Abstract
Objective
To build a predictive tool for assessing both favorable outcome and morbidity in a large series of unruptured aneurysms.
Summary Background Data
Some well-known predictors of clinical outcome for patients with ruptured aneurysms are not useful in forecasting outcome for patients with unruptured aneurysms.
Methods
The authors analyzed 93 patients with a total of 101 unruptured middle cerebral aneurysms who underwent surgical clipping. Intraoperative data was reviewed and seven factors that might influence outcome were identified: 1) aneurysm size >10 mm, 2) presence of a broad neck, 3) presence of intraaneurysmal plaque, 4) clipping of more than one aneurysm during the same surgery, 5) temporary occlusion of the middle cerebral artery, 6) multiple clip applications and repositionings, and 7) use of multiple clips. The entire group of unruptured middle cerebral artery aneurysms was divided into two subgroups on the basis of outcome. Each patient was subsequently analyzed for the Factor Accumulation Index (FAI), the sum of different factors observed in a given patient.
Results
The expected outcome subgroup was represented by 86 patients, with a total of 92 aneurysms, and demonstrated the following results: no factors were found in six patients; FAI of 1: 24 patients; FAI of 2: 23 patients; FAI of 3: 12 patients; FAI of 4: 11 patients; FAI of 5: 8 patients; FAI of 6: one patient; FAI of 7: one patient. Seven patients represented the subgroup of unexpected outcomes with total morbidity of 7.5%. There were no deaths. None of the patients in this subgroup accumulated FAI of 0, 1, 2, or 5; otherwise: FAI of 3: two patients; FAI of 4: two patients; FAI of 6: one patient; FAI of 7: two patients.
Conclusion
It is possible to predict outcome in patients with unruptured middle cerebral artery aneurysm by calculating FAI. The postoperative morbidity increases with an FAI within a range of 3 to 4.
The overall morbidity and mortality associated with the surgical clipping of cerebral aneurysms has been the focus of multiple studies. Estimates of the frequency of incidental intracranial aneurysms range between 5% and 10%, while angiographic studies show a rate from 0.65% to 1%. 1–4 Some of the well-known predictors of clinical outcome in patients with ruptured aneurysms, such as preoperative clinical condition and the amount of subarachnoid blood, are not useful in forecasting the outcome of patients with unruptured aneurysms. There remains no method to predict the outcome in these patients accurately. This is of particular importance because recent reports of the incidence of hemorrhage of aneurysms and the cost associated with screening patients for unruptured aneurysms challenges some of the current management strategies for dealing with aneurysms. Furthermore, newer endovascular approaches offer other treatment options that also require a better understanding of the risks of treatment compared to the natural history. 5
The goal of our study was to build a predictive tool for assessing both favorable outcome and morbidity in a large series of unruptured aneurysms. Our series of middle cerebral aneurysms was selected as a model for this analysis. The middle cerebral artery (MCA) is one of the more common sites of cerebral aneurysms; MCA aneurysms comprise 20% to 25% of intracranial aneurysms. They pose technical problems because of multiplicity, giant size (15%), and the frequent need to reconstruct and preserve the MCA bifurcation. This often makes direct surgical approaches, rather than endovascular methods, the procedure of choice. Because of increased use of endovascular techniques and recent reevaluations of the natural history of cerebral aneurysms, it is appropriate to evaluate current management results against which newer methods will be compared. We reviewed our experience and management strategies for 328 MCA aneurysms in 294 patients, which represents 21.1% of 1,556 operated aneurysms in the senior author’s series.
MATERIALS AND METHODS
Data regarding a total of 740 cerebral aneurysms operated on by the senior author (E.S.F.) during the 1988–1999 time period was collected prospectively and stored in a database. The preoperative data included admission and preoperative Hunt and Hess grade, location of the aneurysm, comorbidities if any, and patient age and gender. This period, which includes almost half of the 1,556 aneurysms in the series, was selected because the cases were managed with a standardized protocol that is still in use. Patients with subarachnoid hemorrhage were operated upon in the first 3 days after hemorrhage, and managed with expanded fluid volume, calcium channel blockers, monitoring of cerebral blood flow velocities with transcranial Doppler (TCD), and standardized anesthetic techniques using inhalational and narcotic agents. All patients were operated upon under moderate hypothermia of 32 to 34°C using inhalational and narcotic agents.
Of the 294 patients, 46 had bilateral MCA aneurysms requiring separate craniotomies. Another 78 patients (26.5%) had an MCA aneurysm as one of multiple aneurysms. Twenty-nine patients (9.9%) had two MCA aneurysms on the same side. Although the incidence of bilateral MCA aneurysms was only 15.7%, the overall occurrence of multiple aneurysms including the MCA was 45.9% (135/294), considerably greater than the expected rate 20% (P < .01).
In the total group of 294 patients with MCA aneurysms, 23 had a hemorrhage from an aneurysm other than the MCA, and 158 patients with multiple aneurysms presented with hemorrhage from the MCA aneurysm (53.8%). There were nine deaths and 29 patients developed increased postoperative neurologic deficits. A favorable outcome occurred in 82% and a combined morbidity and mortality of 12.9% of patients was noted.
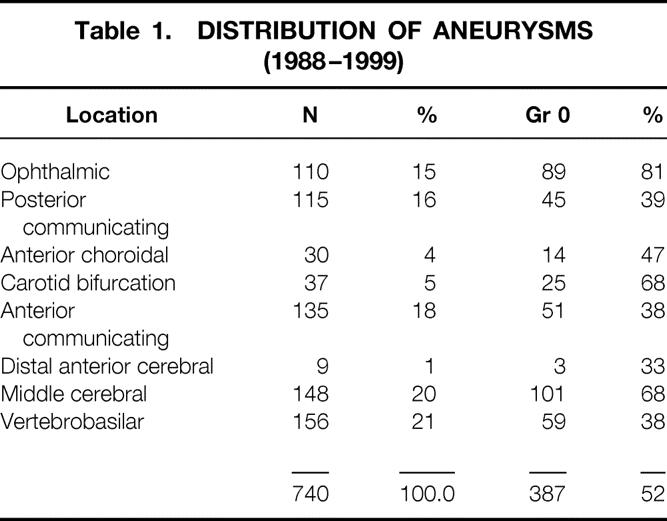
Unruptured aneurysms accounted for 52% (387 aneurysms) of the total of 740 aneurysms treated during this 11-year period. In the period 1988–1999, a total of 93 patients harboring a total of 101 unruptured middle cerebral aneurysms underwent surgical clipping. The unruptured MCA aneurysms chosen for this study represented the largest group, consisting of 101 aneurysms (26.1%) (Table 1).
Table 1. DISTRIBUTION OF ANEURYSMS (1988–1999)
Intraoperative data was reviewed and seven factors that might influence outcome were identified. These factors are listed in Table 2. Each of the intraoperative criteria was analyzed independently.
Table 2. RISK FACTORS

All patients were assessed at the time of discharge and during follow-up visits. Outcome was rated according to five categories:
Excellent: no neurologic deficit, with the patient able to return to preoperative lifestyle.
Good: mild deficit, patient able to return to premorbid work or lifestyle.
Fair: significant neurologic deficit, unable to return to previous work or lifestyle, but able to live at home with minimal or no assistance.
Poor: serious neurologic deficit requiring full-time care.
Death
The entire group of unruptured MCA aneurysms was divided into two subgroups on the basis of outcome. Patients with good or excellent outcomes were considered as the subgroup having an expected outcome, whereas patients with outcomes less than good were considered as having an unexpected outcome. The incidence of the previously mentioned seven independent factors was assessed for each of the two subgroups. Each patient was subsequently analyzed for the Factor Accumulation Index (FAI), the sum of different factors observed in a given patient.
RESULTS
A total of 93 patients harboring 101 unruptured middle cerebral aneurysms had surgery over an 11-year period (1988–1999). Of those, 86 patients, with a total of 92 aneurysms, represented the expected outcome subgroup, and seven patients were in the unexpected outcome group, accounting for a 7.5% morbidity rate in the total group of unruptured MCA aneurysms. There were no deaths in either group. Among the entire group, 34 were men and 59 were women, demonstrating a clear female preponderance. All aneurysms were saccular aneurysms.
Subgroup of Expected Outcome
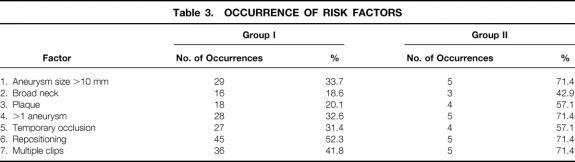
Eighty-six patients harboring a total of 92 unruptured middle cerebral aneurysms comprised this subgroup. The mean age was 53.5 years. Table 3 summarizes the occurrence of the seven risk factors.
Table 3. OCCURRENCE OF RISK FACTORS
The individual patient Factor Accumulation Index (FAI) was calculated as the sum of factors 1 through 7 for each individual patient in the expected outcome subgroup. Those results were as follows: no factors were found in 6 patients; FAI of 1: 24 patients; FAI of 2: 23 patients; FAI of 3: 12 patients; FAI of 4: 11 patients; FAI of 5: eight patients. One patient had a FAI of 6, and one patient had a sum of seven factors (Table 4, Fig. 1).
Table 4. FACTOR ACCUMULATION INDEX (FAI)
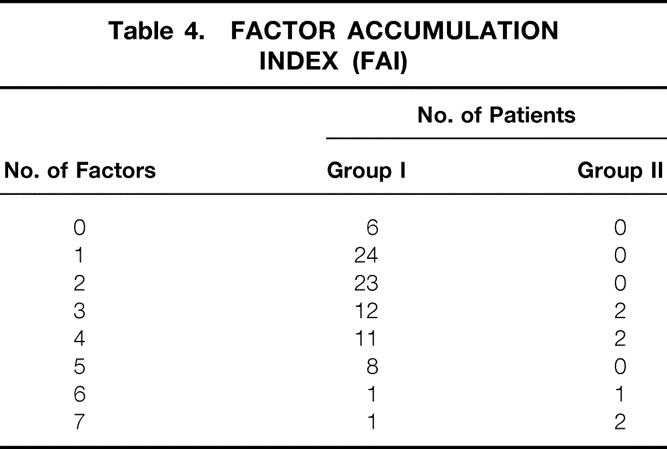
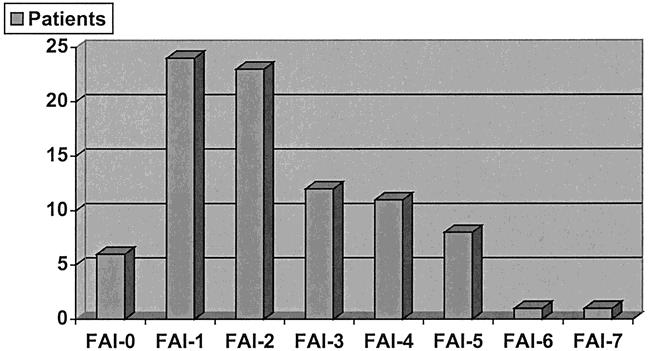
Figure 1. Distribution of Factor Accumulation Index (FAI) in patients with expected outcome.
Subgroup of Unexpected Outcomes
This subgroup was represented by seven patients with an outcome poorer than good. There were three males and four females; mean patient age was 54.5 years (range 15–66). There were no deaths in this subgroup and total morbidity accounted for 7.5% of all unruptured MCA aneurysms. Factor 1 was observed in five patients, Factor 2 in three patients, Factor 3 in four patients, Factor 4 in five patients, Factor 5 in four patients, Factor 6 in five patients, and Factor 7 in five patients.
Each individual patient was similarly analyzed for their FAI, and the following results were obtained. There was no FAI of 0, 1, 2, or 5. An FAI of 3 was found in two patients. Two patients had an FAI of 4. One patient exhibited an FAI of 6, and two patients exhibited an FAI of 7 (Fig. 2). In summary, 75% of Group I patients had an FAI of 3 or less, while all patients in Group II had an FAI of 3 or more.
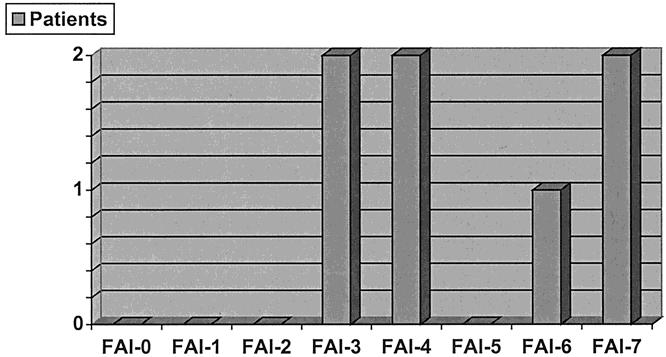
Figure 2. Distribution of Factor Accumulation Index (FAI) in patients with unexpected outcome.
DISCUSSION
Although a few studies suggest that unruptured aneurysms should be left untreated, many surgeons report good results with surgical treatment of unruptured aneurysms. 6–9 For the asymptomatic incidental aneurysm, an outcome of anything worse than good or excellent should be considered unacceptable. Even in the most experienced hands, however, morbidity is never zero.
Regli et al 10 reported one minor complication from clipping of 32 unruptured aneurysms, which was attributed to a 6-minute temporary clipping of the M1 and the two M2 segments during repair of an intraoperative rupture of a 14-mm MCA bifurcation aneurysm. Solomon et al 11 reported a 5% minor complication and 7% major complication rate in 202 unruptured aneurysms, with no major complications in aneurysms smaller than 6 mm. A total of 38 MCA aneurysms were reported in this study.
In a multicenter retrospective study, Wirth et al 12 analyzed the results of surgical treatment of 119 incidental aneurysms <2.5 cm in diameter and reported no mortalities and 6.5% major morbidity. In this series, a middle cerebral aneurysm was present in 37 cases with an operative morbidity of 8.1%. In a metaanalysis of surgical risks for unruptured aneurysms, the senior author (E.S.F.), found an even more favorable outcome in the literature: in 733 patients, there was a morbidity rate of 4.1% and a mortality rate of 1%. 7 These data correlate well with the present study in which we had no mortality and a morbidity rate of 7.5%.
Although our analysis of intraoperative morbidity has some speculative elements, we believe it provides a multifactorial prediction of possible surgical complications. In actuality, operative morbidity differs significantly between individual patients and some of the factors are unpredictable before surgery. In general, it is difficult to show perfect statistical data on a relatively rare entity. It is also difficult to have any meaningful statistics on such subjective criteria as a surgical skills and the enormous variety of vascular pathology in each patient.
The multifactorial analysis of intraoperative findings as an outcome predictor is justified only if each of those factors serves as an independent risk factor. We chose those particular factors based on the senior author’s personal long-term experience with vascular surgery as well as literature reports (Factors 4 and 7). Long-term outcome is known to correlate with patient age, aneurysm size, and location. In Ogilvy and Carter’s proposed grading system for cerebral aneurysms, 13 they implicated 100% of excellent or good outcomes to grade 0 patients <50 years of age with aneurysms <6 mm. They noted a stepwise increase in risk with increased age.
Most vascular neurosurgeons also agree with importance of Factors 1, 2, and 3, and any discussion of intraoperative factors must include temporary occlusion, which has a significant influence on final outcome. In our series, temporary occlusion was used in almost one third of all patients in the expected outcome subgroup (31.4%), having a range of 1.75 to 24 minutes. Only hypothermia was used for neuroprotection. It is a general consensus that temporary occlusion is likely less tolerated in patients with middle cerebral aneurysms in comparison to internal carotid artery (ICA) and anterior communicating artery (ACA) aneurysms. Samson et al 14 reported an infarction rate of 26% in MCA aneurysms in contrast to patients with ICA (7%) and ACA (16%) aneurysms. Lavine et al, 15 in a retrospective review of 49 MCA aneurysms that required temporary occlusion, experienced an overall infarction rate of 22.4%, with a trend toward a decreased infarction rate in those patients who underwent intermittent temporary clip placement and clip placement distal to the lenticulostriate branches. Lavine’s article also supported the conclusion that the MCA is particularly vulnerable to a long occlusion time.
Multiple aneurysms, Factor 4, have been associated with patients harboring MCA aneurysms. 16–18 This rate may be as high as 45% and could be even higher in superior wall aneurysms when compared to inferior wall aneurysms. 3,17,19,20 Wirth et al 12 demonstrated that clipping of single versus multiple incidental aneurysm during the same operation had no correlation with operative morbidity (6/95 vs. 1/12). In contrast, Levine et al 15 showed that the presence of multiple aneurysms is associated with an increased risk of focal infarction. The authors felt that the risk was not related to simultaneous treatment of these lesions rather than the decreased tolerance to cerebral ischemia in patients who developed multiple aneurysms. Patients with multiple aneurysms were also hypothesized as possibly having an increased sensitivity to manipulation of their cerebral microvasculature. In comparison, our findings showed that multiple aneurysms were treated in 32.6% of patients during the same surgery, making it the third most commonly observed factor in the expected outcome group.
It is also our impression that the frequency of occurrence of each particular factor in the expected outcome group indicates the importance of that particular factor in management of these cases. For example, Factor 6 (multiple attempts and clips repositioning) was observed in more than 50% of patients with good or excellent outcomes and in five of seven patients in Group II. We believe that this is a necessary requirement to treat aneurysms of the MCA effectively and not necessarily a cause of complications. The need to reconstruct the bifurcation of the MCA and preserve continuity of the M2 branches is an essential step in successful management of MCA aneurysms.
One limitation of this study is the lack of specificity of each factor for a given patient’s disease. For example, prolonged temporary occlusion in a patient with poor collateral circulation may be a much more important predictor of outcome than in a patient with extensive collaterals. Understanding this, it is still our belief that although no single factor is most important for all patients, the accumulation of factors, FAI, has value in predicting outcome. Factor 7, use of multiple clips, was included in this study due to the results of Tanaka et al. 21 In an analysis of multiple clipping techniques for large and giant ICA aneurysms, this study found that complications might arise from technical aspects of clipping procedure. In an attempt to reconstruct the vessel with multiple and/or fenestrated clips, there may be possible straightening of the artery.
As a final question, can the patient who accumulated less than 3 factors (FAI 0–3) do poorly, and also, what is the chance that a patient who accumulated all seven factors will have a major postoperative stroke? None of our patients in the unexpected outcome group had a FAI of less than 3. The majority of the patients in the expected outcome group had a FAI in a range from 1 to 3. We believe that the fine line in predicting outcome lies between these scores.
Discussion
Dr. Grant Sinson (Philadelphia, Pennsylvania): The traditional approach to treating cerebral aneurysms the past few decades has been aggressive and surgical. That approach has been challenged recently, as you alluded to. One of the challenges has come from our interventional radiology colleagues that have been aggressively recruiting patients by offering a less-invasive procedure to treat aneurysms that has yet-unproven long-term efficacy.
One of the other challenges to this approach has come from the concept of evidence-based medicine. While this is a concept that offers hope to keeping our standards of clinical care high, it can be a double-edged sword, as you made reference in the New England Journal paper. Last year, in response to that paper in the Journal of Neurology, Johnson et al concluded a metaanalysis by saying treatment of small asymptomatic unruptured cerebral aneurysms in patients without a history of subarachnoid hemorrhage worsens clinical outcomes, and thus is neither effective nor cost-effective.
You discussed somewhat the problems with the New England Journal paper, and there were some other design flaws that I think do undermine the validity of the study. Nevertheless, it forces us as a group to critically evaluate our approach to these patients, and I think that you have done an important job in doing that in the data you presented. I have a few specific questions.
Given the limited number of patients, it is difficult to stratify the variables you examined. However, do you feel that the different variables should be weighted somewhat differently based on your experience? For example, do you feel that the presence of plaque in the aneurysm wall is a more important variable than some of the others? Along the same lines, are there other preoperative variables, such as age and other medical conditions, that you factor in in deciding to operate on a patient?
Second, have you changed your intraoperative practices based on these data? For instance, do you use temporary clipping less now, given the fact that you feel that this is a variable?
My final question: You presented outstanding results with 7.5% morbidity in these patients. This compares with the 18% morbidity presented in the New England Journal study. This could be explained by a number of reasons. One, that middle cerebral artery aneurysms are safer to treat than other aneurysms. Alternatively, you just referred simpler aneurysms. The final explanation would be that you are just an excellent surgeon in handling these patients.
If I can assume that you would say it is the latter of those three, then I would ask, do you feel that procedures such as this should be limited to surgeons or medical centers that can demonstrate excellent outcomes and high volumes, as has been suggested not only for other neurosurgical procedures but other subspecialty and other surgical procedures in general?
Presenter Dr. Eugene S. Flamm (New York, New York): One of the reasons for selecting the middle cerebral aneurysms to look at was that they are probably, of all the intracerebral aneurysms, the least suited for endovascular techniques, based on the fact that they are more distal to the origin from the major bifurcation. The middle cerebral artery often requires reconstruction of the bifurcation to preserve it, something that, at least with present techniques, is not as easily done by an endovascular approach. Ultimately, that may change.
As far as weighing the factors, clearly as the list was put up, we ranked the size of the aneurysm. The presence of plaque in the width of the neck we thought would be far more predictive than some of the other ones in terms of number of clips we might apply. In none of them, though, could we get a sense, either looking at the data or trying to analyze it statistically, that one had more significance than the other. The age factors, comorbidities, do play a role, but they seemed to be equally distributed across the two groups.
I would point out that one of the difficulties with this type of an approach is that many of these factors are not determinable preoperatively. A lot of these are observations made at the time of surgery. It is not always possible preoperatively to anticipate that the wall of the aneurysm itself will be thick or that there will be plaque there.
We are recently using 3-D angiography, and we are hoping that that will give us a little better idea of the actual structure and configuration of the aneurysm that may add some predictive value. But right now we are facing the dilemma that the factors are retrospective after we have observed them, only occasionally can we detect calcium or plaque in the vessel, and certainly preoperatively we have no way of knowing whether we are going to use temporary occlusion or multiple clips or multiple repositions.
With regard to temporary occlusion, I have always tried very hard to avoid using that until it is absolutely necessary. There are times when that does occur. But I am just sort of parsimonious with the use of a temporary clip because I know what it does to the intimal lining and the cause of platelet aggregation, which may lead to complications postoperatively. I have grave concern about using temporary clips unless they are necessary or there is no other way of getting around it.
As far as the discrepancy between an individual surgeon’s data and a multicenter study, I think we see that, any time in any field when you pool data like that, there is a broader range. Our own metaanalysis that we published on unruptured aneurysms several years ago, before Johnson’s report, showed a much better outcome, and I certainly would not feel comfortable accepting the data presented in the paper you referred to because it seems at odds with a similar analysis that we carried out.
As far as who should be doing these now and in the future, I think someone who can do it well should be doing it. That becomes a very difficult determinant at the time a patient discovers he or she has an aneurysm, and referral patterns enter into it. In the best of all possible worlds, I suppose only one person should do each of these operations. But I doubt that that will occur.
Footnotes
Correspondence: Arthur A. Grigorian, MD, The Neurological Institute of Central Georgia, 840 Pine St., Suite 880, Macon, GA 31201.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail: grig63@hotmail.com
Accepted for publication April 2000.
References
- 1.Atkinson JLD, Sundt TM Jr, Houser OW, et al. Angiographic frequency of anterior circulation intracranial aneurysms. J Neurosurg 1989; 70: 551–555. [DOI] [PubMed] [Google Scholar]
- 2.Chason JL, Hindman WM. Berry aneurysms of the circle of Willis: results of a planned autopsy study. Neurology 1958; 8: 41–42. [DOI] [PubMed] [Google Scholar]
- 3.McCormick WF. Problems and pathogenesis of intracranial arterial aneurysms. In: Moossy J, Janeway R, eds. 7th Conference: Cerebrovascular Diseases. New York: Grune & Stratton, 1971: 217–231.
- 4.Winn HR, Taylor J, Kaiser DL. Prevalence of asymptomatic incidental aneurysms: review of 4,568 arteriograms. Stroke 1983; 14: 121. [DOI] [PubMed] [Google Scholar]
- 5.International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: risk of rupture and risk of surgical intervention. N Engl J Med 1998; 339: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 6.Chang HS, Kirino, T. Quantification of operative benefit for unruptured cerebral aneurysms: a theoretical approach. J Neurosurg 1995; 83: 413–420. [DOI] [PubMed] [Google Scholar]
- 7.King JT, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg 1994; 81: 837–842. [DOI] [PubMed] [Google Scholar]
- 8.McCormick WF, Acosta-Rua GJ. The size of intracranial saccular aneurysms: an autopsy study. J Neurosurg 1966; 25: 321–368. [DOI] [PubMed] [Google Scholar]
- 9.Wirth FP. Surgical treatment of incidental intracranial aneurysms. Clin Neurosurg 1986; 33: 125–135. [PubMed] [Google Scholar]
- 10.Regli L, Uske A, de Tribolet N. Endovascular coil placement compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysm: a consecutive series. J Neurosurg 1999; 90: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 11.Solomon RA, Fink ME, Pile-Spellman J. Surgical management of unruptured intracranial aneurysms. J Neurosurg 1994; 80: 440–446. [DOI] [PubMed] [Google Scholar]
- 12.Wirth FP, Laws ER Jr, Piepgras D, Scott RM. Surgical treatment of incidental intracranial aneurysms. Neurosurgery 1983; 12: 507–511. [DOI] [PubMed] [Google Scholar]
- 13.Ogilvy CS, Carter BS. A proposed comprehensive grading system to predict outcome for surgical management of intracranial aneurysms. Neurosurgery 1998; 42: 959–970. [DOI] [PubMed] [Google Scholar]
- 14.Samson D, Batjer HH, Bowman G, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery 1994; 34: 22–29. [PubMed] [Google Scholar]
- 15.Lavine SD, Masri LS, Levy ML, Giannotta SL. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: time limitation and advantage of brain protection. J Neurosurg 1997; 87: 817–824. [DOI] [PubMed] [Google Scholar]
- 16.Hacker RJ, Krall JM, Fox JL. Multiple intracranial aneurysms occurring in adults. In: Fox JL, ed. Intracranial Aneurysms. Vol. I. New York: Springer-Verlag, 1983: 36–43.
- 17.Hosoda K, Fujita S, Kawaguchi T, et al. Saccular aneurysms of the proximal (M1) segment of the middle cerebral artery. Neurosurgery 1995; 36: 441–446. [DOI] [PubMed] [Google Scholar]
- 18.Yasargil MG. Multiple aneurysms. In: Yasargil MG, ed. Microneurosurgery. Vol. II. Stuttgart: Georg Thieme-Verlag, 1984: 305–328.
- 19.Ohkuma A, Andou H, Murase S, et al. Cerebral aneurysm at the origin of the lenticulostriate artery: report of two cases. Ann Proc Gifu Prefec Hosp 1992; 13: 9–14. [Google Scholar]
- 20.Yasargil MG. Middle cerebral artery aneurysms. In: Yasargil MG, ed. Microneurosurgery. Vol. II. Stuttgart: Georg Thieme-Verlag, 1984: 124–164.
- 21.Tanaka Y, Kobayashi S, Kyoshima K, Sugita K. Multiple clipping technique for large and giant internal carotid artery aneurysms and complications: angiographic analysis. J Neurosurg 1994; 80: 635–642. [DOI] [PubMed] [Google Scholar]