Abstract
Objective
To determine which patient factors affect the degree of catabolism after severe burn.
Summary Background Data
Catabolism is associated with severe burn and leads to erosion of lean mass, impaired wound healing, and delayed rehabilitation.
Methods
From 1996 to 1999, 151 stable-isotope protein kinetic studies were performed in 102 pediatric and 21 adult subjects burned over 20–99.5% of their total body surface area (TBSA). Patient demographics, burn characteristics, and hospital course variables were correlated with the net balance of skeletal muscle protein synthesis and breakdown across the leg. Data were analyzed sequentially and cumulatively through univariate and cross-sectional multiple regression.
Results
Increasing age, weight, and delay in definitive surgical treatment predict increased catabolism (P < .05). Body surface area burned increased catabolism until 40% TBSA was reached; catabolism did not consistently increase thereafter. Resting energy expenditure and sepsis were also strong predictors of net protein catabolism. Among factors that did not significantly correlate were burn type, pneumonia, wound contamination, and time after burn. From these results, the authors also infer that gross muscle mass correlates independently with protein wasting after burn.
Conclusions
Heavier, more muscular subjects, and subjects whose definitive surgical treatment is delayed are at the greatest risk for excess catabolism after burn. Sepsis and excessive hypermetabolism are also associated with protein catabolism.
The hypermetabolic response to severe burn trauma and sepsis is associated with increased energy expenditure and energy substrate release from protein and fat stores. 1–11 After burn, protein is catabolized, which leads to a loss of lean body mass and muscle wasting. 12–17 Wilmore et al 10 demonstrated that metabolic rate was proportional to body surface area burned. Other investigators, however, have not consistently shown correlations between burn size or other patient characteristics with either hypermetabolism or skeletal muscle proteolysis. 2,3,10 In this study, our aim was to determine those patient characteristics that are predictive of the extent of skeletal muscle catabolism after burn using sensitive modern stable isotope techniques. Such knowledge can direct metabolic therapy to those burn victims at highest risk for impaired wound healing, increased infectious complications, and delayed rehabilitation.
METHODS
The analyses presented here are based on 123 burn subjects who underwent treatment and metabolic studies at the Shriners Burns Hospital for Children—Galveston and The University of Texas Medical Branch—Galveston from March 1996 to November 1999. These subjects were part of several discrete research projects 12–17 carried out during this time. Study designs for the projects were similar in nature, and each was performed under a University of Texas Medical Branch Institutional Review Board-approved protocol. Informed written consent was obtained from each patient or each patient’s guardian before enrollment into the particular study.
Subjects and Clinical Care
All subjects were admitted to the Shriners Burns Hospital for Children or the Truman Blocker Burn Unit at the University of Texas Medical Branch in Galveston, TX, and all were treated in an identical manner by the same team of three burn surgeons. Standard treatment included early excision of the burn wound, systemic antibiotic therapy, and continuous enteral feeding. 18 Within 36 hours of admission, each patient underwent total burn wound excision and grafting with autograft skin and allograft. Patients returned to the operating room when autograft donor sites healed and became available for reharvest (usually 6–10 days). Sequential staged surgical procedures for repeat excision and grafting were undertaken until the wounds were healed.
Each patient received enteral nutrition via nasoduodenal tubes with Vivonex TEN (Sandoz Nutritional Corp., Minneapolis, MN). The composition of Vivonex is 82% carbohydrate, 15% protein, and 3% fat. Daily caloric intake was given at a rate calculated to deliver 1500 kcal per square meter of total body surface area (TBSA) burned + 1500 kcal per square meter TBSA. This feeding regimen was started at admission and continued at a constant rate until the wounds were healed. Caloric intake remained constant throughout the stable isotope infusion periods.
Patients were on bed rest for 5 days after excision and grafting procedures, and ambulated daily thereafter until the next excision and grafting procedure. Of the 123 subjects, 28 were studied twice.
Outcome Measures
The net balance of protein synthesis and breakdown across the leg was taken as the main outcome measure of skeletal muscle protein catabolism. This served as the primary dependent variable for our statistical models. Metabolic rate, measured as resting energy expenditure (REE), was determined concurrently with each protein kinetic study, and this value was compiled along with net balance for statistical analysis.
Independent Variables
The subject demographic variables and hospital course factors listed in Table 1 were recorded. Inhalation injury was defined as evidence of airway injury on bronchoscopy done before the first operation. Body mass index is a value derived from the subject’s weight (kg) divided by the height2 (m2). We defined wound contamination by quantitative cultures of >105 organisms/g of tissue taken surgically or by punch biopsy at the time of the preceding operation. Pneumonia was diagnosed by presence of a new or changing infiltrate on chest radiograph when accompanied by purulent sputum, fever, tachypnea, and leukocytosis.
Table 1. EVALUATED CLINICAL VARIABLES
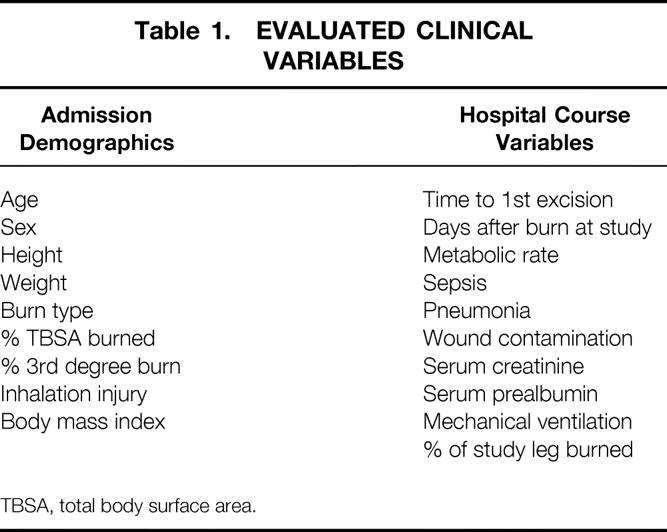
TBSA, total body surface area.
Sepsis was defined using two different methods (Table 2). The first, used by burn clinicians, 18–20 defines enteral feeding intolerance as the inability to tolerate enteral feedings because of abdominal distention, high gastric residuals of >150 cc3/hour, or uncontrollable diarrhea (>100 cc3/24 hrs). The second is a modification of the criteria established by the American Association of Chest Physicians/Society of Critical Care Medicine (AACP/SCCM) Consensus Conference of 1992. 21 The heart rate and respiratory rate parameters were adjusted for age due to the wide spectrum of age-specific normal values encompassed by the subjects included in this study.
Table 2. DEFINITIONS OF SEPSIS
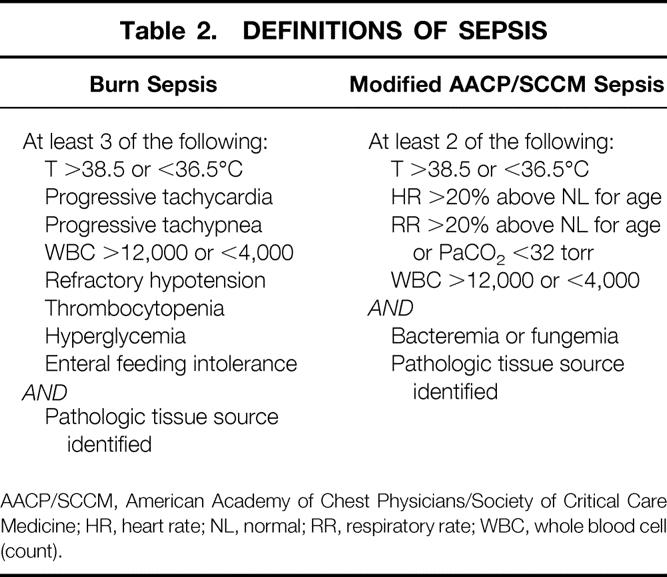
AACP/SCCM, American Academy of Chest Physicians/Society of Critical Care Medicine; HR, heart rate; NL, normal; RR, respiratory rate; WBC, whole blood cell (count).
Stable Isotope Methodology
Study Logistics and Mechanics
The degree of protein catabolism was quantified using stable isotope tracers. Protein kinetic studies were performed beginning between 5 and 7 am on postoperative day 5 from one of the sequential staged excision and grafting procedures. All stable isotope studies consisted of a 5-hour infusion of d5-Phenylalanine, as previously described. 12–17 Briefly, baseline blood samples were obtained from an indwelling femoral arterial or central venous catheter for background amino acid enrichment and systemic indocyanine green concentrations. A primed-constant infusion of L-[ring-2H5]-phenylalanine was given through a central venous catheter for 5 hours using a priming dose of 2 μmol/kg followed by 0.08 μmol/kg/min. Because phenylalanine is neither synthesized nor degraded in the peripheral tissues (it is metabolized only in the liver), measurement across the leg reflects the net balance of protein synthesis and breakdown. Between hours 3 and 4, leg blood flow was determined by indocyanine green infusion into a femoral artery. Blood samples were taken simultaneously from the right and left femoral veins for this determination. Between hours 4 and 5, blood samples were obtained from the femoral artery and ipsilateral femoral vein to determine arteriovenous phenylalanine concentration differences across the leg.
Analysis of Blood Samples
The blood concentration of unlabelled phenylalanine was determined by gas chromatography—mass spectrometry (GCMS) using the internal standard approach and the nitrogen-acetyl-n-propyl esters, as previously described. 22 Briefly, 2 mL of whole blood was immediately mixed with 200 μL of internal standard containing 29.7 μmol/L of [13C6] phenylalanine. The samples were deproteinized with sulfosalicylic acid and centrifuged, and the supernatant was processed to form nitrogen-acetyl-n-propyl esters. The isotopic enrichment of free amino acids in blood was determined on an HP Model 5989 (Hewlett-Packard Co., Palo Alto, CA) by chemical ionization and selected ion monitoring at mass-to-charge ratios of 250:1, 255:1, and 256:1. Finally, indocyanine green concentrations were determined spectrophotometrically at λ = 805 nm on a Spectronic 1001 (Bausch and Lomb, Rochester, NY).
Calculations
Because phenylalanine is neither synthesized nor degraded in the periphery, the difference in concentration of this substrate in the femoral arterial and venous plasma pools reflects the net balance of leg skeletal muscle protein synthesis and breakdown. The net balance (NB) was calculated and standardized for leg volume by the following equation: NB = (CA − CV) · BF
where CA and CV are the blood free amino acid concentrations of the femoral artery and vein, and BF is leg blood flow in cc/min/100 mL leg. Leg blood flow was determined from the following modification of Fick’s equation: Infusion Rate = (CF − CC) · BF
where CF is the femoral venous concentration of indocyanine green and CC is the central (contralateral femoral) venous concentration of indocyanine green. With the infusion rate set at 0.5 mg/min, the equation was solved for leg blood flow (BF). As indicated above, BF was normalized for each patient by leg volume. Subject weight, leg circumference at prescribed points relative to anatomical landmarks, and the distances between these points were used to mathematically model leg volume.
Indirect Calorimetry
Resting energy expenditure (REE) was measured using a Sensor-Medics 2900 metabolic cart (Yorba Linda, CA). All indirect calorimetry measurements were made at 30°C, which is the standard environmental setting for all patient rooms in both our adult and pediatric acute burn ICUs. Composition of inspired and expired gases were sampled and analyzed at 60-second intervals. Values of VCO2, VO2, and REE were accepted when they were at a steady state for 5 minutes. The average REE was calculated from these steady-state measurements.
For statistical comparison, energy expenditure was expressed both as absolute REE and as the percentage of the basal metabolic rate predicted by the Harris-Benedict equation.
Statistical Analysis
Univariate and multiple regression analyses were used to determine the factors influencing catabolism over the natural course of burn injury and its altered pathophysiologic state. The influences of each of the variables listed in Table 1 on the rate of protein catabolism were separately determined by univariate analysis. Backward stepwise selection was employed to find the group of patient factors that best predicted catabolism. In the first step, all factors with a correlation coefficient (r value) of >0.10 by univariate linear regression were entered into the multiple regression model. At each subsequent step, the factor with the largest estimated coefficient of variation was removed until only those variables that were significant remained.
All variables that did not significantly correlate with protein net balance by univariate linear regression were further analyzed. For each of these patient factors, a simple scatter plot was generated (independent variable of interest vs. protein net balance) to see if significant but nonlinear associations with the rate of catabolism were present. When appropriate, Student t tests, one-way, repeated-measures ANOVA with Tukey corrections for multiple comparisons, and nonlinear regressions were used to determine differences and correlations. If no correlations were apparent or no differences were detected after this further analysis, the variable of interest was deemed to be unrelated to the catabolic response after burn. Statistical software (SigmaStat and SigmaPlot, SPSS, Chicago, IL) was used to perform all analyses.
RESULTS
Data from 151 muscle protein studies done in 123 subjects were collected for analysis. Subject demographics are shown in Table 3. The results from the univariate linear regressions are listed in Table 4. Age, sex, height, and weight were all significant predictors of catabolism with negative correlation coefficients, indicating a direct relationship between the magnitude of the independent variable and the extent of catabolism (negative net protein balance). Burn size (%TBSA) and metabolic rate (measured both in terms of absolute REE and as the percentage of the basal metabolic rate predicted from the Harris-Benedict equation) also achieved significance by linear correlation. The strongest single predictor of catabolism was sepsis. The “burn clinician” diagnosis of sepsis correlated more strongly with negative protein balance than sepsis defined by the AACP/SCCM Consensus Conference. In 32 of the 151 studies, the subject undergoing study fulfilled the criteria for both sepsis definitions; in eight instances, the subject fulfilled only the burn sepsis definition, and in 11, only the AACP/SCCM definition.
Table 3. SUBJECT DEMOGRAPHICS
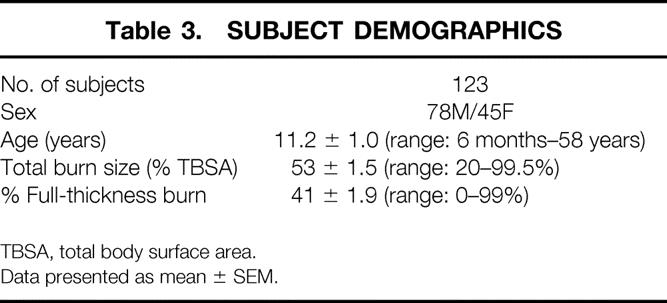
TBSA, total body surface area.
Data presented as mean ± SEM.
Table 4. CLINICAL FACTORS
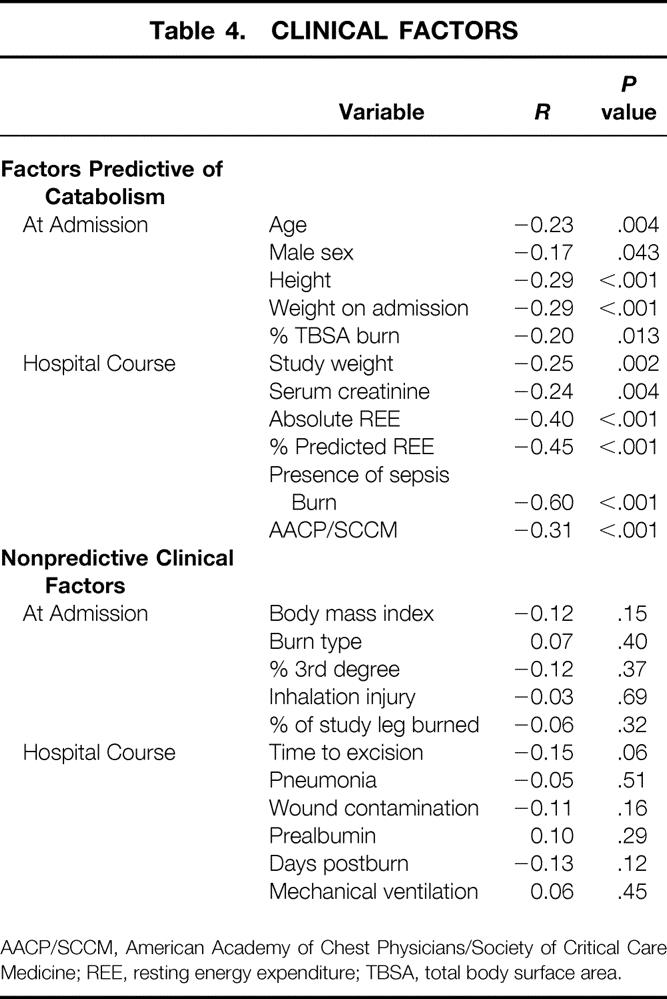
AACP/SCCM, American Academy of Chest Physicians/Society of Critical Care Medicine; REE, resting energy expenditure; TBSA, total body surface area.
Serum creatinine also demonstrated a significant correlation with negative net protein balance; however, none of these 123 subjects was in renal failure at the time of study, and in only one instance was the subject’s serum creatinine over 1.3 mg/dL.
Variables that were not significant independent predictors of a greater degree of catabolism by univariate regression included body mass index (weight/height2, gross anthropometric measure of body fat content), increasing time after burn, burn type (flame, scald, or electrical), area of full-thickness (third degree) burn, area of burn on the study leg, inhalation injury, pneumonia, severe wound contamination, mechanical-assisted ventilation at the time of study, serum prealbumin, and time to excision (Table 4B).
We constructed a multiple regression model to determine the best statistical representation of the catabolic process after burn. In this model, we found the five most significant variables in determining the magnitude of the catabolic response to severe burn were admission weight, percentage of TBSA burned, metabolic rate expressed as the percentage of the predicted energy expenditure, time from burn to the primary excision of the wound, and burn sepsis. The equation describing this relationship follows:
 |
This equation predicted the magnitude of the catabolic process with a correlation coefficient (r) of 0.71, which implies that these factors were able to account for 50% of the variability (r2 = 0.504) in the actual net balance values.
Figure 1 shows how weight upon admission, stratified in 30-kg increments, affects catabolism. Figure 2 shows that after reaching a burn size of 40% TBSA, there is no further consistent increase in protein catabolism. The effect of delay in primary excision of the burn wound is delineated in Figure 3. Figure 4 depicts the correlation between catabolism and measured energy expenditure, expressed as a percentage of the predicted basal metabolic rate.
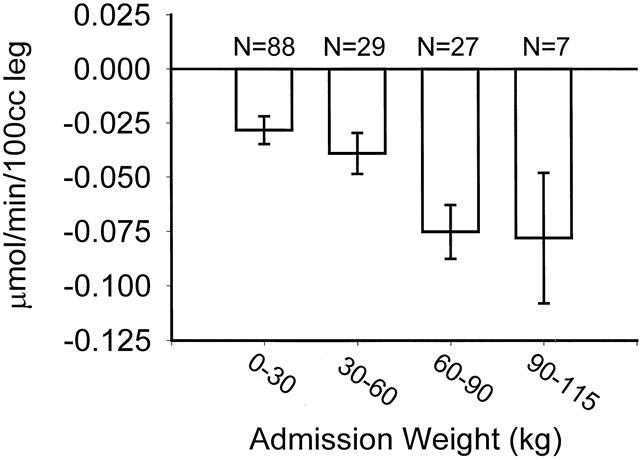
Figure 1. Association between admission weight and negative protein balance. Data presented as mean ± SEM.
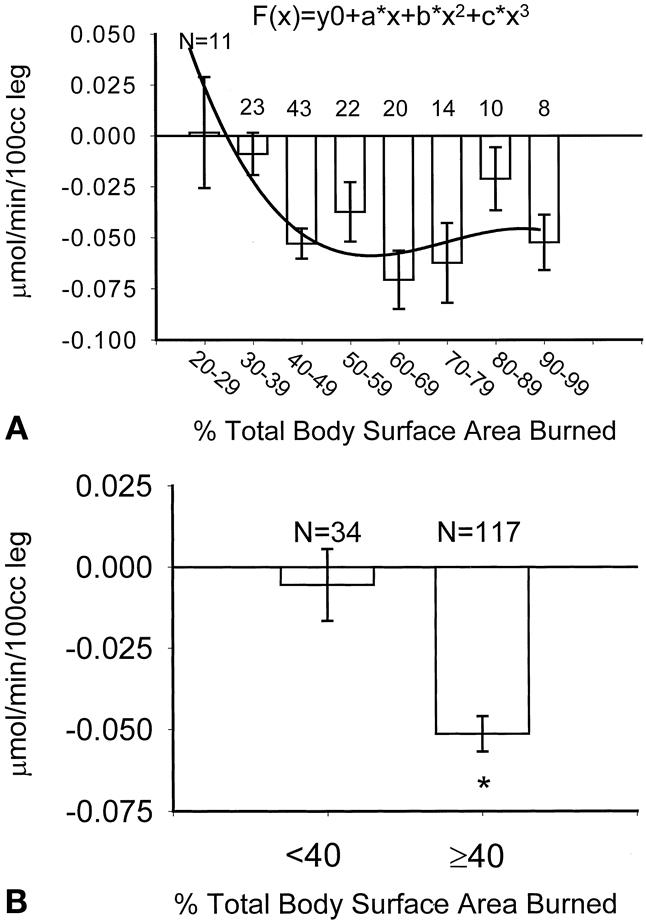
Figure 2. (A) Association between burn size and negative protein balance. Curve represents a “best fit” equation of authors’ data; best fit was a cubic expression as determined by the Akaike information criterion. R = 0.34. (B) Influence of burn size >40% total body surface area on catabolism. Data presented as mean ± SEM. *P = .0001 by Student t test.
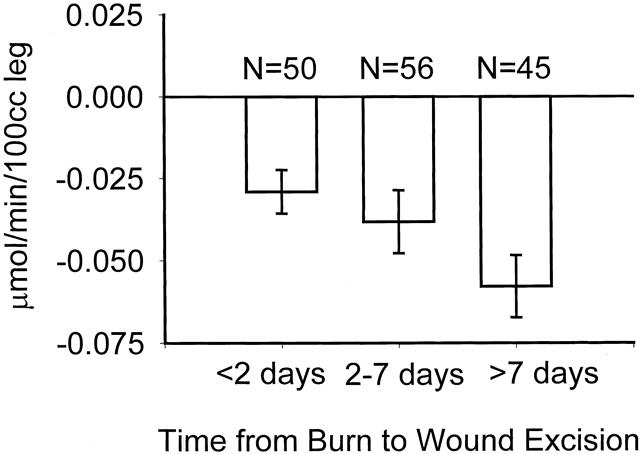
Figure 3. Association between time to primary wound excision and negative protein net balance. Data presented as mean ± SEM.
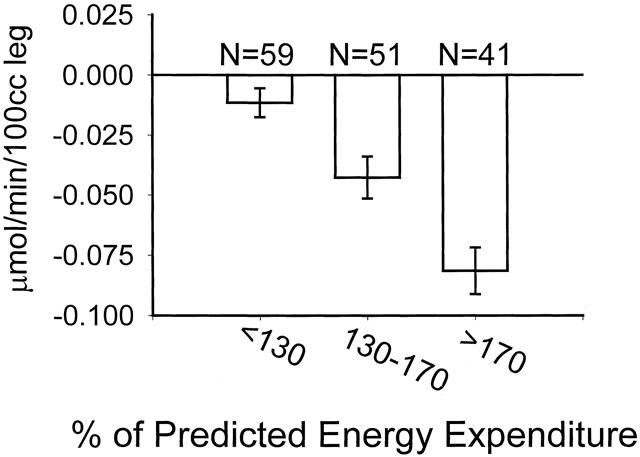
Figure 4. Association between metabolic rate and negative net protein balance. Metabolic rate is expressed as the percentage of energy expenditure predicted by the Harris-Benedict equation (resting energy expenditure/basal metabolic rate). Data presented as mean ± SEM. All groups statistically different (P < .05) from each other by one-way ANOVA followed by Tukey correction for repeated measures.
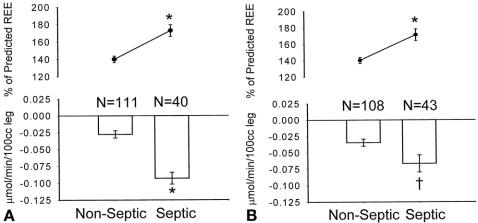
Marked differences in both metabolic rate and protein kinetics were present between septic and nonseptic states. During septic episodes, subjects were more hyperthermic and more likely to experience hypotension, hyperglycemia, enteral feeding intolerance, and thrombocytopenia. They required vasoactive pressors and exogenous insulin more often than subjects studied during nonseptic periods (P < .05). During sepsis, quantitative tissue counts were higher, and the subjects were more likely to have had a positive blood, urine, or sputum culture. It is notable that individuals meeting our criteria for burn sepsis had positive blood cultures only 50% of the time. Subjects diagnosed with sepsis by the AACP/SCCM standards all had bacteremia or fungemia, but almost one fourth did not meet the burn definition of sepsis at the time of study. During episodes of sepsis determined by either set of criteria, there were markedly higher rates of energy expenditure and protein catabolism than during nonseptic episodes (Fig. 5).
Figure 5. (A) Influence of burn sepsis on hypermetabolism and catabolism. Hypermetabolism is expressed as the percentage of energy expenditure predicted by the Harris-Benedict equation. Data presented as mean ± SEM. *P < .0001 by Student t test. (B) Influence of sepsis, as defined by the American Association of Chest Physicians/Society of Critical Care Medicine, on hypermetabolism and catabolism. Hypermetabolism is expressed as the percentage of energy expenditure predicted by the Harris-Benedict equation. Data presented as mean ± SEM. *P < .0001 by Student t T-test.
The effect of age on protein catabolism is illustrated in Figure 6. Clearly, infants do not mount the same catabolic response that older subjects do. The peak age range for protein catabolism is not the most aged group that we studied, but rather the individuals from age 17 to 39—those who were presumably at their peak in terms of physical stature and muscle mass.
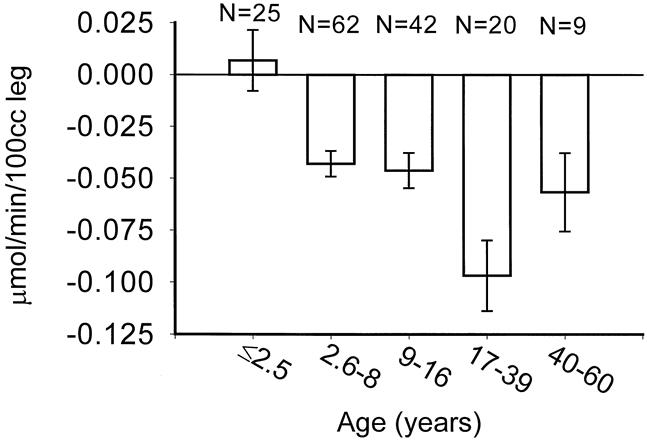
Figure 6. Association between age and negative protein balance. Data presented as mean ± SEM.
A greater degree of protein catabolism appeared in males than in females (Fig. 7). Reexamination of our demographic data revealed no differences in burn size, timing of excision, or incidence of sepsis between males and females. The male subjects included in this study, however, were older (male age 13.2 ± 1.4 years vs. female age 7.8 ± 1.0 years, P < .01), heavier (43 ± 3 kg vs. 28 ± 3 kg, P < .01), and had a higher serum creatinine (0.56 ± 0.04 vs. 0.36 ± 0.02, P < .01). These confounding variables may explain these differences apart from gender.
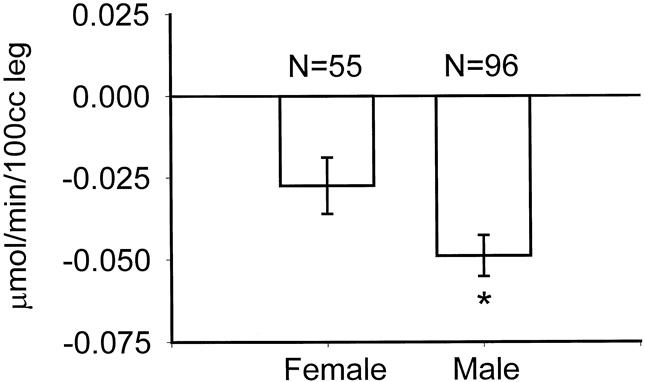
Figure 7. Differences in catabolic response between genders. Data presented as mean ± SEM. *P = .04 by Student t test.
DISCUSSION
Catabolism of lean body mass causes morbidity during acute burn treatment 23–25 and persists for up to 9 months beyond full wound healing, 26 delaying reintegration of the burn victim into society. 27
This study demonstrated that the most predictive variables for an increased catabolic response during acute hospitalization are subject weight, burn size, time from injury to excisional treatment, REE, and the presence of sepsis. We also found that age, male sex, height, and serum creatinine each correlated significantly with the degree of catabolism. Subject weight continued to be a significant variable throughout the hospital course. Serum creatinine correlated directly with negative protein net balance, despite the fact that none of these subjects had clinically relevant renal insufficiency at the time of study. In only one of the 151 study periods did a subject have a serum creatinine over 1.3 mg/dL (a 52 year-old male with a creatinine of 2.58 during a short-lived period of acute tubular necrosis after excision and grafting). The fact that age, sex, height, weight, and creatinine are all significant predictors suggests that total muscle mass may be significant. This inference is further supported by the lack of correlation between body mass index (and index of body fat) and measured rates of protein catabolism. Burn size significantly correlated with the degree of catabolism in a cubic rather than linear fashion (Fig. 2A). Increases in burn size beyond 40% TBSA did not predict a more negative protein net balance.
Time from injury to the primary excision and grafting of the wound was an important predictor of the catabolic response. Early excision of burn wounds has been shown, by our institution and others, to directly benefit patients through reducing blood loss at operation, 28 decreasing overall hospital stay, 29 and limiting serious infectious complications, 30,31 but has not previously been shown to alter the metabolic response. 32
The association between burn-induced hypermetabolism and muscle wasting has been controversial. 3,11 We attempted to control for the effects of circadian rhythms and surgical stress on catecholamine and cortisol levels by performing all indirect calorimetry measurements and protein kinetic studies at the same time of the day on postoperative day 5 after a given serial excision and grafting procedure. Indirect calorimetry measurements were made in a resting state between midnight and 5 am, and all 5-hour stable isotope infusions were begun between 5 and 7 am. We controlled for dietary intake of protein by feeding all subjects the same enteral formula at a rate normalized for each individual based on their body surface area and body surface area burned. With tight control of these factors, which are commonly thought to be confounding influences on protein and amino acid metabolism, our data confirms a strong association between metabolic rate and negative protein net balance.
At the time of the described metabolic studies, only 50% of subjects deemed septic by the surgical service (burn sepsis) had documented bacteremia or fungemia. The AACP/SCCM definition of sepsis was not as strongly predictive of protein catabolism or, for that matter, of general clinical status. Both sets of physiologic criteria, however, did correlate strongly with accelerated rates of both catabolism and hypermetabolism, which is consistent with reports regarding the metabolic response to sepsis and surgical stress in the face of severe illness. 1,8,9,33
Admission patient characteristics that did not significantly correlate with negative protein net balance included the area of full-thickness (third degree) burn, the type of burn, and the presence or absence of an inhalation injury. In these subjects, the area of third degree burn correlated highly with TBSA burned (on average, 80% of each subject’s burns was third degree). The fact that full-thickness burn did not correlate with the catabolic response leads us to conclude that second and third degree skin burns function similarly in precipitating the inflammatory hormonal and cytokine milieu that is responsible for stimulating the protein catabolic response.
Percentage of the study leg burned was not a significant predictor of the cross-leg net protein balance. Our protein kinetic model utilizes leg blood flow, in addition to the difference in substrate concentrations between the arterial and venous plasma pools, to calculate the net protein balance across the leg. In the 1970s, Aulick and colleagues 34,35 reported that leg blood flow increases proportionally to the area of burn wound on the leg, and that most of this increased flow is directed toward the skin. They demonstrated that muscle blood flow was unaffected despite increased flow to the leg as a whole. We believe the difference between these results and ours is that virtually all unburned areas on the legs we studied were used as donor sites. We infer then that donor sites have similar effects in blood flow and catabolism as do burns.
Characteristics of the hospital course that did not correlate with measured catabolism included the requirement for mechanical-assisted ventilation at the time of study, serum prealbumin, and a visceral acute-phase protein reported to be a sensitive marker of acute nutritional status. 36 Pneumonia and severe wound contamination, which at their greatest extents are clearly associated with sepsis, do not independently alter the systemic physiologic response to burn. Muscle catabolism was not dependent on time removed from injury during the acute healing phase. A stable catabolic response in a given individual persists from at least 1 week postinjury to 2 months postinjury in the patients reported here. This complements data we recently reported 26 regarding the prolonged course of catabolism that persists in burns over 40% TBSA for up to 9 months postinjury.
In summary, we have shown that greater burn size, heavier weight upon admission, and longer time to primary wound excision are important initial factors associated with an increased rate of catabolism after burn. Throughout the hospital course, the development of sepsis and severe hypermetabolism also are predictive of a more negative net protein balance. The amount of gross muscle mass appears to correlate with the eventual magnitude of catabolism, but not necessarily with risk of mortality. Successful determination of patients at risk for the greatest degree of muscle wasting is very useful in directing anabolic therapy to improve long-term rehabilitation potential.
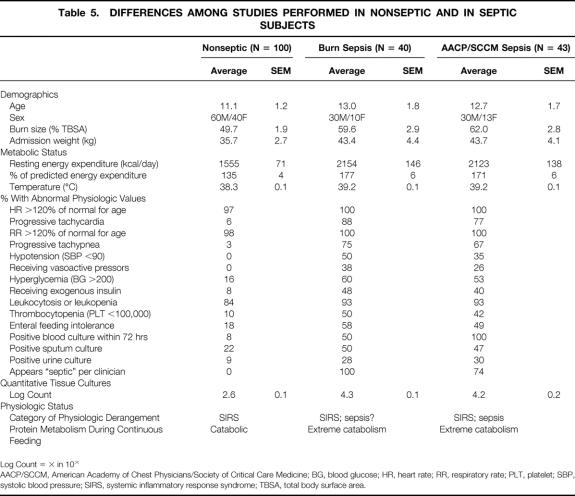
Table 5. DIFFERENCES AMONG STUDIES PERFORMED IN NONSEPTIC AND IN SEPTIC SUBJECTS
Log Count = × in 10×
AACP/SCCM, American Academy of Chest Physicians/Society of Critical Care Medicine; BG, blood glucose; HR, heart rate; RR, respiratory rate; PLT, platelet; SBP, systolic blood pressure; SIRS, systemic inflammatory response syndrome; TBSA, total body surface area.
Discussion
Dr. Douglas W. Wilmore (Boston, Massachusetts): One of the most common responses to critical injury is loss of body protein, first described by Sir David Cuthbertson back in the 1930s, with studies confirmed and extended by Dr. Francis Moore of Boston in the 1940s and 1950s.
In recent years, we have come to appreciate that protein catabolism is related to the extent of injury, the age of the patient, the closure of the wound, the presence or absence of sepsis, and gender. Effects of gender were first described by David Hume, who showed that women did not have as great nitrogen loss as men. Ivan Johnson from England pointed out that depleted patients would lose less nitrogen than would their athletic, robust counterparts. I mention all these points only to emphasize that it is reassuring in this world of constant change that many of the variables that have been described using whole body nitrogen balance techniques have now been specifically related to skeletal muscle protein loss using very elegant stable isotopic techniques. Questions, however, abound.
First, all patients received feedings throughout the study. Were any of the studies performed in nonfed patients? Or were diets compared, such as a high-fat diet versus a high-carbohydrate diet?
Second, when anabolic agents were used, was there a dose-response seen and was a time course evaluated? Can the investigators provide with us pharmacoeconomic data on the efficacy of these agents and give us advice about how to use these agents in our specific metabolic patient situations?
Three, the ability to maintain body protein should be related to some clinical function—improved wound healing, improved immunologic responses, improved growth in children, and improved strength and activity. Specifically, do the authors have data as to the function of skeletal muscle mass, particularly with the anabolic agents? Does grip strength or duration of activity or shortness of convalescence somehow relate to the improvement of net skeletal muscle protein catabolism?
It is only by continuing these important studies in the metabolic response to injury that we will be able to improve patient care in this area.
Presenter Dr. David N. Herndon (Galveston, Texas): In this study, we did not examine anyone in the fasted state. This was chosen specifically to look at catabolism in the normal state for burned patients, which now consists of continuous enteral feeding. However, in 1991 we performed a study with Dr. Dennis Gore looking at the effects of recombinant human growth hormone on protein synthesis and degradation in which the patients were fasting. The values in Dr. Gore’s study were lower than in ours, which is consistent with negative net protein balances found under all conditions while fasting. Our enteral diet in this study consisted of the same enteral formula at a rate normalized to each individual’s total body surface area and area burned. We have recently completed a study in which we varied dietary composition between high-fat and high-carbohydrate feeding in the setting of isocaloric, isonitrogenous enteral formulas, which we are reporting elsewhere; we found that a diet that supplies most of its nonprotein calories as carbohydrate, rather than fat, is anabolic relative to a high-fat diet.
For your second question, to be clear, the data presented in this paper were all derived from burned subjects who had not received anabolic therapy prior to these studies. This paper is, in fact, a summation of control studies done in several anabolic agent research projects over the last 3 years. We have also gathered and summarized all the anabolic therapy data over this time period. This includes use of the following anabolic agents: high-dose and submaximal insulin, IGF-1/IGFBP-3, growth hormone, testosterone, oxandrolone, and propranolol. The use of these drugs was uniformly effective except in children under 21/2 years of age. The least expensive drugs that had salutary effects were propranolol, insulin, and oxandrolone, with propranolol having the most profound effect. Insulin, at both high and low doses, was also very promising, though it entails the clinical risk of hypoglycemia. Oxandrolone is an inexpensive and safe analogue of testosterone that has recently been discovered by the burn community. Expensive drugs, such as growth hormone and IGF-1/IGFBP-3, may no longer be the anabolic agents of choice in light of these new findings. In our studies, we found IGF-1/IGFBP-3 to be very effective in both burned children and adults, but there was a disturbing incidence of peripheral neuropathies in our adult subjects, which we will be reporting on later. In terms of dose response, we did a study with growth hormone at 0.1 mg/kg/day and 0.2 mg/kg/day, and found the 0.1 mg/kg dose not as effective in a limited number of burned children. We therefore selected the 0.2 mg/kg dose for study. Dr. Wilmore, however, has used the 0.1 mg/kg dose effectively in adults.
For your third question, we have recently shown that recombinant human growth hormone given at a dose of 0.2 mg/kg/day throughout hospital stay has a long-term beneficial effect on linear growth (Lancet 1999;354:1789). Height velocity was accelerated between 6 and 24 months postinjury to the level of normal, unburned peers. The untreated burned subject group was retarded in growth, as we had previously reported. In addition, we have recently completed studies on giving recombinant growth hormone in the convalescent period. During convalescence from severe burn, there is a persistent increase in metabolic rate and continued erosion of lean body mass for several months after full healing of all skin wounds. We are analyzing that data now, but preliminarily, there does seem to be an attenuation of the erosion of lean mass during convalescence with growth hormone treatment.
Dr. Palmer Q. Bessey (Rochester, New York): It is comforting that this report reinforces many concepts that have guided us in caring for burn patients over many years, namely, that the metabolic and catabolic responses to injury are influenced by age, sex, body size—which reflects muscle mass—and burn size. It also is of interest that the protein catabolic response, so elegantly demonstrated in this study today, reached a plateau at about 40% to 50% total body surface area burn. That is similar to the patterns we see with hypermetabolism.
The authors suggest that the time to primary excision is another important variable in treating these patients. This is the first study that provides good data supporting that concept. If I understand correctly, when time to excision was an independent predictor of metabolism, it did not quite reach significance but only did so within a multivariate calculation. Is not time to excision in part influenced by how sick the patient is? Early hemodynamic instability, severe respiratory failure and the like, might well delay the time to the operating room. Thus, time to excision might well reflect the severity of illness, just as hypermetabolism and burn size do in a multivariate equation. I wonder if you could elaborate on this point?
The other questions I have relate to strategies we might use to minimize the catabolic draft on the patient’s body protein. Professor Cuthbertson in his early work estimated that the loss of muscle mass from inactivity in severely injured patients approximately equalled the amount lost because of accelerated metabolic muscle breakdown. I wonder if you have any sense about how much inactivity was an influence in the patients you studied. Presumably, inactivity would be related to the extent of burn and severity of illness. Thus, patients with less severe illness and earlier excision would be able to resume activity sooner than more severely ill patients, and thus see earlier reduction in muscle protein breakdown.
John Kinney, of this Association, several years ago emphasized the importance of avoiding overfeeding. In septic patients, he found that overfeeding actually drove hypermetabolism and increased metabolic rate. If I understand it correctly, your nutritional support regimen may provide more calories than many other approaches. For example, a 2-m2 person with a 50% burn would get approximately 4,500 kcal, according to the methods reported here. That would be approximately 50% greater than the amount provided by several other commonly used protocols, which is about 3,000 kcal. Is it possible that modification of the nutritional support you provide might be of some benefit?
Dr. Wilmore pointed out the modifying effect of time on the demonstrated efficacy of anabolic agents. As I understand it, the anabolic agent studies were always performed after the convalescent or control study, therefore at a moderately late stage of the patients’ recovery from burn injury. At that point, they would likely have had lessened degree of hypermetabolism than they would have had during the control study. How much of an effect do you think that played in the observations you made?
Dr. Herndon: Dr. Bessey very appropriately points out that by univariate analysis, the significance of time to excision had a P value of .06. This is just short of the 95% confidence interval usually accepted for statistical significance. In the multiple regression analysis, however, the variability due to the other clinical factors was accounted for and it became one of the five most significant predictors of catabolism. And as Dr. Bessey points out, this is indeed because patients who arrive delayed with unexcised burns are more ill—they have either heavily contaminated wounds or frank burn-wound sepsis. In these patients, however, we are every bit as aggressive in terms of rapid resuscitation and excision of the wound—perhaps even more so. We believe that the treatment for surgical sepsis is aggressive, definitive surgical therapy—the source of sepsis must be removed to avoid continued clinical deterioration and to allow physiologic recovery of respiratory, gut, and immune functions.
Other strategies frequently used to decrease catabolism include exercise and anabolic agents. In terms of exercise, both early mobilization and weight-bearing during the acute wound coverage period, and focused exercise training during convalescence, are likely to be beneficial. We have recently engaged our patients in a 3-month aerobic and anaerobic exercise program during convalescence. A preliminary analysis suggests that this markedly improves strength and decreases loss of muscle mass.
We believe that exercise is an important clinical variable influencing muscle catabolism in critically ill patients. In this particular study, however, all patients were treated in a uniform fashion: bed rest for the first 4 days after each serial excision and grafting procedure, dressings down on the day 4, passive and active exercise on the days 5 and 6, and then return to the operating room for the next grafting on day 7. Bed rest was applied to all subjects identically, and thus was controlled as a potential confounding variable. We do not believe that more exercise during the first 4 days after a split-thickness skin grafting procedure is practical.
Dr. Kinney has indeed cautioned us to avoid overfeeding (Crit Care Med 1982;10(3):163–72). The amount of nutrition supplied daily to this group was 1500 kcal per square meter of body surface area for maintenance plus 1500 kcal per square meter burned. We have derived this formula and shown that it is the caloric intake required to maintain total body weight throughout the acute hospitalization in severely burned patients (J Burn Care Rehabil 1988:9;616). That does not mean that this amount of food maintains lean body mass, but that it maintains total body mass. It may be that these patients are getting fat and still losing muscle mass. Modifications of dietary intake, including combining hypocaloric regimens with anabolic pharmacologic agents, will be investigated by our group in the future. However, the concept of hypocaloric feeding is somewhat worrisome to me—it is very difficult to justify allowing sick, catabolic patients to lose body mass of any sort.
Twenty-eight of these subjects had two studies, and had not received any anabolic agents prior to these stable isotope studies. There was no diminution of the protein catabolic rate between the early and late time periods. This complements data that we recently reported showing that muscle protein catabolism and erosion of lean mass, as measured by serial body composition studies, continue many months after full wound healing and discharge from the hospital.26
Dr. William G. Cioffi (Providence, Rhode Island): These finding corroborate those of Kellman and Pruitt, who reported that hypermetabolism did not increase as burn size increased past 20% to 30% except in patients who are environmentally stressed. Thus, it is not surprising that catabolism has a similar relationship.
I have two methodologic questions and several areas in which I would like to you speculate. First, exercise has a profound effect on catabolism. Although you commented on your exercise program in the rehabilitative state, how did you control for exercise during the acute hospitalizations and during these studies?
Second, since there was no catabolism in patients with less than 40% burn, why did you keep these patients in the study and in your analysis of the data, as they comprise approximately 30% of the population?
This extensive data set has several previously unnoted findings. First is that children less than 21/2 years or less than 15 kg, although hypermetabolic, are not catabolic. Why do you suppose this is true? And why do you suppose anabolic agents have little or no effect in this population?
Second, as previously reported, and Dr. Wilmore alluded to, males had considerably greater catabolism than females, almost two-fold. Was there a difference in outcome either in morbidity or mortality, or, more importantly, as Dr. Wilmore alluded to, in terms of rehabilitation between your male and female patients?
Next, do you have any data on systemic hormone levels in these patients, or systemic IL-6 levels, a cytokine which is noted to correlate with the stress response, but particularly the hormone levels which Wilmore, Bessey, and others have clearly shown to correlate with degree of metabolism?
Finally, in a previous publication you noted that time to excision had no effect on hypermetabolism or catabolism, and yet in this series you note a profound effect in terms of time to excision and complete wound excision. Can you provide an explanation for this discrepancy?
Dr. Herndon: Kellmen, Cioffi, and their colleagues at the U.S. Army Institute for Surgical Research at the Brooke Army Medical Center have concluded that the metabolic response to burn, measured as resting energy expenditure, is independent of burn size beyond 20% TBSA burn when environmental conditions are controlled (Ann Surg 1996:223(4);406–12). This was a very nice physiologic experiment which did show a blunting of the expected increase in metabolic rate with increasing burn size. In fact, I think this study corroborates our findings even more strongly than its authors may suppose. Its only drawback was that it was a relatively small study and enrolled few patients with massive burns (21 of 44 subjects suffered >40% TBSA burns; only 13 subjects >50% TBSA burned). The authors concluded there were no differences at all over the entire spectrum of burn sizes from 20% to 97% TBSA when environmental conditions were controlled. If you look at their data, however, there were definite trends—in fact, their data say with a 90% confidence interval, that burns >40% TBSA do get more hypermetabolic than burns <40% TBSA (160% vs. 150% of basal metabolic rate). So there is a 90% likelihood that they witnessed the same phenomenon we document here (namely a difference in systemic metabolic response to burns <40% TBSA compared with those >40%), but did not recognize this demarcation due to simple, classic type II error.
We included all patients in this analysis for statistical purposes. To gain as much power as possible in our multiple regression, the smaller burns had to be left in with the large burns. There were, in fact, very few studies done on patients with burn sizes from 20% to 30% TBSA (11 of a total of 151 studies).
I am not sure why infants do not mount the same catabolic response as older patients; it may be that some aspect of their immune system or metabolic function is immature and unreactive to the insult, or it may be that this age group is extremely anabolic under normal circumstances, and a zero or slightly positive net balance is relatively catabolic for them. We do not know what a normal net balance is in noninjured infants (continuously fed or not).
The gender differences in this study may have been confounded by the increased size and age of the males studied. There were no differences in morbidity or mortality between genders in this large series. Previously, however, we showed a higher mortality in burned male patients compared with age, weight, and burn size matched females (Lancet 1988;2:1076–7).
Systemic IL-6 is elevated acutely in these patients. We have previously shown catecholamines, corticosteroids, and glucagon to be elevated in this patient population, as Dr. Wilmore and Dr. Bessey previously alluded to.
Time to excision had no effect in a previous study that we performed. In that study, all patients admitted were randomized to excision within 72 hours of the time of admission versus having serial debridements over a period of weeks in our own institution. This study was very small, with only seven subjects in our early, complete excision group and six subjects treated by conservative serial debridement. We did see a trend toward lower metabolic rate in the early excision group here as well, but we did not have the statistical power to demonstrate this difference. This is a much larger series, and I think the increased power has allowed us to document an effect that had been hinted at, but not previously defined.
Dr. Per-Olof J. Hasselgren (Cincinnati, Ohio): I have a question regarding the effect of IGF-1. In some of our studies, we have found that IGF-1 is extremely effective in terms of reversing the catabolic response to burn injury. In contrast, in sepsis, muscles become resistant to IGF-1. My question is: When you analyzed your patients, did you see a difference between burn and burn-infected patients in terms of the response to IGF-1?
Dr. Herndon: We compared this group of protein kinetic studies that were not influenced by anabolic agents with our pool of studies that were performed while patients were receiving anabolic agents, and had these 151 nonanabolic studies from this paper and another 87 pooled anabolic studies. None of our discrete trials using specific agents had large-enough sample sizes to draw conclusions about clinical factors that predict or modulate response to these specific therapies (including our IGF studies). However, when we pooled all these anabolic studies, we were able to discern interesting information that we will report later this year. We saw that anabolic therapy, in general, was efficacious in both septic and nonseptic states.
I should say a word about our definitions of sepsis. Importantly, this particular topic may introduce some selection bias into our analysis. As Dr. Pruitt has cautioned the burn community again and again, it is exceedingly hard to discern sepsis from SIRS in these sick patients. We have used two definitions, the first being the standard of care in the medical world in general (the AACP/SCCM definition), and the second being more commonly used in the burn community and more germane to burn patients in particular. Both definitions predicted catabolism and hypermetabolism in these patients. We have been consistent in our application of these definitions, but I wish to make it clear that we did not study the sickest patients in the spectrum of septic illness. We did not study any moribund patients or patients who needed significant fluid resuscitation, primarily because stable isotope methodology requires the plasma volume to be at a steady state during sampling. We did study ill, septic patients, but all were fairly well compensated on routine critical care support (e.g., continuous IV fluid infusions, pressors, insulin drips, antibiotics).
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): I would like to ask Dr. Herndon to explain two apparent discrepancies. The plateau of catabolism when the burn involved 40% or more of the body surface is certainly not consistent with other studies. Does that represent the fact that you had converted all the unburned surface into donor sites, and were really measuring metabolic rates in patients in whom the skin injury approached 100% of the body surface by that time? Also, how do you explain your finding that time postburn did not affect metabolic rate? Additionally, since physical activity by itself can minimize the proteolysis associated with inactivity, have you observed an effect of physical therapy on the response to the anabolic hormone?
Dr. Herndon: I believe you, Dr. Kellmen, and Dr. Cioffi have witnessed this same phenomenon. The cause of the plateau beyond 40% may well be because all remaining open sites were used as donor sites, and these patients were essentially all 80% to 90% open over their entire body surface area throughout the study periods. It is very interesting to speculate that our mode of treatment may magnify the patient’s metabolic response, but excisional therapy of deep second and third degree burn wounds is the universally accepted best treatment for many reasons other than its metabolic consequences.
These studies were done on postoperative day 5. The requirement for operations at weekly intervals leaves the patients on bed rest for 4 days after every operation. We do exercise these patients between the days 5 and 7 with an active physical therapy program, and have noticed profound effects of physical therapy when given in this weekly fashion over the entire course of the acute surgical treatment of these individuals, which may take 2 to 3 months to complete.
Dr. Stanley M. Levenson (Bronx, New York): Dr. Herndon, during the period of hormonal treatment, in addition to measuring oxygen consumption, did you measure CO2 production, calculate the respiratory quotient, and see how the proportions of protein, fat, and carbohydrate oxidation were modified? Also, I might mention from the historical point of view that it was a little over 50 years ago that John Brash, working with us in the Army Medical Nutrition Laboratory, showed in burned rats with 33% deep burns that testosterone would markedly diminish urinary nitrogen excretion on a fixed nutrient intake. The problem with its use in burn patients was complicated by excess sodium and water retention.
Dr. Herndon: We did determine the respiratory quotient in these patients. And remarkably, though we were using a high-carbohydrate and high-protein mixture, fat is still a primary fuel in these individuals, as lipolysis continues unabated. The respiratory quotients in these patients were similar in all cases and approached 1.0.
Footnotes
Correspondence: David N. Herndon, MD, Shriners Hospitals for Children, 815 Market St., Galveston, TX 77550.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
Supported by SHC Grants #8660 and #8490, NIH Training Grant #2T32GM0825611, NIH Center Grant #1P50GM60338–01, and NIH Grant #5RO1GM56687–03.
There are no financial ties between any of the authors and any corporate entity or product mentioned in this manuscript.
E-mail: dherndon@UTMB.edu
Accepted for publication April 2000.
References
- 1.Bessey PQ, Jiang Z, Johnson D, et al. Post-traumatic skeletal muscle proteolysis: The role of the hormonal environment. World J Surg 1989; 13: 465–470. [DOI] [PubMed] [Google Scholar]
- 2.Ireton-Jones CS, Turner WW, Baxter CR. The effect of burn wound excision on measured energy expenditure and urinary nitrogen excretion. J Trauma 1987; 27 (2): 217–220. [DOI] [PubMed] [Google Scholar]
- 3.Milner EA, Cioffi WG, Mason AD, et al. A longitudinal study of resting energy expenditure in thermally injured patients. J Trauma 1994; 37 (2): 167–170. [DOI] [PubMed] [Google Scholar]
- 4.Cuthbertson DP. Observations on disturbance of metabolism produced by injury to the limbs. Q J Med 1932; 25: 233. [Google Scholar]
- 5.Cuthbertson DP, McGirr JL, Robertson JSM. The effect of fracture of bone on the metabolism of the rat. Q J Exp Physiol 1939; 29: 13. [Google Scholar]
- 6.Young VR, Munro HN. NY-methylhistidine (3-methylhistidine), muscle protein turnover: an overview. Fed Proc 1978; 37: 2291. [PubMed] [Google Scholar]
- 7.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992.
- 8.Monk DN, Plank LD, Franch-Arcas G, et al. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann Surg 1996; 223 (4): 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plank LD, Connolly AB, Hill GL. Sequential changes in the metabolic response in severely septic patients during the first 25 days after the onset of peritonitis. Ann Surg 1998; 228: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilmore DW, Long JM, Mason AD Jr, et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg 1974; 180: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner WW, Ireton CS, Hunt JL, et al. Predicting energy expenditure in burned patients. J Trauma 1985; 25: 11–16. [DOI] [PubMed] [Google Scholar]
- 12.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg 1999; 229: 713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DebRoy MA, Wolf SE, Zhang XJ, et al. Anabolic effects of insulin-like growth factor in combination with insulin-like growth factor binding protein-3 in severely burned adults. J Trauma 1999; 47: 904–11. [DOI] [PubMed] [Google Scholar]
- 14.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg 1999; 229: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects in critically ill burn victims. Crit Care Med 1999; 27: A23. [Google Scholar]
- 16.Hart DW, Wolf SE, Low JFA, et al. The effect of propranolol on catabolic pediatric burn patients. J Burn Care Rehabil 2000; 21 (1): S147. [Google Scholar]
- 17.Hart DW, Wolf SE, Chinkes DL, et al. Anti-catabolism after severe burn: synergism between growth hormone and propranolol. Surg Forum 2000 (in press).
- 18.Wolf SE, Rose JK, Desai MH, et al. Mortality determinants in massive pediatric burns. Ann Surg 1997; 225: 554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf SE, Jeschke MG, Rose JK, et al. Enteral feeding intolerance: an indicator of sepsis-associated mortality in burned children. Arch Surg 1997; 132: 1310–1314. [DOI] [PubMed] [Google Scholar]
- 20.Neely AN, Smith WL, Warden GD. Efficacy of a rise in C-reactive protein serum levels as an early indicator of sepsis in burned children. J Burn Care Rehabil 1998; 19: 102–105. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101 (6): 1644–55. [DOI] [PubMed] [Google Scholar]
- 22.Biolo G, Maggi SP, Williams BD, et al. Increased rates of muscle protein turnover and amino acid transport following resistance exercise in humans. Am J Physiol (Endocrin Metab) 1995; 268 (31): E514–520. [DOI] [PubMed] [Google Scholar]
- 23.Moore FD. Response to starvation and stress. Metabolic Care of the Surgical Patient. Philadelphia: WB Saunders; 1959: 202–275.
- 24.Newsome T, Mason A, Pruitt B. Weight loss following thermal injury. Ann Surg 1973; 178: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason AD Jr. Weight loss in burned patients. J Trauma Inj Infect Crit Care 1979; 19 (11suppl);903–4. [PubMed] [Google Scholar]
- 26.Hart DW, Wolf SE, Mlcak RP, et al. Persistence of muscle catabolism after severe burn. Surgery 2000; 128: 312–319. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe RR. Metabolic responses to burn injury: nutritional implications. In: Herndon DN, ed. Total Burn Care. Philadelphia: WB Saunders; 1996: 217–222.
- 28.Desai MH, Herndon DN, Broemiling L, et al. Early burn wound excision significantly reduces blood loss. Ann Surg 1990; 211: 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herndon DN, Barrow RE, Rutan RL, et al. A comparison of conservative versus early excision therapies in severely burned patients. Ann Surg 1989; 209: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herndon DN, Parks DH. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J Trauma 1986; 26: 149–152. [DOI] [PubMed] [Google Scholar]
- 31.Burke JF, Quinby WC, Bondoc CC. Early excision and prompt wound closure supplemented with immunosuppression. Surg Clin North Am 1978; 58: 1141–1150. [DOI] [PubMed] [Google Scholar]
- 32.Rutan TC, Herndon DN, Van Osten T, Abston S. Metabolic rate alterations in early excision and grafting versus conservative treatment. J Trauma 1986; 26: 140. [DOI] [PubMed] [Google Scholar]
- 33.Gore DC, Jahoor F, Hibbert J, DeMaria EJ. Except for alanine, muscle protein catabolism is not influenced by alterations in glucose metabolism during sepsis. Arch Surg 1995; 130: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 34.Aulick LH, Wilmore DW, Mason AD, Pruitt BA Jr. Influence of the burn wound on peripheral circulation in thermally injured patients. Am J Physiol Heart Circ Physiol 1977; 233: H520–526. [DOI] [PubMed] [Google Scholar]
- 35.Aulick LH, Wilmore DW, Mason AD, Pruitt BA. Muscle blood flow following thermal injury. Ann Surg 1978; 188: 778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingenbleck Y, DeVisscher M, DeNayer P. Measurement of prealbumin as an index of protein calorie malnutrition. Lancet 1972; 2: 106–109. [DOI] [PubMed] [Google Scholar]