Abstract
Objective
To evaluate of the impact of endovascular aneurysm repair on the rate of open surgical repair and on the overall treatment of abdominal aortic aneurysms (AAAs).
Methods
All patients with AAA who were treated during two consecutive 40-month periods were reviewed. During the first period, only open surgical repair was performed; during the subsequent 40 months, endovascular repair and open surgical repair were treatment options.
Results
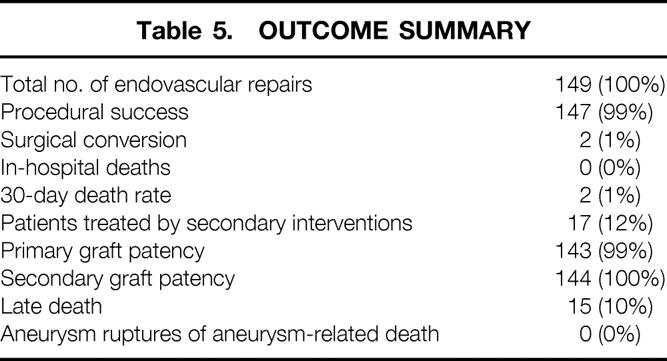
A total of 727 patients with AAA were treated during the entire period. During the initial 40 months, 268 patients were treated with open surgical repair, including 216 infrarenal (81%), 43 complex (16%), and 9 ruptured (3%) aortic aneurysms. During the subsequent 40 months, 459 patients with AAA were treated (71% increase). There was no significant change in the number of patients undergoing open surgical repair and no significant difference in the rate of infrarenal (238 [77%]) and complex (51 [16%]) repairs. A total of 353 patients were referred for endovascular repair. Of these, 190 (54%) were considered candidates for endovascular repair based on computed tomography or arteriographic morphologic criteria. Analyzing a subgroup of 123 patients, the most common primary reasons for ineligibility for endovascular repair were related to morphology of the neck in 80 patients (65%) and of the iliac arteries in 35 patients (28%). A total of 149 patients underwent endovascular repair. Of these, the procedure was successful in 147 (99%), and 2 (1%) patients underwent surgical conversion. The hospital death rate was 0%, and the 30-day death rate was 1%. During a follow-up period of 1 to 39 months (mean 12 ± 9), 21 secondary procedures to treat endoleak (20) or to maintain graft limb patency (1) were performed in 17 patients (11%). There were no aneurysm ruptures or aneurysm-related deaths.
Conclusions
Endovascular repair appears to have augmented treatment options rather than replaced open surgical repair for patients with AAA. Patients who previously were not candidates for repair because of medical comorbidity may now be safely treated with endovascular repair.
Endovascular repair of abdominal aortic aneurysms (AAAs) has been shown in several studies 1–3 to be associated with a death rate comparable to that of open surgical repair but with a lower rate of complications in patients with equivalent or more severe comorbidity (Fig. 1). Consequently, endovascular repair is now extended to many patients who were not considered to be candidates for aneurysm repair in the past. The number of patients with AAA who have been considered unfit for open surgical repair because of severe medical comorbidity has never been defined. Many patients have not been referred by their physicians for evaluation by a surgeon when the perceived perioperative risk of aneurysm repair outweighed the assumed risk of aneurysm rupture. 4 Many such patients have been eventually included in reports of ruptured AAA, in which the rate of patients with previously diagnosed AAA is 12% to 32%. 5 The availability of endovascular repair has made it possible to offer treatment to many of these patients previously thought to be unfit for treatment.
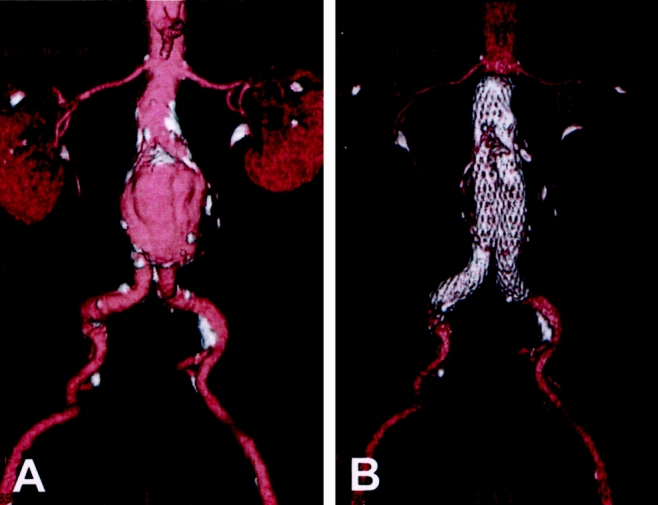
Figure 1. Three-dimensional reconstruction of helical computed tomographic angiography. (A) Preoperative image demonstrating a 6.3-cm infrarenal aneurysm. (B) Postoperative image showing the bifurcated stent-graft in place. Calcifications in the aneurysm wall remain evident.
To evaluate the impact of endovascular repair on the rate of open surgical repair and on the overall treatment of AAA, we reviewed our experience with AAA repair before and after initiation of an endovascular aneurysm repair program at Stanford.
METHODS
All patients who underwent AAA repair at Stanford University Hospital from July 1, 1993, to February 28, 2000, were reviewed. This period consisted of two consecutive 40-month periods. During the first period (July 1, 1993, to October 31, 1996), only open surgical repair of aneurysms was performed. During the subsequent 40-month period (November 1, 1996, to February 28, 2000), endovascular repair was introduced as a treatment option for patients with AAA. Endovascular repair was initially available as part of the AneuRx clinical trial (phase I, II, and III). 1 All aneurysm repairs at Stanford were performed by one of ten vascular surgeons, including full-time faculty and community vascular surgeons. Six of these surgeons participated in the endovascular program and performed open and endovascular aneurysm repair; four surgeons performed only open aneurysm repair. For review of open surgical repair, the surgical registry was reviewed for a diagnosis of “repair of abdominal aortic aneurysm.” Uncomplicated infrarenal aneurysms were classified as such. Juxtarenal, suprarenal, thoracoabdominal, infected aneurysms and those associated with renal or visceral arterial repair, were classified as complex. Ruptured AAAs were counted separately.
Patients referred for possible endovascular aneurysm repair were evaluated with spiral computed tomographic (CT) angiography, magnetic resonance angiography, or contrast angiography and reviewed by a panel of four to six vascular surgeons and two vascular radiologists for considerations related to treatment with the AneuRx stent-graft (Medtronic AVE, Santa Rosa, CA). The clinical data as well as the vascular morphology were reviewed and consensus was reached as to the appropriateness of endovascular repair. Decisions were primarily based on the morphologic features demonstrated on helical CT angiograms, except in patients with severe renal failure, when magnetic resonance angiograms were substituted. Occasionally, further tests, most commonly a high-quality helical CT angiogram or a conventional arteriogram, were requested. The criteria for endovascular repair were based on morphologic requirements for the AneuRx stent-graft: a proximal aortic neck 18 to 26 mm in diameter and at least 10 mm long and an upper limit of 15 mm for the common iliac artery diameter, without excessive tortuosity. Hypogastric artery aneurysms were acceptable and were occluded before endovascular repair. Tortuosity was evaluated in a qualitative fashion. Patients who were considered candidates were prepared for endovascular repair.
A prospective morphologic analysis was performed on a subgroup of patients to characterize the grounds for ineligibility. Patients who were considered unsuitable and were acceptable surgical risks were referred for open repair. Many of these patients returned to their referring institutions and were subsequently followed up and treated there.
After endovascular repair, the hospital course, complications, reinterventions, and survival were recorded prospectively. An analysis comparing comorbidity and postoperative hospital course in the open surgical group and the endovascular group was carried out on a subset of morphologically matched patients. Follow-up after endovascular repair was conducted by serial imaging with CT angiography and duplex ultrasonography within the first month, after 6 and 12 months, and annually thereafter. After each of these examinations, the patients were seen in the outpatient clinic.
Data were analyzed using the Student t test and chi-square test. P < .05 was considered significant.
RESULTS
During a period of 80 months, 727 patients with AAA were treated at Stanford University Hospital. During the first half of this period, 268 patients underwent open repair. Initiation of the endovascular program during the second half of this period increased the total number of patients with AAA treated at Stanford by 71%. The number of open surgical repairs increased insignificantly and the ratio of infrarenal to complex aneurysm repairs remained unaffected (P = .17) (Table 1).
Table 1. ABDOMINAL ANEURYSM REPAIR DURING 2 CONSECUTIVE 40-MONTH PERIODS*
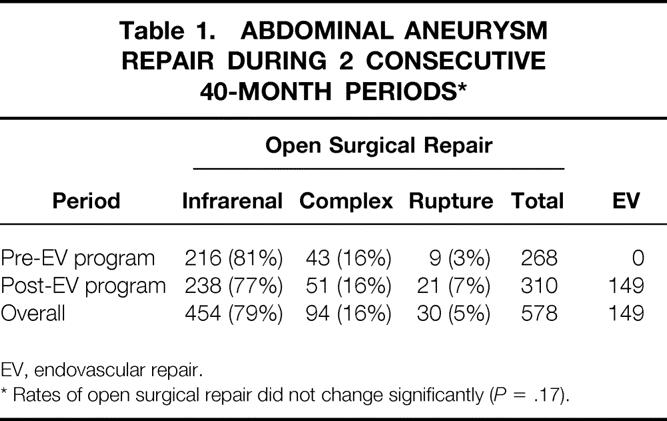
EV, endovascular repair.
* Rates of open surgical repair did not change significantly (P = .17).
Of the 353 patients with AAA referred to Stanford for endovascular repair, 342 (97%) completed the evaluation and 190 (54%) were considered suitable for endovascular repair. The eligibility rate increased significantly from 44% initially to 62% during the second half of the period in which endovascular repair was available (P < .001) (Table 2). After evaluation, 152 patients were found to be morphologically unsuitable for endovascular repair (Fig. 2). In the subset of 123 patients, the grounds for ineligibility were analyzed. The most common primary reason for ineligibility was related to the aneurysm neck, followed by morphology of the common iliac artery (Table 3). Of these, 22 (18%) patients had more than one feature contraindicating endovascular repair.
Table 2. ELIGIBILITY FOR ENDOVASCULAR REPAIR WITH A BIFURCATED AORTIC STENT-GRAFT*
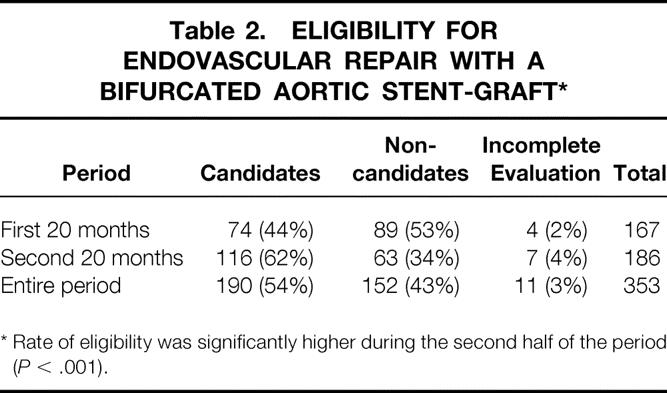
* Rate of eligibility was significantly higher during the second half of the period (P < .001).
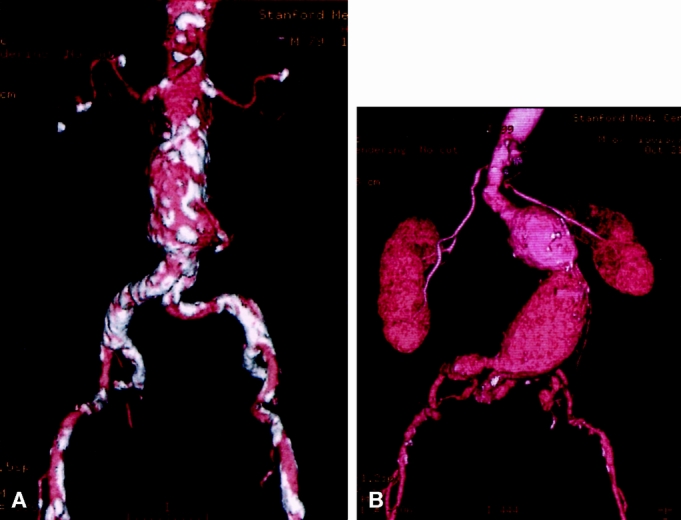
Figure 2. Patients with aneurysm morphology that poses a problem for endovascular repair. (A) Absence of an adequate neck. The aorta is dilated at the level of the renal arteries. (B) Generalized tortuosity with hairpin configuration of the left common iliac artery.
Table 3. PRIMARY REASON FOR INELIGIBILITY FOR ENDOVASCULAR REPAIR*
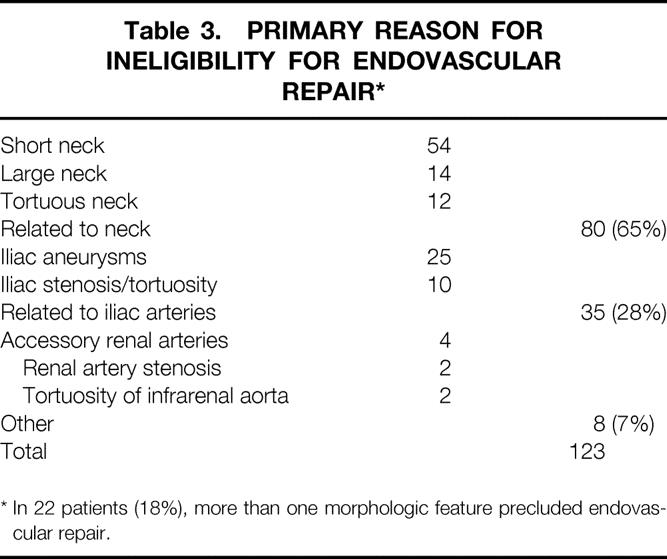
* In 22 patients (18%), more than one morphologic feature precluded endovascular repair.
A total of 149 patients underwent endovascular repair of AAA at Stanford University Hospital. The mean aneurysm diameter in this group was 5.8 ± 9 cm. Of these, 147 (98.7%) procedures were successful and 2 (1.3%) patients underwent uncomplicated conversion to open surgical repair. One patient had an excessively tortuous neck, and the other had small iliac arteries, precluding insertion of the device.
There were no hospital deaths, and two patients (1.3%) died within 30 days. These patients were 45 and 74 years old and had acute myocardial infarction 3 and 4 weeks after an uneventful endovascular repair. Postoperative complications included major complications in 15 (10%) and minor complications in 13 (9%) patients. Major complications included myocardial infarction (4), congestive heart failure (1), early major endoleak (3), femoral pseudoaneurysm, thrombosis, or nerve entrapment (4), colon ischemia treated without surgery (1), transient renal failure (1), and a hemispheric transient ischemic attack (1).
Analysis of patients who met the morphologic criteria for endovascular repair (suitable neck and iliac arteries) on the basis of retrospective review of CT scans was carried out in a subset of 149 morphologically matched patients. Among those, 70 patients had open surgical repair and 79 underwent endovascular repair. The endovascular group was characterized by more medical comorbidities (P < .05) and a higher prevalence of diabetes (P < .05), chronic obstructive pulmonary disease (P < .05), and cerebrovascular disease (P < .05). There was no difference in the 30-day death or complications rate between the open surgery group and the endovascular repair group. However, the severity of complications in the surgical group was considerably greater and was associated with a significantly longer hospital stay compared with the endovascular group (P < .05). There was less blood loss (P < .05), a shorter intensive care unit stay (P < .05), a shorter hospital stay (P < .001), and an earlier return to function (P < .001) in the endovascular group.
After endovascular repair, the endoleak rate at discharge was 36%. It decreased after 1 month to 18% and stabilized thereafter. The follow-up rate was 100% and extended for 12.0 (range 1–39, median 11.5) months. There were 21 secondary procedures in 17 (11%) patients, after a mean period of 13.4 ± 11.3 (median 12.0) months (Table 4). Twenty procedures were performed for treatment of endoleak and one procedure was performed for graft limb occlusion. The primary patency rate was 99% and the secondary graft patency rate was 100%. There were no aneurysm ruptures and no aneurysm-related deaths. Late deaths occurred in 15 (10%) patients after a median of 13.3 (range 4–32) months; death was primarily related to underlying cardiac disease (Table 5).
Table 4. SECONDARY PROCEDURES IN 149 PATIENTS*
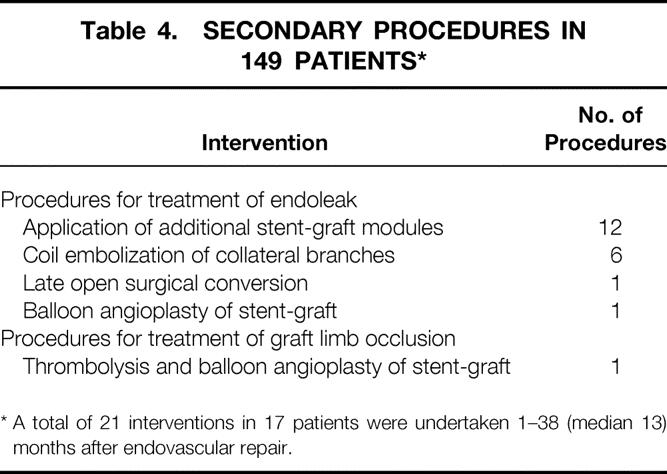
* A total of 21 interventions in 17 patients were undertaken 1–38 (median 13) months after endovascular repair.
Table 5. OUTCOME SUMMARY
DISCUSSION
The AneuRx stent-graft is a modular bifurcated stent-graft made of a woven polyester fabric graft with an exoskeleton of self-expanding nitinol stents. The stent-graft was evaluated in a multicenter clinical trial comparing endovascular repair with standard open surgical repair. 1 The clinical trial demonstrated that patients treated with endovascular devices had a significant reduction in complications, a shorter hospital stay, and an earlier return to function than patients undergoing open surgical repair.
During this study, the number of patients with AAA treated at Stanford University Hospital increased substantially. This appeared to be due primarily to the introduction of the endovascular program. The number and type of open surgical repairs did not change significantly, and the entire volume of endovascular repair represented additional activity. This may reflect increased physician and patient acceptance and referral of patients who previously had not been considered suitable for aneurysm repair.
The greater part of the endovascular experience reported in this study occurred within the framework of the multicenter AneuRx trial. The AneuRx stent-graft was approved by the FDA on September 28, 1999, and no change has been observed since then. However, the wide availability of stent-grafts may change referral patterns; whether such changes will occur remains to be seen.
Endovascular repair has thus far proven to be effective in reducing the aneurysm rupture rate. Aneurysms measuring 5.5 cm in diameter are expected to rupture at an annual rate of 11%. 6 The cohort of patients reported here who underwent endovascular repair had a larger mean aneurysm diameter, and no rupture occurred during 1 year of follow-up. Secondary interventions were performed on 11% of the patients. This rate is probably higher than the rate of reintervention after open surgical repair, but patients after open surgical repair are not routinely followed up with serial imaging studies, and follow-up has generally been less rigorous.
Endovascular aneurysm repair is safe and effective for the treatment of AAA. Rather than replacing open surgical repair, it appears to have augmented the therapeutic options and has made it possible to treat additional segments of the patient population.
Discussion
Dr. Gregorio A. Sicard (St. Louis, Missouri): Since the initial report in 1991 of a successful intraluminal repair of renal abdominal aortic aneurysm by Juan Parodi, this minimally invasive method has gained popularity. In September 1999, a single unit and a modular device, as used by the Stanford group, achieved FDA approval and market release. This important contribution by Dr. Zarins and his colleagues represents a large experience with the first modular device in the U.S. market. These single-center early results are outstanding and will serve as a benchmark for other aortic intraluminal devices that are undergoing FDA trials.
Only two of their 147 devices implanted required conversion, for a 99% technical success—a 1.3% 30-day hospital mortality, and with an average 12-month follow-up, no infections or aneurysm ruptures. Over a 40-month period since their aortic intraluminal program began, Dr. Zarins reports a 71% increase in patients treated for abdominal aortic aneurysms in their institution.
At Washington University School of Medicine–Barnes-Jewish Hospital, we started a similar program in March 1996 and have treated 515 patients with nonruptured aneurysms, of which 42% (220 patients), have undergone the intraluminal technique. Over this 4-year period, we also have seen almost a three-fold increase in patients referred to our institution for aneurysm repair.
Access to multiple devices over the last 12 months, more specifically since the FDA release of new devices, has significantly impacted our approach toward elective treatment of abdominal aortic aneurysms, similar to the Stanford experience. In the first 3 years of our program, only 20% of patients were treated intraluminally, while in the last 12 months, 70% have been treated by this minimally invasive method. I have three specific questions.
Have you observed an increase in patients treated at your institution with the intraluminal grafts over the last 6 months, as the inclusion criteria have been liberalized by the market release of these devices? Could this be a problem as far as results for other centers with less experience than a mature center like yours?
Second, changes in aortic sac morphology associated with aneurysm shrinkage have been well documented. Recent reports of late ruptures from component separation and/or stent-graft migration have raised serious concerns about the long-term durability of this technique. Could you comment on surveillance strategies, and specifically the timing of conversion or adjunctive procedures, to avoid this potentially fatal complication?
Lastly, these devices are very expensive. With competition, we hope that the cost will decrease. Have you carried out a comparative cost analysis between the open and intraluminally treated groups? If you have, what is the impact on the cost of the long-term surveillance testing as well as the need for adjunctive procedures?
Presenter Dr. Christopher K. Zarins (Stanford, California): We have not seen a change in the last 6 months since the device has received market approval. In fact, we may in the future see a decrease as other hospitals in our region start adopting this technology. Patients who were referred to us for endovascular repair from surrounding hospitals are now going to be done locally. As a university referral center, we have traditionally been referred the more difficult and more complex patients for open surgical repairs, while the straightforward aneurysms have been done in local community hospitals.
I think that this same pattern will also develop with the endovascular technique. I think it will rapidly become widely available in most hospitals and you will see that simple, straightforward aneurysms will be done at local hospitals, and you may see that the numbers of patients referred to you may decrease in the future.
Regarding the changes in morphology and the question of late ruptures, clearly this is a very important issue. The experience that we have is a 31/2-year follow-up, and the long-term outcome is not known. In the overall experience with about 1,100 patients in the national multicenter trial, there have been seven late ruptures. Three of these were recently reported and published in the Journal of Vascular Surgery in the March 2000 issue (2000; 31:599–606). In an analysis of these seven patients, two had a known endoleak and were advised to have extender cuffs to seal the endoleaks. They refused recommended treatment; one ruptured 15 months later, the other ruptured 24 months later. The five remaining patients did not have endoleaks. A retrospective analysis of each of these patients showed that each was potentially preventable. By that I mean that a review of the imaging data showed poor device placement or fixation. An extender cuff to correct low placement in the infrarenal neck or a distal extender cuff would have prevented a rupture.
I think that this outlines the importance of continuing, ongoing surveillance of patients with endovascular repair. This is not a technology that one can simply implant and forget. One has to monitor and follow the patients.
Regarding the cost question, this is an important issue. We have looked at the costs of endovascular repair at Stanford and found the average costs are no different from open repair. There is no question that the endovascular device is more expensive than the standard graft we use to treat patients with open surgery. Nonetheless, the hospital length of stay and ICU time is reduced with endovascular repair. But the real difference in the cost lies in the complications. There is a tremendous cost to complications of open repair. For example, if you have a complication with an endovascular repair, you may have a groin issue to deal with, which usually doesn’t cost a lot. If you have open surgery, the magnitude of the complication will result in intensive care, reoperation, a hospital stay of 22 to 30 days. A large part of the overall cost of open surgery resides in the high cost of those patients who have complications. So I think in balance, at least in our own experience, the costs are equivalent.
Dr. Robert W. Hobson II (Newark, New Jersey): Clearly this is a procedure that is going to become a part of the armamentarium of all vascular surgeons. Based on our work with current FDA-approved prostheses and other endovascular procedures, I have three questions to ask Dr. Zarins.
You conclude in your well-written manuscript’s discussion that an endovascular program increases the number of aneurysm cases without diminishing the number of open repairs. However, as innovation becomes routine and referral of straightforward cases decreases, won’t the number of open repairs inevitably decrease? Also, will you begin to expand the treatment of smaller aortic aneurysms with endovascular devices?
Secondly, the number of complex aneurysm repairs, including thoracoabdominal repairs, remains constant at about 16% of your open cases during each of the 40-month periods. Can you speculate about the future of endovascular repair of these complex cases? Are current techniques available for their repair? And what success has been achieved?
Finally, can you comment on the impact of endovascular programs on the training of general and vascular surgeons? A recent report from the American Board of Surgery (ABS) has documented the low volume of aortic surgical cases performed by ABS diplomates recertifying at 10 and 20 years after residency training. As endovascular repair becomes increasingly available across the country, is it reasonable for general and vascular surgeons to repair abdominal aortic aneurysms by open surgical technique without being competent in the performance of endovascular repairs? Will sufficient case material be available for open and endovascular surgical repairs in our training programs?
Dr. Zarins: Your first question regarding whether we will be seeing a decrease in the number of patients undergoing open surgical repair, I don’t think so. We haven’t seen that to date.
The reason I say that is that although many patients will be treated with endovascular techniques, the total number of aneurysms treated will increase. The population is aging. There will be more focus on aneurysms, and doctors will be finding many more aneurysms. Patients who never were considered for repair now may be evaluated and considered for repair. Many of them will be treated with endovascular techniques. Those who are not will wind up needing surgery or being referred for potential surgery. So I think that as the population ages and as the interest in treating aneurysms increases—as I think it will—we will see an increase in both endovascular and open surgical repair.
Regarding whether smaller aneurysms will be repaired, that is an unknown. My guess is that there will be a great interest in this technique among cardiologists, and cardiologists may tend to be more aggressive than surgeons. Perhaps they may be treating 3- to 3.5-cm aneurysms, I don’t know.
What about thoracoabdominal and branch vessel disease? That is an excellent question because there is intensive research right now on mechanisms to treat aneurysms with new technology that will allow attaching branches. So I wouldn’t be surprised if in years to come we will be able to treat thoracoabdominal aneurysms with extensions into the celiac, superior mesenteric, and renal arteries. Currently this is in the developmental phase. Perhaps it will become a reality.
What about training of general and vascular surgeons? This is an important and difficult question. If many of the easy aneurysms are done at community hospitals with stent-grafts, how many will come to the training centers? If there are fewer simple aneurysms and all we see are the complex suprarenal and thoracoabdominal aneurysms, will these be appropriate for general surgery residents?
How will we maintain surgical skills if vascular surgeons all start doing endovascular repairs? Will we remember how to do open surgical repair? We may find that the number of surgeons qualified to do open aneurysm surgery may become an issue in the future. This is something that we need to think about and consider for the future.
Dr. Victor M. Bernhard (Palisade, Colorado): My one area of concern is your incidence of leaks. Your endoleak is about 35%. However, that is quite similar to that which the Guidant/Ancure device has been able to demonstrate. And I was wondering if you want to differentiate between the types of leak and the implications of type 1 or attachment side leaks from the type 2 or the branch leaks in terms of the potential for rupture.
Dr. Zarins: That is an important question. Clearly if there is a type I attachment site endoleak, this needs to be addressed. Also, if there is inadequate attachment of the device, even without an endoleak, this needs definite treatment.
The question about what to do about type II endoleaks with retrograde flow through lumbar arteries or the inferior mesenteric artery is unclear. We have not seen clinical problems from such endoleaks but we are watching and monitoring and very carefully following these patients.
Some people think that the presence or absence of endoleak is primary evidence of the success or failure of endovascular aneurysm repair. Some aggressively embolize side branches to correct endoleaks. However, in our experience endoleaks are poor predictors of future outcome events and do not seem to be good indicators of future adverse events.
Dr. G. Melville Williams (Baltimore, Maryland): I have two questions. I will give you my answers, then I am interested in yours.
Is the stent-graft repair of abdominal aortic aneurysms the vascular surgeon’s equivalent to the lap-chole in general surgery? My answer is no, the reason being that you have added something you don’t know what is going to happen to long-term. So it is quite a different kettle of fish. To the patient it may be the same, until they are out 2 years and are then informed they have to keep coming back.
Second, if you yourself had a 6-cm abdominal aortic aneurysm with ideal anatomy for a stent-graft, would you opt for this or standard surgical repair? My answer to this, being in good health and hoping to live another 15 years, would be, I would want the standard operation because I know the long-term outcome.
Dr. Zarins: Regarding the first question as to whether endovascular repair is analogous to lap-chole, I would say yes and no. Yes, because it requires a new technology, a new way to think about it, indirect visualization, a new conceptual approach which dramatically changes the way you think about aneurysms. But I would say no because it also requires surgical skills. And it requires perhaps a lot of consideration that goes in conjunction with that—access through the femoral arteries, repair of the femoral arteries, dealing with branches such as the internal iliac, perhaps transposing the internal iliac, understanding the physiology of the various branches.
So it is not simply a technique of putting in a device; you have to know about aneurysms. I think that is why the whole area of endovascular aneurysm repair needs to stay in the purview and in the field of vascular surgery and not go into an interventional specialty that some people are considering, such as interventional radiology or cardiology. It requires being a surgeon and knowing about aneurysms. In that sense, I think that clearly the surgical component in the open surgical component is a very important one.
Regarding your question about what I would do if I had a 6-cm aneurysm, I would have an endovascular repair. The decision is a pretty easy one for me because, although we don’t always talk about it, many patients have sexual dysfunction following open surgical repair. We don’t talk about it as much as we should with our patients. And you can say to your younger patients, “You must have an open repair because you are going to live to be 90, and now you are 55 or 60,” but what is he going to do between 55 and 90? So let’s say even at age 70 you need to then have an open surgical repair—well, maybe then you don’t care so much. In any case, I think that the data at this point, at least to me personally, is strong enough that I would go for endovascular repair.
Footnotes
Correspondence: Christopher K. Zarins, MD, Division of Vascular Surgery, Stanford University Hospital, Stanford, CA 94305-5642.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail: zarins@stanford.edu
Accepted for publication April 2000.
References
- 1.Zarins CK, White RA, Schwarten D, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg 1999; 29: 292–305. [DOI] [PubMed] [Google Scholar]
- 2.Brewster DC, Geller SC, Kaufman JA, et al. Initial experience with endovascular aneurysm repair: comparison of early results with outcome of conventional open repair. J Vasc Surg 1998; 27: 992–1003. [DOI] [PubMed] [Google Scholar]
- 3.Moore WS, Kashyap VS, Vescera CL, Quiñones-Baldrich WJ. Abdominal aortic aneurysm: a 6-year comparison of endovascular versus transabdominal repair. Ann Surg 1999; 230: 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederle FA. Risk of rupture of large abdominal aortic aneurysms. Disagreement among vascular surgeons. Arch Intern Med 1996; 156: 1007–1009. [PubMed] [Google Scholar]
- 5.Piotrowski JJ, Akhrass R, Alexander JJ, Yuhas JP, Brandt CP. Rupture of known abdominal aortic aneurysms: an ethical dilemma. Am Surg 1995; 61: 556–559. [PubMed] [Google Scholar]
- 6.Finlayson SR, Birkmeyer JD, Fillinger MF, Cronenwett JL. Should endovascular surgery lower the threshold for repair of abdominal aortic aneurysms? J Vasc Surg 1999; 29: 973–985. [DOI] [PubMed] [Google Scholar]


