Abstract
Objective
To study normothermic extracorporeal liver perfusion (NELP) as a means to preserve livers for transplantation and to reverse warm ischemic injury.
Summary Background Data
The authors provide experimental evidence that successful transplantation after 4 hours of normothermic extracorporeal liver perfusion is possible and as reliable as 4 hours of cold preservation in University of Wisconsin solution. NELP preserves liver function completely and can reverse 60 minutes of warm ischemic injury in non–heart-beating donors.
Methods
Thirty-six German Landrace pigs received transplants in six groups. Group 1 animals received direct transplantation. Group 2 received transplants after 4 hours of cold preservation with University of Wisconsin solution and Group 3 animals after 4 hours of NELP. Group 4 animals sustained 1 hour of warm ischemia before transplantation. Group 5 animals received transplants after 1 hour of warm ischemia and 4 hours of cold preservation and Group 6 animals after 1 hour of warm ischemia and 4 hours of NELP.
Results
All animals receiving livers treated by NELP survived more than 7 days after the transplant (Groups 3 and 6). In contrast, all animals in Group 5 developed primary graft nonfunction within 24 hours after transplantation.
Conclusion
The technique of NELP holds the potential to keep a mammalian liver outside the body completely functional, possibly for more than 4 hours. NELP can be used for liver preservation before transplantation or for the use of organs from non–heart-beating donors.
The goal of organ preservation is to maintain cellular viability and organ function. The success of preservation is proven by primary graft function after transplantation.
The major principle of organ preservation consists of reducing the metabolic activity by lowering the temperature. Metabolic rate shows a 12- to 13-fold decrease when the temperature is reduced from 37°C to 0°C. 1 With the introduction of University of Wisconsin (UW) solution, safe storage of liver grafts for up to 20 hours has been confirmed. 2,3 Because until now it has been the most suitable solution for preservation, UW solution is the standard against which all other modifications in liver preservation must be assessed. 4,5
The technique of isolated liver perfusion for evaluation of preservation methods, either normothermic or at low temperature, has been known for many years. 6–8 Recently we have improved liver perfusion by placing the organ in a waterlogged and sealed chamber. Oscillating pressure profiles imitating the intraabdominal pressures improved perfusion of peripheral lobules significantly. 9 Further, the introduction of simultaneous dialysis of the recirculating perfusate allows for regulation of pH and physiologic electrolyte concentrations. Water-soluble toxins and increased amino acids are removed. 10,11
The potential advantages of normothermic extracorporeal liver perfusion (NELP) versus cold and static preservation are obvious. First, no cold ischemic injury is inflicted. Second, it is possible to monitor liver viability during perfusion by bile production, transaminase release, 12 and metabolic function before transplantation. 13,14 NELP so far has not been shown to allow organ preservation in comparison to cold static preservation. The aim of the present study was primarily to investigate the feasibility of the NELP system for preservation. After 4 hours of normothermic perfusion, livers were transplanted and compared with livers after 4 hours of UW preservation.
Our recent perfusion studies indicated that it is possible to resuscitate livers after warm ischemic injury by the use of NELP. 15 In the present project, we transplanted livers after 60 minutes of warm ischemia and compared the results of NELP with UW preservation and direct transplantation.
METHODS
Thirty-six commercially available Landrace pigs (weight 20–30 kg) were used. Pigs were group-housed in a facility allowing free access to water and twice-a-day feeding of standard pelleted pig chow. Selected weight-matched donor and recipient pigs were fasted 24 hours before transplantation and divided into six groups. Six liver transplantations were carried out in each group.
Study Groups
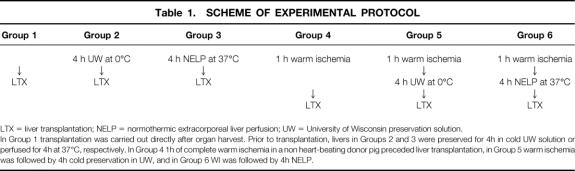
Donor livers were treated as follows (Table 1).
Table 1. SCHEME OF EXPERIMENTAL PROTOCOL
LTX = liver transplantation; NELP = normothermic extracorporeal liver perfusion; UW = University of Wisconsin preservation solution.
In Group 1 transplantation was carried out directly after organ harvest. Prior to transplantation, livers in Groups 2 and 3 were preserved for 4h in cold UW solution or perfused for 4h at 37°C, respectively. In Group 4 1h of complete warm ischemia in a non heart-beating donor pig preceded liver transplantation, in Group 5 warm ischemia was followed by 4h cold preservation in UW, and in Group 6 WI was followed by 4h NELP.
In Group 1, donor and recipient were prepared simultaneously and transplantation was carried out immediately after donor hepatectomy to prevent preservation injury. During the time from hepatectomy to reperfusion, livers were exposed to 36 ± 3 minutes of warm ischemia.
In Group 2, the donor liver was perfused in situ through the portal vein and hepatic artery with UW and preserved for 4 hours at 0°C, followed by liver transplantation. Reperfusion was carried out 35 ± 5 minutes after cold preservation.
In Group 3, the donor liver was explanted and immediately connected to the perfusion circuit without flushing. NELP was carried out for 4 hours at 37°C, followed by liver transplantation. The perfusion setup was as outlined below.
In group 4, the donor hepatic artery, portal vein, suprahepatic vena cava, and bile duct were dissected free from supporting tissue, allowing rapid removal of the liver later. Then the donor was exsanguinated until cardiac arrest occurred. The abdominal walls were approximated and held by clamps. The liver was left in situ completely ischemic for 1 hours. The donor animal was placed on a heating blanket, so body core temperature was kept at more than 35°C during the non–heart-beating period. Meanwhile, the recipient was prepared. The period between hepatectomy in the donor animal and reperfusion of the portal vein in the recipient was 43 ± 4 minutes. The warm ischemic period totaled 103 ± 4 minutes.
In Groups 5 and 6, the donor liver was treated similarly to that in Group 4 until hepatectomy. In Group 5, 1 hour of warm ischemia was followed by 4 hours of cold storage in UW. In Group 6, after 1 hour of warm ischemia, the donor liver was connected without flushing to the NELP system for 4 hours before transplantation.
Normothermic Extracorporeal Liver Perfusion
After hepatectomy in Groups 3 and 6, the liver was prepared for perfusion by inserting spring-perfusion cannulas in the portal vein, infrahepatic vena cava, and hepatic artery. The liver was placed in a sterile plastic bag, which was then immersed in a 37°C warm water bath in a specially designed perfusion chamber. The liver was subjected to intermittent oscillations produced by an external pump at a rate of 15 per minute. The pressure difference was in the range of 0 to 25 cm water. The liver was perfused by the cannulas in the portal vein and hepatic artery. The suprahepatic vena cava was ligated; blood outflow was allowed through the infrahepatic vena cava. According to physiologic conditions, the mean arterial pressure was adjusted to 100 mmHg and the portal venous pressure to 15 cm water. The flow rates measured under these conditions were approximately 150 mL per minute through the hepatic artery and 250 mL per minute through the portal vein. Our perfusion system allows easy control and adjustment of flow rates and perfusion pressures.
The Dideco D701 pediatric hollow-fiber oxygenator (Dideco S.p.A., Mirandola, Italy) was supplied with a mixture of 95% oxygen and 5% carbon dioxide. Bile production was measured continuously. Cholecystectomy was performed in all donor livers; therefore, the bile collected during perfusion reflected the bile produced.
The simultaneous dialysis circuit consisted of two Stöckert roller pumps (Stöckert Instrumente GmbH, München, Germany), a Gambro ALWALL GFS 12 fiber dialyzer (Gambro GmbH, 72373 Hechingen, Germany), and a reservoir containing 10 L dialysate. The perfusate was pumped at a rate of 400 mL per minute through the dialyzer. On the other side of the membrane, dialysate was pumped at a rate of 1 L per minute from the reservoir in a recirculating mode. The volume of perfusate and dialysate was kept constant by control and adjustment of pressures on both sides of the dialyzer. Reperfusion and dialysis were started at the same time. 11
The perfusate consisted of 2 L whole pig blood and 1 L of a balanced electrolyte solution (Jonosteril, Fresenius, Bad Homburg, Germany). Fifteen thousand IE heparin was added at the beginning of reperfusion and 2,500 IE every hour thereafter.
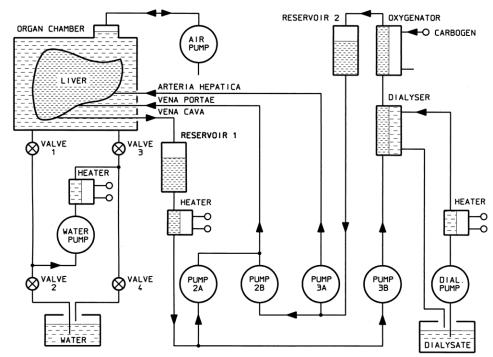
Figure 1 illustrates the extracorporeal liver perfusion circuit consisting of the perfusion chamber, the pump and control unit, an oxygenator, and a dialysis circuit, including a reservoir for the dialysate.
Figure 1. Diagram of the Normothermic Extracorporeal Liver Perfusion Circuit. The liver is perfused via hepatic artery by oxygenated perfusate and via portal vein by mixed venous perfusate. The organ is placed in a sealed chamber that is subjected to intermittent oscillations produced by an air pump. Perfusate is dialysed continuously.
Transplant Technique
Anaesthesia was induced and maintained by fentanyl and metomidate hydrochloride. During surgery, pancuronium was added. Orthotopic liver transplantations were carried out as previously described. 16
A passive venovenous bypass from the portal and infrahepatic vena cava to the right external jugular vein was used. After finishing the anastomosis of the suprahepatic vena cava and portal vein, the livers were reperfused. In accord with clinical practice, the livers were reperfused with whole blood from the recipient. Finally, the infrahepatic vena cava, hepatic artery, and bile duct were anastomosed, in this order.
Light and Electron Microscopy
Liver biopsy samples were taken from all pigs and studied by light and electron microscopy. Liver biopsies were performed at different stages: before dissection; after liver treatment, shortly before reperfusion in the recipient; and 1 hour after reperfusion. Although changes to nonparenchymal cells and hepatocytes must occur during the preservation or ischemic period, 17,18 histologically they become fully manifest only after reperfusion. 1 For this reason we chose to present the results of the third biopsy.
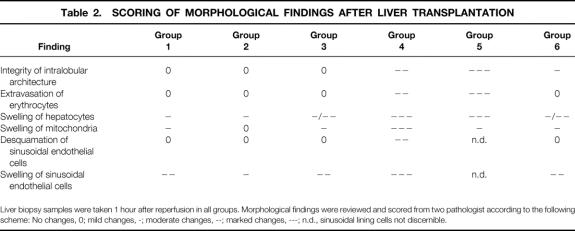
For light microscopy, paraformaldehyde-fixed tissue was stained with hematoxylin and eosin, periodic acid–Schiff, and Masson-Goldner trichrome, according to standards. Electron microscopy probes 1 mm in diameter were fixed with 5% glutaraldehyde in 1/15 mol/L phosphate buffer (pH 7.3) at 4°C for at least 24 hours. After postfixation (2% osmium tetroxide in 1/15 mol/L phosphate buffer), dehydration (graded series of ethanol), and embedding in araldit, ultrathin sections were finally stained with uranyl acetate and lead citrate. Histology was reviewed and scored (Table 2) by two pathologists unaware of the experimental groups.
Table 2. SCORING OF MORPHOLOGICAL FINDINGS AFTER LIVER TRANSPLANTATION
Liver biopsy samples were taken 1 hour after reperfusion in all groups. Morphological findings were reviewed and scored from two pathologist according to the following scheme: No changes, 0; mild changes, -; moderate changes, --; marked changes, ---; n.d., sinusoidal lining cells not discernible.
Analyses
Blood samples were collected for α-GST, ASAT, International Normalized Ratio (INR), and hyaluronic acid 15 minutes, 1, 3, 6, and 12 hours, daily from day 1 until day 4, and at day 7 after reperfusion. Control samples were taken before surgery from donors and recipients for α-GST, INR, and transaminases determination. Control samples for hyaluronic acid analysis were taken from recipients only. Samples were centrifuged and the plasma was collected.
α-GST determination was performed using a porcine-specific α-GST enzyme-linked immunosorbent assay test kit (Hepkit, Biotrin International, Dublin, Ireland). The test procedure is based on an immunometric reaction of α-GST with antibody α-GST IgG, a peroxidase conjugate, and antiporcine α-GST IgG. The resultant color intensity is proportional to the amount of porcine α-GST present in the sample. The assay range is 0 to 100 μg/L, and the detection limit is 0.69 μg/L.
Transaminase activity was analyzed with the Technicon DAX72-System (Bayer Diagnostic GmbH, München, Germany) using photometric standard methods according to the German Society for Clinical Chemistry. The activity is given in units per liter.
INR 19 was analyzed using an STA-coagulation analyzer (Diagnostica Stago, Inc., Parsippany, NJ). The reference range of INR is 1.2 to 0.9 in normal pigs.
The samples for hyaluronic acid determination were frozen at -80°C until analysis. Levels were measured using a radiometric assay kit (Pharmacia HA Test Radioimmunoassay, Pharmacia GmbH, Erlangen, Germany). The test is based on the use of 125I-labeled, specific hyaluronic acid binding proteins (HABP). The unbound 125I HABP is then separated and the radioactivity measured in a gamma-counter. The response is inversely proportional to the concentration of hyaluronic acid in the sample. The concentration is given in μg/L
In Groups 3 and 6, perfusate samples were collected after dialysis of the perfusate before the liver was connected, 5, 15, and 30 minutes, and 1, 2, 3, and 4 hours after reperfusion. Bile was collected continuously.
Statistics
Results are expressed as mean ± SEM. Statistical analysis of differences between Groups was carried out using the Mann–Whitney rank-sum test and where appropriate the Student t test. Changes in one group were examined by the Wilcoxon signed rank test, the Dixon-Mood signed test, and the paired t test. Statistical significance was defined at P < .05.
RESULTS
Primary Graft Function and Survival After Transplantation
After the transplant, all pigs in Groups 1, 2, 3, and 6 maintained functional grafts. No liver failure was observed. In Group 4, one pig developed primary graft nonfunction and died within 18 hours after reperfusion. The other five livers functioned well and allowed long-time survival. In Group 5, all pigs died within 24 hours because of primary graft nonfunction. Reperfusion of the livers in Group 5 was particularly inhomogeneous, with many patchy areas. Primary graft nonfunction was characterized by a diffuse bleeding tendency and inability to wean the pig from the respirator.
Preoperative values of α-GST were in the range of 10.0 ± 1.1 μg/L (n = 36) (Fig. 2). An increase in the α-GST concentration to a peak and then a decrease to preoperative values was observed after successful transplantation. Group 1 peaked (56.1 ± 10.1 μg/L) at 1 hour, Group 2 (58.2 ± 18.4 μg/L) at 15 minutes, Group 3 (75.9 ± 38.3 μg/L) at 6 hours, Group 4 (513 ± 148 μg/L) at 3 hours, and Group 6 (128.7 ± 18.9 μg/L) at 3 hours after reperfusion. In Groups 1 and 2, the control value was reached on the first postoperative day. In Groups 3, 4, and 6, α-GST levels returned to control values on the second day. The α-GST level in group 5 increased continuously, peaking at 12 hours (607 ± 62 μg/L).
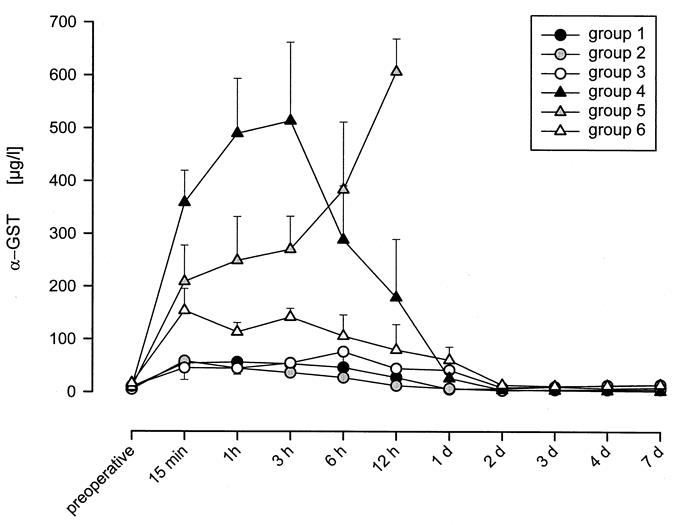
Figure 2. α-GST concentration following liver transplantation in Groups 1-6. Pigs were transplanted in six groups: immediately after donor hepatectomy in Group 1, after 4h of preservation in UW solution at 0 degrees C in Group 2, after 4h of NELP in Group 3, after 1h of WI at 37 degrees C in Group 4, after 1h of WI at 37 degrees and 4h of preservation in UW solution at zero degrees in Group 5, and after 1h of WI at 37 degrees celcious and 4h of NELP in Group 6. An increase of α-GST concentration after reperfusion was followed by a decrease to preoperative values in the range of 10.0 ± 1.1 μg/L. This pattern could be observed in all groups after successful LTX. The release of α-GST was highest in Groups 4 and 5.
The preoperative value of ASAT was 31.5 ± 2.6 U/L (Fig. 3). In Group 1, ASAT activity peaked 6 hours after transplantation at 409 ± 132 U/L. On postoperative day 2, the activity began to decrease, reaching the reference level at day 3. In Group 2, the maximum (372 ± 68 U/L) was reached 12 hours after transplantation, decreasing to the reference level at day 3. In Group 3, the activity increase continued until day 1 (734 ± 366 U/L), returning to normal values at day 4. Group 4 showed the highest activity increase, peaking at day 1 (1,627 ± 506 U/L) and returning to control level at the end of the study period. In Group 5, activity peaked at 1,570 ± 171 U/L at 12 hours. In Groups 4 and 5, the peak was significantly higher than in the other groups. ASAT activity in Group 6 peaked at 603 ± 141 U/L at day 1, returning to control values at the end of the study.
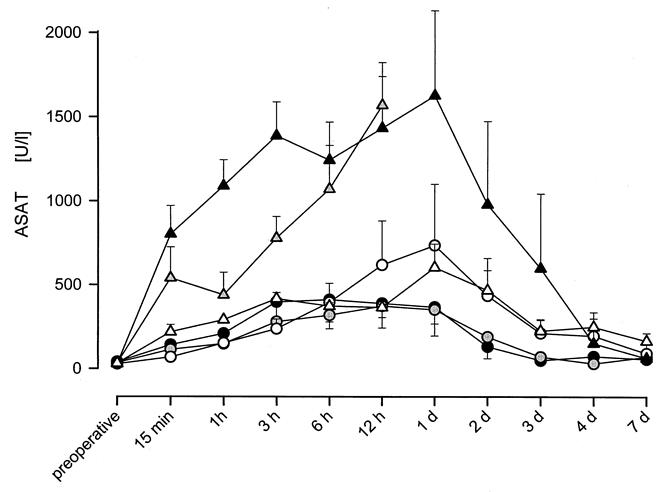
Figure 3. ASAT activity in serum following liver transplantation in Groups 1-6. An increase of ASAT concentration until a maximum and a following decrease to preoperative values in the range of 31.5 ± 2.6 U/l by the end of the study could be measured after successful transplantation. Similar to the release of α-GST (Fig. 2) ASAT activity was highest in Groups 4 and 5.
Preoperative values of INR were in the range of 1.1 to 0.90 (Fig. 4). During the early postoperative period, INR increased in all groups. Twelve hours after reperfusion, INR rose to 1.36 ± 0.074 in group 2 and to 2.97 ± 0.46 in Group 5. Although groups subjected to warm ischemia tended to be associated with higher INR values, differences between groups reached statistical significance only during the first 24 hours. One week after transplantation, values for INR for all groups were on average less than 1.2.
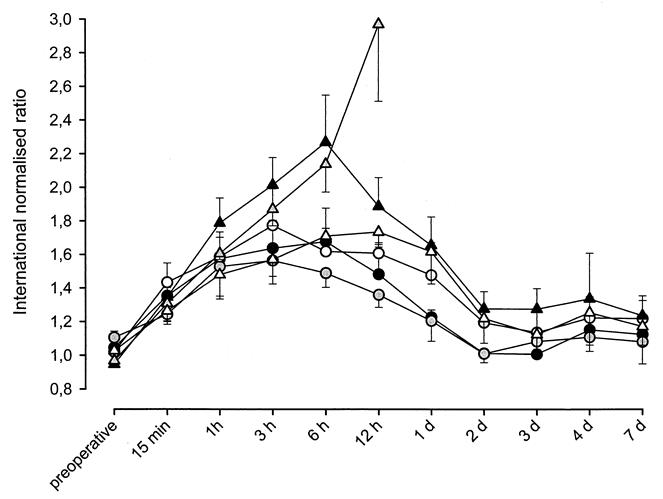
Figure 4. INR following liver transplantation in Groups 1-6. International normalised ratio increased during the early post operative phase in all agroups. 12h after transplantation it was highest in Group 5 with 2.97 ± 0.46. On average INR was higher in groups subjected to WI in Group 2.
Hyaluronic acid concentration increased during the anhepatic phase from a control level of 304 ± 40 μg/L to an average of 1,507 ± 85 μg/L (1,406 ± 279 μg/L in Group 1, 1,668 ± 112 μg/L in Group 2, 1,172 ± 181 μg/L in Group 3, 1,700 ± 227 μg/L in Group 4, 1,918 ± 174 μg/L in Group 5, and 1,177 ± 94 μg/L in Group 6) (Fig. 5). These differences were not statistically significant. In Groups 1, 4, and 6, the concentration of hyaluronic acid remained close to anhepatic levels for 1 to 3 hours after reperfusion. It returned to control levels in Groups 1 and 6 within 12 hours and in Group 4 at day 2. In Group 2, the concentration increased until 3 hours to a maximum of 2,371 ± 392 μg/L. At day 7, the control level was reached. In Group 3, the maximum of 1,552 ± 266 μg/L was reached after 1 hour. The concentration decreased to 1,016 ± 223 μg/L on day 1, remaining there until the end of the study. In Group 5, the concentration of hyaluronic acid increased until 6 hours after reperfusion, peaking at 2,737 ± 899 μg/L.
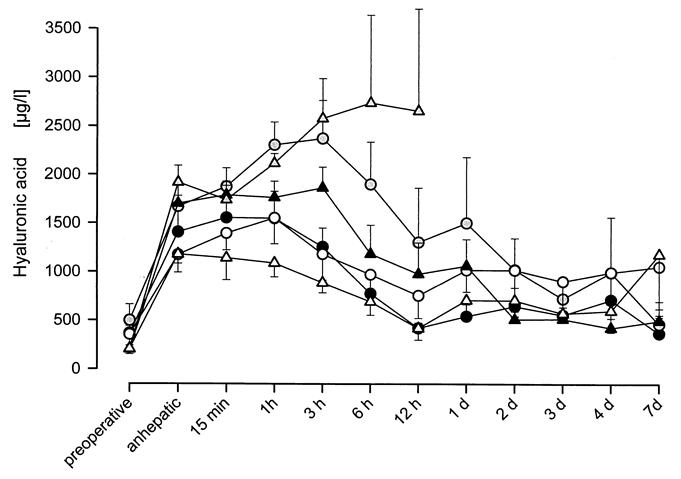
Figure 5. Hyaluronic acid concentration following liver transplantation in Groups 1-6. Hyaluronic acid concentration increased during the anhepatic phase from 304 ± 40 μg/L to an average of 1574 ± 80 μg/L. Resumption of sinusoidal cell function leads to a decrease of hyaluronic acid concentration after reperfusion. Both groups subjected to cold ischemia (i.e., Groups 2 and 5) showed the highest levels of hyaluronic acid concentration after transplantation suggesting that endothelial cells are particularly susceptible to cold ischemia.
Liver Function Parameters During NELP
After 4 hours of perfusion, 100 g liver produced 4.3 ± 0.5 mL bile in Group 3 and 1.8 ± 0.2 mL in Group 6. In Group 3, bile production began 20 minutes after reperfusion; on average, it took 1 hour in Group 6. The amount of bile produced in Group 3 was significantly higher than in Group 6 (P < .0005).
Enzyme release during the first 30 minutes of perfusion was primarily due to the organ condition. Therefore, the perfusate concentration at the end of perfusion was analyzed compared with the value obtained after 30 minutes after reperfusion. In no case was a significant difference measured between the transaminase levels in the perfusate after 30 minutes compared with 4 hours of perfusion.
After 30 minutes, the α-GST concentration was 21.3 ± 4.3 μg/L in Group 3 and 185.6 ± 27.8 μg/L in Group 6 (Fig. 6). The concentration after 4 hours of perfusion was 31.8 ± 6.1 μg/L in Group 3 and 312.7 ± 54.4 μg/L in Group 6. Comparing α-GST concentration at 30 minutes with that at 4 hours, no significant difference was observed either in Group 3 or in Group 6. Comparing the two groups, the concentration differed significantly as a result of ischemic injury before perfusion in Group 6.
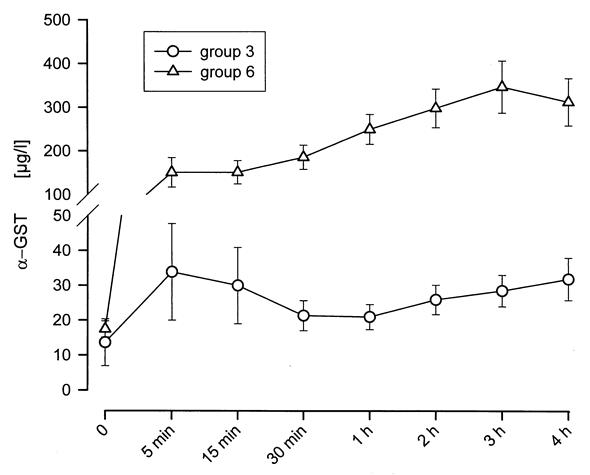
Figure 6. α-GST concentration during 4h of NELP in Groups 3 and 6. Livers were perfused in a specially designed perfusion chamber imitating intraabdominal pressures profiles (figure 1). The introduction of simultaneous dialysis of the recirculating perfusate allows for regulation of pH, physiological electrolyte concentrations and disposal of water soluble metabolites. In Group 3 α-GST concentration did not increase while in Group 6 the increase was insignificant. This indicates that, with reperfusion of the liver in the NELP system, hepatocellular injury had come to a stop.
During perfusion, the ASAT activity increased from 39.8 ± 7.8 U/L at 30 minutes to 57.0 ± 13.0 U/L at 4 hours in Group 3 and from 285.0 ± 55.4 U/L at 30 minutes to 514.3 ± 107.7 U/L at 4 hours in Group 6 (Fig. 7). The activity increase was insignificant within each group but was significant when comparing both groups.
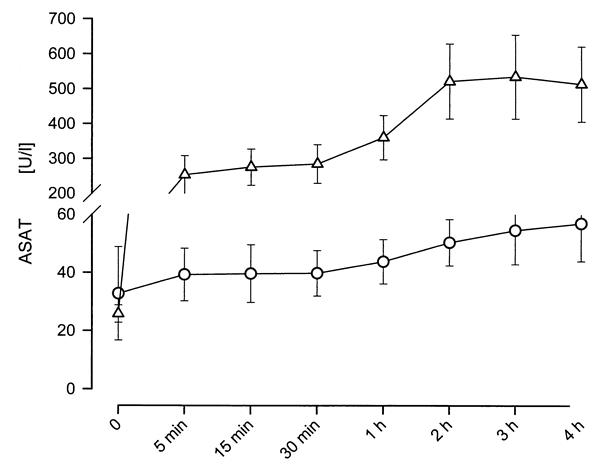
Figure 7. ASAT activity in perusate during 4h of NELP in Groups 3 and 6. The difference between Group 3 and Group 6 after reperfusion reached statistical significance at every given time point. Statistical significance was defined at P < .05. The higher activity of ASAT in Group 6 reflects the amount of hepatocellular injury inflicted by 60 minutes of warm ischemia.
ALAT and LDH activity in the perfusate did not change significantly during perfusion. The ALAT activity after 30 minutes until the end of perfusion was 13.5 ± 0.7 U/L in Group 3 and 37.6 ± 2.3 U/L in Group 6. Lactate dehydrogenase activity after 30 minutes until the end of perfusion was 758 ± 79 U/L in Group 3 and 1,544 ± 82 U/L in Group 6.
The hyaluronic acid concentration in the perfusate decreased continuously from 113 ± 29 μg/L before perfusion to 48 ± 27 μg/L after 4 hours of perfusion in Group 3 (P = .0106) and from 92.6 ± 7.4 μg/L to 16.1 ± 5.4 μg/L in Group 6 (P < .0001) (Fig. 8). There was no significant difference between the groups at any time point.
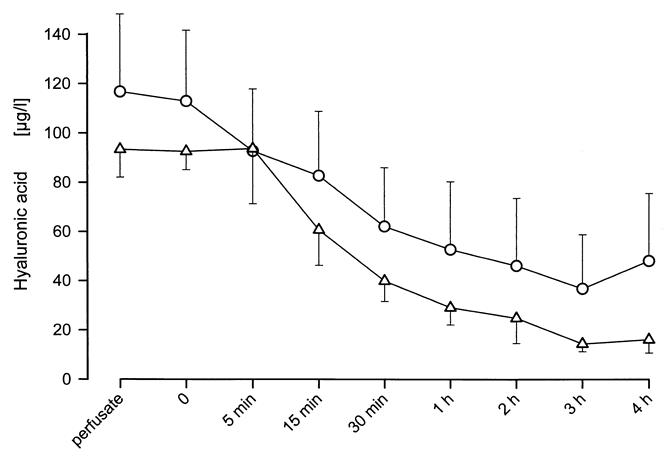
Figure 8. Hyaluronic acid concentration during 4h of NELP in Groups 3 and 6. Hyaluronic acid concentration in the perfusate decreased continuously during perfusion in both groups. Differences between groups were insignificant indicating that 60 minutes of warm ischemia are well tolerated without loss of endothelial cell function.
Light and Electron Microscopic Findings
The control biopsy showed normal liver tissue with regular hepatocellular cytoplasmic organelles and intact sinusoidal lining and space of Disse in all groups. Based on the liver treatment, the results of the third biopsy sample differed significantly.
In Group 1, light microscopy demonstrated a homogeneous cytoplasm of hepatocytes. Lobular architecture and sinusoids were intact. The periportal region was slightly congested, and few scattered necrotic cells were detectable. On electron microscopy, swelling of hepatocytes and mitochondria was seen in the periportal area. Sinusoidal lining cells were swollen without any discontinuities or cell detachment. These morphologic changes were compatible with reversible alterations.
In Group 2, light microscopy demonstrated wide sinusoids and an intact trabecular arrangement. Only discrete periportal cytoplasmic swelling and vacuolization of hepatocytes and sinusoidal endothelial cells were visible. No mitochondrial damage or sinusoidal cell desquamation waspresent. Compared with Group 1, there were minimal differences.
In Group 3, although the intralobular architecture was unchanged, focal congestion, some intrasinusoidal debris, slight hydropic swelling of hepatocytes and sinusoidal lining cells, and a decrease of the mitochondrial matrix were observed. Sinusoidal lining was continuous. None of the microscopic findings in Group 3 showed irreversible damage of the liver.
In Group 4, the light microscopic aspect was dominated by intact lobular and sinusoidal architecture, although some focal areas, especially in the periportal region, showed reduced nuclear staining and cytoplasmic eosinophilia or were necrotic, with focal intralobular collapse of architecture. In areas intact under light microscopy, ultrastructurally hydropic swelling, dilatation of the endoplasmic reticulum, mitochondrial swelling of hepatocytes, and different grades of destruction of continuity of the sinusoidal lining were obvious. Overall, the morphologic findings in this group showed substantial progression in comparison with Group 1. In Group 4, one of the six pigs died 18 hours after transplantation. Liver morphology revealed massive damage, including complete collapse of architecture with cytolytic sinusoidal endothelial cells and hepatocytes.
In Group 5 (Fig. 9), all pigs died within 18 hours and showed progressing dissolution of intralobular architecture, with confluent necrosis and massive bleeding. The residual hepatocytes were extremely hydropic and their endoplasmic reticulum was highly dilated. Sinusoidal lining cells were not discernible. Morphologic aspects in Group 5 suggested irreversible damage.
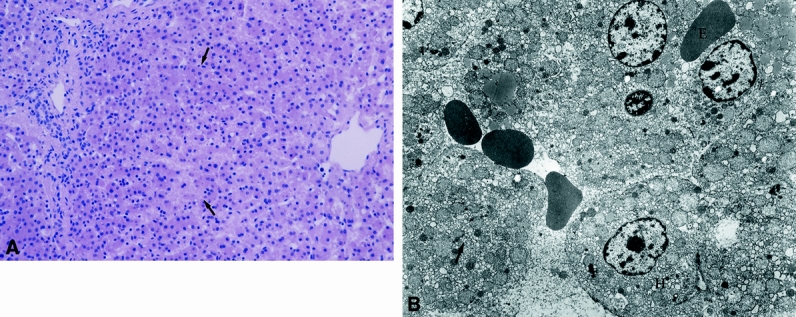
Figure 9. LM (A) and EM (B) of Group 5. Liver tissue in group 5 after 60 minutes of warm ischemia, 4 hours of cold preservation in UW and 1 hour after transplantation shows progressing dissolution of intraobular architecture and confluent necrosis (arrows). (H + E; magnification x 220). On the right side transmission electron micrograph of the same liver tissue demonstrates hydropic (H) or dissolved (arrow) hepatocytes. Bleeding in interepithelial spaces (E). Sinusoidal lining cells were not discernible. (magnification x 4500).
In Group 6 (Fig. 10), intralobular architecture was well preserved. Few necrotic hepatocytes were visible. No extravasation of erythrocytes appeared. Swelling of sinusoidal lining cells, hepatocytes, and mitochondria was discrete. The lumen of sinusoids was narrow and filled with blood cells. None of these alterations indicated irreversible tissue damage. Microscopic evaluation suggested a better overall morphologic condition of these livers than those from either Group 5 or Group 4.
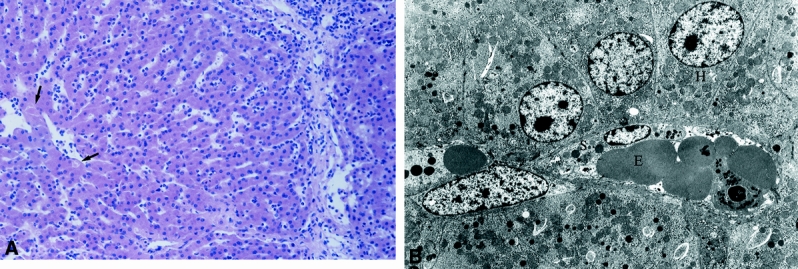
Figure 10. LM (A) and EM (B) of Group 6. Well preserved liver tissue in Group 6 after 60 minutes of warm ischemia, 4 hours of normothermic extracorporeal liver perfusion and 1 hour after transplantation shows sporadic necrosis of hepatocytes (arrows). (H +E; magnification x 220). On the right side, transmission electron micrograph of the same liver tissue reveals swelling of the sinusoidal lining cells (S) and hepatocytes (H). (magnification x 4500).
DISCUSSION
When the transplantation era began, the donor and recipient were prepared simultaneously and transplantation was performed immediately after donor hepatectomy to prevent preservation injury. This era was followed by cold organ preservation, facilitating logistics considerably. 20,21 The introduction of UW solution improved the quality of the cold-preserved grafts and remains the gold standard of organ preservation. 22 The major principle of hypothermic organ preservation is based on the reduction of metabolic activity. Further improvement of UW solution was attempted but without clear clinical impact. 23–26 It is doubtful that further significant improvements of organ-preservation solution will be possible as long as it is based on a static ischemic principle. In contrast to the static method of cold preservation, NELP could be a major step forward if it were possible to maintain the physiologic metabolism of the graft extracorporeally during preservation. 15 To stop the process of biodegradation as it takes place under cold static preservation, a graft needs substrates and must get rid of metabolites. NELP provides substrates and, as long as it is being conducted with simultaneous dialysis, also allows for disposal of metabolites. In this project our aim was to provide experimental evidence that it is possible to keep a liver functional at 37°C outside the body and to transplant it successfully afterward. The perfusate chosen was pig blood from fed pigs, under the assumption that it contains all necessary substrates.
As demonstrated by the survival of all pigs in Group 3, a period of 4 to 5 hours of NELP preserves liver function before transplantation. All grafts were functional and allowed long-time survival.
In contrast to cold static preservation, NELP allows monitoring of liver function before the organ is grafted. Bile production is the most sensitive parameter of liver function. It requires an intact architecture of the liver sinusoid and hepatocyte. 14,27,28 Bile secretion requires several dedicated metabolizing steps and a sufficient supply of adenosine-triphosphate (ATP). 29 During NELP, the amount of bile produced was 4.3 ± 0.5 mL/100 g in Group 3 and 1.8 ± 0.2 mL/100 g in Group 6. No substances stimulating bile secretion were added during perfusion. The amount of bile produced indicated good liver function under NELP. 30 The lower bile level of Group 6 versus Group 3 is attributed to the ischemic injury before NELP. In ischemia, the cellular ATP level decreases, leading to a reduced bile flow rate. Thus, the extent of hepatic injury can be assessed by monitoring the bile flow rate. 31
Comparison of Group 1 and 2 by light and electron microscopy, 1,32 release of transaminases, 33 coagulation factors, 34–36 and metabolism of hyaluronic acid showed similar results. However, levels of hyaluronic acid were highest in Group 2, indicating a slight impairment of endothelial cell function by cold ischemia and confirming the results of previous studies. 37,38
After equilibration of the liver perfusion, release of transaminases came to a standstill and hyaluronic acid was metabolized. From this, we can conclude that after connecting the liver to the NELP system, no further liver damage occurred.
Comparing liver function after the transplant in Groups 1 and 3, similar results were found in terms of pig survival and liver function. This observation underlines our view that NELP maintains liver function without inflicting hepatocellular or endothelial cell injury.
In Groups 4 to 6 before explantation, all livers were subjected to 1 hour warm ischemia. In Group 4, one fulminant hepatic failure occurred; none occurred in Group 6. Further, release of transaminases and INR 39 were lower and metabolism of hyaluronic acid was greater in Group 6 than in Group 4. Light and electron microscopy showed no irreversible damage. In short, these data point to a resuscitating potential of NELP after warm ischemia. This and the monitoring option of livers in NELP might be of particular interest for the use of organs from non–heart-beating donors.
In Group 5, warm ischemia was followed by 4 hours of cold preservation in UW. In no case was this reversible. All pigs in Group 5 died within 24 hours of the transplant with high transaminase levels and destroyed hepatocellular architecture by 1 hour after reperfusion. Transaminase release, INR, hyaluronic acid levels, and morphologic results showed that the combination of hepatocellular injury (60 minutes of warm ischemia) and endothelial injury (4 hours of cold ischemia) was responsible for the lack of reversibility. Because it is generally accepted that reactive oxygen species play a decisive role during organ preservation and reperfusion, 1 recent studies have indicated that cold itself, independent of hypoxia and reoxygenation, leads to apoptosis of cultured hepatocytes and endothelial cells. 40 From these data, we conclude that the damage inflicted on livers by 60 minutes of warm ischemia would be aggravated if followed by cold ischemia. This might not necessarily be the case if the intervals of warm or cold ischemia chosen are shorter, because the degree of preservation damage is time-dependent. 6,41
NELP is a technology that opens numerous application possibilities if it is carried out in a physiologic fashion. Studies are planned to prolong the perfusion interval. NELP may become an alternative to cold static preservation and allow for the use of organs from non–heart-beating donors.
Footnotes
Supported by grants from the Deutsche Forschungsgemeinschaft (project no. DFG SCHO 408/4-2), Sonnenfeld-Stiftung, and Humboldt-Universität Berlin.
Correspondence: PD Dr. Michael R. Schön, Department of Surgery, Charité, Campus Virchow-Klinikum, Humboldt-Universität, Augustenburger Platz 1, 13353 Berlin, Germany. E-mail: michael.schoen@charite.de
Accepted for publication March 27, 2000.
References
- 1.Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation 1992; 53: 957–78. [DOI] [PubMed] [Google Scholar]
- 2.Belzer FO. Clinical organ preservation with UW solution [letter]. Transplantation 1989; 47: 1097–098. [DOI] [PubMed] [Google Scholar]
- 3.Kalayoglu M, Hoffmann RM, D’Alessandro AM,et al. Results of extended preservation of the liver for clinical transplantation. Transplant Proc 1989; 21: 3487–3488. [PubMed] [Google Scholar]
- 4.Sumimoto R, Jamieson NV, Kamada N. Examination of the role of the impermeants lactobionate and raffinose in a modified UW solution. Transplantation 1990; 50: 573–576. [DOI] [PubMed] [Google Scholar]
- 5.Moen J, Claesson K, Pienaar H,et al. Preservation of dog liver, kidney, and pancreas using the Belzer–UW solution with a high-sodium and low-potassium content. Transplantation 1989; 47: 940–945. [DOI] [PubMed] [Google Scholar]
- 6.Bell R, Shiel AG, Dolan P,et al. The evaluation of the isolated perfused liver as a model for the assessment of liver preservation. Aust NZ J Surg 1993; 63: 44–52. [DOI] [PubMed] [Google Scholar]
- 7.Filipponi F, Bacci S, Romagnoli P. Normothermic liver perfusion ex situ: a resuscitation tool for hepatic grafts damaged by warm ischemia. G Chir 1993; 14: 254–258. [PubMed] [Google Scholar]
- 8.Jamieson NV, Sundberg R, Lindell S,et al. The isolated perfused rabbit liver as a model for assessment of organ preservation. Transplant Proc 1988; 20: 996–997. [PubMed] [Google Scholar]
- 9.Neuhaus P, Blumhardt G. Extracorporeal liver perfusion: applications of an improved model for experimental studies of the liver. Int J Artif Organs 1993; 16: 729–739. [PubMed] [Google Scholar]
- 10.Schön MR, Puhl G, Frank J, Neuhaus P. Hemodialysis improves results of pig liver perfusion after warm ischemic injury. Transplant Proc 1993; 25: 3239–3243. [PubMed] [Google Scholar]
- 11.Schön MR, Puhl G, Gerlach J,et al. Hepatocyte isolation from pig livers after warm ischaemic injury. Transplant Int 1994; 7: 159–162. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda T, Yanaga K, Lebeau G,et al. Hemodynamic and biochemical changes during normothermic and hypothermic sanguinous perfusion of the porcine hepatic graft. Transplantation 1990; 50: 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iu S, Harvey PR, Makowka L,et al. Markers of allograft viability in the rat. Relationship between transplantation viability and liver function in the isolated perfused liver. Transplantation 1987; 44: 562–569. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson NV, Sundberg R, Lindell S,et al. A comparison of cold storage solutions for hepatic preservation using the isolated perfused rabbit liver. Cryobiology 1988; 25: 300–310. [DOI] [PubMed] [Google Scholar]
- 15.Schön MR, Hunt CJ, Pegg DE, Wight DG. The possibility of resuscitating livers after warm ischemic injury. Transplantation 1993; 56: 24–31. [DOI] [PubMed] [Google Scholar]
- 16.Calne RY, Yoffa DE, White HJ, Maginn RR. A technique of orthotopic liver translantation in the pig. Br J Surg 1968; 55: 203–206. [DOI] [PubMed] [Google Scholar]
- 17.Lemasters JJ, Bunzendahl H, Thurman RG. Reperfusion injury to donor livers stored for transplantation. Liver Transpl Surg 1995; 1: 124–138. [DOI] [PubMed] [Google Scholar]
- 18.Caldwell Kenkel JC, Currin RT, Tanaka Y, et al. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology 1991; 13: 83–95. [PubMed] [Google Scholar]
- 19.Izumi S, Langley PG, Wendon J,et al. Coagulation factor V levels as a prognostic indicator in fulminant hepatic failure. Hepatology 1996; 23: 1507–1511. [DOI] [PubMed] [Google Scholar]
- 20.Calne RY. Transplantation of the liver. Ann Surg 1978; 188: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Iwatsuki S, van Thiel DH,et al. Evolution of liver transplantation. Hepatology 1982; 2: 614–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamieson NV. Improved organ preservation and its impact on the clinical programme. Eur J Gastroenterol Hepatol 1989; 1: 83–86. [Google Scholar]
- 23.Wahlberg J, Jacobsson J, Tufveson G. Relevance of additive components of University of Wisconsin cold-storage solution. An experimental study in the rat. Transplantation 1989; 48: 400–403. [DOI] [PubMed] [Google Scholar]
- 24.Cheng S, Ragsdale JR, Sasaki AW,et al. Verapamil improves rat hepatic preservation with UW solution. J Surg Res 1991; 50: 560–564. [DOI] [PubMed] [Google Scholar]
- 25.den Butter G, Saunder A, Marsh DC,et al. Comparison of solutions for preservation of the rabbit liver as tested by isolated perfusion. Transplant Int 1995; 8: 466–471. [DOI] [PubMed] [Google Scholar]
- 26.Chiang CH, Hsu K, Yan HC,et al. PGE-1, dexamethasone, U-74389G, or Bt2-cAMP as an additive to promote protection by UW solution in I/R injury. J Appl Physiol 1997; 83: 583-590. [DOI] [PubMed] [Google Scholar]
- 27.Sumimoto K, Inagaki K, Yamada K,et al. Reliable indices for the determination of viability of grafted liver immediately after orthotopic transplantation. Bile flow rate and cellular adenosine triphosphate level. Transplantation 1988; 46: 506–509. [DOI] [PubMed] [Google Scholar]
- 28.Bowers BA, Branum GD, Rotolo FS,et al. Bile flow: an index of ischemic injury. J Surg Res 1987; 42: 565–569. [DOI] [PubMed] [Google Scholar]
- 29.Erlinger S. Bile flow. In: Arias IM, Jakoby WB, Popper H, et al,eds. The liver: biology and pathobiology. New York: Raven Press, Ltd.; 1988: 643–659.
- 30.Mets B, Rose Innes C, Lotz Z,et al. Comparison of in vivo and ex vivo porcine liver function using the same liver. J Hepatol 1993; 17: 3–9. [DOI] [PubMed] [Google Scholar]
- 31.Kamiike W, Nakahara M, Nakao K,et al. Correlation between cellular ATP level and bile excretion in the rat liver. Transplantation 1985; 39: 50–55. [DOI] [PubMed] [Google Scholar]
- 32.Kakizoe S, Yanaga K, Starzl TE, Demetris AJ. Evaluation of protocol before transplantation and after reperfusion biopsies from human orthotopic liver allografts: considerations of preservation and early immunological injury. Hepatology 1990; 11: 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devlin J, Dunne JB, Sherwood RA,et al. Relationship between early liver graft viability and enzyme activities in effluent preservation solution. Transplantation 1995; 60: 627–631. [DOI] [PubMed] [Google Scholar]
- 34.Tiainen P, Hockerstedt K, Rosenberg PH. Hepatocellular integrity in liver donors and recipients indicated by glutathione transferase alpha. Transplantation 1996; 61: 904–908. [DOI] [PubMed] [Google Scholar]
- 35.Schön MR, Akkoc N, Schrem H,et al. Alpha-glutathione-S-transferase is a sensitive marker of hepatocellular damage due to warm or cold ischemia in pig liver transplantation. Transplant Proc 1997; 29: 3036–3038. [DOI] [PubMed] [Google Scholar]
- 36.Shimada M, Yanaga K, Kishikawa K,et al. Prediction of hepatic graft viability before reperfusion: an analysis of effluent from porcine allografts. Transplant Int 1993; 6: 4–7. [DOI] [PubMed] [Google Scholar]
- 37.Adams DH, Wang L, Neuberger JM. Serum hyaluronic acid following liver transplantation: evidence of hepatic endothelial damage. Transplant Proc 1989; 21: 2274. [PubMed] [Google Scholar]
- 38.Itasaka H, Kishikawa K, Suehiro T,et al. Serum hyaluronic acid for the assessment of graft viability in porcine liver transplantation. Surg Today 1994; 24: 719–724. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy M, Ellis AJ, Wendon JA,et al. Use of extracorporeal liver assist device and auxiliary liver transplantation in fulminant hepatic failure. Eur J Gastroenterol Hepatol 1997; 9: 407–412. [DOI] [PubMed] [Google Scholar]
- 40.Rauen U, Polzar B, Stephan H,et al. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species. FASEB J 1999; 13: 155–168. [DOI] [PubMed] [Google Scholar]
- 41.Casavilla A, Ramirez C, Shapiro R,et al. Experience with liver and kidney allografts from non-heart-beating donors. Transplantation 1995; 59: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]