Abstract
Objective
To evaluate randomly the effect of thyrostatic treatment (tiamazole) versus selective (metoprolol) and nonselective β-blockade (propranolol) on whole-body energy metabolism in women with hyperthyroidism.
Summary Background Data
β-blockade is used as an alternative to thyrostatic drugs in the preoperative treatment of patients with hyperthyroidism. β-blockers have well-established symptomatic effects, but in contrast to antithyroid drugs β-blockade is thought to lack direct effects on the increased metabolism in hyperthyroidism.
Methods
Whole-body oxygen consumption and carbon dioxide production was measured in a semiopen canopy system with paramagnetic O2 and infrared CO2 sensors. A constant flow generator and the gas-dilution method for calculation of gas flow were used. Anabolic parameters were body weight, triceps skinfold, and arm muscle circumference.
Results
Tiamazole normalized oxygen consumption and induced signs of anabolism with improved nutritional state. Metroprolol did not affect oxygen consumption. Propranolol reduced elevated oxygen consumption by 54%. Body weight and other anthropometric assessments were stable after specific and nonspecific β-blockade, which also led to symptomatic relief in approximately 90% of the patients.
Conclusion
Tiamazole was the most effective drug to oppose the adverse effects of hyperthyroidism. Therefore, thyrostatic agents are recommended for preoperative treatments of patients with severe catabolic hyperthyroidism. Whenever β-blockers are chosen for treatment of hyperthyroidism, propranolol (β1 + β2) has an advantage because it reduces the metabolic rate, whereas selective β1-blockade seemed to provide only symptomatic relief, related to the normalization of heart rate.
Symptomatic effects of β-adrenoceptor blocking agents are well documented in patients with hyperthyroidism, 1–4 although the mechanism by which β-blockers exert their effects is not fully understood. 5,6 In contrast to antithyroid drugs, β-blockers do not reduce the thyroid hormone secretion, but nonselective β-blockers can decrease the peripheral conversion of free thyroxin (T4) to active triiodothyronine (T3). Previous studies have suggested that the beneficial effects of adrenoceptor blockade in hyperthyroidism are mainly due to the blockade of β1-receptors, 7–9 but nonselective β-blockers are often used for treatment of hyperthyroidism. However, oxygen consumption has been measured in only few hypermetabolic patients receiving β-blockers in previous studies. Small to moderate decreases in oxygen consumption were found in some patients, 10,11 but this phenomenon was not verified in other investigations. 12–14 The aim of the present study was therefore to compare the effects of β-blockers and an antithyroid drug on whole-body oxygen consumption and carbon dioxide production. Anthropometric assessments were performed to assess possible long-term effects of anabolism after the various treatments. The nonselective β-blocker propranolol, the selective β1-blocker metoprolol, and the antithyroid drug tiamazole were used for a random evaluation in women with hyperthyroidism.
PATIENTS AND METHODS
Patients
Surgery is a recommended treatment in young patients with hyperthyroidism in Sweden and several European countries. A prerequisite for safe surgical treatment is medical preparation with either antithyroid drugs or β-adrenoceptor blockade. Therefore, women with diffuse toxic goiter were informed about three treatment alternatives: radioiodine, long-term medication with an antithyroid drug, or surgery after preoperative preparation with either β-blockade or antithyroid treatment. Those who preferred to undergo surgery were asked whether they agreed to be randomized to one of three alternatives of preoperative treatment. After full information was provided, 28 patients in a cohort of 90 women with hyperthyroidism agreed to undergo this randomized treatment. Their ages ranged from 19 to 45 years. All had symptoms and biochemical values typical of hyperthyroidism. None had been treated with antithyroid drugs before the study. Seven patients had been treated with β-blockade for a few weeks before referral. Medication use was stopped in the latter patients for 2 weeks before they were included in the study. Six euthyroid women, age 28 to 65 years, with benign thyroid adenomas agreed to participate in the study as reference patients. They took no medications. The study was approved by the Ethical Committee of Sahlgrenska University Hospital, Göteborg, Sweden. All patients gave informed consent.
Methods
Before randomization, new measurements of serum concentrations of free thyroxin (T4) and triiodothyronine (T3) were obtained. The next morning, after an overnight fast and refraining from smoking, measurements of metabolic and nutrition parameters were made by a trained research nurse who did not know the results of the blood tests. She allocated the patients to one of three treatment groups by a closed-envelope method of randomization. The next day, the patients started to take their preoperative medication. For determination of whole-body metabolism, patients were placed in a calm, silent room in the supine position. Breathing frequency, resting heart rate, oxygen consumption, and carbon dioxide production were measured after a half-hour rest. Indirect calorimetry was performed by a Deltatrac metabolic monitor (Datex, Helsinki, Finland), which is a semiopen canopy system with paramagnetic O2 and infrared CO2 sensors. A constant flow generator and the gas-dilution technique were used to calculate gas flow through the system. All patients lay in the canopy for at least 10 minutes before each measurement was made (duration 30 minutes). 15,16 Whole-body oxygen uptake and carbon dioxide production are presented as mL/min or as mL/min/kg body weight, because normalization to body surface area did not change the results in any direction. Nutritional state was assessed by body weight, triceps skinfold, and arm muscle circumference. All anthropometric measurements were performed in duplicate by the same nurse. The triceps skinfold reflects body fat deposits and was measured at the middle of the relaxed left triceps muscle with the arm in stretched position using a precision metal caliper. Arm muscle circumference was measured at the middle of the left relaxed triceps muscle. 16
Preoperative Medical Treatment
Ten patients were randomized to treatment with the antithyroid drug tiamazole. Four other patients with inadequate symptomatic improvement after treatment with β-blockers were transferred to the group with tiamazole, but they remained in the original randomization group for evaluation. Tiamazole was given in a dosage of 20 mg twice daily for 4 weeks. The dose was then halved and 0.1 mg thyroxine was added as a daily dose.
Ten patients were randomized to treatment with four daily oral doses of 50 mg of the selective β1-blocker metoprolol. The dose was doubled if resting heart rates exceeded 75 beats per minute after 1 week of treatment. Two patients still had an elevated heart rate after 10 days of taking 100 mg/day, and their treatment was withdrawn for 10 days. They underwent another measurement of nutrition and metabolic status and were then transferred to the group receiving tiamazole treatment.
Eight patients were randomized to treatment with the nonselective β-blocker propranolol, which was given at a dosage of 40 mg four times daily. The dose was doubled if resting heart rates exceeded 75 beats per minute after 1 week. Two patients had an elevated heart rate after the 10 additional days of taking 320 mg/day. Their treatment was therefore withdrawn for 10 days, and they underwent another measurement of nutrition and metabolic status and were then transferred for tiamazole treatment.
Patients randomized to β-blockers took their oral morning dose the day of surgery, and if needed they received intravenous injections of β-blockers during surgery. The dose of β-blockers was tapered during 10 days after surgery.
Six euthyroid patients served as controls. They were scheduled for surgery because of benign cold thyroid adenomas and received no medical treatment. They were tested only at the time of diagnosis to define euthyroid reference values for the patients with hyperthyroidism.
Statistics
Results are presented as mean ± SD. All groups were compared using a before-and-after three-group design with the repeated-measures analysis of variance. Statistics are presented as “between groups,” “before and after treatment,” and “interaction between groups and treatment.” All results were evaluated according to intent to treat. The Fisher protected least significant difference (PLSD) test was used post hoc. Within-group differences over time (before vs. after treatment) were tested by a paired t test. P < .05 was regarded as statistically significant;P < .10 was regarded as showing a trend toward statistical significance.
Results
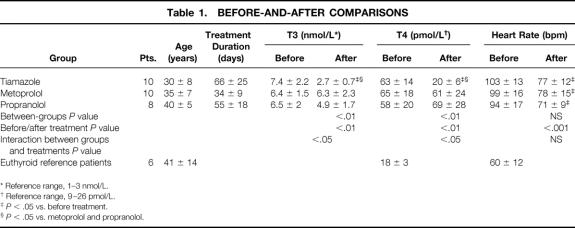
Mean age did not differ among the three groups (Table 1). The duration of medical treatment was longer for patients treated with tiamazole than for those treated with metoprolol (P < .01). Before treatment, no differences were found among the groups in serum concentrations of T3 and free T4. Plasma T3 and free T4 reached normal values only in the tiamazole group (P < .05). Patients in all three groups had significantly elevated resting heart rates before treatment compared with euthyroid patients (P < .05). The resting heart rate in the patients with hyperthyroidism fell from 98 ± 14 to 74 ± 10 (P < .0001) after treatment, with no differences among the groups. The mean breathing frequency per minute in patients receiving tiamazole, metoprolol, and propranolol was 20, 18, and 20, respectively, before treatment. Tiamazole and propranolol decreased breathing frequency (P < .0001); it was unchanged during metoprolol treatment. None of the patients experienced any adverse effect of the medication, and subsequent surgeries were performed without complications.
Table 1. BEFORE-AND-AFTER COMPARISONS
* Reference range, 1–3 nmol/L.
† Reference range, 9–26 pmol/L.
‡P < .05 vs. before treatment.
§P < .05 vs. metoprolol and propranolol.
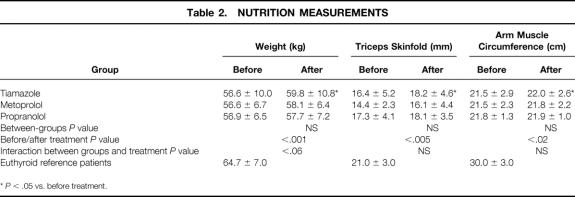
Mean body weight increased significantly in patients receiving tiamazole only. The anthropometric parameters improved in these patients also (Table 2).
Table 2. NUTRITION MEASUREMENTS
*P < .05 vs. before treatment.
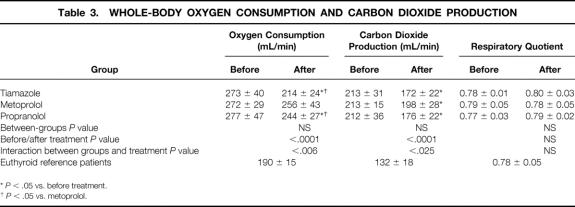
Whole-body oxygen consumption was significantly elevated in all three groups of patients compared with the euthyroid patients (P < .0001;Table 3). Tiamazole and propranolol treatment reduced whole-body oxygen consumption significantly, whereas metoprolol had no such effect. Similar results were obtained when whole-body respiratory gas exchanges were normalized to body weight or to body surface area. Whole-body oxygen consumption in the tiamazole group reached euthyroid levels, because it did not differ from the metabolic rate in the reference patients, whereas propranolol did not normalize the metabolic rate entirely.
Table 3. WHOLE-BODY OXYGEN CONSUMPTION AND CARBON DIOXIDE PRODUCTION
*P < .05 vs. before treatment.
†P < .05 vs. metoprolol.
Whole-body carbon dioxide production decreased significantly in all treatment groups. Respiratory quotients were similar in all groups before and after treatment, suggesting that net substrate oxidations were similar before and after all medical treatments. 16
DISCUSSION
In this study we evaluated the effect of β-blockade and a classic antithyroid drug, tiamazole, on whole-body metabolism and nutritional state in a small group of unselected women with hyperthyroidism. Possible conclusions may be limited because the number of patients was comparatively small. However, several study variables were significantly improved when interaction between groups and treatment were evaluated. The significant group-by-treatment interaction indicated that not all drugs were equally effective in decreasing the metabolic and nutritional parameters. Additional pairwise comparisons indicated that tiamazole was the most effective of the three.
Four patients treated with β-blockade had an inadequate clinical response and were switched to treatment with the antithyroid drug tiamazole as a medical precaution. These patients might represent a more severe disease state, although their serum concentrations of thyroid hormones were similar to those of the other patients. Tiamazole use normalized whole-body oxygen consumption effectively in these patients also. Therefore, β-blockade failed to control hypermetabolism in 4 of 28 (15%) patients with hyperthyroidism. However, all patients had a normal resting heart rate at the end of the study treatment. Normalized serum concentrations of thyroid hormones and confirmed anabolism, as shown by a significant gain in body weight, triceps skinfold, and arm muscle circumference, were observed only in the tiamazole group. Accordingly, whole-body oxygen consumption was also normalized after treatment with the antithyroid drug only, evaluated statistically according to the intent to treat. However, the nonselective β-blocker propranolol also reduced elevated oxygen consumption and carbon dioxide production, whereas treatment with the selective β-blocker metoprolol did not affect oxygen consumption at all.
In the 1960s and 1970s, several studies were published about oxygen consumption after treatment with propranolol. Two showed decreased oxygen consumption by 9% to 12%, 10,11 whereas three others reported no change. 12–14 Selective β-blockade by atenolol caused an 11% decrease in oxygen consumption in one investigation, 11 whereas the combination of β- and α-blockade reduced oxygen consumption by 12%. 17 Most of these earlier investigations involved patients with the Graves variety of hyperthyroidism; one study also included patients with toxic nodular goiter. 11 In previous studies, whole-body oxygen consumption was presented in mL/min, mL/min/kg body weight or mL/min/m2 body surface, making direct comparisons among various studies difficult. Changes in oxygen consumption in previous studies were usually reported as a percentage of whole-body oxygen consumption. In contrast, we present our results on an individual basis in absolute values.
In the recent literature on treatment of hyperthyroidism with β-blockers, only symptomatic effects have been discussed, with nothing about the effects on whole-body oxygen consumption. 6,18 Symptomatic benefits were thought to be due to β1-blockade; this assumption is confirmed by our results because β1-blockade did not affect the metabolic rate but reduced the heart rate significantly. However, the addition of β2-blockade decreased the metabolic rate significantly, which indicates that this effect may be peripheral but is not necessarily substrate-derived, given that respiratory quotient measurements were unchanged in our patients.
Our results demonstrate that only the antithyroid drug tiamazole could normalize whole-body oxygen consumption completely and induce anabolism effectively. These findings confirm that thyrostatic drugs should be preferred for preoperative treatment in catabolic patients with severe hyperthyroidism, although β-blockers may have a more rapid effect for symptomatic relief of hyperthyroidism. Traditionally, both nonselective and selective β1-blockers have been used in large groups of patients for preoperative preparation. 1,18 These drugs have also been used for symptomatic relief in patients with hyperthyroidism during the latency period before antithyroid drugs or radioiodine treatments can control hyperthyroid manifestations. However, whenever β-blockers are chosen for treatment of hyperthyroidism, propranolol (β1 + β2) should be the preferred choice because it has a more direct effect on the hypermetabolism itself than does β1-blockade.
Footnotes
Supported in parts by grants from the Swedish Cancer Society (2014-B98-12XAC), the Medical Research Council (K99-72X-08712-11A, K99-73X-11611-04A), Tore Nilson Foundation, Assar Gabrielsson Foundation (AB Volvo), Jubileumskliniken Foundation, IngaBritt & Arne Lundberg Research Foundation, Swedish and Göteborg Medical Societies, the Medical Faculty, Göteborg University, and Gunnar Nilsson Memorial Foundation.
Correspondence: Svante Jansson, MD, PhD, Dept. of Surgery, Sahlgrenska University Hospital, S-413 45 Göteborg, Sweden. E-mail: Svante.Jansson@dep-surg.gu.se
Accepted for publication May 22, 2000.
References
- 1.Lee TC, Coffey RJ, Currier BM,et al. Propranolol and thyroidectomy in the treatment of thyrotoxicosis. Ann Surg 1982; 195: 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lennquist S, Jortso E, Anderberg B, Smeds S. Beta-blockers compared with antithyroid drugs as preoperative treatment in hyperthyroidism: drug tolerance, complications, and postoperative thyroid function. Surgery 1985; 98: 1141–1147. [PubMed] [Google Scholar]
- 3.Lee KS, Kim K, Hur KB, Kim CK. The role of propranolol in the preoperative preparation of patients with Graves’ disease. Surg Gynecol Obstet 1986; 162: 365–369. [PubMed] [Google Scholar]
- 4.Adlerberth A, Stenstrom G, Hasselgren PO. The selective beta-1-blocking agent metoprolol compared with antithyroid drug and thyroxine as preoperative treatment of patients with hyperthyroidism. Results from a prospective, randomized study. Ann Surg 1987; 205: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feely J, Peden N. Use of beta-adrenoceptor blocking drugs in hyperthyroidism. Drugs 1984; 27: 425–446. [DOI] [PubMed] [Google Scholar]
- 6.Geffner DL, Hershman JM. Beta-adrenergic blockade for the treatment of hyperthyroidism. Am J Med 1992; 93: 61–68. [DOI] [PubMed] [Google Scholar]
- 7.McDevitt DG, Nelson JK. Comparative trial of atenolol and propranolol in hyperthyroidism. Br J Clin Pharmacol 1978; 6: 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murchison LE, Bewsher PD, Chesters MI, Ferrier WR. Comparison of propranolol and practolol in the management of hyperthyroidism. Br J Clin Pharmacol 1976; 3: 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murchison LE, How J, Bewsher PD. Comparison of propranolol and metoprolol in the management of hyperthyroidism. Br J Clin Pharmacol 1979; 8: 581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders J, Hall SE, Crowther A, Sonksen PH. The effect of propranolol on thyroid hormones and oxygen consumption in thyrotoxicosis. Clin Endocrinol 1978; 9: 67–72. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson OR, Karlberg BE, Kagedal B,et al. Non-selective and selective beta-1-adrenoceptor blocking agents in the treatment of hyperthyroidism. Acta Med Scand 1979; 206: 21–25. [DOI] [PubMed] [Google Scholar]
- 12.Howitt G, Rowlands DJ. Beta-sympathetic blockade in hyperthyroidism. Lancet 1966; 1 (7438): 628–631. [DOI] [PubMed] [Google Scholar]
- 13.Georges LP, Santangelo RP, Mackin JF, Canary JJ. Metabolic effects of propranolol in thyrotoxicosis. I. Nitrogen, calcium, and hydroxyproline. Metabolism 1975; 24: 11–21. [DOI] [PubMed] [Google Scholar]
- 14.Zwillich CW, Matthay M, Potts DE,et al. Thyrotoxicosis: comparison of effects of thyroid ablation and beta-adrenergic blockade on metabolic rate and ventilatory control. J Clin Endocrinol Metab 1978; 46: 491–500. [DOI] [PubMed] [Google Scholar]
- 15.Sandstrom R, Drott C, Hyltander A,et al. The effect of postoperative intravenous feeding (TPN) on outcome following major surgery evaluated in a randomized study. Ann Surg 1993; 217: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandstrom R. Total parenteral nutrition in the postoperative period. Department of Surgery, Göteborg, Sweden: Göteborg University; 1995.
- 17.Stout BD, Wiener L, Cox JW. Combined alpha- and beta-sympathetic blockade in hyperthyroidism. Clinical and metabolic effects. Ann Intern Med 1969; 70: 963–970. [DOI] [PubMed] [Google Scholar]
- 18.Vickers P, Garg KM, Arya R,et al. The role of selective beta-1-blocker in the preoperative preparation of thyrotoxicosis: a comparative study with propranolol. Int Surg 1990; 75: 179–183. [PubMed] [Google Scholar]