Abstract
Objective
Clinafloxacin is a novel quinolone with wide activity against the plethora of microorganisms encountered in intraabdominal infections. This trial was performed to examine its clinical efficacy.
Summary Background Data
Clinafloxacin is representative of a new class of quinolones with considerable antimicrobial activity resulting from their mechanisms of action and pharmacodynamics. There is, however, concern about specific potential toxicities, including photosensitivity.
Methods
This prospective, randomized, double-blind trial was conducted to compare clinafloxacin with imipenem/cilastatin as adjuncts in the management of complicated intraabdominal infections.
Results
Five hundred twenty-nine patients were included in the intent-to-treat population, with 312 meeting all criteria for the valid population. Patients with a wide range of infections were enrolled; perforated or abscessed appendicitis was the most common (approximately 50%). One hundred twenty-three of the 150 valid patients treated with clinafloxacin (82%) had successful outcomes, as did 130 of the 162 (80%) treated with imipenem. For the intent-to-treat groups, 219 of 259 patients treated with clinafloxacin (85%) had successful outcomes, as did 219 of 270 patients treated with imipenem/cilastatin (81%). Treatment failure occurred in 39 patients who underwent drainage. There were substantially more gram-negative organisms recovered from the patients with treatment failure who were initially treated with imipenem/cilastatin.
Conclusions
The results of this study clearly demonstrate the safety and efficacy of clinafloxacin in the treatment of a range of intraabdominal infections, and in patients with a broad range of physiologic disturbances.
Antimicrobial therapy is an important element of management of intraabdominal infection, because inadequate therapy results in increased failure rates. 1–4 The infecting flora in intraabdominal infections is well known and consists of facultative and obligate anaerobic organisms, aerobic gram-negative organisms, various streptococci and enterococci, and a plethora of gram-positive anaerobes. 5–7 The synergistic interactions between endotoxin-bearing gram-negative organisms and Bacteroides fragilis define both groups as important targets for antimicrobial therapy. 8
Quinolones have only recently been studied in the management of intraabdominal infection. 9,10 Ciprofloxacin and similar compounds have substantial activity against the gram-negative facultative and aerobic bacilli but lack activity against aerobic gram-positive cocci and obligate anaerobes.
Clinafloxacin is a new quinolone with activity against gram-negative facultative and aerobic bacilli as well as aerobic gram-positive cocci and obligate anaerobes. 11–14 We performed a prospective, randomized, controlled, and double-blinded trial to evaluate this agent in the management of complicated intraabdominal infections. This study also provided a uniform, prospectively generated data set to examine other issues related to the management of intraabdominal infections. We were particularly interested in examining criteria for adequate source control by either surgical procedure or percutaneous intervention. We also verified the use of a surrogate endpoint defined in a prior intraabdominal infections trial.
METHODS
Entry Criteria
This trial was conducted in accord with the Infectious Diseases Society of America/Food and Drug Administration guidelines. 15,16 Patients were eligible for inclusion if they were 18 years or older and showed signs and symptoms of intraabdominal infection, and if surgical or percutaneous drainage of an infectious focus was recently performed or appeared necessary. Key exclusion criteria were little likelihood of survival for more than 48 hours, or an Acute Physiology, Age, and Chronic Health Evaluation (APACHE) II score of more than 30 17; a history of hypersensitivity to the agents being used; other investigational therapy within the previous 30 days; impaired liver function; neutropenia (<1,000 × 106 neutrophils/L); and previous enrollment in the trial. Central nervous system disease, as a risk for seizures, was also an exclusion criterion. Pregnant or breast-feeding women were excluded. Patients with acute renal insufficiency (serum creatinine > 1.5 mg/dL) were excluded.
Patients were entered in this trial either on identification of signs of infection that would likely require intervention or after surgical or percutaneous intervention. Patients were not excluded if they received antibiotics other than the study treatment before intervention if they had a poor clinical response to that treatment. Nonstudy antibiotics were stopped before the study treatment was commenced. For patients enrolled after intervention, no more than single doses of nonstudy therapy were allowed. The protocol was reviewed and approved by each institutional review board, and informed consent was obtained from each patient according to the guidelines of the institution and in accord with the ethical standards of the Helsinki Declaration of 1975.
Each site was provided with a randomization list with a block size of four. One pharmacist was unmasked and monitored patients on a daily basis. Patients received either clinafloxacin 200 mg every 12 hours with placebo infused at 12-hour intervals or imipenem/cilastatin 500 mg every 6 hours. For imipenem/cilastatin, dosage adjustments for renal failure were made according to product labeling. For clinafloxacin, the dose was reduced to 100 mg every 12 hours if the patient’s creatinine clearance was 40 mL/minute or less.
Patient Populations
The modified intent-to-treat (MITT) population comprised all patients who were randomized to treatment and received at least one dose of study drug. The evaluable patient population met the following criteria: entry criteria for the study were satisfied and no exclusion criteria were present, an intraabdominal infection was documented by findings at surgery or percutaneous drainage, and cultures from the abdominal site of infection or blood cultures were positive for pathogenic bacteria. Patients were required to receive 3 days of therapy for the treatment to be considered a failure and 5 days for the treatment to be considered a success.
Outcome Assessment Criteria
Treatment failure was defined as persisting or recurrent infection in the abdomen, documented by the findings at percutaneous or surgical reintervention, or postsurgical wound infection. Patients who received additional antibiotics for undocumented intraabdominal infection were considered invalid successes if there was no evidence of infection by clinical, laboratory, and radiographic criteria. If there was clinical evidence of infection, treatment was considered to be a valid failure. The treatment received by patients with ongoing abdominal infection and who died after 48 hours of treatment was considered a failure.
Assessment of the Adequacy of the Interventional Procedure
The decision to use percutaneous or surgical intervention was made by the treating physician. Lavage of the peritoneal or abscess cavity with antibiotics was prohibited. Use of prosthetic materials as fascial substitutes was prohibited. The decision to close the skin incision was made by the operating surgeon based on the extent of contamination and the risk of subsequent wound infection. Use of drains was similarly at the discretion of the operating surgeon.
All case report forms were initially reviewed to determine the adequacy of the intervention performed. Surgical or percutaneous drainage procedures were deemed adequate for patients without recurrent intraabdominal infection. For surgical procedures, adequacy was defined by drainage of all purulent collections identified on preoperative radiographic examination, and by removal of the source of infection. For percutaneous procedures, adequacy was defined by the absence of subsequent drainage procedures not planned at the time of the initial computed tomography or ultrasound image. Cases not meeting these criteria for adequacy were referred to an expert panel composed of surgeons experienced in the surgical and antimicrobial management of patients with intraabdominal infection, and in analysis of clinical trials data.
Statistical Analysis
Treatment equivalence between clinafloxacin and imipenem/cilastatin was tested using clinical outcome (success or failure) as the primary variable. A categorical modeling procedure provided point estimates (means and variances) for the differences between treatment group clinical outcomes. A two-tailed 95% confidence interval (CI) was constructed from the point estimates using a standard normal approximation. Treatment equivalence was prospectively defined as CI between -15% and +15%.
To assess possible prognostic factors for treatment failure, a logistic regression was performed using clinical outcome as the dependent variable and several possible prognostic factors as independent variables: treatment group, APACHE II score, initial organ site of infection (appendix or all other), polymicrobial infection, presence of diffuse peritonitis, presence of an abscess, postoperative cause, baseline resistant pathogen, previous antibiotics for either prophylaxis or therapy, length of time from hospital admission to study entry 3 days or more, and center enrollment 10 or more.
Analysis was performed using SAS PROC LOGISTIC (SAS Institute, Inc. Cary, NC) with a stepwise variable selection procedure. The significance level for entering a variable into the model was specified at 5%. Eleven of the 312 evaluable patients were excluded from the logistic regression because they had no APACHE II scores.
RESULTS
Five hundred fifty-one patients were entered into this trial. Twenty-two were considered indeterminate because no study medication was administered and insufficient information was provided to determine disease status and outcome. These patients were not further considered, leaving a MITT population of 529 patients.
Two hundred seventeen patients who received study medication were considered not valid, for reasons detailed in Table 1. The most common reason was failure to identify pathogens, usually in the setting of an acute inflammatory disease such as appendicitis. No differences between study arms were identified. This left 312 valid patients for analysis. The demographic characteristics of this population are detailed in Table 2. There were no differences between the treatment groups for the variables examined.
Table 1. REASONS FOR EXCLUDING PATIENTS FROM THE VALID POPULATION
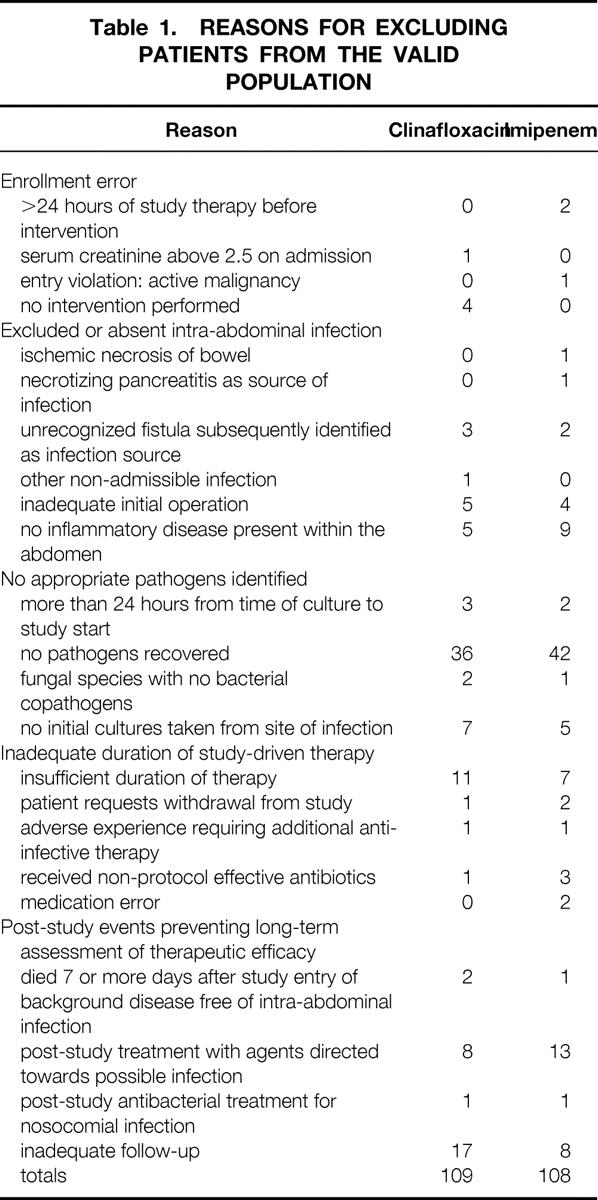
Table 2. DEMOGRAPHIC AND ORGAN SITE TABLE
APACHE, Acute Physiology, Age and Chronic Health Evaluation.
Infectious Pathology
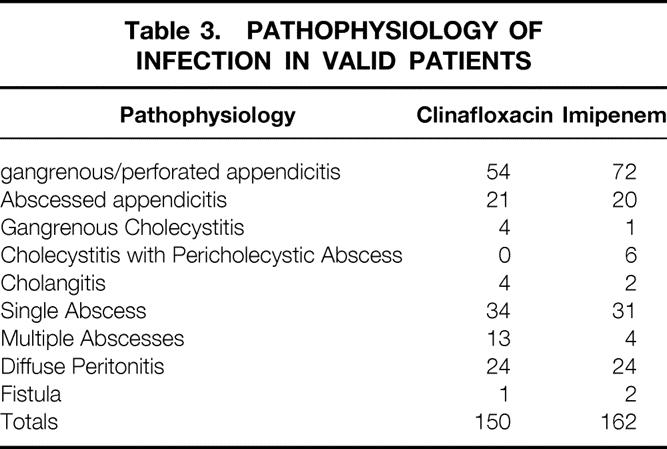
The types of pathology encountered are detailed in Table 3. A total of 82 patients had one or more abscesses. Thirty-four of these were treated percutaneously. For the 22 patients entered into this study because of postoperative abscesses, 14 were treated percutaneously. The outcome of treatment was closely correlated with the type of pathology found. Seventeen of 36 patients with diffuse peritonitis were treatment failures, whereas only 7 of 58 patients with solitary abscesses were failures. The rate of treatment failure was high for patients with multiple abscesses (7/17).
Table 3. PATHOPHYSIOLOGY OF INFECTION IN VALID PATIENTS
Interventions Performed
A high proportion of the patients enrolled in this trial underwent some form of intervention, a consequence of allowing postinterventional therapy before study entry. Only 4 of the 529 patients entered did not undergo some form of intervention.
To be considered valid, patients were required to undergo a drainage procedure. For the 150 valid patients treated with clinafloxacin, 29 were treated percutaneously, 118 underwent surgery, and 3 underwent either an endoscopic or a laparoscopic intervention. Of the 162 valid imipenem-treated patients, 24 were treated percutaneously and 138 surgically. Three were treated either by endoscopy or laparoscopy.
Nine patients were considered to have had inadequate initial interventions after expert panel review. Seven of these were initially treated by percutaneous drainage. In two cases, catheters placed to treat an obstructed and infected biliary system occluded in the immediate postprocedure period. In three other cases, multiple abscesses were initially identified, but not all were drained. In two other cases, extensive diverticular disease involving transmural inflammation was treated percutaneously without resolution until surgery was performed. The laparoscopic failure was inadequate drainage of a periappendiceal abscess. One patient with extensive diverticulitis underwent surgical abscess drainage without colonic resection. Signs and symptoms of infection persisted, and he required reoperation and colectomy. All nine of these patients survived, and all underwent subsequent intervention. In six cases, percutaneous drainage was curative; in three others, surgical treatment was curative.
Microbiologic Findings
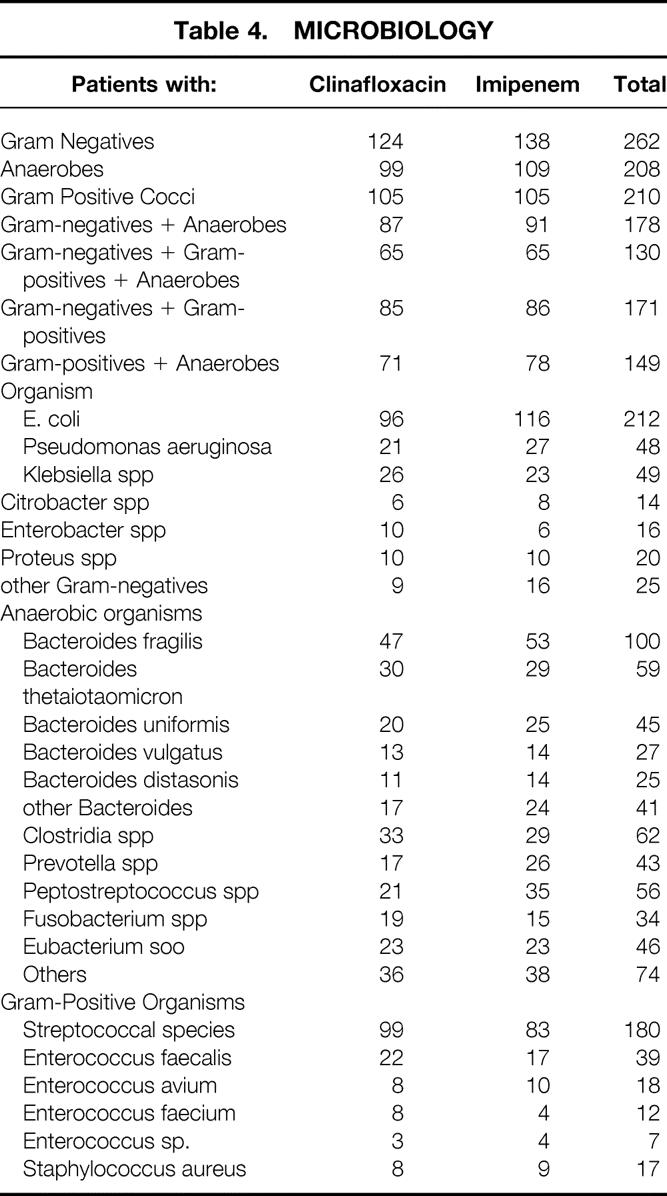
The organisms encountered in this study were similar to those reported in previous studies (Table 4). There was a high incidence of polymicrobial infection, particularly gram-negative organisms and anaerobes with (130/312, 41%) or without (48/312, 15%) gram-positive organisms. Of the 208 patients with anaerobes, 100 (48%) were B. fragilis.
Table 4. MICROBIOLOGY
Clinical Outcome and Treatment Equivalence
Treatment was considered successful in 123 of the 150 valid patients treated with clinafloxacin (82%) and 130 of the 162 (80%) treated with imipenem. For the MITT groups, treatment was considered successful in 219 of 259 clinafloxacin-treated patients (84%) and 219 of 270 imipenem/cilastatin-treated patients (81%). The 95% CI about the difference between the clinical outcomes for the evaluable population (-7%, +10%) and for the MITT population (-3%, +10%) was within predefined (-15%, +15%) bounds for treatment equivalence.
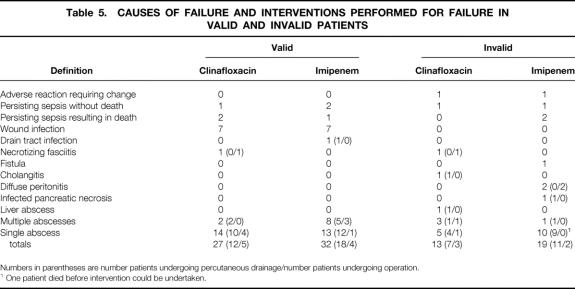
Causes of Treatment Failure
The various bases for treatment failure are presented in Table 5. Treatment failure resulted in a large number of surgical or percutaneous reinterventions. For the valid patients, 9 underwent surgical reintervention and 30 underwent percutaneous treatment. There were no differences between treatment groups. For the invalid group, 23 of the 32 patients in whom treatment was considered a failure underwent surgical intervention (n = 5) or percutaneous drainage (n = 18).
Table 5. CAUSES OF FAILURE AND INTERVENTIONS PERFORMED FOR FAILURE IN VALID AND INVALID PATIENTS
Numbers in parentheses are number patients undergoing percutaneous drainage/number patients undergoing operation.
1 One patient died before intervention could be undertaken.
We reviewed the causes of exclusion for the 23 invalid patients in whom treatment failed and who underwent some form of reintervention for failure. Nine were excluded for an inadequate initial intervention. Two others had fistulas subsequently identified as the source of infection, two had an inadequate duration of study treatment (<3 days), and nine had negative cultures (n = 4), no cultures (n = 3), or no study therapy for more than 24 hours after the initial (on-study) cultures were taken (n = 3). These treatment failures therefore do not include patients with positive cultures who received adequate antiinfective therapy and who then failed to respond. For those patients with either no cultures taken or negative cultures, the surgical findings demonstrated inflammatory but not infectious disease.
Thirty-nine valid patients failed to respond to treatment and underwent either surgical or percutaneous drainage. Fourteen clinafloxacin-treated patients had positive cultures at reintervention (11 abscess cultures, 1 peritonitis fluid culture, and 2 wound cultures). Eighteen imipenem/cilastatin-treated patients had positive cultures (14 abscess cultures, 1 peritonitis fluid culture, and 3 wound cultures).
There were substantially more gram-negative organisms recovered from the patients who failed to respond to treatment and who were initially treated with imipenem (Table 6). The most common was Escherichia coli, followed by Pseudomonas aeruginosa, three Proteus species, and Morganella morganii. The ratio of patients with gram-negative organisms to patients with positive cultures indicated that this was marginally significant (P = .0626). There was no such significance for gram-positive organisms (P = .2635).
Table 6. THE MICROBIOLOGY OF TREATMENT FAILURE
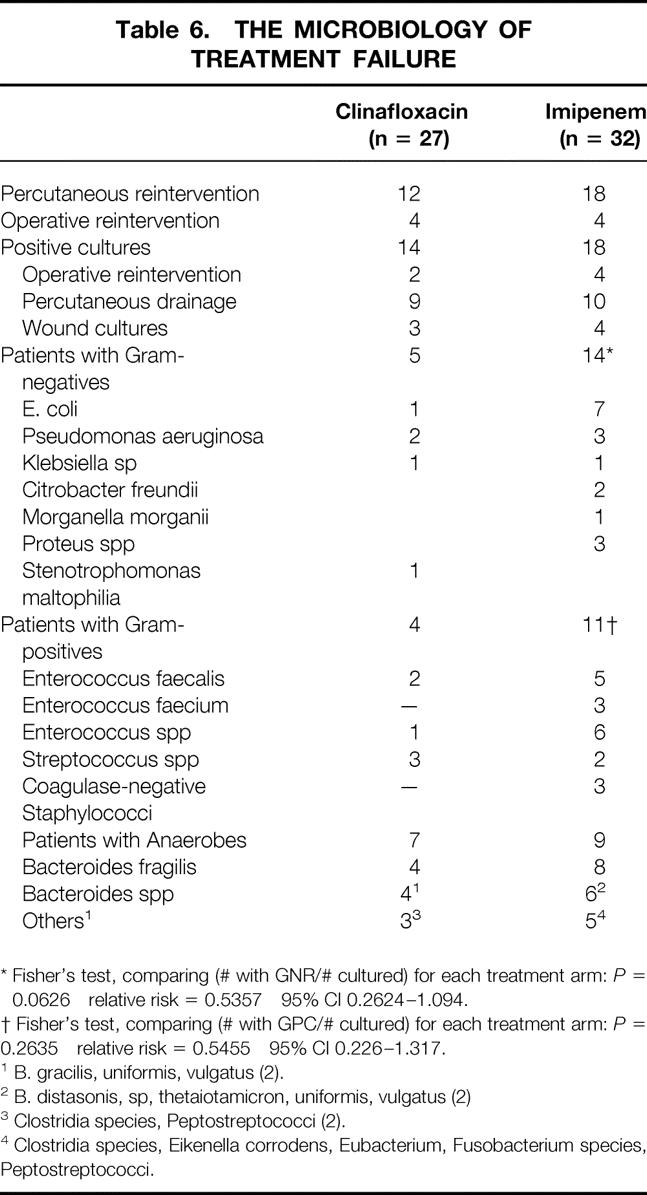
* Fisher’s test, comparing (# with GNR/# cultured) for each treatment arm:P = 0.0626 relative risk = 0.5357 95% CI 0.2624–1.094.
† Fisher’s test, comparing (# with GPC/# cultured) for each treatment arm:P = 0.2635 relative risk = 0.5455 95% CI 0.226–1.317.
1 B. gracilis, uniformis, vulgatus (2).
2 B. distasonis, sp, thetaiotamicron, uniformis, vulgatus (2)
3 Clostridia species, Peptostreptococci (2).
4 Clostridia species, Eikenella corrodens, Eubacterium, Fusobacterium species, Peptostreptococci.
Eight patients treated with clinafloxacin died, as did five treated with imipenem. Five of these patients were considered valid, and death in each case was related to infection. Of the eight invalid patients, death was unrelated to infection in five, and treatment was considered a success in these patients.
Adverse Events
Five hundred twenty-nine patients (259 receiving clinafloxacin and 270 receiving imipenem) were evaluable for safety analysis. Thirty-four percent of clinafloxacin-treated patients and 26% of imipenem-treated patients had at least one adverse event during the study. Most adverse events were mild to moderate, and they most commonly appeared during the first 5 days of study drug administration. The most common adverse events in the clinafloxacin-treated group were diarrhea (7%), hypoglycemia (4%), vaginal moniliasis (3% of women), and nausea (3%). The most common adverse events in the imipenem-treated group were diarrhea (7%), nausea (2%), rash (2%), and abnormal results on liver function tests (2%). Three clinafloxacin-treated patients discontinued therapy because of adverse events (one each of hypotension, hypoglycemia, and rash), as did five imipenem-treated patients (three rashes, and one each of urticaria and allergic reaction). Three clinafloxacin-treated and seven imipenem-treated patients developed Clostridium difficile-associated diarrhea, confirmed by toxin assay or stool culture. No patients had endoscopically documented pseudomembranous colitis.
The two clinafloxacin-specific toxicities known at the beginning of the trial were phototoxicity and hypoglycemia. Four clinafloxacin-treated patients had a phototoxic reaction. These were mild to moderate sunburnlike reactions, and none discontinued treatment because of this event. Twelve clinafloxacin-treated patients and five imipenem-treated patients had laboratory evidence of hypoglycemia. Two clinafloxacin-treated patients and three imipenem-treated patients had documented glucose levels of 50 mg/dL or less. Very low glucose values were reported in one clinafloxacin-treated patient (10 mg/dL) and one imipenem-treated patient (11 mg/dL); neither patient was symptomatic or suffered permanent sequelae.
Overall, 13 patients died. Eight received clinafloxacin and five received imipenem/cilastatin. In seven patients, death was attributed to uncontrolled infection and multisystem organ failure. In six others, death was due to progression of underlying malignancy (n = 3), progressive intestinal infarction (n = 2), or pulmonary embolism (n = 1). There were no differences between study arms.
Logistic Regression
We performed logistic regression using clinical outcomes of the evaluable patient population (success or failure) as the dependent variable with a stepwise variable selection procedure. Only two of the independent variables contributed significantly to prediction of clinical outcome. Presence of diffuse peritonitis was a significant predictor of failure (chi-square = 8.32, P = .0039). The odds ratio for patients with diffuse peritonitis was 0.338 (95% CI, 0.162–0.706). APACHE II score was also a significant predictor for failure (chi-square = 5.66, P = .0174). For each unit increase in APACHE II score, the odd ratio was 0.933 (95% CI, (0.882–0.988). Although treatment failure was significantly less common in patients with gangrenous or perforated appendicitis than in those with other anatomical sources of infection (P = .043, relative risk 0.5754, 95% CI 0.3383–0.9787), this was explained by the lesser severity of illness.
DISCUSSION
Analysis of Results
We undertook this study to evaluate the safety and efficacy of a novel quinolone with activity against the gram-negative, gram-positive, and anaerobic organisms encountered in intraabdominal infections. Our results documented the equivalence of clinafloxacin to imipenem/cilastatin, an agent widely used in the management of intraabdominal infections and in controlled prospective clinical trials of this entity. 18–22
However, it is unlikely that clinical measures will demonstrate differences in comparative trials of broadly active antiinfective regimens. This is particularly true given the multiple factors in addition to antiinfective treatment that determine the outcome from intraabdominal infection. One means of avoiding the impact of these variables is to use an outcome measure more dependent on the antimicrobial activities of the agents under study. Persistence of organisms at a site of treatment failure would seem a direct and valid measure of drug activity.
In a previous trial comparing ciprofloxacin plus metronidazole with imipenem/cilastatin, we found no difference in the broad measure of clinical outcome (recurrent infection) also used in the current study. 9 We did note a substantial difference in the microbiology of failure: we found a high incidence of persisting gram-negative organisms in patients who failed to respond to treatment with imipenem/cilastatin.
The results of the current study prospectively validate that finding. We noted that of the 15 patients initially treated with clinafloxacin who subsequently developed culture-positive recurrent abscesses or wound infections, 5 were infected with gram-negative organisms. Conversely, 14 of 18 patients treated with imipenem/cilastatin who had positive cultures and failed to respond to treatment harbored gram-negative organisms.
This finding is similar to that reported in studies of imipenem/cilastatin that used doses of 500 mg and provided information on the microbiology of treatment failure. 21,23–25 The basis for this is unclear. Many of the persisting isolates were highly susceptible to imipenem. We suspect this effect might be due to the relatively low plasma levels of imipenem dosed at 500 mg. Levels of imipenem or other antiinfectives have not been measured in the residual inflamed tissue after appropriate intervention, and this tissue is most likely the initiating site of recurrent abscess. Studies performed with 1-g doses of carbapenems did not find a similar high level of persisting gram-negative organisms. 10,25–27 These data support the use of the microbiology of treatment failure as a surrogate endpoint.
Analysis of Adequacy of the Initial Intervention
The key determinant of outcome is the procedure performed to deal with the anatomical source and consequence of infection. An adequate surgical procedure is generally agreed on and involves drainage of all fluid collections, closure or resection of any openings into the gastrointestinal tract, and resection of inflamed tissue. The latter aspect of surgical management is the most controversial, and recommendations have ranged from complete peritoneal débridement to attention only to the source of infection. The extent of such inflammation is an important factor limiting the efficacy of percutaneous drainage. 28,29
The primary concern in the context of clinical research is that the adequacy of intervention is an independent variable determining outcome. Particularly with a small number of such patients, there is the very real possibility that such patients would not be evenly distributed by randomization and would therefore skew results. In the absence of clear rules for intervention, we chose to review the procedures performed by using an expert panel. Cases were initially screened by one person (J.S.S.), and patients possibly having had inadequate intervention were referred to this panel for discussion. This process was conducted in a masked manner. We ultimately identified nine patients as having had an inadequate intervention. Although this is a relatively small number, these patients might have an inordinate effect on the outcome analysis in that failure is an uncommon outcome.
The characteristics of these patients were of interest. Most such patients had been treated percutaneously, and the primary problem was identification of patients with extensive inflammatory disease or multiple abscesses possibly or likely requiring surgery to resolve. Because there is currently no way to identify patients with such extensive processes objectively, a consensus panel process is the only recourse.
Enrollment of Patients With Appendicitis
Registration trials of antiinfectives are intended to examine the agent’s safety and efficacy in clinical settings similar to those in which it would be used were it approved for marketing. The range of infections subsumed under the heading of intraabdominal infection and the wide variations in physiologic status of such patients make this a complex task. There has been a bias toward entering patients with substantial physiologic derangement because such patients are currently at greatest risk of treatment failure and because of concerns that certain toxicities may be apparent only in this more severely ill group of patients. However, appendiceal infections are common and provide certain advantages in that a relatively young population, without substantial confounding disease and without risk of death, is studied. A relatively uniform pathology is also encountered in such patients. Studies in the setting of acute appendicitis have identified important differences between various antimicrobial regimens. 2,30,31
The results of the logistic regression analysis bear directly on this subject. Appendiceal infections were not independent predictors of outcome, meaning that the lower failure rates seen with perforated appendicitis were correlated with the severity of illness, not with any unique feature of appendiceal infection.
We therefore conclude that these populations (appendiceal vs. nonappendiceal sources of infection) provide overlapping and complementary information on two questions: the antimicrobial efficacy of the agent in humans, and the safety of the agent across various patient groups. A range of disease processes is necessary because it is likely that different types of infection (e.g., peritonitis vs. abscess, single vs. multiple abscess) and different organ sources of infection (e.g., colon vs. proximal small bowel) present different problems in drug delivery and in the density of pathogens.
We believe it is appropriate to enroll 40% to 60% of the patients in a trial with appendicitis. Sufficient experience is now available with this and other disease entities in clinical trials to allow power calculations based on planned enrollment rates of appendiceal versus nonappendiceal disease. The observed results of the trial would then provide documentation of the accuracy of the power calculations and the adequacy of the sample size used. This upper limit of enrollment of appendicitis would then allow inclusion of patients with nonappendiceal infections, with a range of organ sources, pathology of infection, and physiologic severity. These groups would allow examination of the safety and efficacy of the agent beyond the more homogeneous appendicitis population.
Statistical Analysis
The results of this study were examined using logistic regression. This allows identification of variables other than the antiinfective regimen used that determine outcome from intraabdominal infection. The impact of specific variables on outcome is statistically expressed through weighted outcome prediction models. We identified APACHE II scores and the presence of peritonitis as significant predictors of treatment failure. Patients without diffuse peritonitis had a threefold greater risk of treatment failure. Each unit increase in APACHE II score translated to approximately a 7% increase of odds for failure.
These data are similar to those reported in other studies using this method of analysis. 32–34 The benefit of this form of analysis, however, lies primarily in defining the comparability of the results obtained in this study with those found in other clinical trials. The weightings for these models provide an important means of examining the behavior of the study population. This information provides potential explanations for observed outcome differences based on factors other than treatment. Peritonitis clearly subsumed others, including the presence of either resistant organisms or organisms with higher minimum inhibitory concentrations, including enterococci, prior antimicrobial therapy, and the presence of a postoperative infection.
The specific settings in which an agent becomes the drug of choice must await more widespread (postmarketing) use of the agent. However, based on the results of this trial and the known activities and toxicities of clinafloxacin, this agent would appear most useful for seriously ill patients with mixed-flora infections. In this setting, the wide activity of clinafloxacin would be of likely benefit.
Footnotes
Supported by grants from Parke-Davis Pharmaceutical Research, a division of Warner-Lambert Company.
Correspondence: Joseph S. Solomkin, MD, Department of Surgery, University of Cincinnati, ML #558, Cincinnati, OH 45267-0558. E-mail: joseph.solomkin@uc.edu
Accepted for publication February 25, 2000.
References
- 1.Berne TV, Yellin AW, Appleman MD, Heseltine PN. Antibiotic management of surgically treated gangrenous or perforated appendicitis. Comparison of gentamicin and clindamycin versus cefamandole versus cefoperazone. Am J Surg 1982; 144: 8–13. [DOI] [PubMed] [Google Scholar]
- 2.Yellin AE, Heseltine PN, Berne TV,et al. The role of Pseudomonas species in patients treated with ampicillin and sulbactam for gangrenous and perforated appendicitis. Surg Gynecol Obstet 1985; 161: 303–307. [PubMed] [Google Scholar]
- 3.Solomkin JS, Dellinger EP, Christou NV, Busuttil RW. Results of a multicenter trial comparing imipenem/cilastatin to tobramycin/clindamycin for intraabdominal infections. Ann Surg 1990; 212: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosdell DM, Morris DM, Voltura A,et al. Antibiotic treatment for surgical peritonitis. Ann Surg 1991; 214: 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorber B, Swenson RM. The bacteriology of intraabdominal infections. Surg Clin North Am 1975; 55: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett JG, Onderdonk AB, Louie T, Kasper DL, Gorbach SL. Lessons from an animal model of intraabdominal sepsis. Arch Surg 1978; 113: 853–857. [DOI] [PubMed] [Google Scholar]
- 7.Nichols RL, Muzik AC. Enterococcal infections in surgical patients: the mystery continues. Clin Infect Dis 1992; 15: 72–76. [DOI] [PubMed] [Google Scholar]
- 8.Onderdonk AB, Bartlett JG, Louie T,et al. Microbial synergy in experimental intraabdominal abscess. Infect Immun 1976; 13: 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomkin JS, Reinhart HH, Dellinger EP,et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intraabdominal infections. Ann Surg 1996; 223: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donahue PE, Smith DL, Yellin AE,et al. Trovafloxacin in the treatment of intraabdominal infections: results of a double-blind, multicenter comparison with imipenem/cilastatin. Am J Surg 1998; 176: 53S–61S. [DOI] [PubMed] [Google Scholar]
- 11.Brook I. In vivo efficacies of quinolones and clindamycin for treatment of infections with Bacteroides fragilis and/or Escherichia coli in mice: correlation with in vitro susceptibilities. Antimicrob Agents Chemother 1993; 37: 997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ednie LM, Jacobs MR, Appelbaum PC. Comparative activities of clinafloxacin against gram-positive and -negative bacteria. Antimicrob Agents Chemother 1998; 42: 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs PC, Barry AL, Brown SD. In vitro activities of clinafloxacin against contemporary clinical bacterial isolates from 10 North American centers. Antimicrob Agents Chemother 1998; 42: 1274–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecht DW, Wexler HM. In vitro susceptibility of anaerobes to quinolones in the United States. Clin Infect Dis 1996; 23 (suppl 1): S2–8. [DOI] [PubMed] [Google Scholar]
- 15.Beam TR Jr, Gilbert DN, Kunin CM. General guidelines for the clinical evaluation of anti-infective drug products. Clin Infect Dis 1992; 15 (suppl 1): S5–32. [DOI] [PubMed] [Google Scholar]
- 16.Solomkin JS, Hemsell DL, Sweet R, Tally F, Bartlett J. Evaluation of new anti-infective drugs for the treatment of intraabdominal infections. Clin Infect Dis 1992; 15 (suppl 1): S33–42. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 18.Solomkin JS, Fant WK, Rivera JO, Alexander JW. Randomized trial of imipenem/cilastatin versus gentamicin and clindamycin in mixed flora infections. Am J Med 1985; 78: 85–91. [DOI] [PubMed] [Google Scholar]
- 19.Poenaru D, De Santis M, Christou NV. Imipenem versus tobramycin–antianaerobe antibiotic therapy in intra-abdominal infections. Can J Surg 1990; 33: 415–422. [PubMed] [Google Scholar]
- 20.Christou NV, Turgeon P, Wassef R,et al. Management of intraabdominal infections. The case for intraoperative cultures and comprehensive broad-spectrum antibiotic coverage. Arch Surg 1996; 131: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 21.Brismar B, Akerlund JE, Sjostedt S,et al. Biapenem versus imipenem/cilastatin in the treatment of complicated intraabdominal infections. Scand J Infect Dis 1996; 28: 507–512. [DOI] [PubMed] [Google Scholar]
- 22.Barie PS, Vogel SB, Dellinger EP,et al. A randomized, double-blind clinical trial comparing cefepime plus metronidazole with imipenem-cilastatin in the treatment of complicated intraabdominal infections. Arch Surg 1997; 132: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 23.Barie PS, Christou NV, Dellinger EP,et al. Pathogenicity of the enterococcus in surgical infections. Ann Surg 1990; 212: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brismar B, Malmborg AS, Tunevall G,et al. Piperacillin-tazobactam versus imipenem-cilastatin for treatment of intraabdominal infections. Antimicrob Agents Chemother 1992; 36: 2766–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brismar B, Malmborg AS, Tunevall G,et al. Meropenem versus imipenem/cilastatin in the treatment of intra-abdominal infections. J Antimicrob Chemother 1995; 35: 139–148. [DOI] [PubMed] [Google Scholar]
- 26.Kanellakopoulou K, Giamarellou H, Papadothomakos P,et al. Meropenem versus imipenem/cilastatin in the treatment of intraabdominal infections requiring surgery. Eur J Clin Microbiol Infect Dis 1993; 12: 449–453. [DOI] [PubMed] [Google Scholar]
- 27.Geroulanos SJ. Meropenem versus imipenem/cilastatin in intraabdominal infections requiring surgery. J Antimicrob Chemother 1995; 36 (suppl A): 191–205. [DOI] [PubMed] [Google Scholar]
- 28.Jaques P, Mauro M, Safrit H,et al. CT features of intraabdominal abscesses: prediction of successful percutaneous drainage. AJR. 1986; 146: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 29.Deveney CW, Lurie K, Deveney KE. Improved treatment of intraabdominal abscess. A result of improved localization, drainage, and patient care, not technique. Arch Surg 1988; 123: 1126–1130. [DOI] [PubMed] [Google Scholar]
- 30.Heseltine PNR, Yellin AE, Appleman MD,et al. Perforated and gangrenous appendicitis: an analysis of antibiotic failures. J Infect Dis 1983; 148: 322–329. [DOI] [PubMed] [Google Scholar]
- 31.Gill MA, Cheetham TC, Chenella FC,et al. Matched case–control study of adjusted versus nonadjusted gentamicin dosing in perforated and gangrenous appendicitis. Ther Drug Monit 1986; 8: 451–456. [DOI] [PubMed] [Google Scholar]
- 32.Bohnen JM, Boulanger M, Meakins JL Jr. Prognosis in generalized peritonitis. Arch Surg 1983; 118: 285–290. [DOI] [PubMed] [Google Scholar]
- 33.Ohmann C, Wittmann DH, Wacha H. Prospective evaluation of prognostic scoring systems in peritonitis. Eur J Surg 1993; 159: 267–274. [PubMed] [Google Scholar]
- 34.Burnett RJ, Haverstock DC, Dellinger EP,et al. Definition of the role of enterococcus in intraabdominal infection: analysis of a prospective randomized trial. Surgery 1995; 118: 716–721. [DOI] [PubMed] [Google Scholar]