Abstract
Objective
To evaluate the long-term survival outcomes of a large cohort of liver transplant recipients and to identify static and changing factors that influenced these outcomes over time.
Summary Background Data
Liver transplantation has been accepted as a therapeutic option for patients with end-stage liver disease since 1983, with continual improvements in patient survival as a result of advances in immunosuppression and medical management, technical achievements, and improvements in procurement and preservation. Although many reports, including registry data, have delineated short-term factors that influence survival, few reports have examined factors that affect long-term survival after liver transplantation.
Methods
Four thousand consecutive patients who underwent liver transplantation between February 1981 and April 1998 were included in this analysis and were followed up to March 2000. The effect of donor and recipient age at the time of transplantation, recipient gender, diagnosis, and year of transplantation were compared. Rates of retransplantation, causes of retransplantation, and cause of death were also examined.
Results
The overall patient survival for the entire cohort was 59%; the actuarial 18-year survival was 48%. Patient survival was significantly better in children, in female recipients, and in patients who received transplants after 1990. The rates of retransplantation for acute or chronic rejection were significantly lower with tacrolimus-based immunosuppression. The risk of graft failure and death was relatively stable after the first year, with recurrence of disease, malignancies, and age-related complications being the major factors for loss.
Conclusion
Significantly improved patient and graft survival has been observed over time, and graft loss from acute or chronic rejection has emerged as a rarity. Age-related and disease-related causes of graft loss represent the greatest threat to long-term survival.
Although the technique of liver transplantation was independently described in 1960, 1,2 it was not until 1963 that the first human liver transplantation was performed at the University of Colorado. 3 Between 1963 and 1967, nine such attempts were made worldwide, with poor outcomes; the first meaningful survival of 400 days was not reported until 1967. 4 Under azathioprine, corticosteroid, and antilymphocyte globulin therapy between 1967 and 1980, 170 liver transplants were performed at the University of Colorado, with a 1-year survival rate of 30%. 4 Between 1968 and 1983, 138 liver transplants were performed in Cambridge (UK), with similarly poor outcomes. 5 With the clinical introduction of cyclosporine, 6 and by refining cyclosporine use with the addition of corticosteroids, 7 survival rates after liver transplantation more than doubled.
There have been numerous reports on survival outcomes after liver transplantation with short- to medium-term follow-up. However, only a few reports are available of long-term follow-up. 8–11 The United Network of Organ Sharing (UNOS) began collecting outcome data in October 1987, 12 but the interpretation of this data is limited by the heterogeneity of program practices (e.g., immunosuppression protocols), differences in categorizing causes of liver disease, and lack of uniformity in follow-up. The aims of our study are to examine the long-term outcome after liver transplantation in a large population of patients from a single center with follow-up of up to 18 years and to compare the survival patterns, rate of retransplantation, and causes of death in relation to age, diagnosis, gender, and year of transplantation.
METHODS
The study subjects were 4,000 consecutive patients who underwent liver transplantation between February 1981, when the program was started at the University of Pittsburgh, and April 1998. They received 4,947 allografts. The remaining 601 patients in our overall liver transplant experience, who were excluded from analysis, included 192 patients who received transplants at the VA Medical Center, 59 who received combined liver/intestinal allografts, and 350 who did not have at least 2 years of follow-up. The mean follow-up was 9.4 ± 3.8 years (median 9.6, range 2–18 years). There were 2,172 (54.3%) male patients and 1,828 (45.7%) female patients. To analyze the effect of a given parameter on survival, parameters that varied during the course of patient accrual (e.g., UNOS status) were eliminated from analysis.
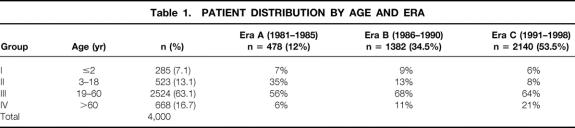
The study population was divided into four age groups based on the recipient’s age at the time of transplant, and into three eras based on the date of the first transplant (Table 1). The three periods coincided with the clinical introduction of cyclosporine (era A), the advent of Orthoclone OKT3 (Ortho, Raritan, NJ) and Viaspan (Dupont, Wilmington, DE) (era B), and the clinical introduction of tacrolimus (era C). The indications for liver transplant in adults and children in these three eras are shown in Table 2.
Table 1. PATIENT DISTRIBUTION BY AGE AND ERA
Table 2. INDICATIONS FOR LIVER TRANSPLANTATION
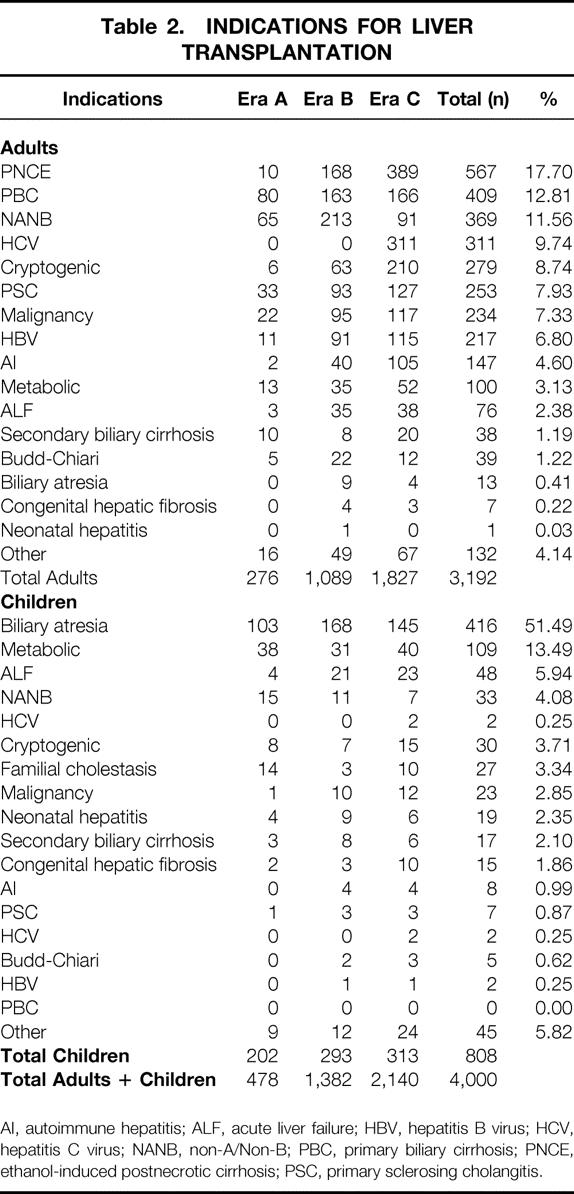
AI, autoimmune hepatitis; ALF, acute liver failure; HBV, hepatitis B virus; HCV, hepatitis C virus; NANB, non-A/Non-B; PBC, primary biliary cirrhosis; PNCE, ethanol-induced postnecrotic cirrhosis; PSC, primary sclerosing cholangitis.
Kaplan-Meier estimates were used to calculate survival curves. Differences in survival curves were compared using log-rank statistics. Differences in proportions were tested using the chi-square test (or the Fisher exact test). A P value less than 0.05 was considered statistically significant. To calculate the relative risk of death, regression analysis was used and 95% confidence intervals were determined.
RESULTS
Patient Survival
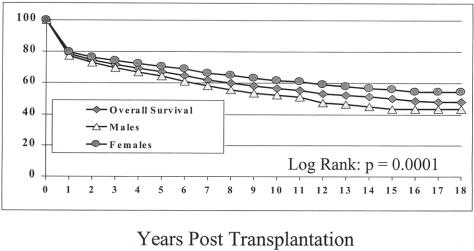
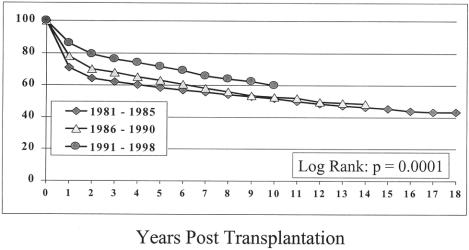
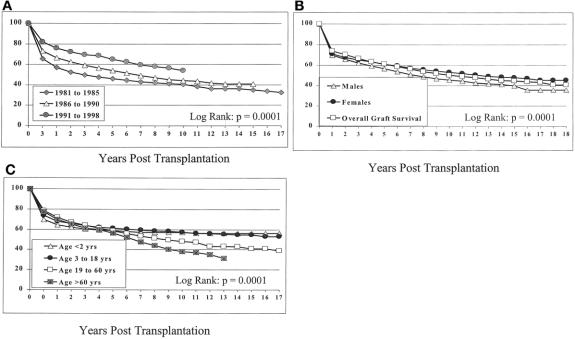
The actuarial patient survival rates for the entire population at 1, 5, 10, 15, and 18 years were 79%, 67%, 57%, 50%, and 48%, respectively (Fig. 1). Although there was little difference in the survival rate in eras A and B, the survival rate in era C was significantly better at 1, 5, and 10 years. Survival rates were 71%, 59%, and 52% for era A, 78%, 63%, and 53% for era B, and 86%, 72%, and 60% for era C, respectively (Fig. 2) (P = .0001).
Figure 1. Overall patient survival with comparison of male and female survival.
Figure 2. Survival according to era of transplantation.
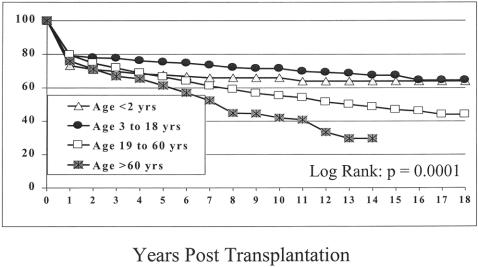
Survival rates for infants (age 2 years or younger, group I), children (age 3–18 years, group II), adults (age 19–60 years, group III), and seniors (age older than 60 years, group IV) were significantly different. The 1-, 5-, 10-, 15-, and 18-year survival rates were 73%, 68%, 66%, 64%, and 64% for group I, 80%, 76%, 72%, 68%, and 65% for group II, and 80%, 67%, 55%, 47%, and 44% for group III. The rates for seniors for 1, 5, and 14 years after the transplant were 76%, 61%, and 30% (Fig. 3) (P = .0001).
Figure 3. Survival according to patient age.
Survival rates for female patients were significantly better than for males, with 1-, 5-, 10-, 15-, and 18-year survival rates of 77%, 64%, 53%, 44%, and 44% for male patients and 80%, 71%, 62%, 56%, and 55% for females (see Fig. 1). These trends were present regardless of the era analyzed (data not shown).
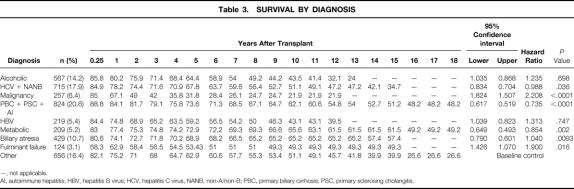
Survival by cause of primary disease is shown in Table 3. The list of diagnoses was condensed into nine categories: alcohol-related liver disease; hepatitis C virus and the older non-A/non-B hepatitis; hepatic malignancies; autoimmune liver diseases, including primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis; hepatitis B virus; metabolic liver diseases; biliary atresia; acute liver failure; and others. Significant differences in survival were noted: patients with biliary atresia, metabolic liver diseases, and autoimmune liver diseases had better survival rates than other patients. There was a significant difference in the survival rate of patients with alcohol-related liver disease in the late posttransplant period (>5 years) versus patients with autoimmune liver diseases (who are approximately age-matched). Similar, although not significant, differences were also seen in the hepatitis C/non-A/non-B recipients after 5 years.
Table 3. SURVIVAL BY DIAGNOSIS
—, not applicable.
AI, autoimmune hepatitis; HBV, hepatitis B virus; HCV, hepatitis C virus, NANB, non-A/non-B; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis.
Causes of Death
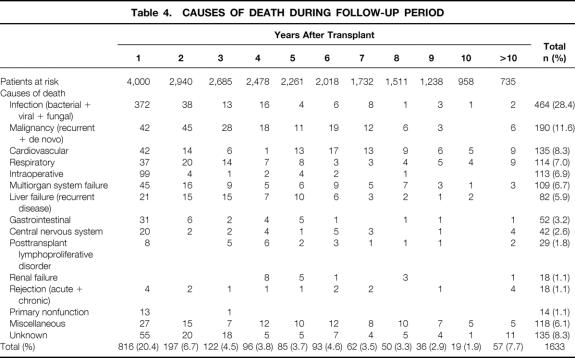
One thousand six hundred thirty-three patients (40.8%) died during the follow-up period. The causes of death at various time intervals from transplant are shown in Table 4. Infection was the most common cause of death at all time points, representing 28.4% of all deaths. This was followed by recurrent or de novo cancers (11.6%) and cardiovascular-related (8.3%) and respiratory-related (7.0%) deaths. Half of all deaths occurred within the first year after the transplant. The death rate after 2 years was 1% to 4% per year (Fig. 4). Death often occurred as a result of age-related complications in groups III and IV (data not shown).
Table 4. CAUSES OF DEATH DURING FOLLOW-UP PERIOD
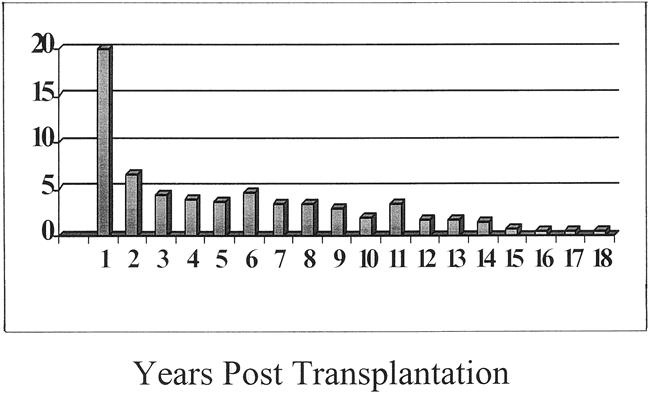
Figure 4. Changes in death rates over time.
Graft Survival
Patient death and retransplantation were considered as graft loss. The overall graft survival rates at 1, 5, 10, 15, and 18 years were 70%, 59%, 49%, 44%, and 41%. Graft survival trends were similar to those for patient survival in terms of era of transplant, gender, and age at time of transplant (Fig. 5).
Figure 5. Graft survival according to (A) era, (B) gender, and (C) age distribution.
Retransplantation
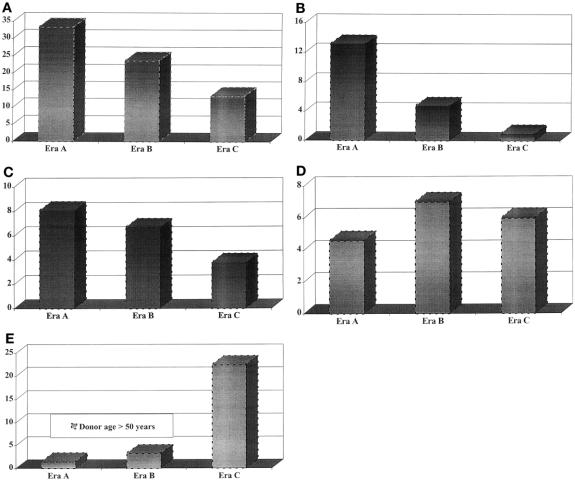
During the follow-up period, 774 patients (19.4%) received two transplants, 148 (3.7%) received three, 20 (0.5%) received four, and 5 (0.13%) received more than four. The rate of retransplantation and the causes of retransplantation in the various eras are shown in Table 5. Overall, the rate of retransplantation declined significantly in each subsequent era, from 33.4% in era A to 23.7% in era B and 13.4% in era C (Fig. 6A) (P = .001). This may in part result from the length of follow-up; however, in all eras, an equal proportion of first retransplants occurred within 30 days after the initial transplant (data not shown). Rejection as a reason for retransplant declined significantly during the past 18 years: 13.2% of patients in era A underwent retransplantation for rejection, and this figure decreased to 4.8% in era B and 1% in era C (see Fig. 6B) (P = .001). The rate of hepatic artery thrombosis fell from 8.1% in era A to 6.7% in era B and 3.8% in era C (see Fig. 6C). Although the rate of primary nonfunction did not appear to change appreciably in eras A, B, and C (see Fig. 6D; this rate was 4.6%, 7.0%, and 6.0%, respectively), the use of donors older than 50 years increased from 1.5% in era A to 3.3% in era B and 22.5% in era C (see Fig. 6E).
Table 5. CAUSES OF RETRANSPLANTATION BY ERA
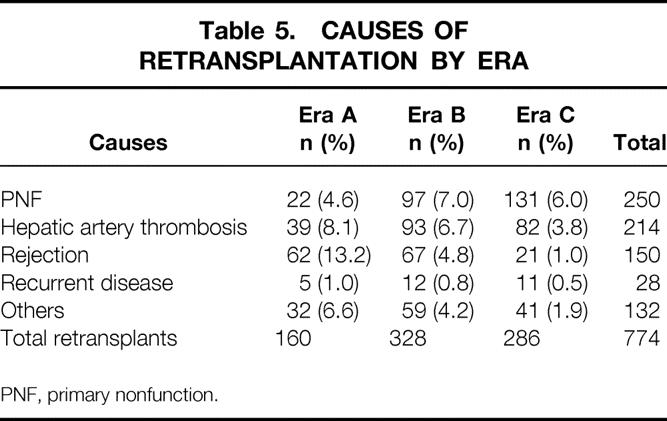
PNF, primary nonfunction.
Figure 6. Changes in retransplantation rates according to (A) era, (B) indication for rejection, (C) indication for hepatic artery thrombosis, and (D) indication for primary nonfunction. Also shown is the increase in the rate of older donor use (E).
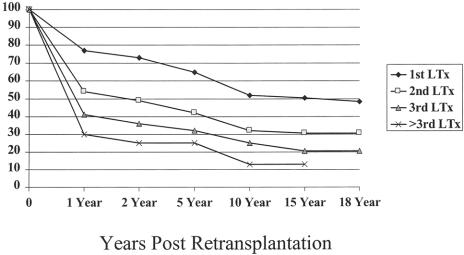
Primary nonfunction and hepatic artery thrombosis were the most common causes of retransplantation in all three eras. Patient survival rates after retransplantation remained considerably poorer compared with primary transplants. Patient survival for those who did not undergo retransplantation was 52% for the first transplant versus 32% for the second, 25% for the third, and 13% for the fourth of more transplant at 10 years (Fig. 7) (P = .001).
Figure 7. Patient survival after one, two, three, and more than three transplants at 1, 2, 5, and 10 years after transplantation.
DISCUSSION
The present study is the largest series of liver transplant patients from a single center with this length of follow-up. Other large series examining survival trends have been reported from the United States, Great Britain, and Canada. 9,11,13–15 This study confirms the results of previous studies, which demonstrate an increased incidence of late graft loss secondary to recurrent diseases, malignancies, and cardiovascular and cerebrovascular accidents, although there are concerning trends in diagnosis-specific survival for patients with alcohol-related liver disease and those with hepatitis C/non-A/non-B. In addition, this study demonstrates the marked impact of the changing face of immunosuppression, with a significant reduction in acute and chronic rejection-associated losses.
With the success of liver transplantation, more patients are being referred for it and more patients older than 60 are considered for transplants. This age group of patients made up the fastest-growing population of transplant patients at our center: the number of patients older than 60 increased by 18-fold from era A to era C. Although they are carefully medically screened before surgery, their long-term survival was significantly less than that of other age groups. After the first year, the major cause of death was from age-related problems, including cardiovascular, respiratory, cerebrovascular, and de novo cancers. 16–18
An increasing demand for liver transplantation has forced many centers to consider the use of older donors. Since 1991, more than 20% of the donors were older than 50 years, traditionally considered as an “expanded” donor and reported to have poorer outcomes. 19,20 This compared with only 1.5% older donors before 1985 and 3% between 1981 and 1990. Despite the use of such donors, the overall survival rate improved significantly from era A to era C. The reasons for these improvements are multiple and include improved diagnostic and therapeutic measures for infectious complications, improved preservation, and the delineation of risk factors that affect outcome. 21,22 However, most notable are the improvements in the immunosuppression regimens. The relatively low number of retransplants or graft failures from acute or chronic rejection may in part be attributed to the newer immunosuppressive agent tacrolimus, which was used for primary therapy in era C and for rescue therapy in era B. 23,24 This finding is compatible with the observation that tacrolimus offers more freedom from rejection and fewer steroid-resistant rejections; the result is that graft loss from rejection has become rare. 25–29
The indications for transplantation in children have not changed appreciably during the past 18 years. However, a changing pattern of indications in adults was apparent. Alcohol-related liver disease is a growing indication and is equaled only by hepatitis C, for which detection became clinically available in 1991. The impact of the various disease processes on long-term outcome is also apparent. In adults, recurrent diseases such as primary hepatic malignancies, steatohepatitis, recurrent alcohol abuse, viral hepatitis, Budd-Chiari syndrome, primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis have been reported and variably affect survival. 30–33 Recurrent disease does not occur to the same extent in children, and this partially accounts for the significantly better long-term survival rate in this population. Even children with the indication of primary hepatic malignancy do better than adults (10-year survival rate of 21% in adults and 50% in children). 34,35
With respect to the specific diagnosis for the transplant, there were notable trends in early versus late survival. This was apparent in patients transplanted for alcohol-related liver disease and hepatitis C/non-A/non-B indications. With alcohol-related liver disease, the overall patient survival rate was not different from other indications, 36 but there was a significantly different pattern of early versus late survival. The early survival rate (0–5 years after the transplant) was better compared with those with other indications, but after 5 years the survival rate was significantly less for those with alcohol-related liver disease. The major cause of patient and graft survival appeared to be nonimmunologic; this did not appear to be related to recidivism. Most late deaths were related to de novo cancer and cardiorespiratory and cerebrovascular events. The risk of death was 2.3 times higher for the alcoholic group of patients beyond 5 years after transplantation.
In patients who underwent transplantation for hepatitis C virus (previously non-A/non-B), the impact of hepatitis C after the transplant is not clear. The almost universal reinfection rate 37 and a high incidence of clinical hepatitis suggests that long-term survival is compromised. Hepatitis C infection after the transplant did not significantly affect the 5-year survival rate in several studies with short follow-up, but it develops into chronic hepatitis in most patients and can develop into cirrhosis in a few patients. 38,39 In this series, the analysis suggests that long-term survival may be reduced by recurrent hepatitis C infection. Although there was a steady decrease in patient and graft survival rates throughout the entire study period, and patient survival was not different up to 5 years, in the period after this there was a disproportionate decrease (although not significant) in survival when compared with other nonviral, nonmalignant indications.
Recently, it was suggested that patients should not undergo liver transplantation if they have a 5-year life expectancy of less than 50% (Dr. John Lake, American Society of Transplantation, personal discussion). Based on the data presented here, patients cannot be excluded based on age, sex, or diagnosis (excluding adult malignancy). Even these criteria are likely to change. Using a multivariate analytic approach, Marsh et al 40 demonstrated that patients with hepatocellular carcinoma without macrovascular invasion and without positive lymph nodes had a more than 50% chance of surviving 5 years after liver transplantation.
Because outcomes after retransplantation do not reach this threshold, one might initially suggest that patients should not undergo retransplantation. However, various analyses have also identified patients who are at higher risk for death after retransplantation, 21,22,41 and this may improve outcomes for these patients also.
CONCLUSIONS
This study demonstrates that liver transplantation continues to mature, with advances in many surgical and medical aspects leading to improved survival rates. An overall patient survival rate of 48% at 18 years supports the conclusion of the NIH Consensus Development Conference of 1983 that liver transplantation is “a therapeutic modality for end-stage liver disease that deserves broader application.” This is particularly true with survival rates in the recent era being significantly better than during earlier periods. With a diminishing rate of retransplantation and lower rates of graft loss from acute or chronic rejection with current immunosuppressive medications, the prospects for improving long-term survival are encouraging. However, recurrence of the original disease and death from age-related problems in the older population will likely limit our ability to achieve maximum survival outcomes.
Acknowledgment
The authors thank Edward Gray for his help in generating the files from the Electronic Database Interface for Transplantation (EDIT) for statistical analysis.
Discussion
Dr. William C. Meyers (Worcester, Massachusetts): This will likely become one of the most quoted papers in transplantation over the next years. The authors have provided us with simple, accurate answers to questions that patients, referring physicians, and others often ask us—the real results of liver transplantation. The authors’ stratifications with respect to survival are straightforward, understandable, and beautifully presented.
The global nature of these statistics is what is important. They take into account all variables, be they the aggressive nature of a donor policy, severity of disease, or types of immunosuppression. It is also particularly appropriate for these numbers to come from our leading liver transplant center for the past two-plus decades.
I have two questions. One is sort of a test of selectivity: Are living-related or other split livers included in these data? Second, how do the authors suggest that we use these data? As gold standards? As middle-of-the-road data? Do they have appropriateness with respect to something like Medicare approval guideline numbers? In other words, what do you see as the limitations of your own data?
Presenter Dr. John J. Fung (Pittsburgh, Pennsylvania): This series does not include our living donor experience. These are cadaveric primary transplants. We have not performed many living donor transplants at Pittsburgh.
I suspect that, like many other series on analysis of long-term outcome, if living donors are to be included, they will need to be stratified in some way due to the differences in severity of patients, of recipient characteristics, as well as differences in preservation and donor quality.
What do I consider as being the principal limitation of the data? As you can imagine, the amount of data that can be presented in such a setting is limited. We have done subset analysis in more detail. For instance, our analysis of patients with malignancy has shown that there are characteristics that are quite favorable, and we have developed models to predict such outcomes.
What can this data be used for? They can be used to influence policies regarding coverage. For example, Medicare continues to deny coverage for patients with hepatocellular carcinoma, even if they have favorable characteristics. Such policymakers need to be educated regarding these advances.
I think the principal limitation of this study is basically that it doesn’t go into details regarding specifics. But I do believe that it does give one an opportunity to look at the evolution of liver transplantation through its entire history.
Dr. Stuart J. Knechtle (Madison, Wisconsin): John, I would like to congratulate you and your colleagues at the University of Pittsburgh for a truly remarkable series of 4,000 liver transplants performed at your center. Since Dr. Starzl and his team, now led by Dr. Fung, established liver transplantation as definitive therapy for end-stage liver disease, your group has pioneered the development of technical aspects of liver transplantation, introduced FK506 as an immunosuppressant, trained many of the liver transplant surgeons in America, and compiled by far the largest series of liver transplants. You are therefore in a unique position to evaluate the long-term outcome of liver transplantation. I have four questions.
First, in view of your observation that infection and cancer account for 40% of deaths long-term, would it not be reasonable to conclude that immunosuppression has been too heavy-handed for optimal long-term management? You and your group have reported a gradual withdrawal of all immunosuppression in a selected series of liver transplant patients with stable graft function. Based on the data you have just shown us, what is your recommendation with regard to long-term management of immunosuppression in liver transplant recipients? How much is just right based on the data you showed?
Secondly, it is clear from your results that compared to the transplanted heart, lung, and kidney, the liver is far less susceptible to chronic rejection, hence the slow rate of graft loss that you showed. Why do you think the liver is relatively less susceptible to chronic rejection?
Thirdly, you identified several recipient groups who have poor long-term outcomes, including patients greater than 60 years of age, patients with alcoholic liver disease, patients with hepatitis B and C, and retransplants. How should these facts influence selection of these particular patient groups for transplantation?
Fourthly, no one knows exactly how long a transplanted donor liver can function. Did you find any adverse effect of transplanting an older donor liver into a very young recipient in terms of long-term function or outcome?
Dr. Fung: Dr. Knechtle, it is an honor for me to have you review the paper also, because the University of Wisconsin has been such a tremendous contributor to the field of liver transplantation and I think has made seminal contributions, one of which has been the use of the UW solution for our preservation. To address your questions:
First issue, reduction of long-term immunosuppression as a way to control the incidence of infection and cancer. As you alluded to, we have been involved in prospective weaning trials. Thus far, we have only prospectively weaned about 100 patients, of which there are now 40 patients who are off immunosuppression. This continues to be a slow process because of the strict requirements to enter that weaning protocol; however, this a trial-and-error study. We need to try to understand what factors are associated with tolerance. The NIH is sponsoring the Immune Tolerance Network, which will focus on assays to predict who will be tolerant. I think larger weaning trials should await this type of effort, either as a way to better predict who can be weaned off immunosuppression or to serve as an adjunct study for scientific purposes. But our policy has been to try to lower the immunosuppression in these patients as quickly as possible. I do believe that the liver has tolerogenic qualities and they do have a lot to do with the chimerism concept that Dr. Starzl has proposed, particularly given the hematopoietic nature of the liver.
Regarding the utility of culling patients at high risk of dying from transplantation, it depends on what society considers as an appropriate threshold. If, as Dr. Jack Lake has proposed, a 50% 5-year survival is the minimum, then patients over the age of 60 can still be transplanted. I think what we need to do as a group of transplant surgeons and physicians is to understand that within “high-risk” groups, such as hepatomas and retransplantation, there are patients who can be transplanted with good outcomes. It is up to us to identify further by these types of analyses what kinds of patients in the high-risk category are going to do well.
Lastly, we have looked at older donor (>50 years) into younger recipients. There is an overall negative impact, but the risk appears to be frontloaded.
Dr. Goran B. Klintmalm (Dallas, Texas): Pittsburgh has not only blazed the path for us technically but also immunologically and not the least in transplant policy. And Dr. Fung, you yourself have taken a tremendous load in this latter feat.
Two of the previous discussants have touched on the question of the groups with poor survivals, the retransplantations. What is now your policy in Pittsburgh? You have never been afraid to take the lead in the past, and I am sure you do that now as well.
Dr. Fung: Thank you for your question about risk factors in retransplants and transplants in elderly patients. We evaluate our elderly physiologically. I think that is probably the only way to do justice to patients in that category. If they are physiologically younger than they appear, then we will transplant them.
From a retransplant standpoint, patients who have reached critical status 2A with renal failure, advanced malnutrition, and other risk factors for which we would not consider primary transplants, would not be retransplanted in the current era.
Dr. Nancy L. Ascher (San Francisco, California): To me the most important information that came out of this analysis was the issue of recurrent disease, in particular those patients with hepatitis B, hepatitis C, and alcoholic liver disease. There have been tremendous strides in treatments that prevent recurrent disease. Can you give the audience some idea of your approach to these patients?
Dr. Fung: Being from San Francisco, you appreciate the impact of viral hepatitis even more than we do with the patients we see in Pittsburgh. Hepatitis C is the fastest-growing indication for liver transplantation in the United States, and we all know that close to 100% of them will become reinfected. With international contributions to understanding how to prevent them, pharmaceutical agents to treat recurrent hepatitis C or prophylaxis hepatitis C, we hope that there will be a way to decrease the long-term graft losses. Having said that, however, we do not see a statistically significant decrease at this point. I still think it is quite a satisfactory survival without worrying too much about recurrent disease.
But alcoholic liver disease was quite surprising in the long-term decrease in survival. It was clear that these patients died primarily of cardiovascular and de novo cancers. When we analyzed the risk factors for them, alcohol and smoking tend to go hand in hand in these patients. I think a more careful follow-up in terms of ear, nose, and throat and digestive follow-up for malignancy is probably one way to either prevent them or catch them at a stage where we can treat them.
Footnotes
Correspondence: John Fung, MD, PhD, 4th Floor Falk Clinic, 3601 Fifth Ave., University of Pittsburgh, Pittsburgh, PA 15213.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail address: fungjf@msx.upmc.edu
Accepted for publication April 2000.
References
- 1.Moore FD, Wheeler HB, Demissianos HV, et al. Experimental whole organ transplantation of the liver and of the spleen. Ann Surg 1960; 152: 374–387. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA Jr, Brock DR, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet 1960; 111: 733–743. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TM, Marchioro TL, Von Kaulia KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet 1963; 117: 659–676. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology 1982; 2: 614–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolles K, Williams R, Neuberger J, Calne R. The Cambridge and King’s College Hospital experience of liver transplantation, 1968– 1983. Hepatology 1984; 4 (1suppl):50S–55S. [DOI] [PubMed] [Google Scholar]
- 6.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979; 2: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 7.Starzl TE, Klintmalm GBG, Porter KA, et al. Liver transplantation with the use of cyclosporin A and prednisone. N Engl J Med 1981; 305: 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon RD, Todo S, Tzakis AG, et al. Liver transplantation under cyclosporine: a decade of experience. Transplant Proc 1991; 23: 1393–1396. [PMC free article] [PubMed] [Google Scholar]
- 9.Abbasoglu O, Levy MF, Brkic BB, et al. Ten years of liver transplantation: an evolving understanding of late graft loss. Transplantation 1997; 64: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 10.Asfar S, Metrakos P, Fryer J, et al. An analysis of late deaths after liver transplantation. Transplantation 1996; 61: 1377–1388. [DOI] [PubMed] [Google Scholar]
- 11.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg 1998; 228: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seaberg EC, Belle SH, Beringer KC, et al. Liver transplantation in the United States from 1987–1998: updated results from the Pitt-UNOS Liver Transplant Registry. Clin Transplant 1998: 17–37. [PubMed] [Google Scholar]
- 13.Amersi F, Farmer DG, Busuttil RW. Fifteen-year experience with adult and pediatric liver transplantation at the University of California, Los Angeles. Clin Transplant 1998: 255–261. [PubMed] [Google Scholar]
- 14.Mirza DF, Gunson BK, McMaster P. Liver transplantation in Birmingham: indications, results, and changes. Clin Transplant 1996: 217–221. [PubMed] [Google Scholar]
- 15.Hemming AW, Cattral MS, Greig PD, et al. The University of Toronto liver transplant program. Clin Transplant 1996: 177–185. [PubMed] [Google Scholar]
- 16.Zetterman RK, Belle SH, Hoofnagle JH, et al. Age and liver transplantation: a report of the Liver Transplantation Database. Transplantation 1998; 66: 500–506. [DOI] [PubMed] [Google Scholar]
- 17.Jain AB, Yee LD, Nalesnik MA, et al. Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using surveillance epidemiologic end result data. Transplantation 1998; 66: 1193–1200. [DOI] [PubMed] [Google Scholar]
- 18.Jain A, Reyes J, Kashyap R, et al. What have we learned about primary liver transplantation under tacrolimus immunosuppression? Long-term follow-up of the first 1000 patients. Ann Surg 1999; 230: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Detre KM, Lombardero M, Belle S, et al. Influence of donor age on graft survival after liver transplantation—United Network for Organ Sharing Registry. Liver Transplant Surg 1995; 1: 311–319. [DOI] [PubMed] [Google Scholar]
- 20.Marino IR, Doyle HR, Erighetti L, et al. Effect of donor age and sex on the outcome of liver transplantation. Hepatology 1995; 22 (6): 1754–1762. [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle HR, Marino IR, Jabbour N, et al. Early death or retransplantation in adults after orthotopic liver transplantation. Can outcome be predicted? Transplantation 1994; 57 (7): 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Facciuto M, Heidt D, Guarerra J, et al. Retransplantation for late liver graft failure: predictors of mortality. Liver Transplant 2000; 6: 174–179. [DOI] [PubMed] [Google Scholar]
- 23.Todo S, Fung JJ, Starzl TE, et al. Single-center experience with primary orthotopic liver transplantation with FK506 immunosuppression. Ann Surg 1994; 220: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fung JJ, Todo S, Jain A, et al. Conversion from cyclosporine to FK506 in liver allograft recipients with cyclosporine-related complications. Transplant Proc 1990; 22: 6–12. [PMC free article] [PubMed] [Google Scholar]
- 25.U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med 1994; 331: 1110–1115. [DOI] [PubMed] [Google Scholar]
- 26.European FK506 Multicentre Liver Study Group. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet 1994; 344: 423–428. [PubMed] [Google Scholar]
- 27.Fung JJ, Eliasziw M, Todo S, et al. The Pittsburgh randomized trial of tacrolimus compared to cyclosporine for hepatic transplantation. J Amer Coll Surg 1996; 183 (2): 117–125. [PMC free article] [PubMed] [Google Scholar]
- 28.Ludwig J, Hashimoto E, Porayko MK, et al. Failed allografts and causes of death after orthotopic liver transplantation from 1985 to 1995: decreasing prevalence of irreversible hepatic allograft rejection. Liver Transpl Surg 1996; 2: 185–191. [DOI] [PubMed] [Google Scholar]
- 29.Jain AB, Fung JJ, Todo S, et al. Incidence and treatment of rejection episodes in primary orthotopic liver transplantation under FK506. Transplant Proc 1991; 23: 928–930. [PMC free article] [PubMed] [Google Scholar]
- 30.Davern TJ, Lake JR. Recurrent disease after liver transplantation. Semin Gastrointest Dis 1998; 9: 86–109. [PubMed] [Google Scholar]
- 31.Neuberger J. Recurrence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis. Liver Transpl Surg 1995; 1 (Suppl): 109–115. [PubMed] [Google Scholar]
- 32.Samuel D, Feray C, Bismuth H. Hepatitis viruses and liver transplantation. J Gastroenterol Hepatol 1997; 12: S335–S341. [DOI] [PubMed] [Google Scholar]
- 33.Hart J, Busuttil RW, Lewin KJ. Disease recurrence following liver transplantation. Am J Surg Pathol 1990; 14 (Suppl): 79–91. [PubMed] [Google Scholar]
- 34.Laine J, Jalanko H, Saarinen-Pihkala UM, et al. Successful liver transplantation after induction chemotherapy in children with inoperable, multifocal primary hepatic malignancy. Transplantation 1999; 67: 1369–1372. [DOI] [PubMed] [Google Scholar]
- 35.Gerber DA, Arcement C, Carr B, et al. Use of intrahepatic chemotherapy to treat advanced pediatric hepatic malignancies. J Pediatr Gastroenterol Nutr 2000; 30: 137–144. [DOI] [PubMed] [Google Scholar]
- 36.Jain A, DiMartini A, Kashyap R, et al. Long-term follow-up after liver transplantation for alcoholic liver disease under tacrolimus. Transplantation (in press). [DOI] [PubMed]
- 37.Mateo R, Demetris AJ, Sico E, et al. Early detection of de novo hepatitis C infection in patients after liver transplantation by reverse transcriptase polymerase chain reaction. Surgery 1993; 114: 442–448. [PubMed] [Google Scholar]
- 38.Boker KH, Dalley G, Bahr MJ, et al. Long-term outcome of hepatitis C virus infection after liver transplantation. Hepatology 1997; 25: 203–210. [DOI] [PubMed] [Google Scholar]
- 39.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med 1996; 334: 815–820. [DOI] [PubMed] [Google Scholar]
- 40.Marsh JW, Dvorchik I, Bonham CA, et al. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer 2000; 88: 538–543. [DOI] [PubMed] [Google Scholar]
- 41.Markmann JF, Markowitz JS, Yersiz H, et al. Long-term survival after retransplantation of the liver. Ann Surg 1997; 226: 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]