Abstract
Objective
To evaluate the short-term outcomes for laparoscopic Roux-en-Y gastric bypass in 275 patients with a follow-up of 1 to 31 months.
Summary Background Data
The Roux-en-Y gastric bypass is a highly successful approach to morbid obesity but results in significant perioperative complications. A laparoscopic approach has significant potential to reduce perioperative complications and recovery time.
Methods
Consecutive patients (n = 275) who met NIH criteria for bariatric surgery were offered laparoscopic Roux-en-Y gastric bypass between July 1997 and March 2000. A 15-mL gastric pouch and a 75-cm Roux limb (150 cm for superobese) was created using five or six trocar incisions.
Results
The conversion rate to open gastric bypass was 1%. The start of an oral diet began a mean of 1.58 days after surgery, with a median hospital stay of 2 days and return to work at 21 days. The incidence of early major and minor complications was 3.3% and 27%, respectively. One death occurred related to a pulmonary embolus (0.4%). The hernia rate was 0.7%, and wound infections requiring outpatient drainage only were uncommon (5%). Excess weight loss at 24 and 30 months was 83% and 77%, respectively. In patients with more than 1 year of follow-up, most of the comorbidities were improved or resolved, and 95% reported significant improvement in quality of life.
Conclusion
Laparoscopic Roux-en-Y gastric bypass is effective in achieving weight loss and in improving comorbidities and quality of life while reducing recovery time and perioperative complications.
Roux-en-Y gastric bypass (RYGBP) has been shown to produce significant weight loss in patients with clinically severe obesity: most studies report a weight loss of 60% to 70% of excess body weight. 1,2 Long-term weight loss has been reported to extend to 10 years and longer by several investigators. 3–5 For these reasons, many surgeons contend that RYGBP is the bariatric procedure of choice for most patients with clinically severe obesity.
Although the perioperative complication rate of RYGBP is acceptable, it is less than ideal. Most bariatric patients have significant comorbidity, increasing their risk of postoperative cardiopulmonary complications. Apart from technical complications, cardiopulmonary and wound-related complications are the most common and most severe. Further, “minor” complications such as ileus and postoperative pain account for average hospital stays ranging from 4 to 8 days. Many investigators would agree that many of the perioperative complications of bariatric procedures are related to the extensive abdominal incisions required only for access. Since the introduction of laparoscopic cholecystectomy in the late 1980s, much has been learned about the profoundly positive impact of laparoscopic surgery on reducing perioperative complications. 6–9 Compared with patients undergoing cholecystectomy, patients undergoing bariatric surgery generally have more comorbidities and require more extensive incisions to complete the surgical procedure. Laparoscopic access for bariatric surgery, thus, may have an even greater impact than laparoscopic cholecystectomy on reducing the perioperative complications related to the conventional access incision.
The purpose of this study was to determine the feasibility and safety of performing RYGBP with laparoscopic access and its impact on perioperative complications.
METHODS
Patients were selected for laparoscopic RYGBP if they met minimal criteria for bariatric surgery proposed by the NIH Consensus Development Panel report of 1991. 10 Patients were further selected on the basis of willingness to undergo the procedure by laparoscopic access, with the risk of conversion to the open method. Initially (first 50 patients), patients were excluded if there was a history of prior major abdominal surgery, coagulopathy, age older than 55, or body mass index (BMI) greater than 50. As experience was achieved, these exclusionary criteria were liberalized significantly. An extensive preoperative evaluation, including history and physical examination, nutritional and psychiatric evaluation, and indicated specialty consultations, was performed on all patients. Laboratory evaluation included complete blood count, serum chemistries, and thyroid function testing. All patients received preoperative abdominal sonography. If gallstones were detected, then laparoscopic cholecystectomy was performed concomitantly.
Patient preparation for surgery consisted of a detailed explanation in written and oral form of the developmental aspect of laparoscopic RYGBP and its benefits and risks, including short- and long-term complications, side effects, nutritional sequelae, and the possibility of conversion to the open procedure. Informed consent was obtained. Preoperative bowel cleansing and perioperative antibiotics were administered (instituted after the first 50 patients). Prophylaxis against venous thrombosis and pulmonary embolus consisted of perioperative pneumatic compression devices and low-dose subcutaneous heparin.
Data were collected prospectively and verified retrospectively, then entered into a customized computer database. Data sources included office charts, follow-up notes, hospital charts, and patient interview. Parameters included patient demographics, comorbidity, surgical time, blood loss, pain medication requirement, hospital stay, recovery, complications, weight loss, change in comorbidity, quality of life changes, and patient satisfaction. Recovery was defined as the number of days after surgery when patients resumed common activities of daily living such as driving, shopping, household activities, and employment.
Follow-up weights were obtained from the University of Pittsburgh Surgical Weight Loss Clinic scale with a capacity of 400 kg. On occasion, official weights were obtained from physician office scales or telephone interview. Telephone callbacks were rejected if weights were inconsistent with recent clinic weights. Weight loss was expressed in terms of mean percentage of excess body weight loss or BMI. Ideal body weight was determined according to the Metropolitan Life Insurance Company 1983 height/weight tables; for a given height, the middle weight for a medium-frame person was chosen as the ideal body weight.
Outcomes related to changes in comorbidities, quality of life, and patient satisfaction were assessed for patients with 1 year or more of follow-up. The Moorehead-Ardelt Quality of Life Questionnaire specific for bariatric surgery was administered according to protocol to assess quality of life changes. 11
Surgical Technique
The surgical technique was a modification of the technique described by Wittgrove et al. 12 The patient was placed in a supine position with the surgeon on the right and the assistant on the left, and two monitors above the patient’s shoulders. After creation of carbon dioxide pneumoperitoneum (15 mmHg) using the Veress needle technique, cannulas (U.S. Surgical, Norwalk, CT) were placed as shown in Figure 1. The operating table was placed in a steep reverse Trendelenburg position. To expose the esophagus and stomach, a 5-mm liver retractor (Genzyme, Tucker, GA) was placed through the inferior right subcostal port, and the left lateral segment of the liver was elevated. Gastric pouch creation was performed as shown in Figure 2. To localize the esophagogastric junction and size the pouch, a Baker jejunostomy tube was inserted orally into the stomach, inflated with saline to 15 mL, and drawn up to the cardia. The use of the Baker tube no longer became necessary after approximately 100 cases and was abandoned. After withdrawing the balloon, a window was created in the lesser omentum near the gastric wall at the lesser curvature. The Endo GIA stapler (U.S. Surgical), 60-mm length and 4.8-mm staples, was inserted and applied three or four times to staple and cut the gastric pouch with three rows of staples on each side. A smaller staple size (3.5 mm) was later substituted to reduce staple line bleeds at the transected stomach.
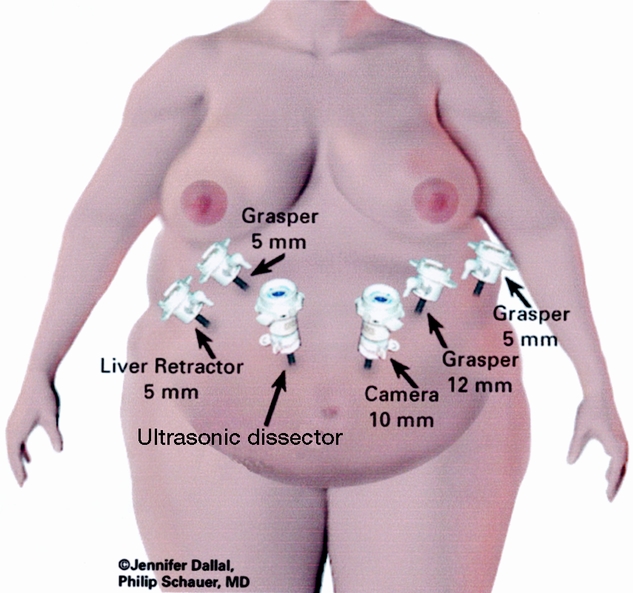
Figure 1. Port placement for laparoscopic Roux-en-Y gastric bypass.
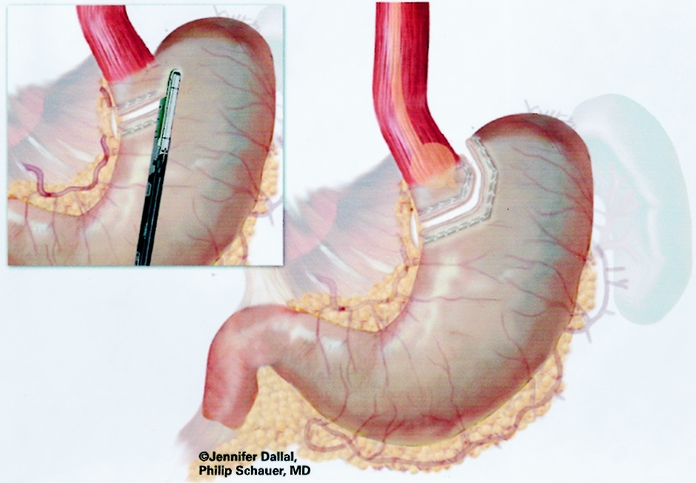
Figure 2. Creation of gastric pouch.
The patient was returned to the supine position to create the jejunojejunostomy. The greater omentum and transverse colon were passed to the upper abdomen to expose the ligament of Treitz. To create the Roux limb, the jejunum was transected with an Endo GIA II stapler (U.S. Surgical), 45-mm length and 3.5-mm staples, at approximately 30 cm from the ligament of Treitz, where a comfortable length of mesentery exists. A smaller staple size (2.5 mm) was later substituted to reduce staple line bleeds at the transected bowel. The jejunal mesentery was then divided with two applications of the Endo GIA II stapler using the vascular load (45-mm length, 2.0-mm staples). A 6-cm length of Penrose drain was sewn to the end of the Roux limb using the Endostitch (U.S. Surgical) (Fig. 3). The Roux limb was then measured 75 cm distally, or 150 cm distally for the superobese, and a stapled side-to-side anastomosis was created with the proximal jejunal limb using one application of the Endo GIA stapler II (60-mm length, 3.5-mm staples). Later, a 2.5-mm staple cartridge was used. The enterotomy sites were stapled closed, and the mesentery of the jejunojejunostomy was sutured closed (Fig. 4).
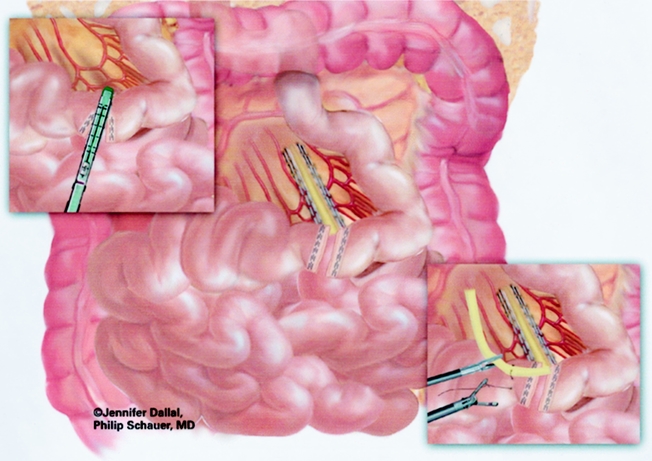
Figure 3. Creation of Roux limb and mesenteric division.
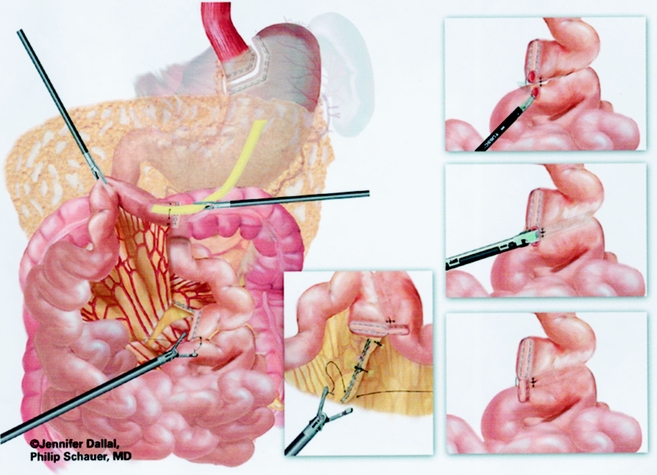
Figure 4. Creation of jejunojejunostomy and closure of mesenteric defect.
A retrogastric–retrocolic tunnel for the Roux limb was then created. Using ultrasonic dissection, a window was created in the mesocolon immediately anterior and lateral to the ligament of Treitz to gain access to the lesser peritoneal sac. The Roux limb was then passed in a retrocolic retrogastric fashion to lie next to the gastric pouch (Fig. 5).
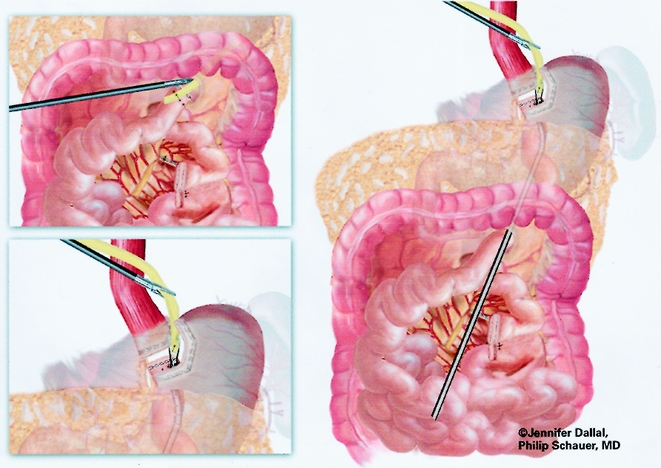
Figure 5. Passage of Roux limb through retrocolic retrogastric tunnel.
The gastrojejunostomy was then created using either an circular end-to-end anastomosis (EEA) stapled technique (first 150 cases) or a linear technique (last 125 cases). The EEA technique is described first. Flexible endoscopy of the gastric pouch was performed, and a long 16-gauge cannula was inserted to allow passage of a wire loop to the pouch. The loop was retrieved with an endoscopic wire snare and brought out the mouth, where it was attached to the anvil of a 21-mm EEA stapler (Endopath, Ethicon Endosurgery, Cincinnati, OH) (Fig. 6). The anvil was then passed through the esophagus into the pouch stem first, and the stem was passed out a small gastrotomy created with electrocautery (Fig. 7). Approximately 15 to 20 cm of the Roux limb was brought up into the upper abdomen, and a longitudinal enterotomy was made on the antimesenteric border of the Roux limb 10 cm from the end. The left upper quadrant cannula site was extended and dilated to allow passage of the airtight 21-mm circular stapler. The circular stapler was advanced through the enterotomy toward the end of the Roux limb. The penetrator was advanced and united with the stem of the anvil. The stapler was closed, discharged, and removed. The enterotomy site was closed with one or two applications of the Endo GIA II stapler (45-cm length, 3.5-mm staples). The gastrojejunostomy anastomosis was closed with interrupted 3–0 Polysorb suture (U.S. Surgical) using the Endostitch, as shown in Figure 8. The gastrojejunostomy and enterotomy site were endoscopically inspected and tested for leakage after insufflation and submerging them in irrigation fluid. In addition, the gastric pouch and Roux limb was irrigated with dilute methylene blue dye to detect leaks.
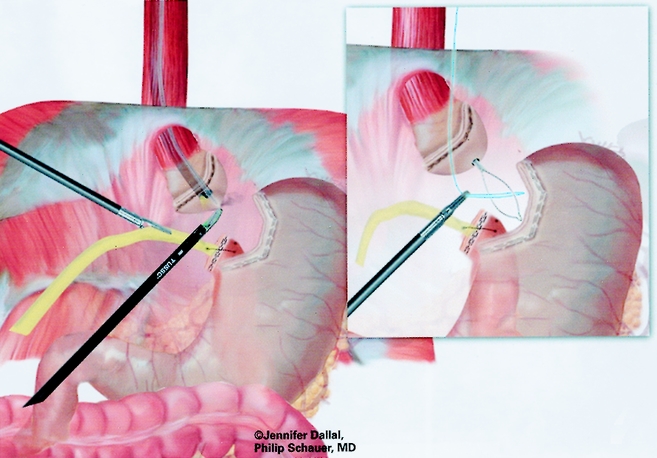
Figure 6. Passage of snare wire for end-to-end anastomosis.
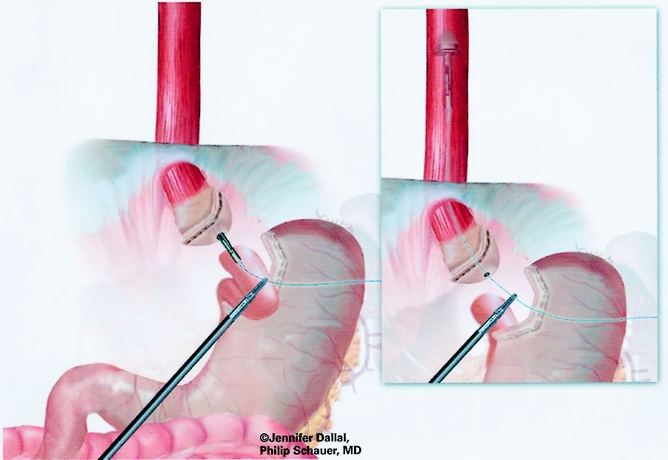
Figure 7. Placement of anvil for end-to-end anastomosis.
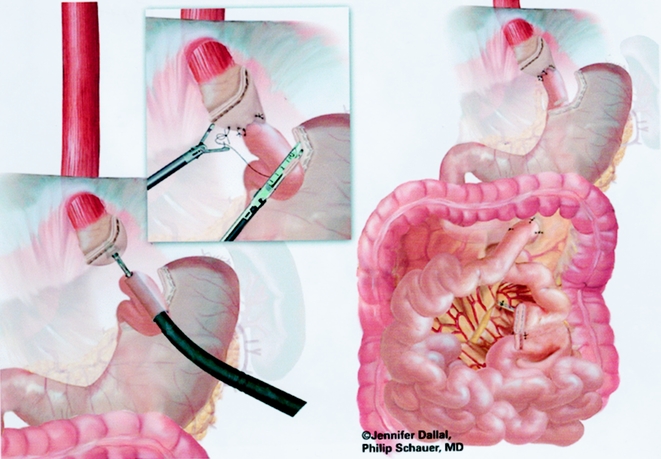
Figure 8. Creation of gastrojejunostomy: circular stapled technique.
An end-to-side gastrojejunostomy technique using an Endo GIA technique was used for the last 125 cases (Figs. 9 and 10). A #10 Jackson-Pratt drain was placed posterior to the gastrojejunal anastomosis and brought out through a right subcostal port site.
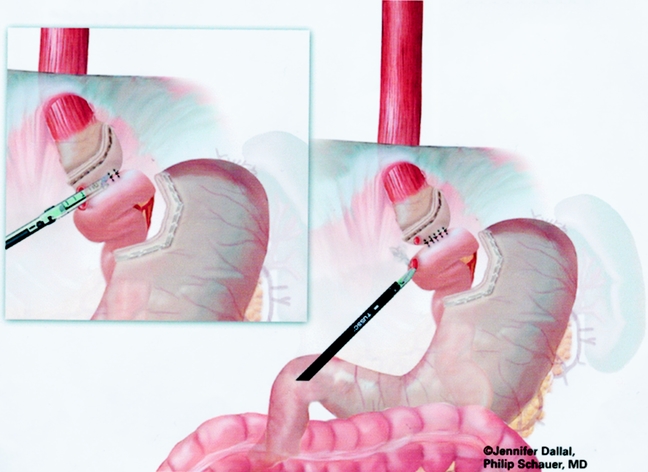
Figure 9. Creation of gastrojejunostomy: end-to-side anastomosis.
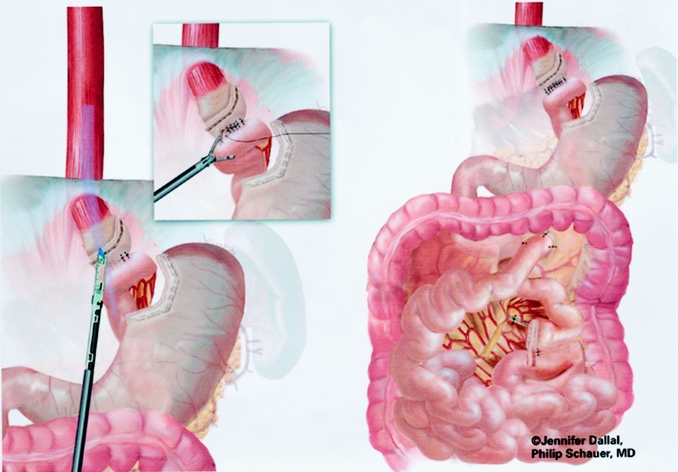
Figure 10. Closure of enterotomy of end-to-side anastomosis.
Postoperative Management
Patients began ambulating on the evening of surgery. Pain management consisted of ketorolac 30 mg intravenously every 6 hours and morphine sulfate intravenously as needed. An upper gastrointestinal series was performed on the morning of the first postoperative day using Gastrografin followed by barium. A clear liquid diet was begun that day, and the patient was discharged from the hospital after demonstrating tolerance of diet and return of bowel function, usually on the second postoperative day. The drain was removed on the 10th postoperative day and the diet was advanced to solid food by the 4th postoperative week. Patient follow-up was scheduled for every 2 months, with laboratory evaluation every 6 months, until weight loss stabilized (usually 1–1.5 years after surgery), then twice per year.
RESULTS
Overview and Demographics
From July 1997 to March 2000, 275 patients underwent attempted laparoscopic RYGBP at the University of Pittsburgh, with a mean follow-up of 9.4 months (range 1–31 months); 104 patients had 1 or more years of follow-up (mean 16.9 months). Follow-up data were obtained in 96% of patients within 3 months of completing this study. Eight patients were lost to follow-up, one patient died of a complication related to surgery, and one patient died of an unrelated cause (homicide). The surgical procedures were performed initially by one attending surgeon (P.R.S.), with the addition of a second attending surgeon (S.I.) after approximately 100 cases. Demographics and surgical risk data are listed in Table 1.
Table 1. DEMOGRAPHICS (n = 275)
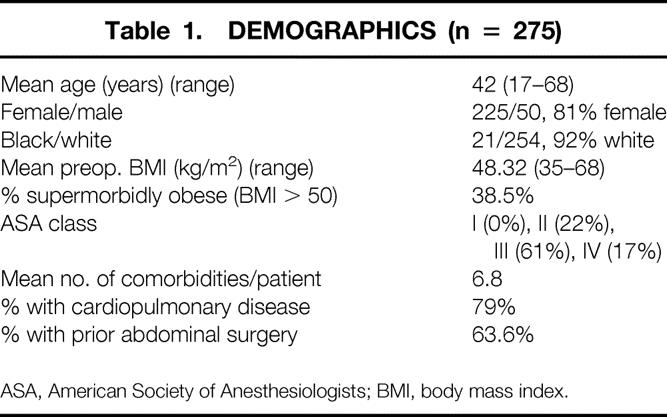
ASA, American Society of Anesthesiologists; BMI, body mass index.
A total of 1,872 comorbidities were identified in the 275 patients (6.8 per patient), and 371 (20%) were newly diagnosed during the preoperative assessment. The most common comorbidities included degenerative joint disease (64%), hypercholesterolemia (62%), hypertension (52%), gastroesophageal reflux disease (51%), depression (41%), hypertriglyceridemia (39%), sleep apnea (36%), fatty liver disease (28%), urinary stress incontinence (24%), type II diabetes (22%), cholelithiasis (17%), and asthma (16%).
Surgical Outcomes
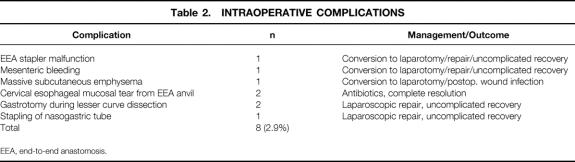
Primary operations performed included laparoscopic RYGBP with a short Roux limb (75 cm) in 118 patients (43%), laparoscopic RYGBP with a long Roux limb (150 cm) in 153 patients (56%), laparoscopic conversion of a failed VBG to RYGBP in 1 patient (0.4%), and laparoscopic to open RYGBP in 3 patients (1.1% conversion rate). Forty-two percent of patients underwent at least one additional concomitant procedure; 18% underwent more than one concomitant procedure. The most common secondary procedures included laparoscopic cholecystectomy (17%), laparoscopic lysis of adhesions (15%), liver biopsy (12%), and umbilical hernia repair (10%). The overall mean operating time was 260 minutes (median 247, range 105–734). For the last 50 patients, the mean operating time decreased to 215 minutes (median 213, range 105–402). The operating time including additional procedures decreased with experience. The mean operative blood loss was 115 mL (median 75 mL, range 25–1,500 mL). Intraoperative complications, including the three conversions to open RYGBP, are listed in Table 2, along with their management and outcome.
Table 2. INTRAOPERATIVE COMPLICATIONS
EEA, end-to-end anastomosis.
Recovery
Indices of recovery are demonstrated in Table 3. Nasogastric suction was used in only the first four patients and was subsequently found to be unnecessary. Postoperative monitoring or treatment in an intensive care unit was required in seven patients (2.5%), with a median stay of 3 days (range 1–14).
Table 3. POSTOPERATIVE RECOVERY (DAYS)
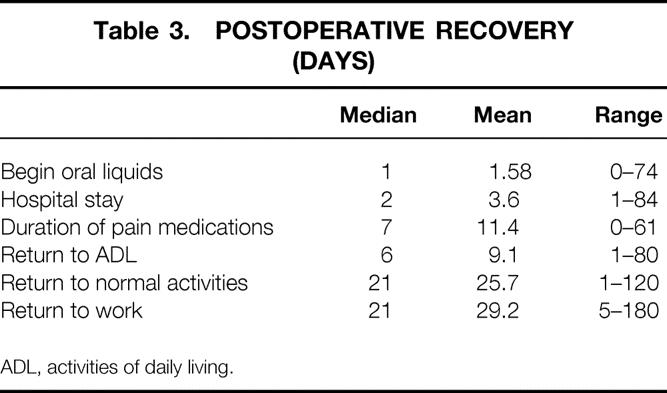
ADL, activities of daily living.
Complications
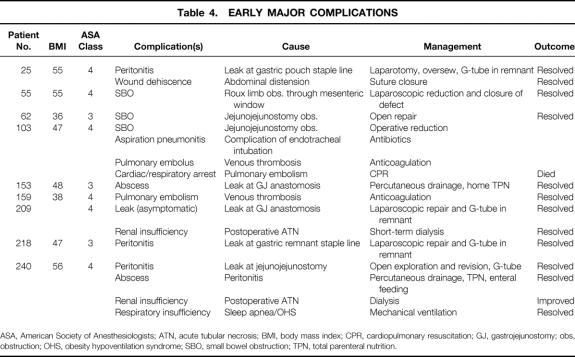
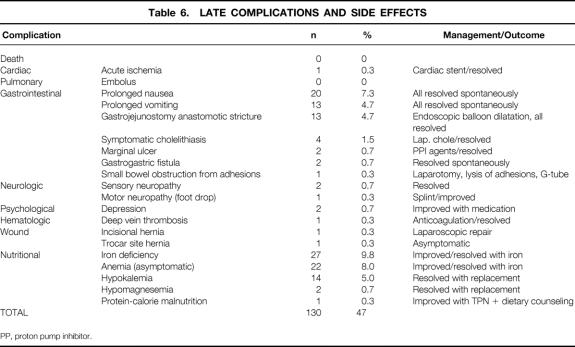
Nine patients had early (<30 days) major complications (3.3%) (Table 4). One of these patients ultimately died as a consequence of a fatal pulmonary embolus, yielding a death rate of 0.4% for this series. Early minor complications and all late complications and side effects are listed in Tables 5 and 6. A total of 27 patients (9.8%) required surgical intervention after the initial operation for the indications shown in Table 7.
Table 4. EARLY MAJOR COMPLICATIONS
ASA, American Society of Anesthesiologists; ATN, acute tubular necrosis; BMI, body mass index; CPR, cardiopulmonary resuscitation; GJ, gastrojejunostomy; obs, obstruction; OHS, obesity hypoventilation syndrome; SBO, small bowel obstruction; TPN, total parenteral nutrition.
Table 5. EARLY MINOR COMPLICATIONS
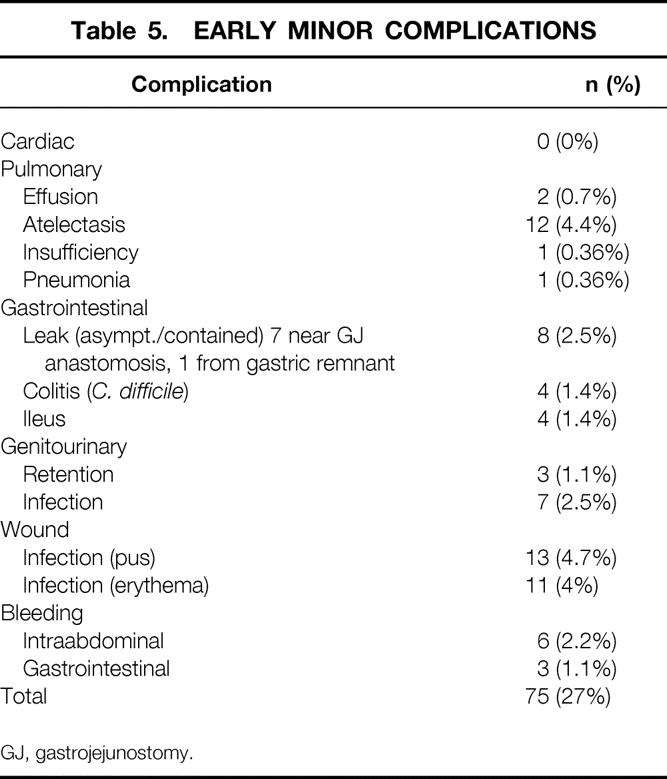
GJ, gastrojejunostomy.
Table 6. LATE COMPLICATIONS AND SIDE EFFECTS
PP, proton pump inhibitor.
Table 7. SURGICAL PROCEDURES AFTER LAPAROSCOPIC ROUX-EN-Y GASTRIC BYPASS
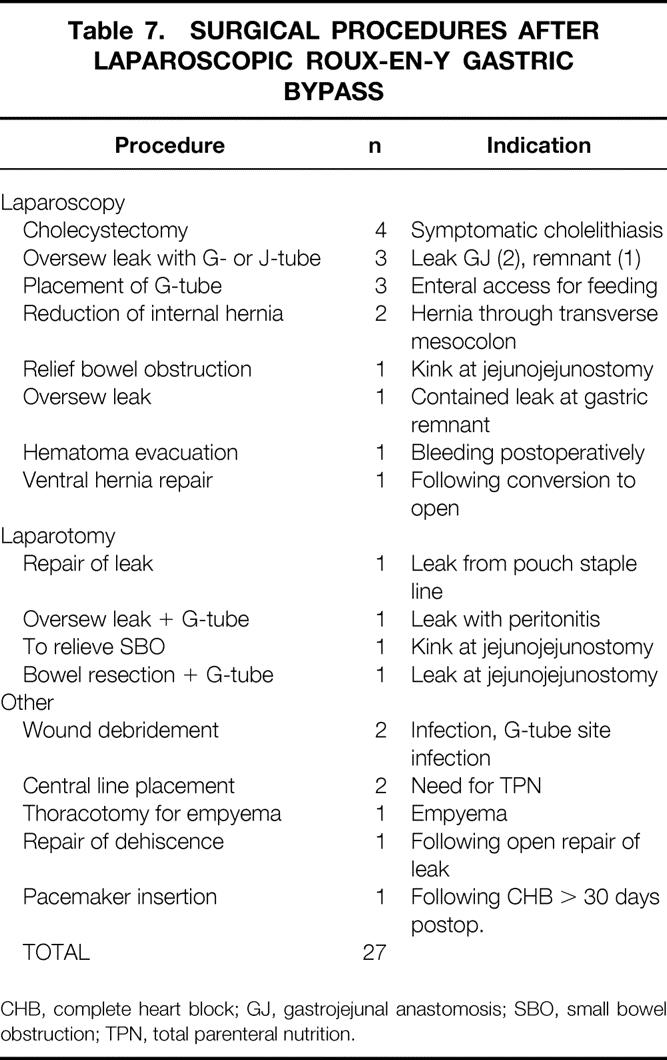
CHB, complete heart block; GJ, gastrojejunal anastomosis; SBO, small bowel obstruction; TPN, total parenteral nutrition.
Noteworthy technical complications included leaks, bowel obstructions, bleeding, and wound infections. A total of 12 gastrointestinal leaks occurred (4.4%); four clinical leaks (1.5%) resulted in either peritonitis or an intraabdominal abscess (see Table 4). Eight subclinical leaks (2.9%) were either asymptomatic or contained (see Table 5). One was identified incidentally by the barium upper gastrointestinal study on postoperative day 1 and was managed by laparoscopic closure with a single suture. One was identified by bile output from the intraabdominal drain on postoperative day 1 and was managed by laparoscopic repair of a small leak at the gastric remnant staple line. Six were identified on postoperative day 8 to 10 by cloudy fluid in the Jackson-Pratt drain and managed by withholding food and fluids for 1 to 2 weeks, with home intravenous fluids (four patients) or by laparoscopic gastrostomy (G-) tube or jejunostomy (J-) tube placement for enteral diversion for 1 to 2 weeks (two patients). Four other patients had radiographic suggestions of contained leaks, but repeat studies suggested that they were radiographic artifacts. Four bowel obstructions (1.5%) occurred, three in the early postoperative period (see Table 4) and one 6 months after surgery secondary to adhesions related to multiple prior surgical procedures (see Table 6). Wound infections characterized by erythema or purulence (see Table 5) occurred almost exclusively at the left upper abdominal trocar site, where the contaminated EEA stapler was withdrawn. In the first 50 patients, the infection rate was 22%. It fell to 10% after routine antibiotic bowel preparation was instituted, and then it fell to 1.5% when a GIA gastrojejunal anastomosis was instituted that allowed removal of the contaminated GIA stapler through a trocar cannula, avoiding contact with the abdominal wall. The trocar site wound infections were managed by oral antibiotics or simple drainage in the outpatient clinic. Except for a laparotomy wound infection (after conversion), no operative débridement or long-term wound packing was required. Postoperative bleeding (see Table 5) occurred in nine patients (3.3%) and was likely related to staple line bleeding (not proven). Intraabdominal bleeding was treated by laparoscopy in one patient (no bleeding site found) and blood transfusion in five patients (one to five units). Gastrointestinal bleeding was identified endoscopically at the gastrojejunal site in one patient. Two patients had lower gastrointestinal bleeding requiring three- and four-unit transfusions before ending spontaneously.
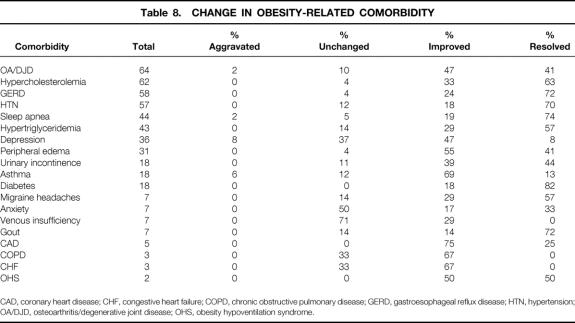
Weight Loss, Comorbidity, and Quality of Life
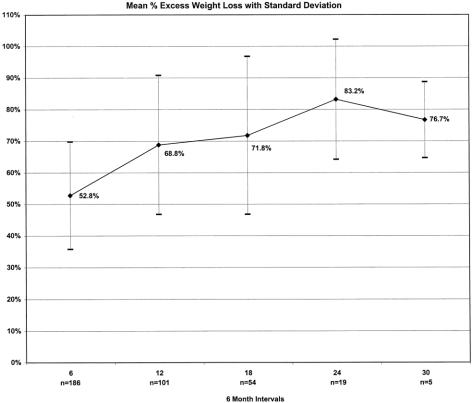
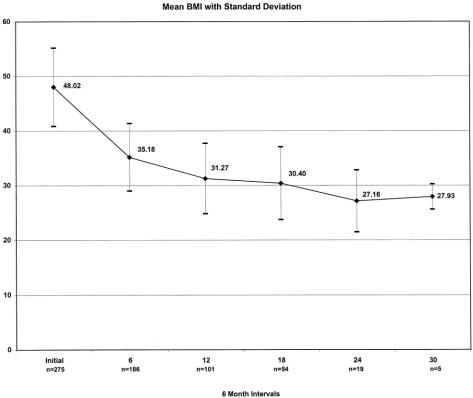
Weight loss, expressed as the mean percentage of excess weight loss and change in BMI, is shown in Figures 11 and 12. Eighty percent of weight measurements were obtained from the same clinic scale; 20% were obtained from telephone callbacks and verified with recent clinic weights. For patients with at least 1 year of follow-up (n = 104), the effect of weight loss on comorbidities and quality of life is demonstrated in Tables 8 and 9. Ninety-seven percent of patients available for follow-up said they would choose laparoscopic RYGBP again if given the opportunity.
Figure 11. Excess weight loss, 0 to 30 months.
Figure 12. Change in body mass index, 0 to 30 months.
Table 8. CHANGE IN OBESITY-RELATED COMORBIDITY
CAD, coronary heart disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; HTN, hypertension; OA/DJD, osteoarthritis/degenerative joint disease; OHS, obesity hypoventilation syndrome.
Table 9. CHANGE IN QUALITY OF LIFE

DISCUSSION
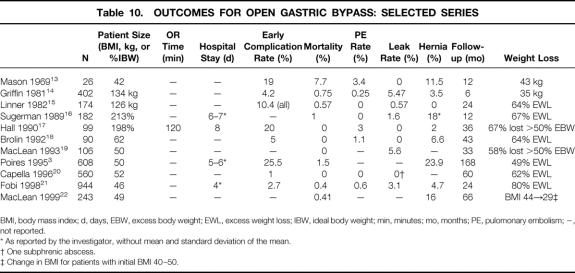
The gastric bypass operation for the treatment of morbid obesity was first described by Mason 13 in 1969 and initially consisted of a loop gastrojejunostomy and a stapled pouch of approximately 10% of gastric volume. During the past 30 years, numerous investigators have introduced modifications, including a Roux-en-Y gastrojejunostomy to prevent bile reflux, a reinforced staple line or an isolated gastric pouch (stapled and divided) to prevent disruption, a smaller pouch (15–30 mL), various lengths of Roux limb segments, and various types of banded pouch outlets. RYGBP has become the surgical procedure of choice for morbid obesity in North America because of its good long-term weight loss, excellent patient tolerance, and acceptable short- and long-term complication rates. Table 10 lists contemporary outcomes for RYGBP performed by many of the investigators who have contributed significantly to the field of bariatric surgery in the past two decades. 3,13–22 New approaches to gastric bypass surgery should be compared to these benchmark outcomes.
Table 10. OUTCOMES FOR OPEN GASTRIC BYPASS: SELECTED SERIES
BMI, body mass index; d, days, EBW, excess body weight; EWL, excess weight loss; IBW, ideal body weight; min, minutes; mo, months; PE, pulomonary embolism; −, not reported.
* As reported by the investigator, without mean and standard deviation of the mean.
† One subphrenic abscess.
‡ Change in BMI for patients with initial BMI 40–50.
A laparoscopic approach to RYGBP may offer benefits that have been shown to occur with other recently introduced laparoscopic procedures, including a reduction in postoperative pain and complications, a shorter hospital stay, and faster recovery. High-risk morbidly obese patients with multiple comorbidities may in particular benefit from a less invasive approach because they are more vulnerable to cardiopulmonary and wound-related complications.
A laparoscopic approach to RYGBP was first described by Wittgrove et al. 12 Their technique involves creation of a 15- to 30-mL gastric pouch isolated from the distal stomach, a 21-mm stapled, circular anastomosis, a 75-cm retrocolic, retrogastric Roux limb, and a stapled side-to-side jejunojejunostomy. They have reported on their experience with 75 patients with 3 to 30 months of follow-up. 23 The operating time was 159 to 343 minutes. The mean hospital stay and recovery time were 2.8 days (range 2–75) and 15 days (range 7–30), respectively. Excess weight loss at 12 to 30 months was 81% to 95%. The incidence of major complications was 11%, and the leak rate was 4/75 (5%). There were no deaths. Most comorbidities, such as hypertension and non-insulin-dependent diabetes mellitus, were either eradicated or significantly improved. They have recently reported on their experience with 500 patients with a 5-year follow-up, demonstrating similar results and excess weight loss in the 70% to 80% range. 24 Other investigators have reported various laparoscopic approaches to gastric bypass with similar benefits but relatively short follow-up. 25–27
In this study, 275 patients underwent laparoscopic RYGBP with an acceptable early complication rate (3.3% major, 27% minor), a low conversion rate (1%), a short median hospital stay (2 days), and rapid recovery (21 days). The excess weight loss at 24 and 30 months was 83% and 77%, respectively, and resulted in significant improvement in comorbidities and quality of life.
Surgical complications in our series appeared to be comparable to those in the open series (see Table 10). Our early major complications were predominately related to sepsis from anastomotic leaks, pulmonary embolus, and bowel obstructions. The overall incidence of these complications appears consistent with reports in the literature, although a clear incidence of bowel obstruction is not reported. Major cardiopulmonary complications were noticeably absent. Our strategy of leak prevention included a two-layer closure of the gastrojejunostomy without tension, intraoperative endoscopic evaluation, early postoperative radiographic evaluation, patient counseling regarding warning symptoms, and close follow-up in the first 3 weeks after surgery.
An unusual number of subclinical leaks were uncovered in this series; in other series, they may be either not recognized (and resolve) or not reported as clinically important. Whether they are a result of the laparoscopic anastomotic techniques (EEA and GIA), or just early detection of subclinical leaks that would otherwise go undetected, is not clear. Our nonoperative management approach for these subclinical leaks appears to have been reasonable, because none resulted in subsequent peritonitis or abscess, although one patient developed a gastrogastric fistula that spontaneously closed. The three early postoperative bowel obstructions are for the most part preventable and not necessarily related to the laparoscopic technique. Since the occurrence of the Roux limb herniation through the mesocolon defect, we have routinely closed the defect with sutures to the jejunum and have not encountered another occurrence. Since the occurrence of the two partial obstructions at the afferent side of the enteroenterostomy, we have placed an antiobstruction suture as described by Brolin 28 and have not seen another similar obstruction.
Postoperative bleeding (3.3%) from intraabdominal or gastrointestinal sources may have been a result of our stapling technique. Since we downsized to 3.5-mm staples on the stomach and 2.5-mm staples on the small bowel, we have had only one postoperative bleed in the last 100 patients that required transfusion. The type, incidence, and severity of late complications or side effects appear to be similar to those encountered for open gastric bypass.
Our data, supported by the other series of laparoscopic gastric bypass operations, suggest notable differences in the incidence of some important nontechnical complications between open and laparoscopic techniques. Wound-related complications of open RYGBP, including wound infections, incisional hernias, and dehiscences, are common and account for significant short- and long-term problems and cost. In one study of open RYGBP that specifically evaluated for wound complications, the incidence was 15%, and nearly one third were serious enough to delay hospital discharge. 29 In that same study, the rate of incisional hernia, which nearly always requires surgical repair, was 16.9%. Our data suggest that the laparoscopic approach significantly reduces both the incidence and severity of these troublesome wound complications.
Another potential advantage of the laparoscopic approach for gastric bypass is the reduction in cardiopulmonary complications, which occurred after the introduction of laparoscopic cholecystectomy. 6,7 Although cardiopulmonary complications after open RYGBP are rare, they are an important contributor to major complications and death. 30 Further, patients with obesity-related respiratory dysfunction have a fivefold increase in perioperative death after open RYGBP. 30 Because of the relatively low frequency and inconsistent reporting, direct comparison of rates of cardiopulmonary complications between this series and open series is not feasible. Thus, we can make no significant conclusions regarding decreased rates of cardiopulmonary complications with the laparoscopic approach. The relatively uncommon requirement for intensive care unit stay (2.5%) in our series suggests that cardiopulmonary compromise may be less common after laparoscopic gastric bypass.
Comparing indices of recovery between open and laparoscopic approaches is difficult because relatively few open gastric bypass studies include precise information on such indicators as operating time, hospital stay, and duration of convalescence. The laparoscopic approach appears to result in rapid recovery in terms of the indices evaluated in our study, allowing patients to return to full activity quickly. This is particularly important in the morbidly obese patient, who is often limited in activities of daily living before surgery and thus may have significant difficulty after surgery as well.
The important outcomes relating to the goal of bariatric surgery such as weight loss and improvement in comorbidities and quality of life appear to be equally favorable for laparoscopic gastric bypass. Long-term follow-up, however, will be necessary to confirm that the laparoscopic approach is equally enduring. Because the surgical principles are the same for both approaches, the long-term outcomes are likely to be similar.
The laparoscopic approach is technically challenging but with experience can be mastered. As with most complex laparoscopic procedures, the learning curve is steep, and long operating times are required. Wittgrove et al. 12 have found that with experience, operating times can be reduced to close to those for open RYGBP. Our operating times have gradually decreased with experience and approach those for open RYGBP. The laparoscopic approach is technically more difficult in superobese patients, especially those with extensive abdominal fat. Our current limit is a BMI of 70; this is due primarily to inadequate instrument length and poor exposure in these patients with extensive intraabdominal fat. Finally, the laparoscopic approach may be exceedingly difficult in patients with enlarged livers because of inadequate exposure of the esophagogastric junction.
Surgeon preparation is an important key to success with this challenging, advanced laparoscopic procedure. The surgeon must first be familiar with management of the bariatric patient, including appropriate indications for surgery, preoperative evaluation, perioperative management, and long-term follow-up care. Advanced laparoscopic skills, including two-handed technique and laparoscopic stapling and suturing, are required. Animal laboratory experience and preceptoring by an experienced surgeon may be helpful. Both fundamentals of bariatric surgery and advanced laparoscopic surgery should be mastered before performing laparoscopic gastric bypass.
CONCLUSION
The results of this study indicate that laparoscopic RYGBP is technically feasible and safe. It is associated with a low rate of perioperative complications, a short hospital stay, and rapid recovery compared with the expected results of open RYGBP. It is, however, a technically formidable operation requiring long operating times and a steep learning curve. Laparoscopic RYGBP is a promising bariatric procedure with potentially significant advantages over the open approach, but further evaluation is necessary to determine long-term weight loss and complications.
Discussion
Dr. Harvey J. Sugerman (Richmond, Virginia): This is the first large series of laparoscopic gastric bypass procedures from an academic medical center. I was initially skeptical about the safety of performing the gastric bypass procedure laparoscopically. But having seen you do this with two procedures, on two occasions, live, in front of an audience of surgeons, and completing both cases, including turnaround time, by 3 pm, I became a believer. Even more impressive is your willingness to tackle superobese patients with a BMI of 50 or greater. At our center, we started with a hand-assisted approach to learn how to do the operation under the leadership of Drs. DeMaria and Schweitzer in the first 25 cases, but now have performed the operation in 48 gastric bypass patients completely laparoscopically. I agree that the weight loss can be superimposed on the open technique. But we have limited our eligibility criteria to a BMI less than 50, primarily because we have had trouble with instruments that are too short. Could you address this issue?
As with other laparoscopic procedures, it is crucial that the operation be equivalent to the open approach. My primary concern has been that all three potential internal hernia defects must be closed laparoscopically; these are at the jejunojejunostomy, the opening in the mesocolon, and the opening between mesocolon and the jejunal mesentery, the so-called Petersen hernia. You weren’t closing these potential hernia sites initially, but one of your few disasters occurred as a consequence of this complication. What are you doing now?
Although laparoscopic vertical banded gastroplasty is possible and laparoscopic adjustable silicone gastric banding is being performed in huge numbers of patients in Europe, I agree with Dr. Schauer that, from evidence-based medicine through several randomized prospective trials, the gastric bypass is by far the most effective procedure, and I support your primary use of this procedure.
I am concerned with laparoscopic surgeons jumping into bariatric surgery without the full commitment these patients require. They need to know how to perform the procedures both open and laparoscopically. They need to know the importance of long-term follow-up, the risks of severe thiamine deficiency, the need for iron, calcium, B12, and multivitamin supplementation, et cetera. The patients need dietary counseling before and after surgery to help them optimize and maintain weight loss. But as you have also pointed out, it can also be a problem for bariatric surgeons to jump into laparoscopic procedures without adequate laparoscopic training. This is a very tough operation, I can tell you, for me personally. Perhaps you can expand upon that issue.
You suggest that one of the major advantages of the procedure is a shorter length of hospital stay and that most average open gastric bypass LOS is between 4 and 8 days. In our center, patients are discharged an average of 3.6 days after their open gastric bypass, and in many instances on the second day. This is the same as your series. Thus, I don’t think a decreased LOS will offset the increased cost of laparoscopy. I believe the major advantage will be to the patient and the health insurance companies years down the road with a markedly decreased need for incisional hernia repair or lysis of adhesions for small bowel obstructions. However, neither we, the surgeons, nor the hospital are being paid the increased costs associated with the laparoscopic procedure. Dr. Schauer, is the University of Pittsburgh giving you any grief about the increased cost of the operation at a time when all of us are being asked to reduce expenses? Have you been able to do anything about reimbursement on this issue?
Lastly, you predict that this approach will decrease cardiopulmonary morbidity. I am not sure I buy that hypothesis. Your data don’t really support it. Five percent of your patients developed severe atelectasis, with pleural effusions, pulmonary insufficiency, and pneumonia in other patients as well. We have shown that centrally obese patients already have an increased intraabdominal pressure. A prolonged laparoscopic procedure will increase this pressure further, pushing the diaphragm superiorly for a long time, compressing the lung. Proving your hypothesis will require a randomized, prospective trial comparing open to laparoscopic gastric bypass.
In summary, this paper will provide an even further impetus for laparoscopic bypass surgery. We now need the surgical equipment companies to provide the instruments we need to facilitate the procedure and optimize its safety.
Presenter Dr. Philip R. Schauer (Pittsburgh, Pennsylvania): It’s true, Dr. Sugerman, patients with a BMI greater than 50 are much more difficult to do. The operations take longer and the exposure is more difficult. We have learned some ways to get around some of these problems such as a large liver. We also are working with companies to develop longer instruments and longer scopes. It will be a matter of time before we can actually take on most superobese patients. Our current limit right now is a BMI of about 70.
We did have a bowel obstruction early in our series resulting from herniation of the Roux limb through the defect in the transverse mesocolon. We are now closing that defect in all three that you have mentioned.
Your third question related to the very important issue of training and experience. The laparoscopic surgeon with little bariatric training must learn what bariatric surgeons have to teach, and vice versa. This operation is technically very challenging and is one of the more difficult laparoscopic procedures to perform. It is very easy to get into trouble. So bariatric surgeons must learn and develop the appropriate laparoscopic skills.
The issue of cost always comes up. This initial study did not deal with it. It is probably expensive, considering the cost of the current instrumentation and the initial increase in operating time due to the steep learning curve. But I think there may be some cost savings, related not only to a reduction in subsequent repair of incisional hernias, but also to a reduction in ICU stay. In our series, only about 2.5% of patients required ICU stay, including high-risk patients and patients who had complications. In terms of reimbursement, there is no code now for laparoscopic bariatric surgery. It is going to probably take a few years for that to develop.
Dr. Walter J. Pories (Greenville, North Carolina): Only a few years ago there was wide agreement that the gastric bypass could never be done by laparoscopy. The patients were just too big, exposure was just too difficult, and it would be impossible to pull the gut way up behind the colon to the cardia. Furthermore, these patients, well known for their compromised cardiopulmonary status, would never tolerate a pneumoperitoneum. Well, this afternoon we have heard that the gastric bypass can be done with outcomes every bit as good and in some ways better than the open approaches.
I have watched Dr. Schauer operate. He is not only unusually facile, he has also developed an approach that is elegantly safe and amazingly efficient. The first operation I observed, on a patient who weighed over 400 pounds, took about 2 hours. The operation on the second patient took 15 minutes longer.
It is time that more surgeons address the epidemic of obesity. Of the 5 million Americans who are morbidly obese, most are plagued by comorbidities that limit the length and quality of their lives. For most, surgery is the only effective therapy; medical approaches almost always fail.
Our experience with 831 patients done with the open approach followed rigorously for as long as 16 years demonstrates a durable weight loss of 100 pounds. No other approach, be it diets or drugs or exercise or behavioral modification, can produce such results.
In a diabetic patient [slide], the results are dramatic. Based on our patients, the surgical approach restores euglycemia in 83% of diabetics. That means a total remission of diabetes. It restores euglycemia in 99% for those who have impaired glucose tolerance. It prevents progression of occult diabetes. And in a matched group, it improved the mortality rate in the morbidly obese diabetics from 4.5% to 1% per year.
I think we can safely conclude now after 16 years that diabetes in the morbidly obese is a surgical disease. There is only one caveat: bariatric surgery is not as easy as it seems. It is not just the operation that is hard. The workup, the perioperative care, and the follow-up are also demanding. I urge you to offer this service at your institution. And if you do, learn it first from Dr. Schauer. You won’t find a finer example.
Dr. Schauer: Dr. Pories makes a number of points with which I must agree. Thank you.
Dr. Lloyd D. Maclean (Montreal, Quebec, Canada): On a scale of 1 to 10 of ascending complexity, I think a laparoscopic cholecystectomy would be 3 and I suspect this operation would be something like 8 or 9. I commend the authors for their very low morbidity and even lower mortality rate. It is an accomplishment.
My reason for rising is that I have been able to follow 89% of patients in a group of 243 followed a minimum of 3 years to a maximum of 8.4 years with a mean of 5.5 years. There is good and bad news, I think, if you follow these patients long enough.
The good news is in the morbidly obese patients. There we found that 93% got a satisfactory result—that is, a BMI less than 35 kg/m2. Only 7% failed (that is, they failed to lose at least 50% of their excess weight), and 60% got an excellent result (that is, a BMI < 30 kg/m2).
The results in the superobese are quite different, especially those who are followed a long time, and there is definite weight gain in all these operations. In the superobese, 57% in our experience got a satisfactory result (i.e., BMI < 35 kg/m2), and 26% surprisingly are excellent (i.e., BMI < 30 kg/m2), and we were unable to predict that preoperatively.
We remain skeptical about lengthening the intestinal bypass. We have done a series with a short and a long loop. While it is statistically significant, I suspect it won’t be clinically so.
I have three or four very brief questions. We think pouch size is critical to this operation. We make a very small pouch of 10 to 15 cc, and make it so it is in the dependent position so it empties and doesn’t enlarge with time. We think those are important points. We have found iron deficiency very difficult to look after in the long haul, even with prophylactic iron by mouth. Would you agree that the quality of life is correlated to the approximation to normal weight in these patients? That certainly has been our experience. Before I sit down, I have got to admit we have a 16% rate of ventral hernia. For that reason alone I think this is probably the way to go.
Dr. Schauer: We agree with Dr. MacLean regarding the importance of making a very small pouch. Regarding the issue of quality of life and how that relates to ultimate weight loss, I think it is important for us first to define what constitutes a “good” result. Actual weight loss may not necessarily be the most important factor. Instead, it may be the impact of the operation on the patient’s quality of life and the change in their comorbidities. There may be some patients who may have less weight loss, 30% or 40% of excess weight, but may still have a significant improvement in quality of life and in their comorbidities. Therefore, we may need to develop quality of life indicators more specific for these patients.
Dr. Robert Brolin (New Brunswick, New Jersey): First, although your results are superb, the one chink perhaps in the otherwise shining armor is that of the leak rate. In your experience and that of others who do laparoscopic gastric bypass, the leak rate of close to 5% is significantly higher than that published with the open approach. I wondered if you had any insight as to why this might be the case.
Second, I wonder if you see this approach paralleling that of cholecystectomy—that is, do you think that the laparoscopic approach to serious morbid obesity will eventually replace the open approach?
Dr. Schauer: When we started, Dr. Brolin, our biggest fear was having a leak. That is one of the most common, dreaded complications of this operation. So we began a strategy to prevent leaks and find them very early if possible. This involved checking the gastrojejunal anastomosis intraoperatively with an endoscope, looking for air leaks before we finished the operation. Before closing, we placed a drain posterior to the anastomosis. The day after surgery, we obtained an upper GI study. Postoperatively, we followed our patients very, very carefully. I think what happened was that we probably picked up some of these subclinical leaks that may otherwise have gone undetected. All of these subclinical leaks, which were 8 of the 12 leaks we had, were managed either by keeping the patient NPO for a few days and on IV antibiotics, or in a couple patients, by placing laparoscopic G-tubes in the gastric remnant and feeding them enterally for a few days. None of those resulted in any long-term complications, except one patient who had a gastrogastric fistula develop after a subclinical leak, but that fistula resolved. So perhaps we were picking up leaks that might not have ever become clinically significant and thus not reported in other series.
Your second question related to the issue of will the laparoscopic approach completely overtake the gastric bypass? I think it is going to take more outcome studies to provide adequate comparisons with the open approach. It is also going to take time for the bariatric surgical community and the laparoscopic surgical community to learn the other community’s trade so that they can become proficient in both bariatric surgical principles and laparoscopic techniques. It might take a few years for all this to happen.
Dr. Bruce D. Schirmer (Charlottesville, Virginia): Dr. Schauer, 79% of your patients had cardiovascular disease preoperatively and 17% were ASA class IV. What are your criteria for postoperative surgical intensive care monitoring of your patients?
Second, patients who lose weight, whether by dietary or surgical means, have about a 30% incidence of gallstone formation with rapid weight loss of over 50 pounds. You have chosen to take gallbladders out in patients with preoperatively proven gallstones. Are you contemplating either offering prophylactic cholecystectomy or Actigall? Have you seen any patients in your series already who have returned with postoperative gallstones that needed cholecystectomy?
Twenty-eight percent of your patients had fatty liver at the time of surgery. Did you actually see any patients with cirrhosis? Did the biopsies show any patients with nonalcoholic hepatitis? If so, what happened to those patients?
My final question has to do with the role of endoscopy in this operation, as a bariatric surgeon. I know you have changed your technique now to using a GIA stapler for the proximal anastomosis. Do you still do intraoperative endoscopy? I would like you to comment on the role of the bariatric surgeon as an endoscopist.
Dr. Schauer: Regarding the ICU stay, Dr. Schirmer, we carefully evaluated our patients preoperatively, obtained the appropriate consultations with the cardiology and pulmonary service, and tuned them up as best we could. In the vast majority of cases, we found that the patients just didn’t need postoperative ICU care. However, the higher-risk patients did have monitoring in a step-down unit as opposed to an ICU.
In terms of the gallstone issue, we evaluated all our patients preoperatively for gallstones. If they were present, we generally removed the gallbladder at the time of surgery. If they were not present, we offered them either postoperative Actigall or a watch-and-wait approach. Many of the patients to whom we offered Actigall refused to take it because of the side effects. In the series so far, we have had four patients who have come back and developed symptomatic gallstones. We removed their gallbladder laparoscopically. Interestingly, when we went back, there were very, very few adhesions in the abdomen.
Regarding the issue of liver disease, we have been pretty progressive at performing liver biopsies and evaluating this particular problem. In a few patients, we had biopsies come back with fibrosis and hepatitis. For the most part, they have really been subclinical. Our approach has been to watch these patients carefully. Many of them were diagnosed for the first time, and we sent them to our liver specialist at the University of Pittsburgh to evaluate chronically.
Regarding endoscopy, I think it is a very important and helpful adjunct to the operation. In all of these cases, we performed intraoperative endoscopy to check for leaks. In a few patients, we did find intraoperative air leaks, small little bubbles, at the gastrojejunal anastomosis; in these cases, we oversewed the microleaks, and for the most part those patients did quite well. I think out of about seven or eight who had intraoperative air leaks, one or two went on to develop a subclinical postoperative leak.
Dr. Bruce M. Wolfe (Sacramento, California): I note in the manuscript that your protocol calls for an upper GI to be done on the first postoperative day, which presumably is why you detected these subclinical leaks. Did you also detect the leaks that later became a major clinical problem? Most clinically apparent leaks of GI anastomoses are detected at day 4 or beyond. One of our great concerns is that the patient will already be at home when the leak occurs, and a delay in getting them back and appropriately treated may arise.
Dr. Schauer: For about the first 225 patients, Dr. Wolfe, our postoperative upper GI study was negative for leaks, including those patients that did develop a clinical leak, so we were going to abandon the upper GI studies. On the very next case, we had an incidental leak identified by the upper GI studies despite a negative intraoperative check. The patient was totally asymptomatic. We were able to take her back to surgery that same day. We closed the leak laparoscopically with a single stitch which proved sufficient. So at this point I am reluctant to abandon using the postop upper GI because it did help us, at least in that one patient.
Dr. Henry Buchwald (Minneapolis, Minnesota): I believe it was an unnecessary ploy to start your paper with a list of the complications of open bariatric surgery—a quoting of exaggerated and not unusual percentages for complications. There are many series that report data for open bariatric surgery with far, far, far less than the complication rates you projected—e.g., 5% incisional hernia rate, 5% wound infections, and so on.
I would like to reiterate what Dr. Brolin said, that 1.3% and 4.4% overall leak rates for laparoscopic surgery are very high in comparison to the open technique. I would like to compliment you at the same time, since these leak rates are extremely low for the laparoscopic technique.
Pulmonary emboli, I believe the cause of your one fatality, may be increased by the laparoscopic technique because you have the patient in a reversed Trendelenburg position for several hours under conditions of increased intraabdominal pressure. Could you please comment on this?
Having said what I have said, I do believe that laparoscopic bariatric surgery is indeed the wave of the future. And you are certainly one of the leaders in this field.
Finally, can you just give us a little clue as to what those minor complications were that exceeded 50% in your series?
Dr. Schauer: Dr. Buchwald, I agree with you that the issue of venous thrombosis and pulmonary embolism in the morbidly obese patient is a very important subject. I think today we still don’t know all the answers—for example, what anticoagulant agents to use and at what doses. In our program, we were, I think, quite aggressive in using pneumatic compression devices as well as subcutaneous heparin. We used 5,000 U twice a day. Perhaps we are underdosing our patients. I think you are right about the issue of pneumoperitoneum decreasing venous return. Several studies have documented a significant decrease in venous return during a laparoscopic procedure. However, there may be some benefits of laparoscopy—for example, patients ambulate quite quickly after laparoscopic surgery—that may counteract some of those defects. In our lab, we looked at the issue of postoperative hypercoagulable states and found that in a swine model, animals undergoing a laparoscopic cholecystectomy had a relative decrease in their hypercoagulable state compared to animals that had a laparotomy with cholecystectomy. This issue really is unresolved yet and there are arguments on both sides of that issue.
Regarding the long-term complication rate of 50%, that was—as you will see in the manuscript—quite an inclusive list. We included everything from postoperative nausea to side effects that most would consider transient and typical for open gastric bypass as well. Most of these were quite minor and had very little impact on the overall quality of life of the patient.
Footnotes
Correspondence: Philip R. Schauer, MD, Dept. of Surgery, University of Pittsburgh, Presbyterian University Hospital, C-800, 200 Lothrop St., Pittsburgh, PA 15213-2582.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail: schauerpr@msx.upmc.edu
Accepted for publication April 2000.
References
- 1.Sugerman HJ, Kellum JM, Engle KM, et al. Gastric bypass for treating severe obesity. Am J Clin Nutr 1992; 55: 560S–566S. [DOI] [PubMed] [Google Scholar]
- 2.Benotti PN, Forse RA. The role of gastric surgery in the multidisciplinary management of severe obesity. Am J Surg 1995; 169: 361–367. [DOI] [PubMed] [Google Scholar]
- 3.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy of adult-onset of diabetes mellitus. Ann Surg 1995; 222: 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yale CE. Gastric surgery for morbid obesity. Complications and long-term weight control. Arch Surg 1989; 124: 941–946. [DOI] [PubMed] [Google Scholar]
- 5.Pories WJ, MacDonald KG Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-year follow-up. Am J Clin Nutr 1992; 55 (suppl 2): 582S–585S. [DOI] [PubMed] [Google Scholar]
- 6.Williams LF Jr, Chapman WC, Bonau RA, et al. Comparison of laparoscopic cholecystectomy with open cholecystectomy in a single center. Am J Surg 1993; 165: 459–465. [DOI] [PubMed] [Google Scholar]
- 7.Buanes T, Mjaland O. Complications in laparoscopic and open cholecystectomy: a prospective comparative trial. Surg Lap Endo 1996; 6: 266–272. [PubMed] [Google Scholar]
- 8.Schauer PR, Luna J, Ghiatas AA, et al. Pulmonary function after laparoscopic cholecystectomy. Surgery 1993; 114: 389–399. [DOI] [PubMed] [Google Scholar]
- 9.Schauer PR, Sirinek KR. The laparoscopic approach reduces the endocrine response to elective cholecystectomy. Am Surgeon 1995; 61: 106–111. [PubMed] [Google Scholar]
- 10.Gastrointestinal surgery for severe obesity. National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 1992; 55: 615S–619S. [DOI] [PubMed] [Google Scholar]
- 11.Moorehead MK, Oria HE. Bariatric analysis and reporting outcome system (BAROS). Obes Surg 1998; 8: 487–499. [DOI] [PubMed] [Google Scholar]
- 12.Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg 1994; 4: 353–357. [DOI] [PubMed] [Google Scholar]
- 13.Mason EE, Ito C. Gastric bypass. Ann Surg 1969; 170: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffen WO, Bivins BA, Bell RM, Jackson KA. Gastric bypass for morbid obesity. World J Surg 1981; 5: 817–822. [DOI] [PubMed] [Google Scholar]
- 15.Linner JH. Comparative effectiveness of gastric bypass and gastroplasty. Arch Surg 1982; 117: 695–700. [DOI] [PubMed] [Google Scholar]
- 16.Sugerman HJ, Londrey GL, Kellum JM, et al. Am J Surg 1989; 157:93–102. [DOI] [PubMed]
- 17.Hall JC, Watts JM, O’Brien PE, et al. Gastric surgery for morbid obesity: the Adelaide Study. Ann Surg 1990; 211: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brolin RE, Kenler HA, Gorman JH, Cody R. Long-limb gastric bypass in the superobese: a prospective randomized trial. Ann Surg 1991; 215: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLean LD, Rhode BM, Sampalis J, Forse RA. Results of the surgical treatment of obesity. Am J Surg 1993; 165: 155–162. [DOI] [PubMed] [Google Scholar]
- 20.Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass. Am J Surg 1996; 171: 74–79. [DOI] [PubMed] [Google Scholar]
- 21.Fobi MAL, Lee H, Holness R, Cabinda D. Gastric bypass operation for obesity. World J Surg 1998; 22: 925–935. [DOI] [PubMed] [Google Scholar]
- 22.MacLean LD, Rhode B, Nohr CW. Late outcome of isolated gastric bypass. Ann Surg 2000; 231: 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittgrove AC, Clark GW, Schubert KR. Laparoscopic gastric bypass, Roux-en-Y: technique and results in 75 patients with 3–30 months follow-up. Obes Surg 1996; 6: 500–504. [DOI] [PubMed] [Google Scholar]
- 24.Wittgrove AC, Clark GW, Schubert KR. Laparoscopic gastric bypass, Roux-en-Y: the results in 500 patients with 5-year follow-up. Obes Surg 2000; 10: 233–239. [DOI] [PubMed] [Google Scholar]
- 25.Lonroth H, Dalenback J, Haglind E, Lundell L. Laparoscopic gastric bypass. Another option in bariatric surgery. Surg Endosc 1996; 10: 636–638. [PubMed] [Google Scholar]
- 26.Gagner M, Garcia-Ruiz A, Arca MJ, Heniford TB. Laparoscopic isolated gastric bypass for morbid obesity. Surg Endosc 1999; S19: S6. [Google Scholar]
- 27.Nguyen NT, Ho Hung S, Palmer L, Wolfe B. A Comparison of laparoscopic versus open gastric bypass for morbid obesity. J Am Coll Surg 2000; 191: 149–155. [DOI] [PubMed] [Google Scholar]
- 28.Brolin RE. The antiobstruction stitch in stapled Roux-en-Y enteroenterostomy. Am J Surg 1995; 169: 355–357. [DOI] [PubMed] [Google Scholar]
- 29.Kellum JM, DeMaria EJ, Sugerman HJ. The surgical treatment of morbid obesity. Curr Prob Surg 1998; 35: 796–851 [DOI] [PubMed] [Google Scholar]
- 30.Mason EE, Tang S, Renquist KE, et al. A decade of change in obesity surgery. Obes Surg 1997; 7: 189–197. [DOI] [PubMed] [Google Scholar]