Abstract
Objective
To compare patients with gallbladder cancer presenting for therapy with and without prior operation elsewhere to determine if an initial noncurative procedure alters outcome.
Summary Background Data
Nihilism has traditionally surrounded treatment of gallbladder cancer, particularly since the majority of cases are discovered during exploration for presumed gallstone disease when unsuspected cancers cannot be handled definitively and tumor is often violated.
Methods
Presentation, operative data, complications, and survival were examined for 410 patients presenting between July 1986 and March 2000. In particular, the 248 patients presenting for therapy after prior operation elsewhere were compared with the remainder who presented without prior operation to determine if an initial noncurative procedure alters outcome.
Results
Overall Outcome: 51 patients were inoperable, 92 were subjected to exploration and biopsy only, 135 to noncurative cholecystectomy, 30 to surgical bypass, and 102 to potentially curative resections consisting of portal lymph node dissection and liver parenchymal resections. Operative mortality was 3.9%. T-stage predicted likelihood of distant metastases and resectability. Median survival for resected patients was 26 months and 5-year survival was 38%, and for patients not resected, 5.4 months and 4% (P < .0001). Effect of Prior Operation: 22 patients subjected to potentially curative resection as the first surgical procedure were compared to 80 patients resected after prior exploration elsewhere. Mortality, complication, and long-term survival were the same. By multivariate analysis (Cox regression), resectability and stage were independent predictors (P < .001) of long-term survival, but prior surgical exploration was not.
Conclusion
Unresected gallbladder cancer is a rapidly fatal disease. Radical resection can provide long-term survival, even for large tumors with extensive liver invasion. Long-term survival can be achieved for patients presenting after prior noncurative surgical exploration.
Alfred Blalock wrote in 1924 that “in malignancy of the gallbladder when a diagnosis can be made without exploration, no operation should be performed, inasmuch as it only shortens the patient’s life.”1 This pessimistic view of gallbladder cancer is understandable because this rapidly growing tumor has a propensity for early dissemination. Furthermore, due to the proximity of the gallbladder to the liver and major vasculature, extensive hepatic resections are often required to eradicate local disease. Therefore, until recently most reports of cure of gallbladder cancer occurred “by accident” after complete excision of early stage disease by simple cholecystectomy for gallstones. In a review of the literature up to 1978, Piehler and Crichlow 2 reported only a 5% 5-year survival and a median survival of 5 to 8 months for 5,836 cases. Even for the 25% who were treated by resection with curative intent, only 16.5% survived 5 years. These results were echoed recently by a report from the French Surgical Association Survey of 724 carcinomas of the gallbladder, in which Cubertafond et al 3 reported a median survival of 3 months, 5-year survival of 5%, and 1-year survival of 14%. A review of gallbladder cancer from Australia revealed a 12% 5-year survival rate, with all survivors having stage I or II disease. 4 These dismal results reinforced the nihilism that surrounds this disease.
George Pack was the first to advocate radical liver resection as treatment for gallbladder cancer, 5 reporting in 1955 on the first three gallbladder cancers treated by right hepatic lobectomy and portal lymph node dissection. This radical approach was not embraced until recently, however, because of the risk of such extensive surgery. As experience with liver resection for other cancers has demonstrated increasing safety of liver resections and biliary reconstructions, such major resections are increasingly performed for gallbladder cancer. 6–10 Data accumulated over the last decade have clearly suggested the utility of such a radical approach for selected patients with gallbladder cancer. However, there are many unresolved questions. Are such radical resections warranted for advanced stage disease? Which of the many staging systems for gallbladder cancer are the most useful for prediction of prognosis, for selection of patients for adjuvant therapy, and for stratification of patients in clinical trials? Most importantly, because a large number of patients are discovered to have gallbladder cancer during operative therapy for presumed gallstone disease, are reoperative radical resections warranted after prior noncurative cholecystectomy? The current study addresses these question using data from a tertiary cancer referral center.
MATERIALS AND METHODS
All patients seen at the Memorial Sloan-Kettering Cancer Center (MSKCC) in the 13-year period between July 1986 and March 2000 were identified in the Department of Surgery’s prospective hepatobiliary database. At MSKCC, care for gallbladder cancer is planned under the auspices of a multidisciplinary Hepatobiliary Disease Management Team, and new patients are discussed twice weekly at clinical-radiologic staging conferences. During this period, 410 patients were seen for consideration of therapy. Data for these patients were then extracted from the database and hospital and office charts, as well as interviews with patients. Data examined included demographics (including age, gender); clinical history, including history of prior therapy; pathology; hospital course, including complications; and outcome. Follow-up was by personal contact with the patient, the patient’s family, or the attending physician.
In particular, the 248 patients presenting for therapy after prior operation elsewhere (127 open cholecystectomies, 85 laparoscopic cholecystectomies, 36 other procedures) were compared with the remainder who presented without a prior operation to determine if prior noncurative procedure altered outcome. Of note, there is evidence that tumors classified as T2 (through the muscularis mucosa) or deeper (T3, T4) require an operation more extensive than a cholecystectomy for a potentially curative result. Therefore, unlike most series in the literature, T2, T3, or T4 gallbladder cancers treated by simple cholecystectomy are not considered curative resections in this report.
Definitions
Nomenclature for extent of resection is as follows. A right trisegmentectomy refers to resection of Couinaud’s segments 11 4, 5, 6, 7, 8; a left trisegmentectomy refers to resection of segments 2, 3, 4, 5, 8; a right lobectomy is resection of segments 5, 6, 7, 8; and a left lobectomy is resection of segments 2, 3, 4. An extended cholecystectomy includes resection of segments 4B and 5. Lymph node sampling or lymph node dissection consists of resection of lymphatic tissues in the porta hepatis, portal-caval, and high parapancreatic areas. When bile duct resection was performed, the goals were usually to eradicate tumor at the cystic duct–common duct junction, and to facilitate the portal-lymph node dissection.
Clinical staging of disease was performed by AJCC staging 12 and by modified Nevin staging criteria. 6,13 The major difference in these staging criteria is the difference in significance attributed to nodal metastases and extensive direct liver invasion. In the AJCC staging system, N1 metastases for T1–T3 cancers is classified as stage III, while nodal metastases weigh more heavily in the modified Nevin classification and are classified as stage IV. For tumors that invade more than 2 cm into the liver (T2), the AJCC system classifies these as stage IV and equates them with patients with metastatic disease; the modified Nevin classifies these as stage III, distinct from distant metastases. While this report demonstrates the modified Nevin staging as the most predictive of prognosis, T staging by AJCC criteria is the most practical for day-to-day use, because most patients will present after simple cholecystectomy when only the extent of penetration through the gallbladder wall is certain. T2 tumors are those that have invaded through the muscularis layer into perimuscular connective tissue, but not through the serosa. T3 tumors are those penetrating through the serosa into an adjacent organ, but less than 2 cm into the liver. T4 tumors are those with greater than 2 cm invasion into the liver or those that involve more than one adjacent organ.
Statistics
The chi-square test or Fisher exact test, where appropriate, were used for univariate comparisons. For univariate survival analysis, survival plots were by Kaplan-Meier, and comparisons by log-rank. Multiple logistic regression was used to incorporate all of the explanatory variables in the same model. 14 Using multiple logistic regression in the SPSS Statistical package (SPSS, Inc., Chicago, IL), prognostic factors were determined for the hazard rate of complications. Differences were considered significant at the P = .05 level. All deaths within 30 days of surgery or during the perioperative hospitalization were considered surgical mortality.
RESULTS
Demographics
In the 13-year study period, 410 total patients with gallbladder cancer were seen at MSKCC. The median age for the entire group was 65 years, with a gender distribution of 137 males (33%) and 273 females. Two hundred and eighty-nine patients (70%) have died, with median survival for nonsurvivors of 6.6 months.
Presentation
Symptoms
The most common presenting symptom was pain (n = 264, 64%). Jaundice was also common and occurred in 151 patients (37%). These symptoms were often indistinguishable from gallstone disease, and many patients were submitted to operations with the presumed diagnosis of symptomatic cholelithiasis, choledocholithiasis, Mirizzi syndrome, or acute cholecystitis. Weight loss (10%) was less common than in some previous studies, 15–17 possibly due to the fact that this was a population that presented at a tertiary referral center. A palpable mass was the presenting symptom in 5% of patients.
Prior Interventions
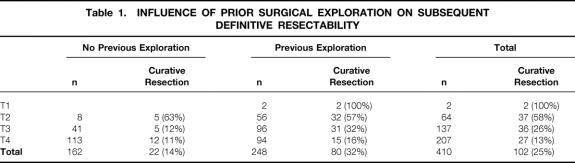
One hundred and twenty-three patients presented without prior intervention; 39 others presented after percutaneous biopsy or endoscopic manipulation, for a total of 162 patients who presented without prior surgical intervention. A total of 248 patients presented after previous surgical exploration: 212 after prior cholecystectomy (127 open cholecystectomies, 85 laparoscopic cholecystectomies), and 36 after surgical exploration without cholecystectomy. As summarized in Table 1, there were only two patients who presented after cholecystectomy with T1 (mucosal) tumors. The majority of patients, with T2 tumors, presented after previous cholecystectomy.
Table 1. INFLUENCE OF PRIOR SURGICAL EXPLORATION ON SUBSEQUENT DEFINITIVE RESECTABILITY
Definitive Therapy
Of the 162 patients presenting without previous surgical therapy, 51 were found by imaging to have unresectable disease and were not subjected to surgical exploration. Of the remaining 111 patients presenting without previous surgical intervention, 48 were proven by intraoperative biopsy to have unresectable disease and laparotomy was abandoned. Eighteen patients with nonresectable disease had a palliative cholecystectomy, while 19 were treated with a biliary bypass and four with a gastrojejunostomy. Twenty-two patients were treated with a potentially curative procedure.
Of the 248 patients presenting after previous surgical intervention, two had had a definitive simple cholecystectomy for T1 cancers; 76 others were found by further work-up to have nonresectable disease, and 28 more were not offered further therapy or refused further surgical therapy. One hundred forty two patients were subjected to new surgical exploration; 55 patients were subjected only to an operative biopsy that documented unresectable disease. Seven unresectable cases were treated by surgical bypasses (five gastrojejunostomies, two biliary bypasses). The remainder were treated by potentially curative resections (Table 2).
Table 2. OPERATIVE PROCEDURES FOR GALLBLADDER CANCER PERFORMED WITH CURATIVE INTENT
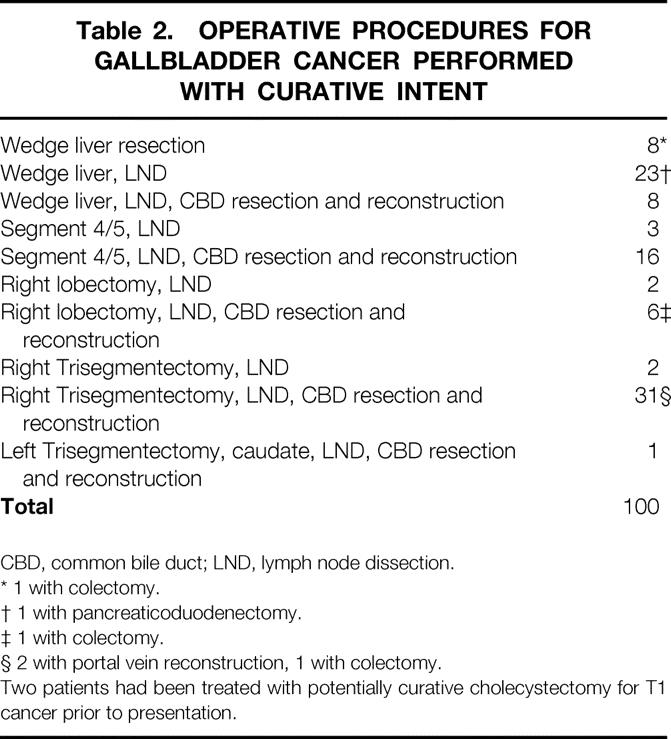
CBD, common bile duct; LND, lymph node dissection.
* 1 with colectomy.
† 1 with pancreaticoduodenectomy.
‡ 1 with colectomy.
§ 2 with portal vein reconstruction, 1 with colectomy.
Two patients had been treated with potentially curative cholecystectomy for T1 cancer prior to presentation.
Prior surgical exploration was not associated with a lower likelihood of definitive curative resection, when compared to patients with no prior operation (see Table 1). In fact, patients without prior surgical exploration were less likely to be resected. One explanation for this is the greater likelihood for biliary or vascular involvement by tumor in those patients presenting without prior surgical exploration. These patients had a higher rate of jaundice (54% vs. 25% in patients with prior surgical exploration), and required larger liver resections for extirpation of tumor (54% required lobectomy or more for resection of tumor versus 37% in patients presenting after prior surgical exploration). In addition, those presenting after a prior surgical exploration have had the benefits of surgical staging prior to referral, and for any given T-stage are therefore likely to be a better selected population. This possibility is substantiated by the fact that as a group, patients without prior exploration were more likely to have metastatic disease (69% versus 43%, P = .0001 by chi-square).
Of note, the two patients with T1 tumors presenting after simple cholecystectomies were not offered any other therapy. Twenty-eight patients with more advanced disease presenting after simple cholecystectomy without radiologic signs of unresectable disease also were not subjected to further therapy, either because the treating physician did not offer additional therapy or because the patients refused. These included 16 patients with T2 cancers, eight patients with T3 disease, and four cases of T4 cancers. These 28 cases were not considered curatively resected patients in the analysis of outcome.
The procedures performed as resections with curative intent are listed in Table 2. These included 58 cases treated with minor (<lobectomy) liver resections, and 42 treated with a lobectomy or a trisegmentectomy. Ten cases were treated without portal lymph node dissection, while the remainder were treated with a lymphadenectomy. Additional procedures performed on these patients included common bile duct excisions (n = 62), colectomy for direct tumor extension (n = 3), portal vein reconstruction (n = 2), and one pancreaticoduodenectomy.
Pathologic Considerations
The distributions of histologic subtypes in the current report (Table 3) is similar to previous publications from other centers. 18 Adenocarcinomas predominate. The majority of tumors were moderately well differentiated. The T-stage of disease correlated with the likelihood of nodal metastases and with peritoneal dissemination.
Table 3. PATHOLOGIC CONSIDERATIONS
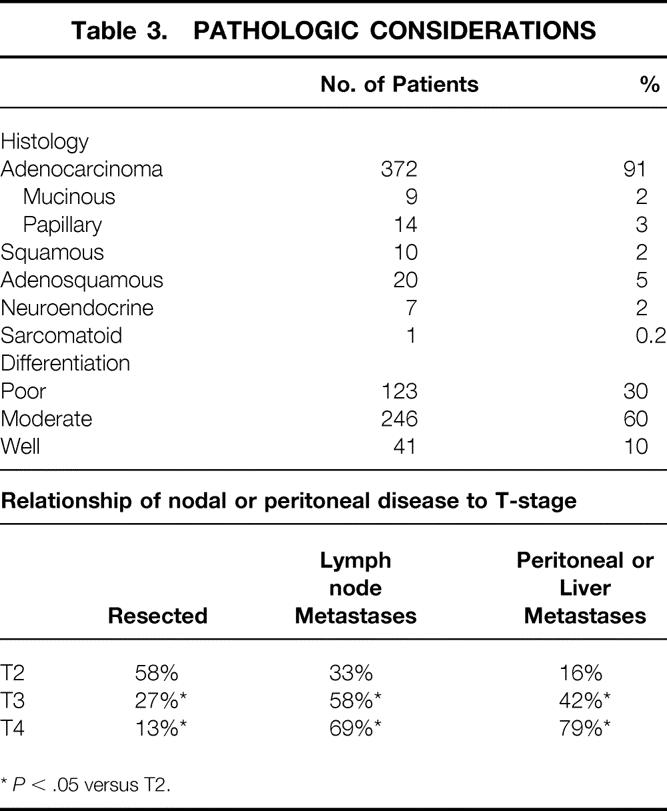
*P < .05 versus T2.
Outcome of Resected Patients
Perioperative Results
Median operative time was 300 minutes (range 64–530). Median hospital stay was 10 days (range 4–41). Forty-five complications occurred in 29 patients after potentially curative resections, including four deaths (Table 4). Two deaths were due to hepatic failure, and two were due to pneumonia and pulmonary failure. All deaths occurred in patients subjected to resection of a lobe of liver or more (Table 5). Preoperative jaundice or operative blood loss over 1 L were additional risk factors for death.
Table 4. COMPLICATIONS AFTER RESECTION FOR GALLBLADDER CANCER
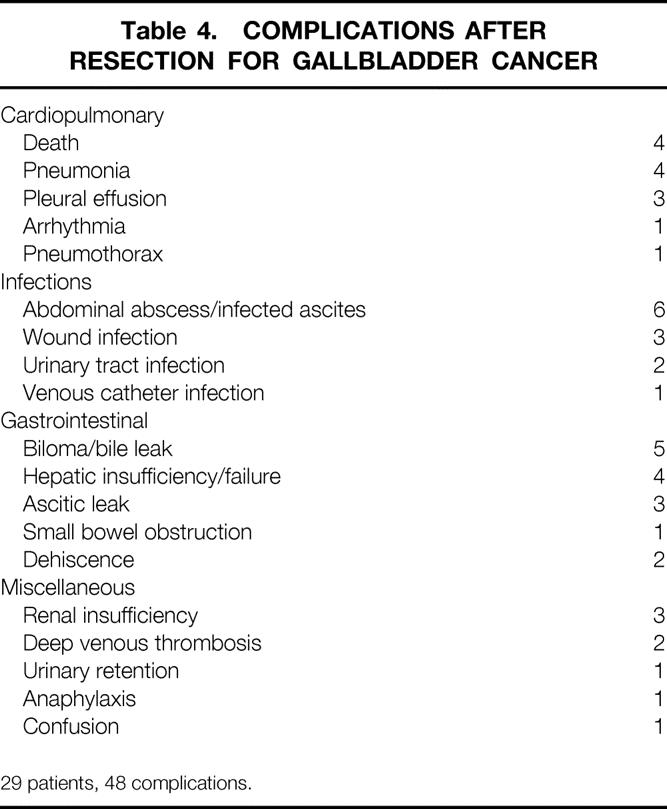
29 patients, 48 complications.
Table 5. FACTORS PREDICTIVE OF COMPLICATION OR DEATH AFTER RESECTION
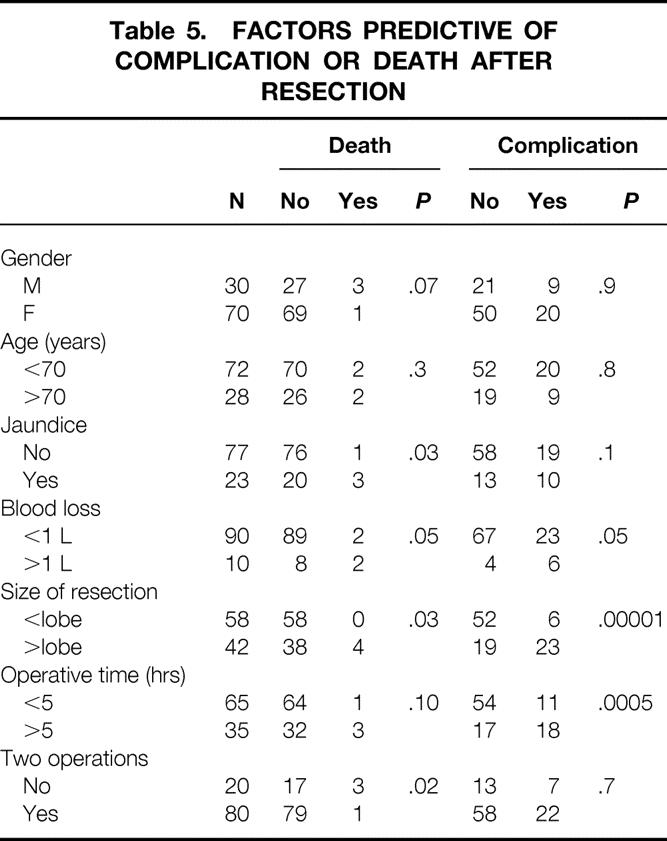
Intraabdominal collections were the most common complication (n = 11), consisting of sterile bilomas in five and abscesses in six (see Table 4). There were 12 instances of infectious complications, and eight instances of wound complications (infection, ascitic leak, or dehiscence). Blood loss over 1 L, resection of a lobe of liver or more, and operative time over 5 hours were factors associated with a higher risk of complications (see Table 5).
Long-Term Results After Resection
There is no doubt that resection has a major influence on outcome. Figure 1 demonstrates survival for patients resected with curative intent, compared with those treated with noncurative surgery or with no surgery. Median survival for those treated nonoperatively was 5.4 months and actuarial 5-year survival was 4%. Those treated with noncurative operation had a median survival of 8.0 months and an actuarial 5-year survival of 3%. Of the 308 patients treated without curative resection, only four were actual 5-year survivors. Three of these four patients have since died. For the 100 patients treated with curative intent, the median survival was 26 months and the actuarial 5-year survival was 38%. Twenty of these 100 patients are actual 5-year survivors to date.
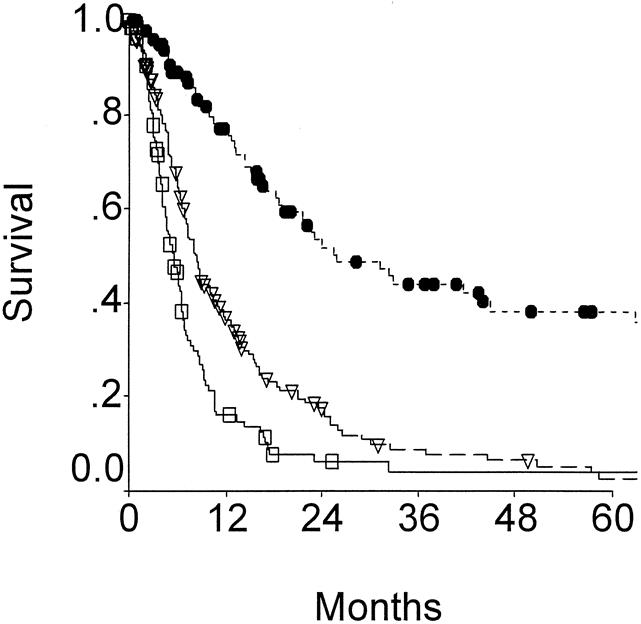
Figure 1. Overall survival for patients treated with no surgery (open box), simple cholecystectomy or bypass (open triangle), or resection (solid circles). Patients treated by surgical resection clearly demonstrated much improved outcome compared to those treated without surgery (P < .0001).
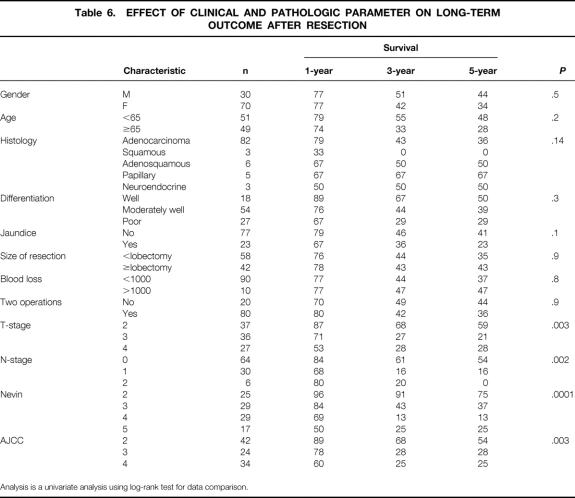
Table 6 lists the variables analyzed as potential predictors of adverse long-term outcome. Factors most influential on outcome by univariate analysis included T-stage (Fig. 2) and N- stage (Fig. 3). Patients with T2 tumors had a significantly more favorable outcome than patients with T3 or T4 tumors, but advanced T-stage did not preclude long-term survival. To date, five patients each in the T3 and the T4 groups have been actual 5-year survivors. In fact, even though patients with T4 tumors were less likely to be resected (see Table 1), outcome of resected patients with T4 tumors were similar to those for patients with resected T3 tumors (see Fig. 2). Patients with nodal metastases had a poor outcome (see Fig. 3). Of the 36 patients with node-positive disease treated by resection with curative intent, only two survived more than 5 years, and both subsequently died from recurrent disease.
Table 6. EFFECT OF CLINICAL AND PATHOLOGIC PARAMETER ON LONG-TERM OUTCOME AFTER RESECTION
Analysis is a univariate analysis using log-rank test for data comparison.
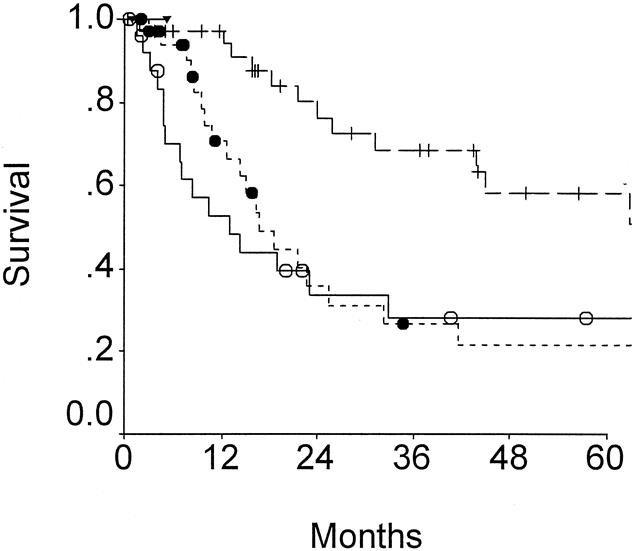
Figure 2. Survival according to T-stage of disease for patients resected of gallbladder cancer. T2 (cross), T3 (solid circles), and T4 (open circles) are compared (P = .003).
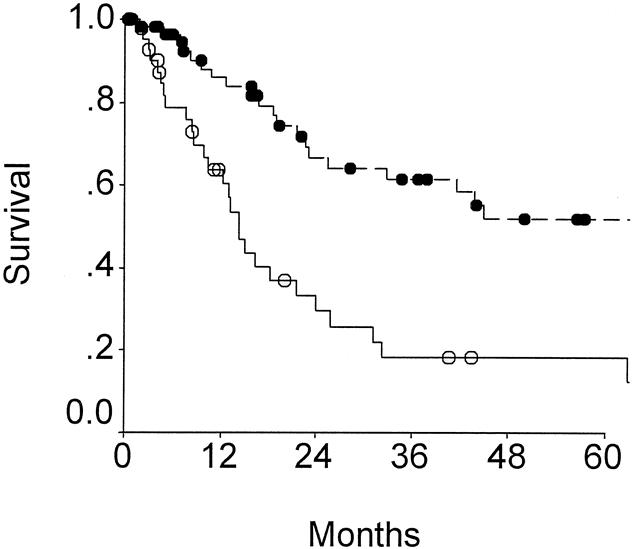
Figure 3. Outcome according to node status for patients resected of gallbladder cancer, showing positive (n = 36; open circles) and negative (n = 64; solid circles) for nodal metastases (P = .002).
Both the AJCC staging system (Fig. 4A) and the modified Nevin staging systems (see Fig. 4B) predicted long-term outcome. Due to the poor prognosis of nodal metastases, the modified Nevin staging appears to be superior. The Nevin staging system places greater weight on node-positive disease and less weight on direct liver extension by tumor than the AJCC or the Japanese Biliary staging systems.
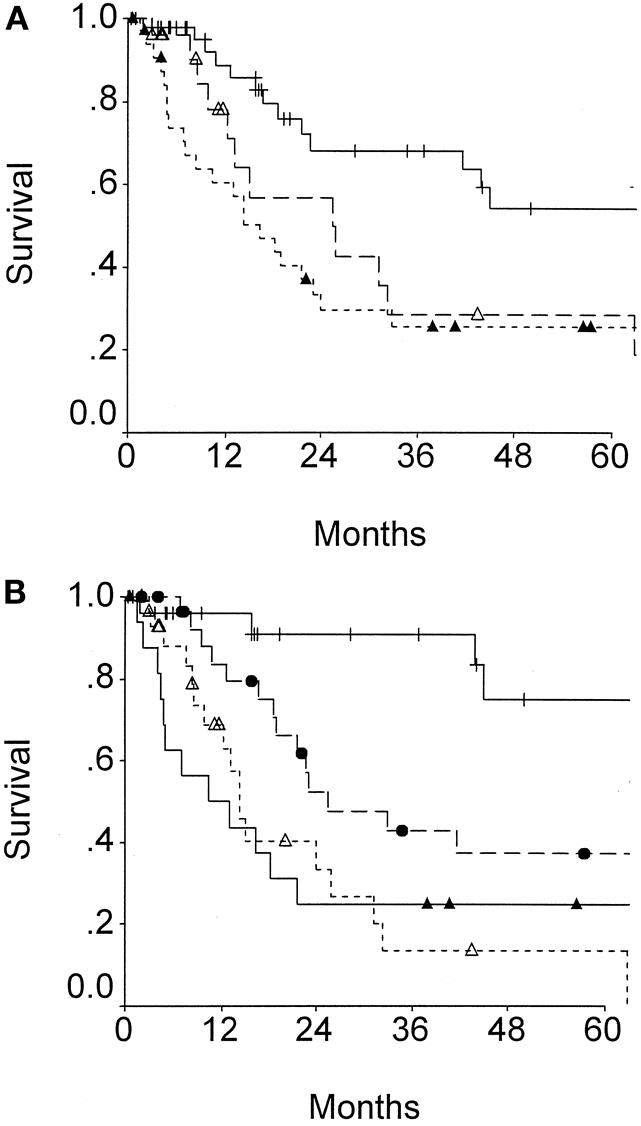
Figure 4. Survival of patients after resection for gallbladder cancer according to (A) TNM or (B) modified Nevin staging. (A) TNM stage 2 (cross), stage 3 (open triangles), or stage 4 (solid triangles);P = .003. (B) Modified Nevin stage 2 (cross), stage 3 (solid circles), stage 4 (open triangles), and stage 5 (solid triangles);P = .0001.
When multivariate analysis is performed using the variables deemed significant by univariate analysis as covariates, N-stage of disease and T-stage of disease remained independent predictors of adverse long-term outcome (Table 7). Prior exploration was not a predictor of adverse outcome.
Table 7. MULTIVARIATE ANALYSIS FOR PREDICTORS OF LONG-TERM OUTCOME FOR RESECTED PATIENTS

Reresection After Prior Noncurative Surgical Therapy
The current data also support the hypothesis that a radical reresection is reasonable therapy for the patients presenting after prior surgical therapy. Survival for those patients with T2 tumor treated with a radical reresection was compared to those 16 potentially curable patients not offered reresection or who refused reresection (Fig. 5). Five-year survival for those subjected to reresection was 61%, compared with 19% for those treated with simple cholecystectomy. The numbers of potentially resectable patients with T3 or T4 cancers who were not treated with radical reresection are too small for firm conclusions. However, of the eight patients with potentially curable T3 tumors treated with simple cholecystectomy, only one patient remains alive (no evidence of disease at 10 months). All four patients with radiographically resectable T4 cancers treated solely by cholecystectomy died within 11 months.
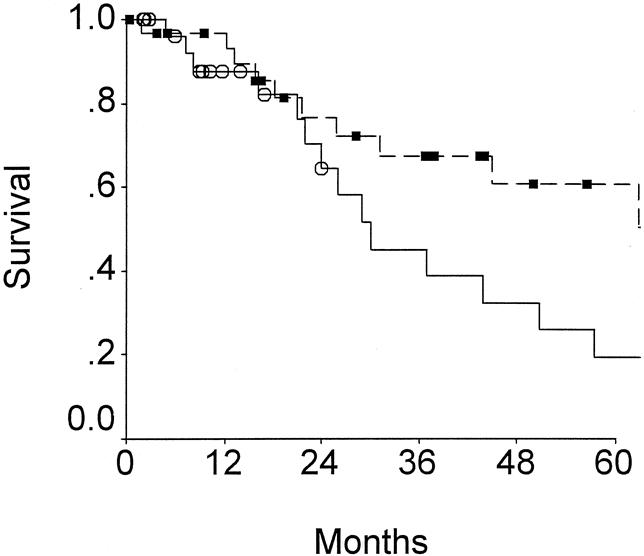
Figure 5. Outcomes of patients with T2 gallbladder cancers. Patients undergoing radical resection (box) are compared to patients undergoing cholecystectomy (open circle) (P < .05).
Survival after radical resection for patients presenting without prior surgical intervention and for patients presenting after prior surgical exploration are compared in Figure 6. There is no difference in outcome between those treated with one operation versus those treated with two operations. This should not be interpreted as indicating an equivalency of single versus staged resectional therapy, because the group presenting without prior surgery is a group with more cases of advanced disease (50% stage IV disease versus 18% in the group with prior surgery; 45% presenting with jaundice versus 16% in the group with prior exploration). When multivariate analysis is performed using modified Nevin staging, presentation with jaundice, size of liver resection, and two operations as covariates, Nevin staging and jaundice were independent predictors of outcome (see Table 7). Prior exploration was not a predictor of adverse outcome.
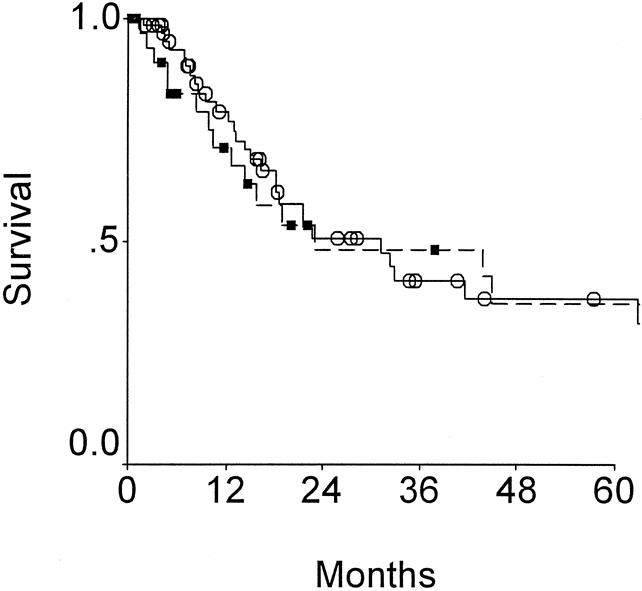
Figure 6. Effect of prior surgery on outcome for those patients resected with curative intent for gallbladder cancer. Survival for patients presenting with no prior surgical therapy (circles) are compared to those presenting for definitive therapy after prior surgical exploration (squares) (P = NS).
DISCUSSION
Much controversy exists about the optimum surgical procedure for treatment of gallbladder cancer. Recommendations have ranged from simple cholecystectomy to ultraaggressive resections consisting of combined major liver resection and pancreaticoduodenectomy. 19 The controversy exists because until recently, the operations required for complete resection of cancer were associated with high morbidity and mortality. 7,9 The reported complication rate after resection for gallbladder cancer has ranged from 5% to 54%7 and mortality from 0 to 21%. 6,7,19 In a multiinstitutional review of 1,686 gallbladder cancer resections, 7 cholecystectomy was associated with a 12.8% complication rate and 2.9% mortality, extended cholecystectomy with 22% morbidity and 2.3% mortality, and hepatic lobectomy with 48% morbidity and 8% mortality. Recently, however, increasing numbers of reports have documented increasing safety of extensive resections. Extended procedures are now associated with an operative mortality of less than 5%. 6,10,20–22 The current report is further evidence of the safety of extended resection. In this series of aggressively treated patients, only 29% suffered complications and there was only 4% mortality. These are acceptable figures for potentially curative therapy for a disease that, when unresected, is associated with a 5-month median survival.
Extended resections were once thought unnecessary for T2 tumors. These tumors by definition remain subserosal but have invaded through the muscular layer. It would at first seen logical that a simple cholecystectomy could be adequate treatment for T2 tumors. However, the hepatic plane of dissection in a simple cholecystectomy is generally a subserosal plane between the liver and gallbladder, where tumor may be violated. In the review of Yamaguchi and Tsuneyoshi, 23 25 patients had tumor extending into the subserosal layer, and 11 of these had positive microscopic margins after simple cholecystectomy. Additionally, T2 tumors have been reported to have an incidence of regional nodal metastases as high as 43%22 In the current study, the incidence of nodal metastases for T2 tumors is 33% (see Table 3). The most reasonable therapeutic operation for T2 gallbladder cancer therefore is radical cholecystectomy 16 to include a liver resection and a regional lymphadenectomy of the hepatoduodenal ligament. The utility of such a radical approach has been supported by data demonstrating that simple cholecystectomy for T2 tumor is associated with a 5-year survival of 20% to 40%, 8,23–25 while radical resections were associated with a 5-year survival upward of 80%. 8,10,22,25 Data from patients with T2 tumors in the current study are further support. Those with radiographically resectable disease but not subjected to repeat resection after simple cholecystectomy had a 5-year survival of 19%, while those treated with radical resections had a 5-year survival of 61% (see Fig. 5). Radical resection is not only safe but reasonable cancer therapy for those with T2 gallbladder cancers.
Greater controversy exists concerning treatment of locally advanced gallbladder cancer. In a number of collected reviews and multicenter collected series, surgical resection for T3 or T4 tumors rarely resulted in long-term survival. 2–4 In one series, 4 there were no long-term survivors among patients treated for T3 or T4 tumors, and the median survival for these patients was only 46 days. In most of these series, advanced disease was treated by limited resection, and the dismal results were as much due to the biology of the cancers as the difficulties in clearing locally advanced disease without resorting to a major hepatic resection. The last decade has provided much support for an aggressive approach by confirming the possibility for long-term survival after radical resection of locally advanced disease. Onoyama et al 26 recently reported a 44.4% 5-year survival for stage III disease after extended cholecystectomy. Other series employing radical resections have reported a 25% to 50% 5-year survival for patients with stage III and IV disease. 6,10,22,27 In the current series, the 5-year survival for T3 and T4 patients after radical resection was 21% and 28% respectively, with five actual 5-year survivors in each group. These data combine to justify a radical approach for T3 and T4 disease.
Many staging systems have been advocated for the classification of gallbladder cancer. The most useful are the modified Nevin system 6,13 and the AJCC/UICC TNM staging system. 28 The Japanese Biliary Surgical Society system 26 is also important because of the extensive literature on gallbladder cancer originating from Japan. All three systems weigh depth of liver involvement, extent of nodal metastases, and presence of distant metastases. The major difference is the relative emphasis of nodal metastases. Data from a number of studies originating from Japan has indicated the possibility of long-term survival after resection of node-positive gallbladder cancers. 8,9,29,30 Onoyama 26 reported a 5-year survival rate of 60% for patients having metastatic disease to N1 nodes. Shirai et al 8 reported a 45% 5-year survival for patients with positive regional nodes, documenting 9 patients surviving more than 5 years after radical resection. Western studies have reported few long-term survivors among patients with documented nodal metastases. 10,24,31 The current study also reports a dismal outcome for patients with lymphatic metastases. Whether this difference in outcome between Japanese and Western centers is due to a difference in anatomy, biology, or surgical technique is unknown. The data from the Japanese studies would support use of the classification system of the Japanese Biliary Surgical Society or the AJCC/UICC TNM staging system, where N1 disease is classified as stage II and stage III disease respectively. Data in the current study, and indeed from most Western series, would be more supportive of the modified Nevin classification. Nevin 13 originally classified patients into five stages, based primarily on the thickness of invasion, and combined patients with direct liver extension or distant metastasis into stage V. This staging system was later modified by Donahue et al, 6 whereby tumors with contiguous liver invasion were reclassified as stage 3 and noncontiguous liver involvement as stage 5. Stage 4 continued to include lymph node metastasis. A patient with bulky direct invasion of liver is more likely to be cured than a patient with nodal metastases. The modified Nevin classification is much more useful in selection of patients for adjuvant therapy and for stratification of patients in clinical trials.
A significant number of gallbladder cancers will present for definitive therapy after prior cholecystectomy. This is particularly common for early-stage tumors, where most cancers are diagnosed upon pathologic analysis after simple cholecystectomy. For patients treated initially with open cholecystectomy, past publications indicate that a radical reresection is warranted for tumors with a depth of penetration equal or greater than T2. Shirai et al 32 reported the 5-year survival rate after radical reresection for T2 tumors to be 90%, compared to 40% after simple cholecystectomy alone. De Aretxabala 25 reported the 5-year survival of patients treated by radical reresection for T2 tumors to be 70%, which is significantly improved over the 20% 5-year survival for 8 patients treated by simple cholecystectomy. The results of the current study are similar, and are strong support for such a reoperative approach. The current data add significantly to the previous studies, not only because of the large number of patients undergoing reoperative radical resection, but also because the study represents a significant experience in the era of laparoscopic resection. The results demonstrate that such reresections are reasonable after laparoscopic cholecystectomy. The only change in operative approach for the laparoscopically discovered gallbladder cancer is the need for excision of laparoscopic port sites during the reoperative procedure because of the possibility of port-site dissemination of disease. 33 This propensity for growth of gallbladder cancer with surgical wounds is not peculiar to the laparoscopic wound, and was well described for the laparotomy wound by Pack in his landmark publication in 1955. 5 The port-site excisions, however, are likely to be more a staging procedure than therapeutic. In the current series, all three patients who had microscopic cancer found during pathologic examination of the excised port sites have manifested subsequent peritoneal recurrence.
There have been suggestions that prognosis for patients subjected to two operations is less favorable than for patients treated with a single procedure. Gall et al 27 reported a median survival of 42 months for patients undergoing a curative resection at the first operation versus 13 months for those requiring two operations. Our current study demonstrates no difference in long-term survival after curative resection between those treated with one operation versus those treated with two. This should not be interpreted as indicating an equivalency of the two clinical courses, because the group presenting without prior surgery is a group with more cases of advanced disease. These data, however, do indicate that reoperative radical therapy is associated with the same long-term outcome that justifies resection of patients presenting without previous surgery. Furthermore, this outcome is sufficiently favorable, and the outcome of incomplete resection is sufficiently dismal, to justify radical reresection of incompletely treated gallbladder cancer.
Unresected gallbladder cancer is clearly a rapidly fatal disease. Radical resection is indicated for early-stage (T2) tumors, but can result in long-term survival even for tumors with bulky liver involvement (T3 or T4). Gallbladder cancer discovered during or after cholecystectomy for presumed gallstone disease deserves consideration for radical reresection. The high incidence of recurrence, particularly local recurrence, encourages active investigation of adjuvant therapy. Retrospective studies would suggest chemoradiation as a promising adjuvant for study 34–37 Because of the rarity of this cancer, however, clinical trials for this disease should be performed through cooperative multicenter efforts so that results may be accumulated with sufficient expedience to improve the care of these patients within our academic lifetime.
Discussion
Dr. Ronald K. Tompkins (Los Angeles, California): Dr. Fong and his associates have presented a detailed analysis of one of the largest single-institution experiences with gallbladder cancer in the United States. Their data clearly indicate that in selected patients even with advanced disease, radical resection offers significantly greater chance of survival than less radical procedures or nonoperative therapy.
While trying to convince us that gallbladder cancer is not a dismal disease, it remains a fact that only 14% of their 162 patients referred for initial operation were able to undergo potentially curative resections. Conversely, 32% of the patients referred after prior operation were able to undergo operation with curative intent. This latter group, as has been pointed out by Dr. Fong, was better selected by the referring surgeons, demonstrated by the fact that 70% with no prior operation had T4 lesions, whereas only 38% of previously operated patients had the same stage of disease.
The authors have pointed out very accurately in their manuscript the selection of these patients for resection. So we are not comparing each group with the other, we are comparing only those who were able to be resected in each group. I have some questions for the authors.
Have you identified a time period following prior operation where you might not reoperate due to an increased likelihood of intraabdominal spread of the tumor? In other words, is delay in referral to a tertiary center such as yours a risk factor in your data group?
Second, how do you manage the port sites in those patients referred after laparoscopic cholecystectomy where cancer is found?
Finally, do you resect segment I (caudate lobe) in any of your procedures?
Presenter Dr. Yuman Fong (New York, New York): We have been very, very aggressive about reresecting these patients. The median time to reresection after a prior operation is approximately 30 days. Therefore, when the patients present for reevaluation, we reimage, stage the patient, and take them to the operating room in a very short time. Very few patients have come in a long period of time after their prior operation, so we don’t have enough data to address the impact of waiting prior to reexploration.
In terms of port sites, what we do now is to reexcise all of the port sites at the time of a radical reresection. The residents actually refer to this operation as a radical umbilicalectomy. In the beginning, I hoped that this would be a therapeutic procedure, but of the three cases in which I have had microscopic disease in the port sites, all three patients have recurred within the abdomen sometime subsequently. So I believe it is more a staging procedure at this point. I am hoping that when we discover a good adjuvant therapy for these patients, microscopic evaluation of the port sites will be one way of selecting patients for adjuvant therapy.
In terms of the resecting the caudate lobe, unlike cholangiocarcinoma, where we find the need in about a third of the cases to resect the caudate lobe in order to clear disease, in gallbladder cancer we found a much lower incidence of caudate vessels or caudate duct invasion. In this series, there was only one patient who required caudate lobe resection to remove disease.
Dr. Robert M. Beazley (Boston, Massachusetts): We have all dealt with gallbladder carcinoma, but I am very impressed with your results. Unfortunately in my experience, the only ones I think I have had good luck with have been the ones the pathologists told me about a week later, the T1 lesions. So I commend you for your accomplishments.
Fortunately, this disease is relatively uncommon. Reading between the lines in your paper, the message I carry away is that these patients are best managed at centers by teams like yours. If that is the case and I stumble upon a gallbladder carcinoma, Dr. Fong, are there things I should or should not do before I close the abdomen or pull out the laparoscope? Or should I not be in the abdomen? Could you give us some guidance on that score?
What is the role of ultrasound in this disease and in what percentage of your nonreferral patients was the diagnosis made by ultrasound? Might that modality in some patients avoid a nontherapeutic, useless intervention?
Each of your patients received some type of liver surgery, with almost half receiving major liver resections. You have shown that nodal disease is a more adverse finding than liver extension. Would you describe your nodal dissection? Do you remove the common bile duct to do a more complete nodal resection or is the removal of the common bile duct done more in keeping with the amount of liver that is resected?
Does survival correlate with the number or distribution of lymph nodes in your experience? In other words, is the cystic duct lymph node involvement really bad? Or is it worse if you have three or four nodes?
Did any of the advanced T3 and T4 tumors receive adjuvant therapy, and if so what type of treatment?
Dr. Fong: In terms of what to do to avoid confronting a gallbladder cancer within the operating room, I think the ultrasound, as you mentioned, is a very, very good tool. If there is a sessile polyp, or if there are indications of a mass within the gallbladder, the patients should not undergo laparoscopic cholecystectomy. They should not be brought to the operating room without the intent for a curative operation.
I actually have a big file of scans, collected over the years, that were done preoperatively prior to a laparoscopic cholecystectomy or an open cholecystectomy that have demonstrated the tumors prior to an operation performed as treatment for gallstone disease. I think vigilance in the examination of preoperative images for cholelithiasis is most important.
In terms of major liver resection, the reason that we perform a major resection derives from the aggressive philosophy of Dr. Leslie Blumgart, the senior member of our team. As he has long pointed out, when we go to resect these tumors, particularly after a prior operation, there will be scar in the entire porta hepatis and it will be very difficult to tell what is scar and what is tumor. If we are attempting a potentially curative resection, we try to clear all areas that may have tumor. Frozen sections are not practical because of the large number of frozen sections needed. Therefore, when we go back on a reresection, we almost always end up having to take out a lobe or a trisegment just to clear the entire area that may be tumor.
In terms of the common bile excision, the reason we do that is because it does afford us a much better lymphadenectomy in the porta hepatis. The other reason we do it is because in bulky disease there is danger of bleeding from the portal vein immediately behind the bile duct. If the bile duct is cut and reflected upward, there is much better visibility of the portal vein. If you need to do a portal vein reconstruction, the visibility is much, much better with the common bile duct cut. That is the other reason that we routinely cut the bile duct, reflect it up, look behind for vascular invasion and to prepare for repair or reconstruction.
In terms of distribution of nodes in relation to outcome, we have had patients with only a single positive cystic duct node who have recurred. We have never had a long-term disease-free survivor from node-positive disease even in the cystic duct node. The two actual 5-year survivors with node-positive disease were both positive in the cystic duct node. Nevertheless, when we see a node-positive patient, we wish there were proven adjuvant therapies for their treatment.
Finally, in terms of the adjuvant therapy, 22 of these patients had adjuvant therapy: three of them had just radiation therapy, three had just chemotherapy, and the rest had chemoradiation. They were not done in any systematic fashion. Most of the people who had adjuvants were the node-positive patients. And certainly no firm conclusions can be made from the nature or the way we gave the adjuvant therapy. I certainly believe that this is a very important area for study. I am hoping that a cooperative oncology group such as the American College of Surgeons’ Oncology Group will set up a national study to allow us to address the question of whether chemoradiation will be important in this group of patients. Certainly one single center is not going to be able ever to answer that question, because over a 13-year period we resected 100 patients. Therefore, this has to be a multicenter study for us to decide whether adjuvant therapy is important.
Dr. William C. Meyers (Worcester, Massachusetts): These authors have chronicled in a huge series an important and presently underappreciated advance in hepatobiliary surgery. As with bile duct cancer, gallbladder cancer can be much more curable than previously thought. The improved safety of aggressive hepatic resection now makes this possible.
I was surprised several years ago when we found that five of 10 patients on whom I had performed a radical aggressive resection for gallbladder cancer were still alive and well without recurrent disease. All these patients had also undergone adjuvant chemotherapy and external radiation.
These results and similar ones for selected patients with bile duct cancer led us to dramatically change our operative approach for many of these patients. We now explore these patients, looking mainly for the clean, uninvolved liver, resect the rest of the liver, and perform hepaticojejunostomies as necessary. With gallbladder cancers specifically, we usually perform an aggressive bed resection and lymph node dissection as a minimum—like you, Dr. Fong. For bile duct cancer in particular, we have abandoned our old approach of simply trying to dissect up high enough to get above the tumor. A similar attitude has developed for gallbladder cancer.
Dr. Fong, two questions: One, do you generally agree with my comments with respect to our approach to these tumors, and do you perceive gallbladder cancer and bile duct cancer as being similar in nature? And would you comment on how many patients are presently out there treated by only what you have described as a nihilistic approach and who might benefit from your aggressive surgery?
Dr. Fong: In terms of similarities to cholangiocarcinoma, certainly the operations are very similar. Some of the differences include a lower incidence of caudate lobe involvement. Cholangiocarcinomas almost always require a lobectomy or a trisegmentectomy for removal, simply because the confluence of hepatic ducts will be involved by definition. Those are the major technical differences.
In terms of biologic differences, I think of cholangiocarcinomas as a less aggressive disease. There is a lower likelihood of peritoneal metastasis and there is longer term survival in terms of nonresectable disease than in gallbladder cancer. So biologically I think they are somewhat different.
It also then comes back to our philosophy of treating the patients in terms of palliative treatment. Because when I encounter a patient with nonresectable cholangiocarcinoma and jaundice, I almost always bypass them if I have already made an incision to explore them. For gallbladder cancer, I rarely bypass them and prefer to ask a radiologist to stent the patient, simply because the lifespan for nonresectable disease is so short that a bile leak from the bypass will result in the patient spending a major portion of the rest of his life in the hospital. So I think of the two diseases as biologically different, but technical aspects of resecting them are very similar.
Dr. Henry A. Pitt (Milwaukee, Wisconsin): Drs. Fong and Blumgart have nicely documented that 25% of patients with gallbladder cancer are candidates for radical resection and that this procedure is warranted even when a prior exploration and cholecystectomy have been performed. This analysis began 14 years ago; therefore, I have a number of questions related to the current management, which certainly has evolved over the years.
First, would you comment on the role of laparoscopy in staging these patients, especially those with no prior surgery or patients with T4 lesions who have a less than 15% chance of being resectable?
Second, would you comment further on the extent of lymphadenectomy and the need for performing a pancreaticoduodenectomy to achieve an adequate lymph node clearance? A number of Japanese groups have advocated both liver and pancreatic resections, but the reported morbidity and mortality has been quite high.
Third, would you comment further on the role of palliative surgery? You mentioned your philosophy with respect to biliary bypass. I agree that in many of these patients a biliary bypass may be very difficult. What about the need for bypassing the duodenum because of duct extension of the tumor? Is there a role for an ethanol splanchnicectomy in palliating pain in these patients, as we have shown is important in pancreatic cancer patients?
Finally, I would like to agree with you completely on the need for multiinstitutional trials on the role of adjuvant chemoradiation, and I hope that we will be able to achieve that goal through the American College of Surgeons Oncology Group.
Dr. Fong: In terms of the laparoscopy, if somebody presents without prior surgery, we now routinely perform laparoscopy for these patients. And we have about a 50% yield on the laparoscopy because peritoneal disease is such a big component of the spread of this cancer. However, if somebody presents with a prior resection, we do not perform a further laparoscopy because of the dense adhesions that usually are present within 30 days of the prior surgery.
Now, one side issue from that is that because everybody is worried about port site recurrence, why put a laparoscope in? I don’t believe wound recurrences are particular to the laparoscopic port sites, I believe gallbladder cancer grows in any wound that it gets into. George Packs’ original description in 1955 shows that these tumors grow in normal wounds just fine, and I have seen it numerous times in open cholecystectomy wounds. In terms of the extent of lymphadenectomy, we believe we should do a portal lymphadenectomy and a high peripancreatic lymphadenectomy. We do a full Kocherization, we take the lymph nodes in the high retropancreatic area, but we do not go further. Only in one of these cases did we do a pancreaticoduodenectomy along with a liver resection, and that was in a very young patient. There is data from the Japanese literature advocating pancreaticoduodenectomy, but I do not believe that the data in terms of morbidity and mortality is justified by the long-term outcome that is presented.
In terms of palliative therapy, I have already mentioned that when the patients are jaundiced, we prefer to drain the patients percutaneously. But in terms of the gastrojejunostomy that may be necessary in these patients, we only do that if the patients are symptomatic. We have no experience in using an ethanol splanchnicectomy.
In terms of the trial, I am hoping that all of you who treat this disease will participate in a trial that we will put together through the American College of Surgeons Oncology Group to examine chemoradiation as an adjuvant for this disease.
Dr. John Terblanche (Cape Town, South Africa): Dr. Fong, as a pessimist up until today, I am convinced by your data that there is a need for an aggressive approach in highly selected cases. But I think the audience needs to recognize that you are presenting a highly selected group of patients. My question is whether there is a difference in the outcome in the postlaparoscopic cholecystectomy patients who had the gallbladder damaged and/or spillage of the catheter and those that did not?
Dr. Fong: It is very difficult to determine who has had a damaged gallbladder. That is because oftentimes we can’t even get the prior scans from the other hospital and the only thing that will arrive is a single slide that shows gallbladder cancer. So I cannot answer that question. Certainly when I reoperate on somebody and I see gallstones in the abdomen, I am very worried about further spread of cancer.
Dr. Lawrence W. Way (San Francisco, California): The most curable gallbladder cancers are the small ones that may not be detectable on cursory external examination of a stone-filled gallbladder. Many small unsuspected cancers are first discovered by pathologists in gallbladders removed for gallstone disease. Because this occurs several days postoperatively, the most convenient time for extending the operation by removing liver and lymph nodes during the initial cholecystectomy has passed. In fact, these so-called incidental cancers should more accurately be thought of as overlooked cancers, for they stem from the practice of submitting gallbladder specimens to the pathologist unopened. Most could be detected if gallbladders were immediately inspected for the presence of focal wall thickening or raised patches of mucosa, submitting suspicious areas for frozen section examination. Looked at in this way, incidental gallbladder cancers represent a defect in practice. It is interesting to speculate on whether the results of treatment would improve if this defect were corrected.
I have two questions. First, can you define the term “unresectability based upon imaging studies,” which was one of your reasons not to operate? Secondly, were any patients cured in whom a segment of the portal vein had to be removed?
Dr. Fong: In terms of unresectability, the imaging studies have gotten so good that we oftentimes can totally determine major vascular encasement without invasive angiography. So normally we will perform a repeat Doppler ultrasound and/or an MRCP.
The most common reason that we find proven unresectable disease without further surgery is portal vein encasement or vascular involvement in such a way that a tumor is not removable. On occasion, we have found clear nodal disease that is in the retropancreatic area that we believe is not curable and have proven by needle biopsy of these areas. In terms of portal vein resection, there were two cases of portal vein resection that were done with these hundred patients.
Footnotes
Correspondence: Yuman Fong, MD, Hepatobiliary Surgery Service, Dept. of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
Supported in part by NIH grants RO1CA76416, RO1CA72632, and RO1CA61524 (to YF).
E-mail: FongY@MSKCC.org
References
- 1.Blalock AA. A statistical study of 888 cases of biliary tract disease. Johns Hopkins Hosp Bull 1924; 35: 391–409. [Google Scholar]
- 2.Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet 1978; 147: 929–942. [PubMed] [Google Scholar]
- 3.Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder: results of the French Surgical Association Survey [see comments]. Ann Surg 1994; 219: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkinson DS. Carcinoma of the gall-bladder: an experience and review of the literature [Review]. Austr N Z J Surg 1995; 65: 724–727. [DOI] [PubMed] [Google Scholar]
- 5.Pack GT, Miller TR, Brasfield RD. Total right hepatic lobectomy for cancer of the gallbladder. Ann Surg 1955; 142: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue JH, Nagorney DM, Grant CS, et al. Carcinoma of the gallbladder: does radical resection improve outcome? Arch Surg 1990; 125: 237–241. [DOI] [PubMed] [Google Scholar]
- 7.Ogura Y, Mizumoto R, Isaji S, et al. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg 1991; 15: 337–343. [DOI] [PubMed] [Google Scholar]
- 8.Shirai Y, Yoshida K, Tsukada K, et al. Radical surgery for gallbladder carcinoma. Ann Surg 1992; 216: 565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouchi K, Owada Y, Matsuno S, Sato T. Prognostic factors in the surgical treatment of gallbladder carcinoma. Surgery 1987; 101: 731–737. [PubMed] [Google Scholar]
- 10.Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer: implications for staging and management [Review]. Ann Surg 1996; 224: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couinaud C. Bases anatomiques des hepatectomies gauche et droite reglees. J Chirurg 1954; 70: 933–966. [PubMed] [Google Scholar]
- 12.Fleming ID, Cooper JS, Henson DE, et al. AJCC Cancer Staging Manual. 5th ed. Philadelphia: Lippincott-Raven; 1997.
- 13.Nevin JE, Moran TJ, Kay S, King R. Carcinoma of the gallbladder: staging, treatment, and prognosis. Cancer 1976; 37: 141–148. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life tables (with discussion). J R Statist Soc B 1972: 187–220. [Google Scholar]
- 15.Perpetuo MD, Valdivieso M, Heilbrun LK, et al. Natural history study of gallbladder cancer: a review of 36 years experience at M.D. Anderson Hospital and Tumor Institute. Cancer 1978; 42: 330–335. [DOI] [PubMed] [Google Scholar]
- 16.Thorbjarnarson B, Glenn F. Carcinoma of the gallbladder. Cancer 1959; 12: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 17.Burdette WJ. Carcinoma of the gall bladder. Ann Surg 1957; 145: 832–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 1995; 75: 171–190. [DOI] [PubMed] [Google Scholar]
- 19.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatopancreatoduodenectomy for advanced carcinoma of the biliary tract. Hepato-Gastroenterology 1991; 38: 170–175. [PubMed] [Google Scholar]
- 20.de Aretxabala X, Roa I, Burgos L, et al. Gallbladder cancer in Chile: a report on 54 potentially resectable tumors. Cancer 1992; 69: 60–65. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura S, Sakaguchi S, Suzuki S, Muro H. Aggressive surgery for carcinoma of the gallbladder. Surgery 1989; 106: 467–473. [PubMed] [Google Scholar]
- 22.Matsumoto Y, Fujii H, Aoyama H, et al. Surgical treatment of primary carcinoma of the gallbladder based on the histologic analysis of 48 surgical specimens. Am J Surg 1992; 163: 239–245. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi K, Tsuneyoshi M. Subclinical gallbladder carcinoma. Am J Surg 1992; 163: 382–386. [DOI] [PubMed] [Google Scholar]
- 24.Oertli D, Herzog U, Tondelli P. Primary carcinoma of the gallbladder: operative experience during a 16-year period. Eur J Surg 1993; 159: 415–420. [PubMed] [Google Scholar]
- 25.de Aretxabala X, Roa IS, Burgos LA, et al. Curative resection in potentially resectable tumours of the gallbladder. Eur J Surg 1997; 163: 419–426. [PubMed] [Google Scholar]
- 26.Onoyama H, Yamamoto M, Tseng A, et al. Extended cholecystectomy for carcinoma of the gallbladder. World J Surg 1995; 19: 758–763. [DOI] [PubMed] [Google Scholar]
- 27.Gall FP, Kockerling F, Scheele J, et al. Radical operations for carcinoma of the gallbladder: present status in Germany. World J Surg 1991; 15: 328–336. [DOI] [PubMed] [Google Scholar]
- 28.Beahrs OH, Henderson DE, Hutter RV, Myers MH. Gallbladder. In: Manual for Staging of Cancer. Philadelphia: JB Lippincott; 1988: 93–98.
- 29.Yamamoto M, Onoyama H, Ajiki T, et al. Surgical results of operations for carcinoma of the gallbladder. Hepato-Gastroenterology 1999; 46: 1552–1556. [PubMed] [Google Scholar]
- 30.Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder: an appraisal of a radical second operation after simple cholecystectomy. Ann Surg 1992; 215: 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boerma EJ. Towards an oncological resection of gall bladder cancer [Review]. Eur J Surg Oncol 1994; 20: 537–544. [PubMed] [Google Scholar]
- 32.Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder: an appraisal of a radical second operation after simple cholecystectomy. Cancer 1988; 62: 1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer 1998; 83: 423–427. [DOI] [PubMed] [Google Scholar]
- 34.Todoroki T. Radiation therapy for primary gallbladder cancer [Review]. Hepato-Gastroenterology 1997; 44: 1229–1239. [PubMed] [Google Scholar]
- 35.Todoroki T, Iwasaki Y, Orii K, et al. Resection combined with intraoperative radiation therapy (IORT) for stage IV (TNM) gallbladder carcinoma. World J Surg 1991; 15: 357–366. [DOI] [PubMed] [Google Scholar]
- 36.Hanna SS, Rider WD. Carcinoma of the gallbladder or extrahepatic bile ducts: the role of radiotherapy. Can Med Assoc J 1978; 118: 59–61. [PMC free article] [PubMed] [Google Scholar]
- 37.Vaittinen E. Carcinoma of the gall-bladder: a study of 390 cases diagnosed in Finland 1953—1967. Ann Chirurg Gynaecol Fenniae 1970; 168 (suppl): 1–81. [PubMed] [Google Scholar]