Abstract
Objective
To determine prospectively the immunologic response and adverse clinical events in surgical patients exposed to bovine thrombin during cardiac surgical procedures.
Summary Background Data
Topical bovine thrombin is used extensively as a hemostatic agent during cardiovascular surgery. Antibodies developing after exposure to bovine thrombin have been anecdotally associated with hemorrhagic complications.
Methods
One hundred fifty-one patients undergoing cardiac surgical procedures were prospectively recruited for this study before surgical exposure with topical bovine thrombin. Immunoassays were used to determine antibody levels against both bovine and human coagulation proteins before and after exposure to bovine thrombin. Alterations in coagulation assay parameters and adverse clinical events were followed in all patients enrolled in the study.
Results
Baseline elevated antibody levels to one or more bovine coagulation proteins were observed most frequently in patients with a prior history of a surgical procedure during which bovine thrombin is frequently used. More than 95% of patients developed a seropositive response to bovine coagulation proteins, and 51% manifested elevated antibody levels to the corresponding human coagulation proteins after bovine thrombin exposure. Postoperative coagulation abnormalities were more common in patients with antibodies to human coagulation proteins. Patients with multiple elevated antibody levels to bovine proteins before surgery were more likely to sustain an adverse clinical outcome after surgery. Using a logistic regression model, the adjusted odds ratio for sustaining an adverse event with multiple elevated antibody levels to bovine proteins before surgery was 5.40.
Conclusions
Bovine thrombin preparations are highly immunogenic and appear to be associated with an increased risk for adverse clinical outcomes during subsequent surgical procedures. The clinical safety of these commonly used preparations needs to be reassessed, and reexposure to these agents should likely be avoided.
In the United States, an estimated 250,000 patients undergo coronary artery bypass grafting (CABG) and more than 60,000 patients undergo cardiac valve surgery annually. 1,2 As many as 7% to 12% of these patients require a second (or subsequent) procedure. 3,4 Topical thrombin preparations have been used as hemostatic agents during cardiovascular surgery for years and may be applied as a spray, a paste, or a component of fibrin glue. 5 All single-component thrombin preparations currently in use in the United States are prepared from bovine plasma. 5
Patients exposed to topical thrombin preparations may develop antibodies to bovine thrombin, factor V, and various other proteins found in these preparations. 6–9 Antibody development is generally detected as a prolonged thrombin clotting time, because this assay frequently uses bovine thrombin. 10 In some cases, these antibodies cross-react with the corresponding human coagulation proteins, specifically factor V, resulting in hemorrhagic complications. 6,11
Few studies have addressed the frequency with which these antibodies develop after exposure to bovine thrombin and how commonly antibody development results in adverse clinical events. Bänninger et al 12 described prolonged thrombin clot times and low factor V levels in 11 of 24 patients treated with fibrin glue during cardiovascular surgery. All 11 patients had received fibrin glue-treated prosthetic valves or aortic grafts. In contrast, the 13 patients who underwent CABG did not develop abnormal results on coagulation studies. 12 None of the patients developed hemorrhagic complications. Carroll et al 13 described 21 patients treated with a fibrin sealant prepared with bovine fibrinogen and bovine thrombin, all of whom developed antibodies to bovine fibrinogen, thrombin, and factor V. Cross-reacting antibodies to human thrombin and factor V were observed in most patients, but none of the patients developed a clinically significant complication. 13 No studies have prospectively investigated the development of antibodies after exposure to bovine thrombin alone.
In this study we prospectively collected serologic, hematologic, and clinical outcomes data in 151 patients undergoing cardiac surgical procedures before and after exposure to bovine thrombin. From these data we sought to address four questions: What is the incidence of anticlotting factor antibody development in patients exposed to bovine thrombin? Does the type of surgery, amount of bovine thrombin administered, or history of prior surgery influence the risk of developing anticlotting factor antibodies? How frequently do antibodies directed against bovine coagulation proteins cross-react with the corresponding human proteins? Are these anticlotting factor antibodies associated with an increased risk for the development of postoperative complications?
METHODS
Patients
All patients undergoing CABG or cardiac valve surgery at Duke University Medical Center were eligible for this study. Exclusionary criteria included abnormal baseline coagulation study results that did not occur because of anticoagulant therapy, and enrollment in a separate study that precluded inclusion in this study. Informed consent was obtained from all patients, as approved by the Institutional Review Board at Duke University Medical Center and in accordance with the ethical standards of the Helsinki Declaration of 1975.
Patients were stratified into those undergoing CABG and those undergoing cardiac valve replacement surgery, because patients undergoing the latter were potentially exposed to a greater amount of bovine thrombin during surgery. 12 Baseline information recorded for each patient included all previous surgical procedures, medications, and comorbid conditions (specifically, diabetes mellitus, hypertension, prior myocardial infarction, stroke, tobacco use, peripheral vascular disease, chronic obstructive pulmonary disease, ulcer disease, congestive heart failure, and a family history of coronary artery disease). Prior surgical procedures during which a patient may have been exposed to topical bovine thrombin included median sternotomy for CABG or cardiac valve replacement, carotid endarterectomy, peripheral vascular surgery, total hip or knee replacement, or diskectomy. Patients who had undergone one of these procedures in the past 10 years were identified as potentially having been exposed to topical bovine thrombin.
No changes in the attending physician’s practice were requested. The decision to administer topical bovine thrombin was made by the surgeon during surgery. Based on clinical practice at Duke University Medical Center, thrombin use was recorded as follows: sternal paste (approximately 4,000 units thrombin), thrombin-soaked Gelfoam collars (approximately 5,000 units thrombin), direct thrombin spray (approximately 5,000–20,000 units thrombin), and as a component of fibrin glue (approximately 20,000 units thrombin).
Clinical information documented and evaluated throughout the patient’s hospital stay included adverse events, medications and blood products used, and clinical laboratory results obtained as part of the patient’s regular medical care. Adverse events included hemorrhagic complications, including postoperative bleeding reported as excessive or necessitating surgical intervention, unanticipated nonsurgical bleeding (e.g., gastrointestinal bleeding), or blood product transfusion more than 2 days after surgery; thromboembolic complications; wound healing complications (e.g., prolonged wound healing, wound infection); and death. Isolated blood product transfusion within the first 48 hours after surgery was not included as an adverse event. Additional complications developing after discharge from the hospital were reviewed when the patient was contacted 4 to 8 weeks after surgery.
Plasma and serum samples were obtained before surgery and at 24 to 48 hours, 4 to 7 days, and 4 to 8 weeks after surgery. Samples were processed as described 14 and stored at -80°C until analyzed.
Reagents
Purified human and bovine prothrombin, thrombin and factor V, and bovine factor Va, were obtained from Haematologic Technologies, Inc. (Essex Junction, VT). The topical bovine thrombin preparation used was Thrombogen (Johnson & Johnson Medical, Inc., Arlington, TX). The murine monoclonal antibody 6A5 is an IgG1 antibody that binds to the second C-type domain of human factor V. 15 All other reagents were obtained from Sigma Chemical Corp. (St. Louis, MO).
Immunologic Testing
Antibodies to topical thrombin were detected by enzyme-linked immunosorbent assay (ELISA). Briefly, topical thrombin was diluted 1:100 in phosphate-buffered saline (PBS), pH 7.4, coated onto microtiter plate wells, and incubated overnight at 4°C. The wells were blocked with 0.25% (wt/vol) nonfat dry milk/0.2% Tween 20 in PBS (blocking buffer) and then incubated with 50 μL of a 1:10 dilution of patient serum in blocking buffer for 1 hour at 37°C. Bound IgG and IgM were detected as previously described. 16
The normal range was determined with 50 normal persons, recruited from laboratory and clinic personnel. An elevated antibody level was defined as greater than two standard deviations above the normal mean. Each ELISA plate included two normal donors and two patients with known antibodies to the topical thrombin preparation (patients R.S. and M.J. 6) as negative and positive controls, respectively. Each plate also included wells incubated with all reagents except for the diluted serum, which provided the background absorbance that was subtracted from all results.
Antibodies to bovine factor V and factor Va were identified as described above, except that the microtiter plate wells were coated with 50 μL of 5 μg/mL bovine factor V or factor Va instead of topical thrombin. Antibodies to bovine prothrombin were identified as described previously. 16 Antibodies to purified bovine thrombin were determined as described for antibodies to prothrombin, except that purified bovine thrombin (5 μg/mL) was used to coat the microtiter plate wells.
Antibodies to human factor V were identified by coating microtiter plate wells with the murine monoclonal antihuman factor V antibody 6A5 (50 μL of 2.5 μg/mL overnight at 4°C). The wells were washed and blocked, after which they were incubated with 50 μL of 5 μg/mL human factor V. The wells were then incubated with a 1:10 dilution of patient plasma for 1 hour at 37°C. Bound IgG was detected as described above. Antibodies to human prothrombin and thrombin were identified as described for the corresponding bovine proteins.
Coagulation Testing
All coagulation assays were performed in duplicate on an ST4 mechanical coagulometer (Diagnostica Stago, Asnieres-sur-Seine, France). Duplicate samples that differed by more than 10% were repeated. Prothrombin times were assessed using the thromboplastin MDA Simplastin L (Organon Teknika, Durham, NC), which had an international sensitivity index of 2.0. Activated partial thromboplastin times were performed with the auto-aPTT reagent (Organon Teknika). Thrombin clot times were determined with bovine thrombin (Thrombogen). Normal ranges were established for each assay using the 50 normal donors, and a prolonged result was defined as greater than two standard deviations above the mean.
Statistical analysis
The usual chi-square test for homogeneity or the Fisher exact test was used to compare the proportions across study groups. The logistic regression model was used to adjust the odds of developing an adverse event based on the presence of multiple elevated antibody levels at baseline when the presence of comorbid conditions was considered.
RESULTS
A total of 176 patients were enrolled between April 1996 and May 1997. Twenty-five patients did not complete the study for these reasons: death before completion of testing (n = 9), surgery not performed (n = 2), thrombin not used during the procedure (n = 1), and incomplete laboratory testing (n = 13). Of the 151 patients who completed the study, 109 underwent CABG and 42 underwent valve surgery (Table 1). Clinical characteristics of the two patient subsets were similar with the exceptions of diabetes mellitus and prior myocardial infarction, both of which were more common in patients undergoing CABG. Twenty-seven patients (19 undergoing CABG and 8 undergoing valve surgery) had undergone one of the surgical procedures in the preceding 10 years during which topical thrombin is frequently used. There was no difference in the amount of thrombin administered to patients undergoing CABG or valve surgery, nor was an increased amount used in patients who had undergone a prior surgical procedure (data not shown).
Table 1. PATIENT CHARACTERISTICS
* Surgical procedures performed within the preceding 10 years; median sternotomy (13 patients), neurosurgery or orthopedic surgery (8), or peripheral vascular surgery (6).
† Other modalities include direct thrombin spray, thrombin-soaked Gelfoam collars, and fibrin glue. Most of these patients received sternal paste in addition to a second modality.
Baseline Antibody Levels
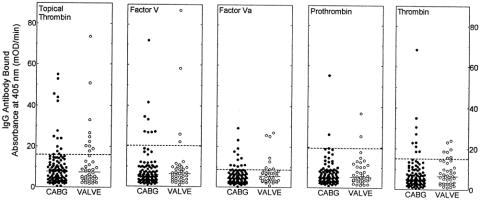
Before surgery, the numbers of patients with elevated IgG antibody levels to the topical thrombin preparation or bovine factor V, factor Va, prothrombin, and thrombin were similar in both the CABG and valve surgery groups (Fig. 1). However, when preoperative antibody levels of the test patients were compared with serum samples collected from 50 normal donors, the following differences in antibody profiles were observed. First, in patients undergoing valve surgery, 10 of 42 (23.8%) had baseline elevated antibody levels to the topical bovine thrombin preparation, compared with 3 of 50 (6%) normal donors (P = .018). However, eight of the valve surgery patients had previously undergone surgical procedures during which bovine thrombin is frequently administered; five of these eight patients had preoperative antibodies. If these patients were excluded, 5 of 34 preoperative valve patients (14.7%) had elevated antibody levels; this did not differ significantly from the normal donor population (P = .2597). Further, in this group of patients, 8 of 42 (19%) had preoperative elevated antibody titers to bovine factor Va compared with 1 of 50 (2%) normal donors (P = .01). Second, when prior surgical history was evaluated as a potential risk factor for having preoperative antibody titers against clotting factor antigens in the whole study group, 27 patients were identified who had undergone surgical procedures in the preceding 10 years in which possible exposure to bovine thrombin was highly likely. Of these 27 patients with probable prior exposure to bovine thrombin, 9 (33%) had preoperative antibody titers against two or more bovine antigens tested. This was in contrast to 6 of 124 patients (4.8%) who were positive for preoperative antibodies against two or more bovine antigens with no obvious surgical history of bovine thrombin exposure (P = .0001). These data suggest that undergoing prior surgical procedures in which bovine thrombin is often used results in immunologic sensitization to bovine antigens, with persistent detectable antibody titers often years after the initial exposure. Although this distinction may seem academic at first glance, we observed that patients with preoperative antibody titers against multiple bovine antigens had the highest risk for major complications after cardiovascular surgical procedures.
Figure 1. Baseline (preoperative) IgG binding to bovine coagulation proteins. IgG binding to the topical thrombin preparation, bovine factor V, factor Va, prothrombin, and thrombin was detected by enzyme-linked immunosorbent assay. Each symbol represents the result for an individual patient. Patient results are separated according to the type of surgery performed (coronary artery bypass grafting [CABG] or valve replacement surgery). The dashed horizontal lines represent two standard deviations above the means for the normal donor population. The solid horizontal lines represent the median values for the two patient groups being compared.
Antibody Response After Exposure to Topical Bovine Thrombin
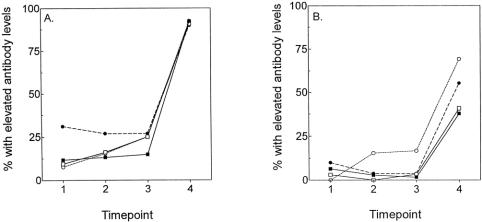
By postoperative day 4 to 7, there was a slight increase in the number of patients with elevated IgG antibody levels for patients who received more than sternal paste (Fig. 2). By 4 to 8 weeks after surgery, however, more than 90% of all patients (including those who were positive at baseline) had elevated IgG antibody levels to the topical thrombin, independent of type of surgery or thrombin dose. In contrast, only approximately half the patients manifested elevated IgM antibody levels by 4 to 8 weeks, with no significant difference between the CABG and valve surgery groups.
Figure 2. Development of antibodies to topical thrombin preparation. Patients were stratified into four groups: coronary artery bypass grafting, treatment with sternal paste only (n = 77; -▪-); coronary artery bypass grafting, treatment with sternal paste and additional thrombin (n = 32; -□-); prosthetic valve surgery, treatment with sternal paste only (n = 29; -•-); and prosthetic valve surgery, treatment with sternal paste and additional thrombin (n = 13; -⊖;). The four time points are before surgery, 24 to 48 hours after surgery, 4 to 7 days after surgery, and 4 to 8 weeks after surgery. (A) Percentage of patients with elevated IgG antibody levels. (B) Percentage of patients with elevated IgM antibody levels.
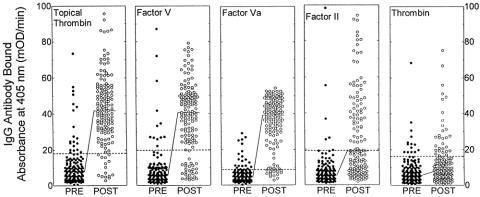
Mean IgG antibody levels against all of the bovine coagulation proteins analyzed were elevated by 4 to 8 weeks after surgery versus preoperative levels (Fig. 3). Most patients developed elevated IgG antibody levels to bovine factor V and factor Va (80.7% and 90.7%, respectively). Fewer patients developed antibodies to bovine prothrombin or thrombin (47% and 20.5%, respectively). A prior surgical procedure was not associated with an increased risk of developing elevated antibody levels to bovine factor V, factor Va, or prothrombin, but was associated with elevated antibody levels to bovine thrombin (10/27 patients with prior surgery vs. 21/124 patients without;P = .02). Overall, of the 105 patients with completely normal antibody levels at baseline, 99 (94.3%) developed elevated antibody levels to one or more bovine coagulation protein after surgery.
Figure 3. Development of elevated antibody levels to topical thrombin preparation, bovine factor V, bovine factor Va, bovine factor II, and bovine thrombin. Data are presented for all 151 patients combined. The dashed horizontal lines represent two standard deviations above the means for the normal donor population. The solid horizontal lines represent the median values for the two patient groups being compared. PRE, before thrombin exposure; POST, after thrombin exposure.
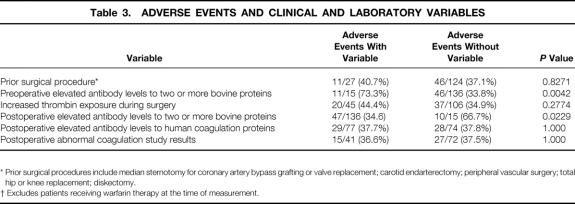
Seventy-seven patients (51%) had elevated antibody levels to human coagulation proteins after surgery, predominantly against factor V, thrombin, or both (Fig. 4). Elevated antibody levels to human proteins did not occur more frequently in patients who had been treated with increased amounts of topical thrombin or who had undergone a prior surgical procedure (data not shown). Almost all patients with elevated antibody levels to human factor V or prothrombin had elevated antibody levels to the corresponding bovine proteins (92% and 100%, respectively). In contrast, 4 of 11 patients with elevated antibody levels to human thrombin did not have elevated antibody levels to either bovine prothrombin or thrombin.
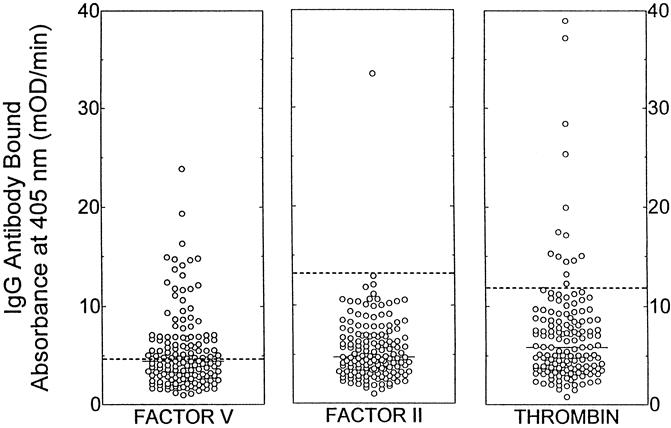
Figure 4. Elevated antibody levels to human coagulation proteins. IgG binding to human factor V, prothrombin, and thrombin was detected by enzyme-linked immunosorbent assay. Data are presented for all patients at 4 to 8 weeks after surgery. The dashed horizontal lines represent two standard deviations above the means for the normal donor population. The solid horizontal lines represent the median values for the patients.
Coagulation Abnormalities
At 4 to 8 weeks after surgery, 24 patients (of 137 tested) were receiving warfarin therapy. Excluding these 24 patients, 41 of the remaining 113 had one or more abnormal coagulation test results (36.3%). Twelve of these patients had multiple abnormal test results, 12 had isolated prolonged prothrombin times, and 14 had isolated prolonged activated partial thromboplastin times. Elevated antibody levels to one or more of the human coagulation proteins studied were identified in 23 patients with abnormal coagulation test results (56.1%). There was no correlation between prolonged coagulation studies and the type of surgical procedure performed, the amount of thrombin applied, or the presence of elevated antibody levels to one or more bovine proteins. Only eight patients had a prolonged bovine thrombin clot time. All eight of these patients had elevated antibody levels to the topical thrombin preparation, purified bovine thrombin, or both. None had elevated antibody levels to human prothrombin or thrombin.
Adverse Clinical Outcomes
Fifty-seven patients had a postoperative complication (Table 2). No increased risk for an adverse outcome was observed with a history of a prior surgical procedure, use of an increased amount of thrombin during surgery, presence of elevated antibody levels to bovine or human coagulation proteins after surgery, or abnormal coagulation study results (Table 3). In contrast, there was a significant increase in the risk for an adverse postoperative outcome for patients who had elevated antibody levels to two or more bovine proteins before surgery. Similar results were obtained if hemorrhagic events were considered in isolation, with a significant increase in risk only for patients with elevated antibody levels to multiple bovine proteins before surgery (P = .02). None of the variables was associated with an increased risk for thromboembolic events, wound complications, or death as isolated clinical manifestations (data not shown).
Table 2. POSTOPERATIVE COMPLICATIONS
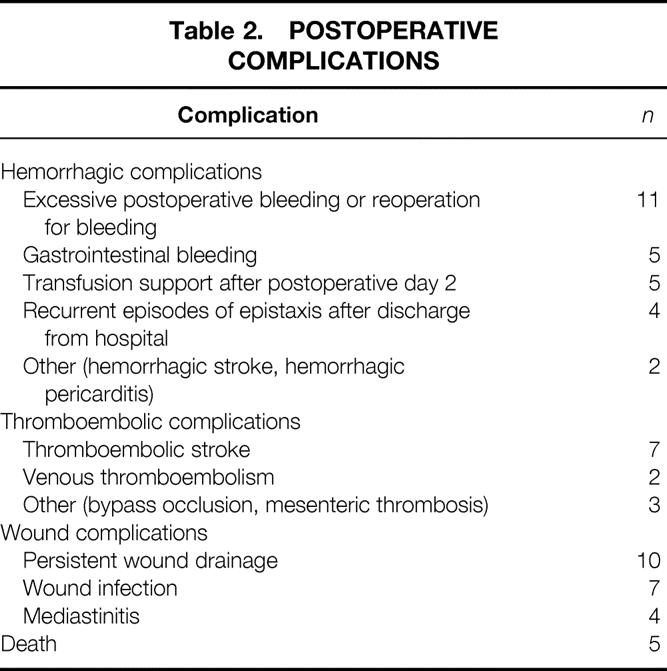
Table 3. ADVERSE EVENTS AND CLINICAL AND LABORATORY VARIABLES
* Prior surgical procedures include median sternotomy for coronary artery bypass grafting or valve replacement; carotid endarterectomy; peripheral vascular surgery; total hip or knee replacement; diskectomy.
† Excludes patients receiving warfarin therapy at the time of measurement.
The unadjusted odds ratio for developing an adverse event when multiple elevated antibody levels were present at baseline was 5.38 (95% confidence interval: 1.62–17.8). These results were also evaluated in light of comorbidities present at baseline. Each of 10 conditions was analyzed as predictors of any adverse event using a logistic regression model. Both hypertension (P = .03) and ulcers (P = .01) were found individually to be significantly related to an adverse event finding. However, when either or both of these comorbid conditions was used in a regression model with the presence of multiple elevated antibody levels at baseline, the latter was still quite significant (P < .01). Under these circumstances, the adjusted odds ratio for developing an adverse event with elevated baseline antibody levels was 5.40 (95% confidence interval: 1.54–18.8).
DISCUSSION
Antibodies that bind to the prothrombinase complex, prothrombin, or thrombin may develop as spontaneous autoantibodies, 17,18 as alloantibodies in patients with inherited deficiency states after treatment with factor concentrates or plasma preparations, 19,20 or in previously normal patients treated with topical bovine thrombin preparations. 6,11 Clinical manifestations include hemorrhagic complications, 6,11,18,21–23 thromboembolic complications, 24–29 or asymptomatic laboratory abnormalities. 10,17
The prevalence of elevated antibody levels to bovine or human coagulation proteins in our normal population was similar to the reported frequencies of anticardiolipin antibodies (4–8%) and spontaneous autoantibodies to factor VIII (17%) in normal donor studies. 30–32 In contrast, we observed that patients with a history of a prior surgical procedure during which topical thrombin is often administered were significantly more likely to have elevated IgG antibody levels to bovine coagulation proteins at baseline (9/27 vs. 6/124 patients, P = .0001). Although this suggests that these patients had previously been treated with bovine thrombin, this cannot be proven by this study because prior thrombin exposure could not be documented.
Our results demonstrate that topical bovine thrombin, even if administered in relatively limited amounts, is extremely immunogenic. Most patients developed antibodies to bovine factor V and factor Va, confirming the presence of this coagulation protein in the topical thrombin preparation used during the study. Thirty-one patients (20.5%) developed an antibody specific for purified bovine thrombin, suggesting that the use of more highly purified bovine thrombin preparations will likely still result in an immune response. Only 7% of the patients developed a prolonged bovine thrombin clot time, indicating that this assay is not an effective screen for the development of bovine thrombin-induced antibodies. Slightly more than half the patients also developed elevated antibody levels to human coagulation proteins, predominantly factor V.
Adverse clinical complications in the postoperative period did not appear to be associated with the development of elevated antibody levels to bovine or human coagulation factors. However, adverse clinical outcomes were most frequently seen in patients with multiple elevated antibody levels to bovine proteins before their current exposure to topical thrombin. Because most patients with multiple elevated antibody levels before surgery had previously undergone a surgical procedure during which bovine thrombin is frequently used, we suspect that these patients were most likely treated with bovine thrombin during the earlier procedure. These data suggest that secondary exposure to bovine thrombin during surgery may result in an associated immunologic response that results in alterations of normal hemostasis leading to adverse clinical events in the postoperative period.
Given the extensive use of bovine thrombin as a topical hemostatic agent, the population at risk for both primary and secondary exposure to this agent is potentially enormous. Our results clearly illustrate that bovine preparations of thrombin are highly immunogenic and that patients who have preoperative antibodies directed against bovine antigens are at increased risk for postoperative complications. Important questions unanswered by this report include the impact of elevated antibody levels on noncardiovascular surgical procedures, and whether reexposure to bovine thrombin is required for an adverse outcome after a subsequent surgical procedure in patients with preoperative antibodies to bovine thrombin. Although highly purified bovine thrombin preparations may be less immunogenic, this has not been systematically investigated, and the safety of these preparations in patients who have been previously exposed to “less purified” preparations of bovine thrombin is unknown. Human thrombin preparations have been used outside the United States for several years, but their safety in patients previously treated with bovine thrombin, especially those who developed antibodies to human thrombin, is also unknown. These issues suggest that the widespread use of bovine thrombin as a topical hemostatic agent should be reconsidered and that the dynamic interplay between immunologic exposure to hemostatic proteins (bovine or possibly human) and postoperative adverse events requires careful study.
Acknowledgments
The authors thank the staff of the Duke Clinical Coagulation Laboratory for sample collection and technical assistance. They also thank Martin L. Lee, PhD, for assistance with statistical analysis and Robert W. Anderson, MD, William H. Kane, MD, PhD, B. Gail Macik, MD, Roger Lundblad, PhD, Edward D. Gomperts, MD, Laura A. Navalta, Karen R. Baker, RN, and Thomas F. Slaughter, MD, for their input and advice at various stages of this project.
Footnotes
Supported by grants from Baxter Healthcare Corporation (T.L.O., J.H.L.), the American Red Cross (T.L.O.), the American Heart Association (J.H.L., T.L.O.), and the Department of Surgery, Duke University Medical Center. Dr. Ortel is a Clinician Scientist Initiation Grant Awardee (91-149) from the American Heart Association and Genentech, Inc., and a Pew Scholar in the Biomedical Sciences. Dr. Lawson is a Clinician Scientist Awardee from the American Heart Association and Genentech, Inc. (95004380). Presented in part at the XVIth Congress of the International Society on Thrombosis and Haemostasis, Florence, Italy, June 6–12, 1997; the 39th Annual Meeting of the American Society of Hematology, San Diego, California, Dec. 5–9, 1997; and the Third Annual Conference on Emerging Applications of Tissue Sealants, San Diego, California, May 2–3, 1998.
None of the authors of this manuscript have any financial interest in the surgical products discussed in this manuscript, any of the corporations that provided support for the study, or any other products that may be influenced by the publication of this work.
Correspondence: Jeffrey H. Lawson, MD, PhD, Box 2622, Dept. of Surgery, Duke University Medical Center, Durham, NC 27710. E-mail: lawso717@acpub.duke.edu
Accepted for publication March 23, 2000.
References
- 1.Anderson GM, Grumbach K, Luft HS,et al. Use of coronary artery bypass surgery in the United States and Canada. Influence of age and income. JAMA 1993; 269: 1661–1666. [PubMed] [Google Scholar]
- 2.Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med 1996; 335: 407–416. [DOI] [PubMed] [Google Scholar]
- 3.Edwards FH, Clark RE, Schwartz M. Coronary artery bypass grafting: the Society of Thoracic Surgeons National Database experience. Ann Thorac Surg 1994; 57: 12–19. [DOI] [PubMed] [Google Scholar]
- 4.Tyers GF, Jamieson WR, Munro AI,et al. Reoperation in biological and mechanical valve populations: fate of the reoperative patient. Ann Thorac Surg 1995; 60 (suppl 2): S464–S468. [DOI] [PubMed] [Google Scholar]
- 5.Alving BM, Weinstein MJ, Finlayson JS,et al. Fibrin sealant: summary of a conference on characteristics and clinical uses. Transfusion 1995; 35: 783–790. [DOI] [PubMed] [Google Scholar]
- 6.Ortel TL, Charles LA, Keller FG,et al. Topical thrombin and acquired coagulation factor inhibitors: Clinical spectrum and laboratory diagnosis. Am J Hematol 1994; 45: 128–135. [DOI] [PubMed] [Google Scholar]
- 7.Rapaport SI, Zivelin A, Minow RA,et al. Clinical significance of antibodies to bovine and human thrombin and factor V after surgical use of bovine thrombin. Am J Clin Pathol 1992; 97: 84–91. [DOI] [PubMed] [Google Scholar]
- 8.Cmolik BL, Spero JA, Magovern GJ,et al. Redo cardiac surgery: late bleeding complications from topical thrombin-induced factor V deficiency. J Thorac Cardiovasc Surg 1993; 105: 222–228. [PubMed] [Google Scholar]
- 9.Chouhan VD, De la Cadena RA, Nagaswami C,et al. Simultaneous occurrence of human antibodies directed against fibrinogen, thrombin, and factor V after exposure to bovine thrombin: effects on blood coagulation, protein C activation and platelet function. Thromb Haemost 1997; 77: 343–349. [PubMed] [Google Scholar]
- 10.Flaherty MJ, Henderson R, Wener MH. Iatrogenic immunization with bovine thrombin: a mechanism for prolonged thrombin times after surgery. Ann Intern Med 1989; 111: 631–634. [DOI] [PubMed] [Google Scholar]
- 11.Zehnder JL, Leung LLK. Development of antibodies to thrombin and factor V with recurrent bleeding in a patient exposed to topical bovine thrombin. Blood 1990; 76: 2011–2016. [PubMed] [Google Scholar]
- 12.Bänninger H, Hardegger T, Tobler A,et al. Fibrin glue in surgery: frequent development of inhibitors of bovine thrombin and human factor V. Br J Haematol 1993; 85: 528–532. [DOI] [PubMed] [Google Scholar]
- 13.Carroll JF, Moskowitz KA, Edwards NM,et al. Immunologic assessment of patients treated with bovine fibrin as a hemostatic agent. Thromb Haemost 1996; 76: 925–931. [PubMed] [Google Scholar]
- 14.Moll S, Ortel TL. Monitoring warfarin therapy in patients with lupus anticoagulants. Ann Intern Med 1997; 127: 177–185. [DOI] [PubMed] [Google Scholar]
- 15.Ortel TL, Quinn-Allen MA, Keller FG,et al. Localization of functionally important epitopes within the second C-type domain of coagulation factor V using recombinant chimeras. J Biol Chem 1994; 269: 15898–15905. [PubMed] [Google Scholar]
- 16.Ortel TL, Moore KD, Quinn-Allen MA,et al. Inhibitory anti-factor V antibodies bind to the factor V C2 domain and are associated with hemorrhagic manifestations. Blood 1998; 91: 4188–4196. [PubMed] [Google Scholar]
- 17.Nesheim ME, Nichols WL, Cole TL,et al. Isolation and study of an acquired inhibitor of human coagulation factor V. J Clin Invest 1986; 77: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Spada AR, Skalhegg BS, Henderson R,et al. Fatal hemorrhage in a patient with an acquired inhibitor of human thrombin. N Engl J Med 1995; 333: 494–497. [DOI] [PubMed] [Google Scholar]
- 19.Fratantoni JC, Hilgartner M, Nachman RL. Nature of the defect in congenital factor V deficiency: study in a patient with an acquired circulating anticoagulant. Blood 1972; 39: 751–758. [PubMed] [Google Scholar]
- 20.Mazzucconi MG, Solinas S, Chistolini A,et al. Inhibitor to factor V in severe factor V congenital deficiency. A case report. Nouv Rev Fr Hematol 1985; 27: 303–305. [PubMed] [Google Scholar]
- 21.Bajaj SP, Rapaport SI, Fierer DS,et al. A mechanism for the hypoprothrombinemia of the acquired hypoprothrombinemia-lupus anticoagulant syndrome. Blood 1983; 61: 684–692. [PubMed] [Google Scholar]
- 22.Bajaj SP, Rapaport SI, Barclay S, Herbst KD. Acquired hypoprothrombinemia due to nonneutralizing antibodies to prothrombin: mechanism and management. Blood 1985; 65: 1538–1548. [PubMed] [Google Scholar]
- 23.Lawson JH, Pennell BJ, Olson JD, Mann KG. Isolation and characterization of an acquired antithrombin antibody. Blood 1990; 76: 2249–2257. [PubMed] [Google Scholar]
- 24.Kapur A, Kelsey PR, Isaacs PET. Factor V inhibitor in thrombosis. Am J Hematol 1993; 42: 384–388. [DOI] [PubMed] [Google Scholar]
- 25.Koyama T, Saito T, Kusano T, Hirosawa S. Factor V inhibitor associated with Sjogren’s syndrome. Br J Haematol 1995; 89: 893–896. [DOI] [PubMed] [Google Scholar]
- 26.Kamphuisen PW, Haan J, Rosekrans PCM, Van der Meer FJM. Deep-vein thrombosis and coumarin skin necrosis associated with a factor V inhibitor with lupus-like features. Am J Hematol 1998; 57: 176–178. [DOI] [PubMed] [Google Scholar]
- 27.Vaarala O, Puurunen M, Manttari M,et al. Antibodies to prothrombin imply a risk of myocardial infarction in middle-aged men. Thromb Haemost 1996; 75: 456–459. [PubMed] [Google Scholar]
- 28.Palosuo T, Virtamo J, Haukka J,et al. High antibody levels to prothrombin imply a risk of deep venous thrombosis and pulmonary embolism in middle-aged men. A nested case-control study. Thromb Haemost 1997; 78: 1178–1182. [PubMed] [Google Scholar]
- 29.Arnaud E, Lafay M, Gaussem P,et al. An autoantibody directed against human thrombin anion-binding exosite in a patient with arterial thrombosis: effects on platelets, endothelial cells, and protein C activation. Blood 1994; 84: 1843–1850. [PubMed] [Google Scholar]
- 30.Shi W, Krilis SA, Chong BH,et al. Prevalence of lupus anticoagulant and anticardiolipin antibodies in a healthy population. Aust NZ J Med 1990; 20: 231–236. [DOI] [PubMed] [Google Scholar]
- 31.Vila P, Hernandez MC, Lopez-Fernandez MF, Battle J. Prevalence, follow-up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb Haemost 1994; 72: 209–213. [PubMed] [Google Scholar]
- 32.Algiman M, Dietrich G, Nydegger UE,et al. Natural antibodies to factor VIII (anti-hemophilia factor) in healthy individuals. Proc Natl Acad Sci USA 1992; 89: 3795–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]