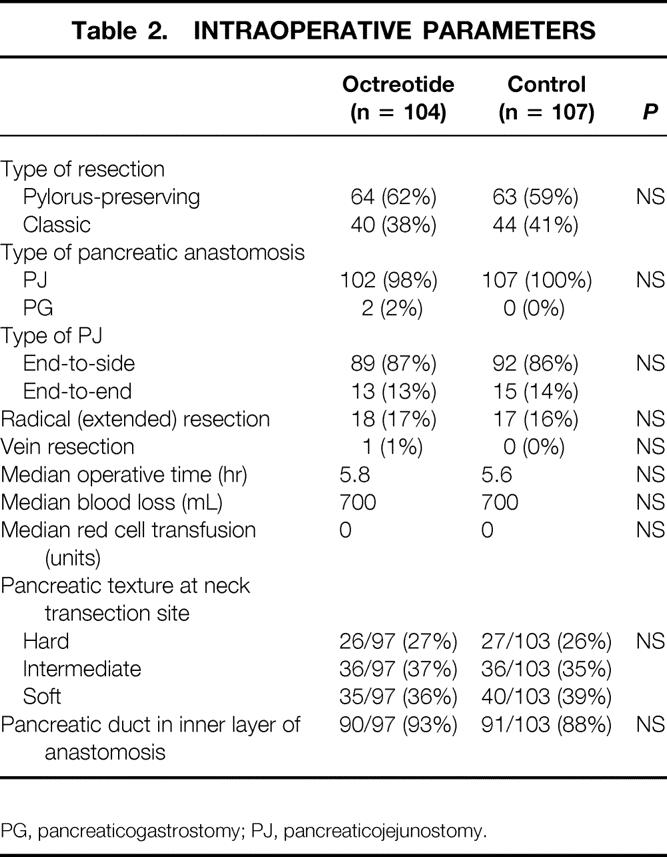
PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy.
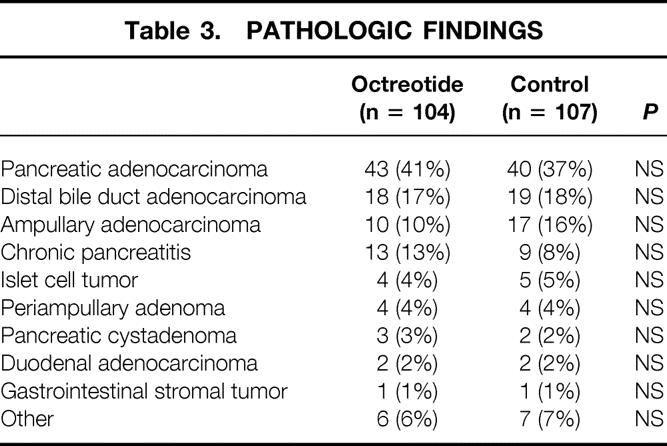
Table 3 depicts the final pathologic analyses of the resected specimens. The two groups were comparable in terms of pathology, with the most common findings being adenocarcinomas of the pancreas, distal common bile duct, and ampulla, followed by chronic pancreatitis.
Table 3. PATHOLOGIC FINDINGS
Complications
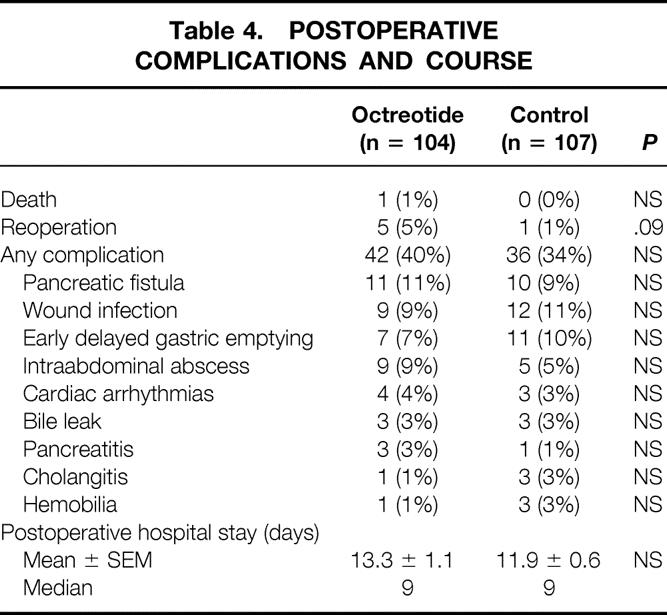
The postoperative complications and course are shown in Table 4. There was one death in the octreotide group, a woman who died after reoperation for ischemic bowel on postoperative day 11. Five additional patients required reoperation during their index admission—three for fascial dehiscence, one for postoperative bleeding, and one for a pancreatic anastomotic leak. The reoperation rate was 5% in the octreotide group and 1% in the control group (P = .09). Overall, 37% of all the patients had one or more postoperative complications, distributed as 40% in the octreotide group and 34% in the control group. The three most common postoperative complications were pancreatic fistula, wound infection, and early delayed gastric emptying. The complication rates were similar between the groups, as was the length of postoperative hospital stay (median 9 days).
Table 4. POSTOPERATIVE COMPLICATIONS AND COURSE
Pancreatic Fistula
The overall incidence of pancreatic fistula was 10% (21/211), which was similar between the octreotide group (11% 11/104) and the control group (9% 10/107). When the pancreatic fistula rate was examined as a function of surgeon-described pancreatic texture, there was a strong association: 0% (0/53) for hard pancreatic texture, 3% (2/72) for intermediate pancreatic texture, and 25% (19/75) for soft pancreatic texture (P < .0001). Table 5 depicts the complications as a function of pancreatic texture. Although complications were more common with soft texture, octreotide had no influence on the pancreatic fistula rate for any pancreatic texture.
Table 5. PANCREATIC FISTULA AND PANCREATIC TEXTURE
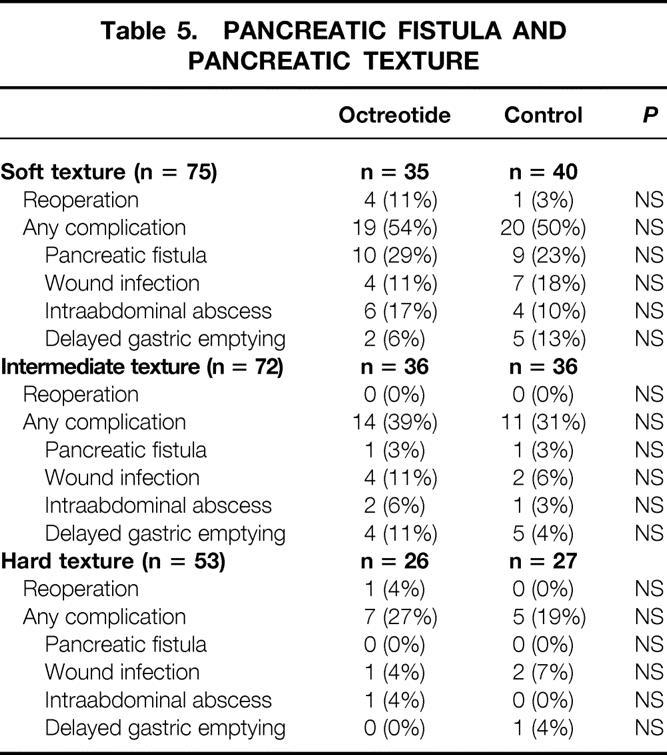
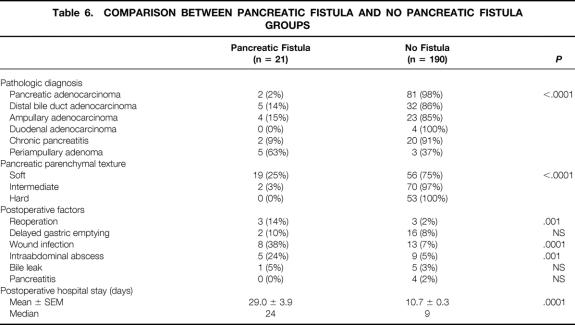
Table 6 shows a comparison of multiple postoperative factors for the 21 patients with a pancreatic fistula versus the 190 without a pancreatic fistula. Many factors are significantly different between these two groups, including specimen pathology, pancreatic texture, reoperation rate, incidence of wound infection and intraabdominal abscess, and postoperative length of stay. Of the 21 patients with a pancreatic fistula, 11 were in the octreotide group and 10 were in the control group. The mean postoperative length of hospital stay was 38 ± 6 days for the 11 patients receiving prophylactic octreotide (median 35, range 14–74) and significantly shorter (19 ± 2 days) for the 10 patients in the control group (median 18 days, range 8–32 days;P = .01).
Table 6. COMPARISON BETWEEN PANCREATIC FISTULA AND NO PANCREATIC FISTULA GROUPS
A stepwise regression analysis was performed to determine the association of pancreatic fistula with pancreatic texture and pathologic diagnosis. Soft pancreatic texture was strongly associated with pancreatic fistula (odds ratio 18.6, P = .0002). Pathologic diagnosis was not associated with a pancreatic fistula when comparing other diagnoses with the entities of pancreatic and bile duct adenocarcinoma and chronic pancreatitis (odds ratio 1.4, P = .52).
Adverse Effects
No adverse reactions to the study drugs (octreotide and saline control) were observed. No patient asked to be withdrawn from the study.
Cost Issues
This study was performed with the expert assistance of the Investigational Drug Service of the Johns Hopkins Hospital. The cost of carrying out this study, including study initiation costs, randomization, and inventory management, totaled $22,815. At the time of the study, the cost for one 250-μg dose of octreotide was $61. The cost for the octreotide used in the 211 evaluable patients (104 receiving octreotide) was $139,568. Thus, the total cost of this study was $162,383.
Currently, the patient charge per dose of octreotide is $27 for one 100-μg dose and $64 for one 250-μg dose. If octreotide were used prophylactically for 22 perioperative doses, the patient charges would be $594 for the 100-μg dose and $1,408 for the 250-μg dose. The elimination of octreotide prophylaxis would save not only these patient charges but also the pharmacy and nursing time needed to dispense and administer each dose. Further, the elimination of octreotide would reduce patient discomfort (elimination of a subcutaneous injection three times a day).
DISCUSSION
This prospective, randomized, double-blinded, placebo-controlled, single-institution trial was designed to evaluate the efficacy of prophylactic octreotide in patients undergoing elective pancreaticoduodenal resection. The primary endpoints analyzed were complications, death, and length of hospital stay. During the 2-year study period, we recruited, randomized, and analyzed 211 patients, all of whom underwent pancreaticoduodenectomy. This represents the largest number of patients in such an octreotide trial, all of whom have undergone one operation: pancreaticoduodenectomy. As shown in Tables 1 through 3, the octreotide and control groups were comparable when tabulating patient demographics, preoperative factors, intraoperative parameters, and pathologic findings. Patient outcomes were assessed by physicians and study nurses not aware of the patient’s group (octreotide or control). All data were collected and entered into our pancreaticoduodenectomy database, and all patients were discharged from the hospital before we broke the randomization code used to assign patients to the octreotide or control group. Using these techniques, potential bias in evaluating outcomes was eliminated.
The results appear clear: prophylactic octreotide does not decrease the rates of postoperative pancreatic fistula, other complications, or death, nor does it decrease the length of postoperative hospital stay. This study corroborates the earlier study by Lowy et al, 28 who similarly reported a lack of efficacy of octreotide in pancreaticoduodenectomy. In that single-institution study from the M.D. Anderson Cancer Center, 110 patients undergoing pancreaticoduodenectomy for presumed malignancy were evaluated. Nearly 50% of the patients received preoperative chemoradiation therapy, 58% received intraoperative radiation therapy, and 19% underwent reoperative resection. Further, although the study was prospective and randomized, no placebo control was used. The study has received criticism because of the relatively small number of patients (n = 110), the fact that nearly all patients had cancer (96%), and the fact that many underwent chemoradiation or reoperation, and because of the lack of a placebo control. Nonetheless, the primary endpoint in the study was clinical pancreatic fistula, and the observed rates were 12% in the octreotide group and 6% in the control group. Moreover, the overall rates of pancreatic fistula (clinical plus biochemical pancreatic fistula) were 28% in the octreotide group and 21% in the control group. Clearly, both the current study from Johns Hopkins and the earlier study from M.D. Anderson fail to support the use of octreotide for patients undergoing pancreaticoduodenectomy.
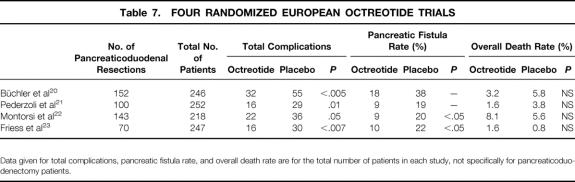
There are four previously published, multiinstitution prospective randomized trials from Europe 20–23 and one meta-analysis using the European data 27 that have been interpreted as showing a benefit to the use of octreotide in elective pancreatic surgery. Each of the four trials evaluated more than 200 patients, used octreotide at a dosage of 100 μg three times a day, and reported significant reductions in overall complications. Two of the four trials also reported significant reductions in the pancreatic fistula rate with the use of octreotide (Table 7). However, none of these European trials included only pancreaticoduodenal resections, and the rates of pancreatic fistula in the placebo groups were high in all four trials (≥19%). In the first trial by Büchler et al, 20 152 of the 246 patients underwent pancreaticoduodenectomy, and the overall pancreatic fistula rate in the placebo group was high at 38%. In the second trial by Pederzoli et al, 21 100 of the 252 patients underwent pancreaticoduodenectomy, and the overall pancreatic fistula rate in the placebo group was 19%. In the third trial by Montorsi et al, 22 143 of 218 patients underwent pancreaticoduodenectomy, and the overall pancreatic fistula rate in the placebo group was 20%. In the fourth trial by Friess et al, 23 70 of 247 chronic pancreatitis patients underwent pancreaticoduodenectomy, and the overall pancreatic fistula rate in the placebo group was 22%. Only in the Montorsi trial did the authors analyze the rates of pancreatic fistula specifically in pancreaticoduodenectomy patients, finding no differences between octreotide and placebo. 22 None of the other three trials evaluated pancreaticoduodenectomy patients alone with respect to endpoints, and it may be inappropriate to extrapolate their data to the setting of pancreaticoduodenectomy.
Table 7. FOUR RANDOMIZED EUROPEAN OCTREOTIDE TRIALS
Data given for total complications, pancreatic fistula rate, and overall death rate are for the total number of patients in each study, not specifically for pancreaticoduodenectomy patients.
A meta-analysis performed by Rosenberg et al 27 used data from the four European trials to evaluate the potential for octreotide to reduce complications and costs in patients undergoing pancreatic resection. Two of the three authors of this paper were employed by Novartis Pharmaceuticals Canada, and the analysis was financially supported by Novartis (makers of octreotide). In this cost-effectiveness analysis, which calculated cost savings in Canadian dollars, it was determined that the use of octreotide would save $853 to $1,642 per patient when used in a theoretical cohort of 100 patients, sparing 16 patients complications. Of course, this meta-analysis relies exclusively on the European data, does not analyze different types of pancreatic surgery (e.g., pancreaticoduodenectomy, distal pancreatectomy, enucleation), and was supported by a pharmaceutical company that manufactures the drug being analyzed. Obviously, the conclusions from the current study and that of Lowy et al 28 stand in direct contrast to the meta-analysis, in that octreotide has not been observed to protect against pancreatic fistula or other complications. Thus, its use would not be cost-effective, but rather would be associated with the increased cost of purchasing, dispensing, and administering an unnecessary drug.
It has been suggested that the prophylactic use of octreotide may have a particular benefit in patients at high risk for pancreatic complications after elective pancreatic resection. 20 In the setting of pancreaticoduodenal resection, these high-risk patients are typically considered to be those lacking pancreatic parenchymal fibrosis, specifically patients with a soft (normal) pancreatic texture undergoing resection for duodenal cancer, ampullary tumors, cystic neoplasms, or islet cell tumors. Certainly the current data (see Tables 5 and 6) confirm an association between pancreatic texture and pancreatic fistula rate: the pancreatic fistula rate was 0% for hard pancreatic texture, 3% for intermediate pancreatic texture, and 25% for soft pancreatic texture (P < .0001). However, the pancreatic fistula rate was not influenced by the presence or absence of octreotide. Further, octreotide therapy did not influence the rates of other complications, such as wound infection, intraabdominal abscess, delayed gastric emptying, or total complications.
Pancreatic fistula remains an important, common complication after pancreaticoduodenectomy, occurring in 10% of patients. The current data indicate that the occurrence of a pancreatic fistula is most highly associated with a soft pancreatic parenchymal texture. When a pancreatic fistula occurs, it is associated with a higher incidence of reoperation, wound infection and intraabdominal abscess formation, and an increased length of hospital stay (median 24 days vs. 9 days for no fistula). Unfortunately, octreotide use does not influence the incidence of pancreatic fistula or any other complications after pancreaticoduodenectomy. Further, our data for the 21 patients with a pancreatic fistula indicate that the 11 patients receiving prophylactic octreotide in whom a fistula developed had a significantly longer hospital stay than the 10 patients not receiving octreotide (mean postoperative stay 38 vs. 19 days;P = .01). We have no explanation for this observation and caution that the numbers of patients with fistula are small, with a wide range in the length of stay.
Another issue that deserves mention is the dosage of octreotide chosen for this study. The four European studies used octreotide at a dosage of 100 μg three times a day. The study by Lowy et al 28 used 150 μg three times a day. We chose to use a higher dosage of 250 μg three times a day. This dosage is at the upper limit of recommended dosing, and it remains within the usual therapeutic window for reducing pancreatic exocrine secretion, presumably the mechanism for preventing postoperative pancreatic fistula. We are aware of no physiologic rationale why octreotide at a dosage of 300 μg/day would be beneficial, but 750 μg/day would show no effect. We interpret our data, and the past reports of Lowy et al 28 and Montorsi et al, 22 as indicating no role for octreotide as prophylaxis in patients undergoing pancreaticoduodenectomy. Eliminating the use of octreotide in this setting would produce a cost savings of $1,408, which is the patient charge in our hospital for a 7-day (22-dose) perioperative course of octreotide at 250 μg/dose.
Octreotide (or its newer long-acting analogs) may have some beneficial effect in patients undergoing other forms of elective pancreatic surgery. For example, the study by Montorsi et al 22 suggests that octreotide may reduce the pancreatic fistula rate in patients undergoing distal pancreatectomy or tumor enucleation. 22 A small study by van Berge Henegouwen et al 31 in healthy volunteers suggested that octreotide may reduce the incidence of delayed gastric emptying, although such an effect was not seen in our study. Further, a small study by Benedetti et al 32 suggested that octreotide prophylaxis may reduce the incidence of complications after pancreatic transplantation. Additional large, well-designed trials are needed to evaluate the role of octreotide in various clinical settings.
In conclusion, these data demonstrate that the prophylactic perioperative use of octreotide does not reduce the incidence of pancreatic fistula, total complications, or death after pancreaticoduodenectomy. Prophylactic octreotide also does not influence the postoperative length of hospital stay. The accumulated evidence from this study and others indicates that prophylactic use of octreotide in this setting should be eliminated, at a considerable cost savings.
Acknowledgments
The authors thank the Johns Hopkins Hospital Investigational Drug Service for their assistance with this study and the many Johns Hopkins Hospital physicians and nursing personnel who participated in the surgical care of these patients.
Discussion
Dr. Murray F. Brennan (New York, New York): There has been a great deal of opinion articulated about the value of prophylactic octreotide in altering morbidity and mortality, and in the present trial with over 200 patients randomized, there was no benefit. For proponents of this therapy, it is really hard to imagine that octreotide would not help. There were previous trials, as you heard, which showed significant decreases in overall morbidity, and in fistula rate in two of the four trials. So the question then is not why, but why is this a negative trial?
First, when analyzing trials from any specialist institution, the control rate of a particular complication would be expected to be less than in other groups, particularly multiinstitutional trials. This is very true here—the overall complication rate between 30 and 40% and an in-hospital mortality a very impressive .5%. In addition, they had a remarkably low incidence of serious complications such as fistula, 11% and 9%, and reoperation, 5% and 1%. This is reflected in a median postoperative stay. In other trials the pancreatic fistula rate, as you heard, ranged between 19% and 38%. In the present study with a fistula rate of 10%, it would require approximately 500 patients in each group to lower that rate to 5%. Dr. Yeo, Dr. Cameron, you are damned by your own abilities. For the merely mortal, there may still be some small benefit to octreotide.
Secondly, the patients also received erythromycin to promote gastric emptying and H2-receptor antagonists as prophylaxis for stress, and it is possible they may have helped to decrease complications.
The authors confirm what has long been felt, that the soft pancreas is associated with a greater fistula rate. This is interesting but somewhat difficult to understand in patients who undergo, as they do here, a duct-to-mucosa anastomosis. Dr. Yeo, should it not be the strength of the mucosal anastomosis rather than the overall texture of the pancreas? I would be interested in your comments.
Rather surprisingly to me, the drainage tubes used by the authors were of the Relia-Vac type. This creates very considerable pressure—indeed a pressure in excess of arterial pressure, when the drain is stripped. We have avoided these for many years, thinking that the pressure generated was far too high. But again, given the superb results of the present team, that would seem to be inappropriate, although my own concerns about the overall value of drains remains.
The authors’ conclusions are clear. In their patients, prophylactic octreotide does not decrease the rate of postoperative fistula formation, consistent with the observations from M.D. Anderson.
So in summary, the authors have a well-designed prospective randomized trial, done in a single institution, one of the few that can do such trials. Because of the expertise of the authors, it may be that their complication rate is sufficiently low that no adjunct is likely to lower the complication rate.
I have three brief questions. One interesting question for me is the use of Relia-Vac drains and the pressure they generate. Two, what about the soft pancreas? Is it really the soft pancreas? Did you have a mucosa-to-mucosa anastomosis?
Three, pancreatic fistula is described as obvious disruption or drainage of 50 mL of amylase-rich fluid after postoperative day 10. Here the median hospital stay was 9 days. At least half the patients had been discharged prior to the time of assessment of the fistula. I assume the drains have been removed, or that a visit to the home was continued in the study. Is it possible to demonstrate that the primary biologic effect that you were looking for—i.e., decrease in pancreatic drainage fluid—was identified by simply analyzing the amount of drainage fluid created?
Presenter Dr. Charles J. Yeo (Baltimore, Maryland): I will make two comments and then try to answer your questions. First, to be fair, the definition of pancreatic fistula that we use is fairly conservative—i.e., 50 mL after day 10—and the definitions used by the European trials were very liberal—meaning 10 cc of fluid out the drain after postoperative day 3. I think part of the discrepancy in the data go back to those definitions. We believe our definition describes a clinical leak, while the liberal definition describes a biochemical leak. Our personal belief is that a biochemical leak is usually clinically unimportant and that those drains can be removed with no sequelae. Therefore the distinction becomes important, which is why we use the definition that we do.
The other issue you talked about was erythromycin, and could erythromycin be playing a role in the reduction of complications. We use erythromycin not as a prophylactic antibiotic, but because of its role as a motilin agonist to prompt gastric emptying. As our data show, our delayed gastric emptying rate is very low. We do use antibiotics for prophylaxis against wound infection, but personally I don’t think erythromycin is having any influence on pancreatic fistula rates.
All the drains we used were closed-suction round silicon drains. Most were Relia-Vac drains. We also use round Jackson-Pratt drains. The devices used as reservoirs are either the bellows-type system, which most of us are familiar with, or the grenade-type system. It is my impression that after several hours of being in place, the suction really holds, because there is tissue ingrowth and adjacency of serosal surfaces. I don’t believe there is terribly high pressure being exerted on the viscera.
The issue of leak rates and the pancreatic texture is very important. Our analysis shows that soft texture is the most predictive. When we analyzed for pathologic diagnoses, it was not as predictive. The soft gland does not hold stitches well. It is like trying to sew Jello to the bowel. And although we tried to do a duct-to-mucosa anastomosis for the inner layer, and were able to accomplish that in over 90% of patients, with these soft glands, you can often see the stitches pull through as they are placed—usually by our chief residents. I think it is a matter of getting good tissue apposition and having good tissues to hold together.
The other issue, of course, is that soft glands, as typically are seen in duodenal cancers, have normal pancreatic parenchyma and a normal output of exocrine secretions, so they are more likely to have a higher risk of leakage. The other issue that you asked, Dr. Brennan, dealt with drain output. In this study, we didn’t measure the volume of drain output. We had done that for a previous study. It is very tedious to do and somewhat unreliable, so we used pancreatic fistula as an endpoint. Most patients have no evidence of fistula and have their drains removed on postoperate days 4, 5, or 6.
Dr. Andrew L. Warshaw (Boston, Massachusetts): I would like to call attention to the fact that the advantage of receiving a manuscript in advance is much mitigated by having to follow Dr. Brennan in the discussion. Dr. Yeo, this really is an excellent study. I will resist the temptation to call it “Yeoman’s Work.” It shows—conclusively as far as I am concerned—that there was no demonstrable benefit from perioperative octreotide in preventing pancreatic fistulas and other complications of mortality after pancreaticoduodenectomy.
The question is why your results and conclusions are different from those of the European multicenter trials. As you recognize, your findings may not be extrapolated from pancreaticoduodenectomy to other pancreatic operations, especially distal pancreatectomy, which in experiences like our own is significantly more likely to lead to a pancreatic fistula than pancreaticoduodenectomy. This difference in trial design may in part explain the discrepancy between the present findings and the prior European trials that included all pancreatic resections.
I note and commend your group on your extremely low fistula rate in both the control and the trial group. In fact, you projected in the trial design a fistula rate of 15% in calculating your statistics, but you beat that by a fair amount with an actual average of under 11%. Your fistula rate in both groups is one of the best ever reported. As Dr. Brennan points out, this may be particularly important. It reflects not only on the statistics of the study, but it also emphasizes that octreotide or anything else may not be able to effect improvement of an already excellent outcome. Perhaps octreotide would have a significant benefit in operations performed by less-experienced and less-skillful surgeons. That factor also could impact the positive effect observed in the multicenter trials from Europe.
Your leaks were almost entirely confined to the soft pancreatic texture group. There were fewer than 1% in the 125 patients with a hard or intermediate pancreatic substance, whereas it was 25% (19 out of 75) with soft pancreas. The numbers get to be a little small when you do subgroup analyses, but even in the high-risk group, there was no beneficial effect of octreotide.
The question is, what do you recommend in the future? Are there other methods for preventing a pancreatic fistula from a soft pancreas that you have not addressed in your study? Do you use a transanastomotic stent in the small pancreatic duct group, which usually correlates with a soft pancreas? You alluded to fibrin sealants. There are several glues and sealants being tested now. Might these make a difference?
Dr. Yeo: The first question deals with how these data are able to be used by non–high-volume surgeons, and are they really conflicted with the European trials. A previous trial by Lowy et al from M.D. Anderson analyzed 110 patients. Of the four European trials, only one (Montorsi et al) analyzed pancreaticoduodenectomy patients alone, with 143 patients. Neither trial had differences in the fistula rates in pancreaticoduodenectomy patients. We evaluated 211 patients. So, of the published prospective randomized trials, there are roughly 464 patients dealing only with pancreaticoduodenectomy, and the fistula rates are not any different. I think this information speaks to the issue of whether this is just a phenomenon in a high-volume center or whether it can be applicable to medium- or even lower-volume surgeons.
I would caution you, though, to do a self-assessment of your fistula rates. It really depends on your patient population. If you are operating in a cancer center largely on malignancies, then you are going to have mainly intermediate- and hard-texture glands and you should have very few pancreatic fistulas. If, on the other hand, you do a lot of periampullary adenomas or islet cell tumors (typically with soft texture), then you are going to be faced with a much higher fistula rate. So self-assessment is important.
The issue of how to change the fistula rates: good surgical technique, based upon good Halstedian principles—careful suturing, proper mobilization of the pancreatic remnant, et cetera—is important. We do not routinely use transanastomotic stents.
We do have a study that we are in the process of initiating which will look at the issue of fibrin glue to seal the anastomosis. Again, the issue arises that one must assess for the different types of pancreatic textures—only about a third of the patients that we do have soft texture, and they have a 25% leak rate. So obviously the numbers of patients that we need to enroll into the whole study are going to be quite high, in order to show a benefit to fibrin glue.
Dr. J. Patrick O’Leary (New Orleans, Louisiana): My question is quite simple. In this study, finds of a soft pancreas was a predictor of postoperative fistula. Did you actually correlate the histology of the pancreas away from the tumor to see if it was in fact normal? I would guess hardness of the pancreas has to do with the presence or absence of chronic inflammation. You have the ideal circumstance to tell me what a soft pancreas is histologically.
Dr. Yeo: We are referring to the texture of the pancreas at the pancreatic neck transection site, the site that is just ventral to the portal vein-superior mesenteric vein axis. Soft texture equates to a normal pancreas at the pancreatic neck. This is seen in patients with islet cell tumors, periampullary adenomas, duodenal cancers— typically patients who have not had pancreatitis, do not have tumors, either ampullary, distal bile duct, or pancreatic cancers that are obstructing the pancreatic duct. In fact, texture is the most powerful predictor—not pathology alone, but the texture as judged by the surgeon at the time of the pancreatic reconstruction. I think all of us who do pancreatic surgery would agree on what a soft, normal pancreas is.
Dr. Kenneth W. Warren (Boston, Massachusetts): Many years ago, I reported 348 cases of pancreatoduodenectomy; we divided them into two 10-year groups. In the second group, we had an incidence of pancreatic leak in only four patients; in only one did we think it was contributory to death. We did use stents in some of these patients with small ducts. I want to ask, how many of the patients that leaked had a direct duct-to-mucosa anastomosis? I have always practiced this. I think you can do it irrespective of the size of the duct.
I will say one word about the soft parenchyma. If you are going to do a duct-to-duct anastomosis, as Dr. Brennan suggested, that should be the key to the incidence of fistula formation. With respect to the softness, it is the only place in surgery where large bites should be used, and the knots should not be tied tightly.
Dr. Yeo: I am not sure I can follow-up Dr. Warren. I thank him for his historical and technical comments. The answer to your question is that 90% of our anastomoses were done with the pancreatic duct incorporated into the inner layer. Thus, 90% of patients had a duct-to-mucosa anastomosis.
Dr. Lawrence W. Way (San Francisco, California): An important aspect of this study is how pancreatic fistula is defined. I agree with Dr. Yeo’s approach, which is to use a definition that restricts the subsequent analysis to clinically important leaks.
I have two questions. First, 20 to 30 years ago, when the mortality rate of pancreaticoduodenectomy averaged 20%, pancreatic fistula was one of the major causes of death. Nowadays, pancreatic fistula seems to produce considerably less morbidity. For example, we reported a recent 10-year experience (Arch Surg 1989;124:778) from our medical center in which pancreatic fistula was the cause of no postoperative deaths nor reoperations. Thus, the importance of this problem seems to be decreasing along with its incidence. How serious were the pancreatic fistulas in your patients? Do you agree that the consequences have lessened as much as I suggested?
Second, it should be possible to reanalyze your data using the various definitions of pancreatic fistula adopted by others who have written on this subject. If that were done, how would your results compare with the other studies you mentioned?
Dr. Yeo: The second question first. We didn’t reanalyze our data based on other definitions because it is fairly common early postop to have drainage with elevated amylase. The volume and amylase content typically decline. Our belief is that early amylase-rich drainage, particularly at a low volume, is clinically irrelevant and not important, so we haven’t reanalyzed our data.
To get down to what happens when you get a fistula: Twenty-one patients in this study had a fistula. The patients who had a fistula had a mean stay in the hospital of 29 days, compared to a mean stay of 11 when they didn’t. The patients who had a fistula stayed longer, their hospital costs were higher, and they had other complications.
Once a patient gets a fistula, if they are clinically stable we allow the drains to remain in place. Fully 85% to 90% of such patients require no further intervention, the drains just simply remain in place a few more days. Not all of them are even put on TPN at this point. Many are allowed to eat. And some patients, a minority, have been sent home with the drains in place to be removed as an outpatient. So definitely our management has evolved. Only one patient in this whole series required reoperation for a pancreatic leak. So I believe that your observation is correct—in many cases, pancreatic leaks are not as problematic as they were years ago.
Dr. Jonathan E. Rhoads (Philadelphia, Pennsylvania): When I was interested in doing Whipple’s, we tried anastomosing the stump of the pancreas to the back of the stomach. And we liked the operation. The stomach wall was thicker, we made an opening in it which would fit the pancreas precisely, and one could put one row of sutures in through the open end of the stomach to the mucosa, and another row around the outside, and had—theoretically at least—the advantage that the pancreatic juice was inactivated as it flowed into the acid-containing stomach. I wondered if the authors had a series with the gastric anastomosis and what sort of a rate leak they would get. We thought we had fewer leaks and came to like the procedure a good deal.
The only other question I would ask is that when you do such a precise analysis as we have listened to, which is very impressive, one has to remember that it only determines the result at a particular dosage of the drug. I think you said some of the Europeans had given the drug 3 times a day. I wondered how you arrived at and had confidence in the particular dosage which you selected.
Dr. Yeo: I would like to thank Dr. Rhodes. He clearly stands as a giant in this field, and he wrote a seminal paper in the 1960s dealing with pancreaticogastrostomy (Am J Surg 1967;113:85–90).
In this particular series, only two patients had a gastric anastomosis. We have previously, in front of this group in 1995, presented a prospective randomized trial where we looked at pancreaticojejunostomy versus pancreaticogastrostomy. That trial had 145 patients in it, was prospective and randomized, and the fistula rates were identical. I would point out that when you have a leak from the pancreaticogastrostomy, it can make it difficult to feed the patient postop, because their ingested food may leak out these drains, because the leak is right in the stomach. So, we have actually gotten away from pancreaticogastrostomy except in rare cases where the gland is very large and bulky, and simply won’t fit into the side of the jejunal limb.
Dr. Rhoads asked another very perceptive question about the drug dosing. We picked a dose of 250 μg 3 times a day. The European trials used 100 μg 3 times a day and the M.D. Anderson trial used 150 μg 3 times a day. All these doses are within the therapeutic window for octreotide as far as its role in the suppression of exocrine secretion. We chose the higher dose because of a little bias on our part. We suspected that this was going to be a negative trial and we wanted to be sure that we had given enough of a dose so one of the criticisms wouldn’t be, well, what if you had used a higher dose? So that explains the dosing.
Dr. Larry C. Carey (Tampa, Florida): Octreotide is a painful injection. I wonder if there was a problem in maintaining the blindness of your prospective trial because patients often complain bitterly about the pain of octreotide injection. Secondly, was it necessary to stop the octreotide because of patient reluctance to continue?
Dr. Yeo: We intentionally used a volume of octreotide of only 0.25 mL, which is smaller than some of the typically used volumes, in order to get around the injection-site pain issue. Octreotide does hurt a bit more than saline. No patient requested to be withdrawn or terminated from this study because of pain at the injection site. There were also no other adverse reactions to the octreotide that we identified in this study.
Dr. Bruce M. Wolfe (Sacramento, California): As you indicated, the rationale for the use of octreotide is to diminish exocrine secretion. In the fasting state, there is essentially no stimulation of exocrine secretion—it is when feeding is resumed that exocrine secretion resumes. How aggressive are you in trying to resume oral feeding and do you use enteral feeding, particularly in patients who have advanced malnutrition before operation?
Dr. Yeo: As far as the refeeding issue goes, we typically remove nasogastric tubes on postoperative day 1, usually on the morning of the day after surgery. Then patients are allowed sips of clear liquids on postoperative days 1 and 2, and then usually a clear liquid diet by postoperative day 3, and a regular diet by postoperative day 4 or 5. We have been amazed at how well that is tolerated. Many patients now are being discharged on days 7, 8, or 9. In fact, our critical pathway targets days 8 or 9 as the discharge day.
We do not routinely use jejunostomy or gastrostomy tubes. In fact, I can’t remember the last time we put in a feeding jejunostomy tube on any of these patients. We simply use the GI tract after we have reconstructed it, giving the patients oral intake relatively early.
Footnotes
Correspondence: Charles J. Yeo, MD, Dept. of Surgery, Johns Hopkins Hospital, Blalock 606, 600 N. Wolfe St., Baltimore, MD 21287-4606.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
Supported in part by NIH grants RO1-CA56130 and P50-CA62924.
E-mail: cyeo@jhmi.edu
Accepted for publication April 2000.
References
- 1.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, outcomes. Ann Surg 1997; 226: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strasberg SM, Drebin JA, Soper NJ. Evolution and current status of the Whipple procedure: an update for gastroenterologists. Gastroenterology 1997; 113: 983–994. [DOI] [PubMed] [Google Scholar]
- 3.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995; 221: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 1995; 130: 295–300. [DOI] [PubMed] [Google Scholar]
- 5.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165: 68–73. [DOI] [PubMed] [Google Scholar]
- 6.Yeo CJ. Management of complications following pancreaticoduodenectomy. Surg Clin North Am 1995; 75: 913–924. [DOI] [PubMed] [Google Scholar]
- 7.Madiba TE, Thomson SR. Restoration of continuity following pancreaticoduodenectomy. Br J Surg 1995; 82: 158–165. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith HS, Ghosh BC, Huvos AG. Ligation versus implantation of the pancreatic duct after pancreaticoduodenectomy. Surg Gynecol Obstet 1971; 132: 87–92. [PubMed] [Google Scholar]
- 9.DiCarlo V, Chiesa R, Pontiroli AE, et al. Pancreaticoduodenectomy with occlusion of the residual stump by neoprene injection. World J Surg 1989; 13: 105–111. [DOI] [PubMed] [Google Scholar]
- 10.Gall FP, Gebhardt C, Meister R, et al. Severe chronic cephalic pancreatitis: use of partial duodenopancreatectomy with occlusion of the pancreatic duct in 289 patients. World J Surg 1989; 13: 809–817. [DOI] [PubMed] [Google Scholar]
- 11.Hiraoka T, Kanemitsu K, Tsuji T, et al. A method for safe pancreaticojejunostomy. Am J Surg 1993; 165: 270–272. [DOI] [PubMed] [Google Scholar]
- 12.Kingsnorth AN. Duct to mucosa isolated Roux loop pancreaticojejunostomy as an improved anastomosis after resection of the pancreas. Surg Gynecol Obstet 1989; 169: 451–453. [PubMed] [Google Scholar]
- 13.Matsumoto Y, Fujii H, Miura K, et al. Successful pancreatojejunal anastomosis for pancreatoduodenectomy. Surg Gynecol Obstet 1992; 175: 555–562. [PubMed] [Google Scholar]
- 14.Kapur BML. Pancreaticogastrostomy in pancreaticoduodenal resection for ampullary carcinoma: experience with thirty-one cases. Surgery 1986; 100: 489–493. [PubMed] [Google Scholar]
- 15.Mason GR, Freeark RJ. Current experience with pancreatogastrostomy. Am J Surg 1995; 169: 217–219. [DOI] [PubMed] [Google Scholar]
- 16.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg 1995; 222: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klempa I, Schwedes U, Usadel KH. Verhutung von postoperativen pankreatitischen Komplikationen nach Duodenopankreatekomie durch Somatostatin. Chirurgie 1979; 50: 427–432. [PubMed] [Google Scholar]
- 18.Kohler E, Beglinger C, Dettwiler S, et al. Effect of a new somatostatin analogue on pancreatic function in healthy volunteers. Pancreas 1986; 2: 154–159. [DOI] [PubMed] [Google Scholar]
- 19.Bauer W, Briner U, Doepfner W, et al. SMS 201–995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci 1982; 31: 1133–1140. [DOI] [PubMed] [Google Scholar]
- 20.Büchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg 1992; 163: 125–131. [DOI] [PubMed] [Google Scholar]
- 21.Pederzoli P, Bassi C, Falconi M, et al. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Br J Surg 1994; 81: 265–269. [DOI] [PubMed] [Google Scholar]
- 22.Montorsi M, Zago M, Mosca F, et al. Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery 1995; 117: 26–31. [DOI] [PubMed] [Google Scholar]
- 23.Friess H, Beger HG, Sulkowski U, et al. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. Br J Surg 1995; 82: 1270–1273. [DOI] [PubMed] [Google Scholar]
- 24.Yeo CJ. Does prophylactic octreotide benefit patients undergoing elective pancreatic resection? J Gastrointest Surg 1999; 3: 223–224. [DOI] [PubMed] [Google Scholar]
- 25.Friess H, Büchler MW. Efficacy of somatostatin and its analogues in pancreatic surgery and pancreatic disorders. Digestion 1996; 57 (suppl 1): 97–102. [DOI] [PubMed] [Google Scholar]
- 26.Berberat PO, Friess H, Uhl W, Büchler MW. The role of octreotide in the prevention of complications following pancreatic resection. Digestion 1999; 60 (suppl 2): 15–22. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg L, MacNeil P, Turcotte L. Economic evaluation of the use of octreotide for the prevention of complications following pancreatic resection. J Gastrointest Surg 1999; 3: 225–232. [DOI] [PubMed] [Google Scholar]
- 28.Lowy AM, Lee JE, Pisters PWT, et al. Prospective randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg 1997; 226: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron JL. Atlas of Surgery, vol 1. Philadelphia: Decker/Mosby-Year Book; 1990: 400–409.
- 30.Yeo CJ, Barry MK, Sauter PK, et al. Erythromycin accelerates gastric emptying following pancreaticoduodenectomy: a prospective, randomized placebo-controlled trial. Ann Surg 1993; 218: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Berge Henegouwen MI, van Gulik TM, Akkermans LMA, et al. The effect of octreotide on gastric emptying at a dosage used to prevent complications after pancreatic surgery: a randomized, placebo controlled study in volunteers. Gut 1997; 41: 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benedetti E, Coady NT, Asolati M, et al. A prospective randomized clinical trial of perioperative treatment with octreotide in pancreas transplantation. Am J Surg 1998; 175: 14–17. [DOI] [PubMed] [Google Scholar]