Abstract
Objective
To review more than a decade of experience with complete repair of tetralogy of Fallot (TOF) in neonates at the University of Michigan; to assess early and late survival, perioperative complications, and the incidence of reoperation; and to analyze patient, procedural, and morphologic risk factors to determine their effects on outcome.
Summary Background Data
Palliation of TOF with systemic-to-pulmonary artery shunts has been the accepted standard for symptomatic neonates and infants. Complete repair has traditionally been reserved for infants older than 6 months of age because of the perception that younger and smaller infants face an unacceptably high surgical risk.
Results
A retrospective review from August 1988 to November 1999 consisted of 61 consecutive symptomatic neonates with TOF who underwent complete repair. Thirty-one patients had TOF with pulmonary stenosis, 24 had TOF with pulmonary atresia, and 6 had TOF with nonconfluent pulmonary arteries. The mean age at repair was 16 ± 13 days, and the mean weight was 3.2 ± 0.7 kg. Before surgery, 36 patients were receiving an infusion of prostaglandin, 26 were mechanically ventilated, and 11 required inotropic support. Right ventricular outflow tract obstruction was managed with a transannular patch in 49 patients and a right ventricle-to-pulmonary artery conduit in 12. Cardiopulmonary bypass time averaged 71 ± 26 minutes. Hypothermic circulatory arrest was used in 52 patients (mean 38 ± 12 minutes). After cardiopulmonary bypass, the average intraoperative right/left ventricular pressure ratio was 55% ± 13%. There were no new clinically apparent neurologic sequelae after repair. The postoperative intensive care unit stay was 9.1 ± 8 days, with 6.8 ± 7 days of mechanical ventilation. There was one hospital death from postoperative necrotizing enterocolitis on postoperative day 71 and four late deaths, only one of which was cardiac-related. Actuarial survival was 93% at 5 years. Follow-up was available for all 60 hospital survivors and averaged 62 months (range 1–141 months). Twenty-two patients required a total of 24 reoperations at an average interval of 26 months after repair. Indications for reoperation included right ventricular outflow tract obstruction (19), branch pulmonary artery stenosis (11), severe pulmonary insufficiency (4), and residual ventricular septal defect (1). The 1-month, 1-year, and 5-year freedom from reoperation rates were 100%, 89%, and 58%, respectively.
Conclusions
Complete repair of TOF in the neonate is associated with excellent intermediate-term survival. Although the reoperation rate is significant, this is to be expected with the complex right ventricular outflow tract and pulmonary artery anatomy seen in symptomatic neonates and the need for conduit replacement in patients with TOF with pulmonary atresia.
The first complete description of tetralogy of Fallot (TOF) is credited to the French physician Etienne Fallot, who published his findings in 1888. 1 It was not until 1945, however, that the first surgical treatment for TOF was performed by Alfred Blalock at Johns Hopkins University. 2 Several innovative systemic-to-pulmonary shunt procedures were soon developed, 3,4 followed by the first successful \. intracardiac repair using human cross-circulation by Lillehei and Varco at the University of Minnesota in 1954. 5 The first successful repair using a pump oxygenator was performed by Kirklin at the Mayo Clinic 1 year later. 6 Numerous contributions have been made in the management of this defect since these initial pioneering efforts, including one-stage versus two-stage repair, transannular patching, conduit insertion, and transatrial repair, to name a few. In the past decade, the interest in earlier repair has increased. This interest, however, has been tempered by dated perceptions regarding the risk of this approach in neonates, including the effects of cardiopulmonary bypass, right ventriculotomy, and pulmonary insufficiency. As the surgical risk for many congenital anomalies has been reduced with improved intraoperative and postoperative care, it is becoming apparent that there are many benefits associated with early repair versus palliation. These benefits include promotion of normal growth and development of organs, elimination of hypoxemia, the ability to minimize or avoid right ventricular muscle excision, and decreased late dysrhythmias.
The initial presentation of the patient with TOF depends on the degree of right ventricular outflow tract (RVOT) obstruction. Most commonly, cyanosis is mild at birth and gradually progresses with age as the obstruction increases as a result of increasing hypertrophy of the right ventricular infundibulum. A few patients, however, have significant cyanosis at or shortly after birth. In this group, the RVOT obstruction is nearly always due to a hypoplastic pulmonary valve annulus with or without severe right ventricular infundibular obstruction or hypoplasia. Cyanosis is constant in these patients because of the fixed nature of the obstruction to pulmonary blood flow. Patients with atresia of the pulmonary valve and main pulmonary trunk are dependent on a patent ductus arteriosus or systemic aortopulmonary collateral arteries for pulmonary blood flow.
The purpose of this review is to evaluate our policy of complete repair in this select population of symptomatic neonates. Early and late survival, perioperative complications, and the incidence of reoperation were assessed. Patient, procedural, and morphologic risk factors were analyzed to determine their effects on outcome.
METHODS
Patient Population
The medical records of 61 consecutive neonates who underwent complete repair for TOF at C.S. Mott Children’s Hospital, University of Michigan Health System, from August 1988 to November 1999 were reviewed. This current review expands and extends an earlier study from our institution and includes the patients reported in that paper. 7 Informed consent was obtained for participation in the study. The scientific merit and ethical standards of the study were reviewed and approved by the University of Michigan Institutional Review Board.
Thirty-one patients had TOF with pulmonary stenosis (TOF/PS), 24 had TOF with pulmonary atresia (TOF/PA), and 6 had TOF with nonconfluent pulmonary arteries. All patients had either duct-dependent pulmonary blood flow or unacceptable levels of systemic arterial hypoxemia requiring surgical intervention. Patients with multiple aortopulmonary collateral arteries or associated complete atrioventricular septal defects were excluded from the study.
During the period of this review, eight neonates underwent initial palliation rather than primary repair (two with TOF/PS, six with TOF/PA). Four had significant physiologic compromise before the initial operation that made definitive repair unsafe. Two of these patients had intraabdominal catastrophes requiring exploratory laparotomy with enteric diversion. Another had suffered two cardiac arrests after cardiac catheterization with hemodynamic instability, and one was small for gestational age (2.3 kg). Additional reasons for initial palliation rather than repair included complex pulmonary artery anatomy with widely divergent nonconfluent pulmonary arteries (1), anomalous left coronary artery in an unstable patient (1), and individual surgeon preference early in our experience (2).
Surgical Techniques
All patients underwent repair using hypothermic cardiopulmonary bypass with or without intermittent periods of circulatory arrest. A single dose of cold blood cardioplegia (20 mL/kg) was administered at the time of aortic cross-clamping and was repeated if the aortic cross-clamp time exceeded approximately 30 minutes. Right ventricular infundibular obstruction was relieved by division of parietal and septal extensions of the infundibular septum without muscle excision (Fig. 1). The ventricular septal defect was closed using a transventricular (n = 23) or transatrial (n = 38) approach, as deemed most appropriate by the surgeon. In general, the transventricular approach was used in patients requiring ventriculotomy for conduit placement, and a transatrial approach was used when a limited transannular outflow patch was inserted. If present, a patent foramen ovale was left open. In the 31 patients with TOF/PS, the obstruction at the level of the pulmonary annulus was relieved with a transannular patch in 29 and a right ventricle-to-pulmonary artery (RV-PA) conduit in two. In the 24 patients with TOF/PA, the obstruction was relieved with a transannular patch in 15 and an RV-PA conduit in nine. In this group, a transannular patch was selected where there was continuity between the main pulmonary trunk and the right ventricle, and conduits were placed only when the main pulmonary trunk was atretic. In the six patients with TOF with nonconfluent pulmonary arteries, the obstruction at the level of the pulmonary annulus was relieved with a transannular patch in five and an RV-PA conduit in one. The nonconfluent pulmonary artery was divided from its systemic arterial origin and anastomosed primarily to the contralateral vessel. 8 Right and left ventricular pressures were measured at the completion of the repair by direct needle puncture.
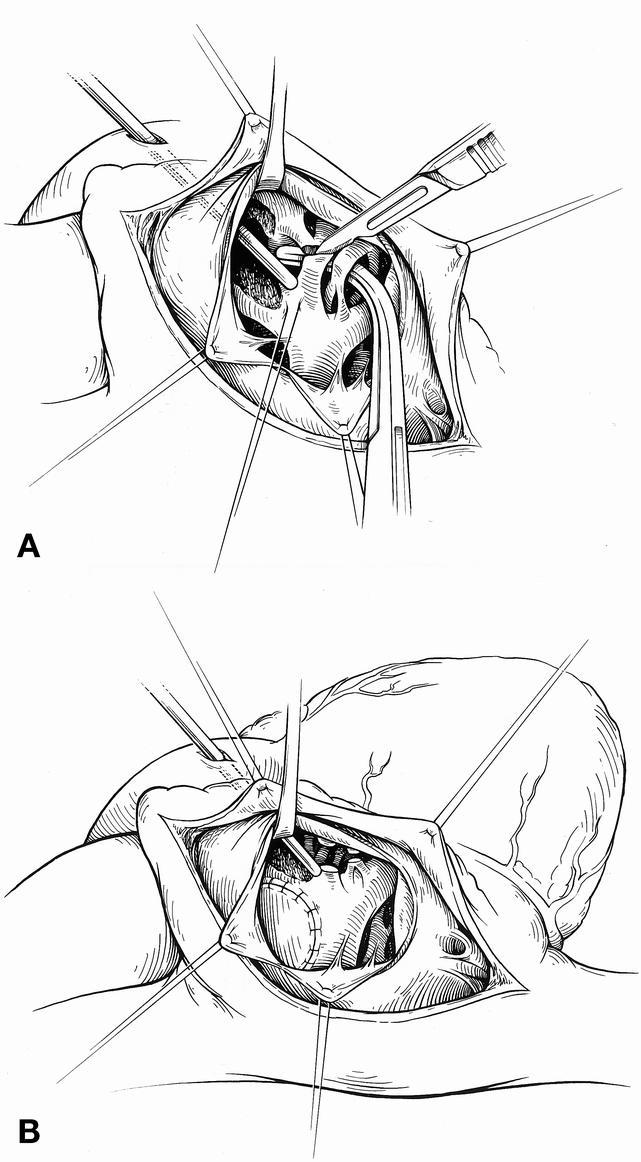
Figure 1. The surgical anatomy as viewed through a right atriotomy. (A) Division of the obstructing muscle bundles along the anterior limb of the septal band. Division of these muscle bundles is usually sufficient to relieve the outflow tract obstruction when repair is performed early. (B) View through the right atriotomy and tricuspid valve after patch closure of the ventricular septal defect. The ends of the divided muscle bundles can be seen.
Follow-Up
Before hospital discharge, all patients underwent a standard 12-lead electrocardiogram, a chest x-ray, and a transthoracic echocardiogram with Doppler examination of the heart and great vessels. Follow-up data regarding survival and reoperation were obtained from the inpatient medical record, direct family contact, and correspondence with the referring cardiologist.
Statistical Analysis
The medical record of each patient was reviewed to determine the morphologic, clinical, procedural, and follow-up data. Standard methods were used to obtain the mean, median, and standard error for continuous values as indicated. Group data are presented as the mean plus or minus standard deviation of the mean, and proportional data are presented with their 95% confidence limits. Multiple regression analysis was performed to establish whether correlation existed between groups and events. Actuarial survival and reoperation rates were derived by the methods of Kaplan and Meier.
RESULTS
Preoperative Data
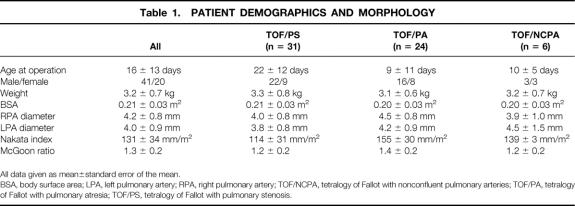
There were 41 boys and 20 girls. The average age at the time of repair was 16 ± 13 days (range 0–43 days). The mean weight at the time of repair was 3.2 ± 0.7 kg, with a mean body surface area of 0.21 ± 0.03 m2. Twenty-six patients required preoperative mechanical ventilation. Thirty-six patients were receiving an infusion of prostaglandin at some time before repair. Eleven patients required inotropic support before repair. The average systemic arterial oxygen saturation on room air was 83% ± 8%. The diameter of the right pulmonary artery averaged 4.2 ± 0.8 mm, and that of the left pulmonary artery averaged 4.0 ± 0.9 mm. The mean Nakata index was 131 ± 34 mm/m2, and the mean McGoon ratio was 1.3 ± 0.2 (Table 1). There were no differences between morphologic subgroups.
Table 1. PATIENT DEMOGRAPHICS AND MORPHOLOGY
All data given as mean±standard error of the mean.
BSA, body surface area; LPA, left pulmonary artery; RPA, right pulmonary artery; TOF/NCPA, tetralogy of Fallot with nonconfluent pulmonary arteries; TOF/PA, tetralogy of Fallot with pulmonary atresia; TOF/PS, tetralogy of Fallot with pulmonary stenosis.
Surgical Data
Cardiopulmonary bypass time averaged 71 ± 26 minutes, with an aortic cross-clamp time of 45 ± 15 minutes. Hypothermic circulatory arrest was used in 52 patients (mean 38 ± 12 minutes). The average right/left ventricular pressure ratio at the completion of repair was 0.55 (range 0.3–0.88). Forty-nine patients underwent repair with a transannular patch and 12 with an RV-PA conduit. There were no intraoperative deaths. Delayed sternal closure was used in 10 patients.
Postoperative Data
There was one hospital death, yielding a hospital survival rate of 98.4% (95.2–100%). This patient, who also had Noonan syndrome, underwent repair on day of life 19. His postoperative course was notable for the development of necrotizing enterocolitis with subsequent multiple organ system failure, of which he ultimately died 2 months after his initial cardiac repair. One other neonate had necrotizing enterocolitis and acute renal failure after surgery and recovered completely. Other postoperative complications included supraventricular tachycardia (1), diaphragmatic paralysis requiring plication (1), chylothorax requiring thoracic duct ligation (1), respiratory failure requiring tracheostomy in association with CHARGE syndrome (1), and pyloric stenosis requiring pyloromyotomy (1).
The total postoperative hospital stay averaged 19 days (range 6–200 days). Although not significant, there was a trend toward a decreased hospital stay. During the first 5 years of this review, the average postoperative hospital stay was 23 days; during the later 5 years, it averaged 16 days. The postoperative intensive care unit stay was 9.1 ± 8.3 days (n = 37), with mechanical ventilation for 6.8 ± 7 days (n = 39).
Discharge transthoracic Doppler echocardiography demonstrated a hemodynamically significant residual ventricular septal defect in 1 patient, residual left pulmonary artery stenosis in 11, and an average peak instantaneous pressure gradient across the RVOT of 13.1 ± 21.2 mmHg. No patient had complete heart block or more than mild tricuspid regurgitation.
Follow-Up Data
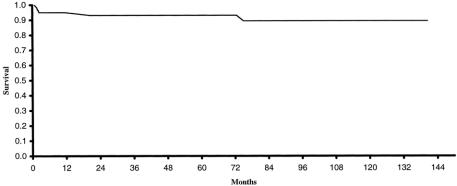
Follow-up data were available for all 60 patients who survived to hospital discharge. Follow-up ranged from 1 to 141 months, with an average of 62 months. There were four late deaths. One patient with severe cerebral palsy and developmental delay died of pneumonia 6 years after repair. Two patients died suddenly at home 2 months after repair. Autopsy findings for both of these patients were consistent with sudden infant death syndrome. The final patient had a ventricular fibrillatory arrest complicated by severe anoxic brain injury after conduit replacement at age 21 months. Three deaths occurred in patients with TOF/PA and one in a patient with TOF/PS. Two patients were repaired with RV-PA conduits and two were repaired with a transannular patch. The actuarial survival rate was 93% at 5 years (Fig. 2).
Figure 2. Actuarial survival for all neonates undergoing complete repair of tetralogy of Fallot, August 1988 to November 1999, at the University of Michigan (n = 61).
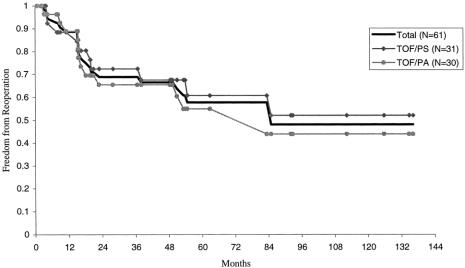
A total of 22 patients required reoperation. Two of these patients required 2 reoperations each, thus yielding a total of 24 reoperations (Table 2). The most common indication for reoperation was recurrent RVOT obstruction (n = 19). Eleven patients with prior transannular patches underwent placement of an RV-PA conduit. Five patients with prior RV-PA conduits had outgrown their original conduit and required replacement. Three patients underwent additional muscle division or excision from the RVOT. Three patients with prior transannular patches and severe pulmonary insufficiency underwent insertion of a pulmonary valve. Of the patients undergoing reoperation for RVOT obstruction, 10 also had concomitant augmentation of the left pulmonary artery. Only one patient required reoperation solely for bilateral pulmonary artery stenoses. Concomitant closure of four patent foramen ovales and one residual ventricular septal defect was performed. One patient requiring two reoperations underwent the procedures 2 years and 9 years after the initial repair. Both operations were for RV-PA conduit change for recurrent stenosis. The other patient requiring reoperation twice underwent RVOT muscle resection and left pulmonary artery augmentation 2 years after repair, followed by pulmonary valve placement 10 years after the initial repair for severe pulmonary insufficiency. The time to reoperation ranged from 3 to 85 months (average 26 months). The 1-month, 1-year, and 5-year freedom from reoperation rates were 100%, 89%, and 58%, respectively (Fig. 3). Although not significant, patients with TOF/PS had a slightly higher freedom from reoperation rate at 5 years compared with patients with TOF/PA (61% vs. 55%).
Table 2. INDICATIONS FOR REOPERATION
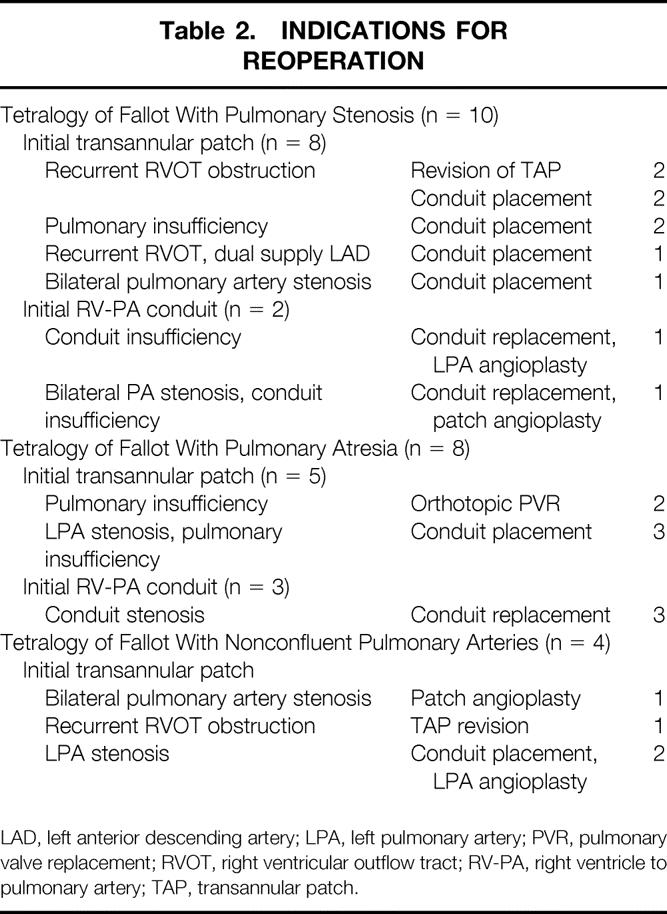
LAD, left anterior descending artery; LPA, left pulmonary artery; PVR, pulmonary valve replacement; RVOT, right ventricular outflow tract; RV-PA, right ventricle to pulmonary artery; TAP, transannular patch.
Figure 3. Freedom from reoperation after complete repair of tetralogy of Fallot in the neonate, August 1988 to November 1999, at the University of Michigan.
DISCUSSION
Although TOF was described more than 100 years ago, the past two decades have demonstrated an improved understanding of the varied features of this defect and their sequelae after repair. In particular, an appreciation of the consequences of longstanding RVOT obstruction has led to an increased interest in earlier complete repair. In one study, Seliem et al 9 examined the effect of early relief of RVOT obstruction on right ventricular morphology in relation to the age at the time of repair. Among their patients who underwent repair before 6 months of age, both absolute right ventricular wall thickness and the wall thickness-to-transverse dimension ratio decreased significantly after repair. In contrast, patients who underwent repair after 6 months of age showed no significant change in these findings. Early resolution of right ventricular hypertrophy and fibrosis are believed to be important in decreasing the incidence of late right ventricular dysfunction and ventricular arrhythmias.
Despite the physiologic rationale and clinical evidence that early repair may avert some of the long-term complications associated with longstanding right ventricular hypertrophy, concern has remained regarding the safety of primary repair in the neonate. Of particular concern is the need for a transannular patch, which was formerly considered a contraindication to complete repair in young infants. This risk factor has now been neutralized. 7,10–12 Additional concern regarding the use of cardiopulmonary bypass in infants has also been refuted. In a study comparing the complication rate from cardiopulmonary bypass used during repair compared with palliative procedures in neonates undergoing a variety of congenital heart procedures, it was found that bypass was not an independent determinant of survival. 13 Effective repair combined with proper diagnostic techniques and postoperative management was cited as a critical determinant of survival.
Results of repair of TOF in the neonate have improved dramatically during the past decade. A review of TOF repair from 1973 to 1988 demonstrated a hospital mortality of 18.5%. 14 A 26-year retrospective review revealed a decrease in the mortality of primary repair in all age groups from 11.1% before 1990 to 2.1% after 1990. 15 Two additional series reported a similar mortality of 3% for repair in both infants and neonates. 16,17 The mortality in our series of repair involving only neonates (younger than 30 days of age) demonstrates a highly comparable hospital mortality of 1.6%. Although we reported in a prior review that the placement of a valved homograft conduit was an independent risk factor for perioperative death, 7 this risk has not been supported in this larger series. Focused analysis of this youngest age group with the most severe RVOT obstruction demonstrates results equivalent to those previously published, which often include older patients, and reinforces the safety of early repair. The operative mortality is not increased, and the benefits of early physiologic repair as previously defined can be maximized.
Although the risk of perioperative death in this age group is low, concern remains regarding the need for reoperation. In our series, among those requiring reoperation, most exhibited recurrent/residual RVOT obstruction. Within this group, 53% had concomitant left pulmonary artery stenoses requiring augmentation. These patients were included in our previous review of neonatal repair for TOF, in which we found that the most common indication for reoperation was correction of left pulmonary artery stenosis. 7 As discussed in that review, close attention to the ductus insertion site with patch augmentation of the proximal left pulmonary artery is important to reduce recurrent stenosis. In fact, since the aggressive attention to the ductus insertion site, we have noted a reduction in recurrent left pulmonary artery stenosis requiring surgical intervention. The right/left ventricular pressure ratio remains a valuable indicator of reoperative risk. An intraoperative ratio greater than 0.7 was an independent risk factor for reoperation (P < .02).
In this series, 42% of patients initially repaired with RV-PA conduits required conduit replacement. This rate is comparable to the findings of other studies, in which repair of infants (first 3 months of life) with TOF was associated with a 47% conduit replacement rate. 18 Obviously, freedom from reoperation in a series of symptomatic neonates, all of whom had either pulmonary atresia or severe hypoplasia of the pulmonary valve annulus, must be viewed in its proper context. Older patients, even those undergoing elective repair in early infancy, have more favorable RVOT anatomy. No series of routine elective repair in asymptomatic neonates has been reported. Although the reoperation rate is considerable in this series, the complication and death rates from reoperation were low. Given the complexity of the anatomy involved in neonates who are symptomatic secondary to severe RVOT obstruction, as well as the use of prosthetic material with limited growth potential, it is not surprising that reoperation rates are significant. Further reduction in the reoperation rate is limited by the morphology of the disease.
In conclusion, this retrospective review of complete repair of TOF in the neonate is the largest documented single-center experience. The time interval reviewed represents an era of increased interest in early repair for many forms of complex congenital heart disease. The objective of this review was to confirm the safety of a policy of primary repair in neonates and to evaluate potential risk factors associated with adverse outcomes. The low hospital and late mortality reinforce our belief that neonatal repair is a reasonable option without prohibitive risk. In addition, the financial environment of medicine encourages maximization of cost to patient benefit. An analysis of the associated hospital cost for one-stage versus two-stage repair for TOF demonstrated a significant reduction in total hospital cost and total hospital stay when primary repair was performed. 19 These data provide a benchmark for comparison with protocols of early palliation followed by later repair in a challenging group of symptomatic neonates with complex TOF.
Discussion
Dr. Richard A. Jonas (Boston, Massachusetts): At Children’s Hospital in Boston, we have pursued a philosophy of early primary repair for tetralogy for many years. Last year in Circulation we reported a survival of 92%, very similar to your report, at 5 years, of 99 consecutive neonates and infants under 3 months of age who underwent surgery between 1998 and 1996.
At next month’s AATS meeting in Toronto, we will describe more than 20 years of follow-up for patients who underwent primary repair of tetralogy in infancy by Dr. Castaneda in the 1970s. There were no late deaths among 52 operative survivors. Perhaps surprisingly, the incidence of freedom from reoperation was 91% for patients who had a transannular patch and freedom from reoperation was 80% for those who had a nontransannular patch. I have two questions.
I note that all of your patients who had an outflow patch had a transannular patch. Should we infer that you have a very aggressive philosophy regarding transannular patch placement, or do you have a conservative approach as to who undergoes surgery? In other words, can we infer that your patient population is at the extreme end of the anatomical spectrum? Leading on from that, in view of the outstanding results that you describe here for symptomatic neonates, do you think you might relax your criteria and operate on nonsymptomatic patients earlier in life who have tetralogy?
Secondly, we believe that it is very important to leave the foramen ovale open to allow right-to-left decompression in the early postoperative period. Neonates tolerate cyanosis very much better than an elevated CVP. Can you please expand for us how you handle the foramen ovale in your patient population?
Presenter Dr. Jennifer C. Hirsch (Ann Arbor, Michigan): In regards to your first question, I think it is a combination of both. We are very aggressive with placing a transannular patch in these neonates. In general, those with a Z score less than −2 undergo placement of a transannular patch. It should be noted that the patch is very limited in size and usually involves just the annulus and several millimeters on either side. We place the patch within a commissure to avoid disrupting the pulmonary valve annulus itself.
In regard to the preoperative status of these neonates, most of these patients are very cyanotic secondary to severe right ventricular outflow tract obstruction. The severity of this obstruction explains why most of our patients do undergo transannular patch placement. So, I think it is a combination of both an aggressive approach with transannular patches as well as the severe symptoms of the patients needing surgery in the first month of life.
In regard to neonates not undergoing primary repair, we had only eight patients during our study period who underwent systemic to pulmonary artery shunt placement. Thus, the majority of neonates that present to us with symptoms do undergo early primary repair.
As for the patent foramen ovale, we also leave it open. In the patient with a large ASD, we narrow the defect in size to several millimeters to allow release of pressure on the right side of the heart.
Footnotes
Correspondence: Edward L. Bove, MD, Section of Cardiac Surgery, University of Michigan School of Medicine, 1500 E. Medical Center Dr., Mott F7380, Ann Arbor, MI 48109-0223.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail: elbove@umich.edu
Accepted for publication April 2000.
References
- 1.Fallot E. Contribution a l’anatomie pathologique de la maladie bleue (cyanose cardiaque). Marseille Med 1888; 25: 77ff. [PubMed] [Google Scholar]
- 2.Blalock A, Taussig HB. The surgical treatment of malformation of the heart in which there is pulmonary stenosis or pulmonary atresia. JAMA 1945; 128: 189. [DOI] [PubMed] [Google Scholar]
- 3.Potts WJ, Smith S, Gibson S. Anastomosis of the aorta to a pulmonary artery. JAMA 1946; 132: 627. [DOI] [PubMed] [Google Scholar]
- 4.Waterston DJ. Treatment of Fallot’s tetralogy in children under one year of age. Rozhl Chir 1962; 41: 181. [PubMed] [Google Scholar]
- 5.Lillehei CW, Cohen M, Warden HE, et al. Direct visualization intracardiac surgical correction of the tetralogy of Fallot, pentalogy of Fallot and pulmonary atresia defects. Ann Surg 1955; 142: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirklin JW, DuShane JW, Patrick RI, et al. Intracardiac surgery with the aid of a mechanical pump-oxygenator system (Gibbon type): report of eight cases. Mayo Clin Proc 1955; 30: 201. [PubMed] [Google Scholar]
- 7.Hennein HA, Mosca RS, Urcelay G, Crowley DC, Bove EL. Intermediate results after complete repair of tetralogy of Fallot in neonates. J Thorac Cardiovasc Surg 1995; 109: 332–344. [DOI] [PubMed] [Google Scholar]
- 8.Shanley CJ, Lupinetti FM, Shah NL, et al. Primary unifocalization for the absence of intrapericardial pulmonary arteries in the neonate. J Thorac Cardiovasc Surg 1993; 106: 237–247. [PubMed] [Google Scholar]
- 9.Seliem MA, Wu YT, Glenwright K. Relation between age at surgery and regression of right ventricular hypertrophy in tetralogy of Fallot. Pediatr Cardiol 1995; 16 (2): 53–55. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Kirklin JW, Blackstone EH, et al. Effect of transannular patching on outcome after repair of tetralogy of Fallot. Ann Thorac Surg 1989; 48: 783. [DOI] [PubMed] [Google Scholar]
- 11.Kirklin JW, Blackstone EH, Jonas RA, et al. Morphologic and surgical determinants of outcome events after repair of tetralogy of Fallot and pulmonary stenosis. J Thorac Cardiovasc Surg 1992; 103: 706. [PubMed] [Google Scholar]
- 12.Kirklin JW, Blackstone EH, Kirklin JK, et al. Surgical results and protocols in the spectrum of tetralogy of Fallot. Ann Surg 1983; 198: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turley K, Mavroudis C, Ebert PA. Repair of congenital cardiac lesions during the first week of life. Circulation 1982; 66 (2pt2):I214–I219. [PubMed] [Google Scholar]
- 14.DiDonato RM, Jonas RA, Lang P, et al. Neonatal repair of tetralogy of Fallot with and without pulmonary atresia. J Thorac Cardiovasc Surg 1991; 101 (1): 126–137. [PubMed] [Google Scholar]
- 15.Knott-Craig CJ, Elkins RC, Lane MM, et al. A 26-year experience with surgical management of tetralogy of Fallot: risk analysis for mortality or late reintervention. Ann Thorac Surg 1998; 66: 506–511. [DOI] [PubMed] [Google Scholar]
- 16.Reddy VM, Liddicoat JR, McElhinney DB, et al. Routine primary repair of tetralogy of Fallot in neonates and infants less than three months of age. Ann Thorac Surg 1995; 60 (6suppl):S592–596. [DOI] [PubMed] [Google Scholar]
- 17.Touati GD, Vouhe PR, Amodeo A, et al. Primary repair of tetralogy of Fallot in infancy. J Thorac Cardiovasc Surg 1990; 99: 396–402. [PubMed] [Google Scholar]
- 18.Perron J, Moran AM, Gauvreau K, et al. Valved homograft conduit repair of the right heart in early infancy. Ann Thorac Surg 1999; 68: 542–548. [DOI] [PubMed] [Google Scholar]
- 19.Ungerleider RM, Kanter RJ, O’Laughlin M, et al. Effect of repair strategy on hospital cost for infants with tetralogy of Fallot. Ann Surg 1997; 225: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]