Abstract
Objective
To summarize the authors’ laparoscopic experience for paraesophageal hernia (PEH).
Summary Background Data
Laparoscopic antireflux surgery and repair of small hiatal hernias are now routinely performed. Repair of a giant PEH is more complex and requires conventional surgery in most centers. Giant PEH accounts for approximately 5% of all hiatal hernias. Medical management may be associated with a 50% progression of symptoms and a significant death rate. Conventional open surgery has a low death rate, but complications are significant and return to routine activities is delayed in this frequently elderly population. Recently, short-term outcome studies have reported that minimally invasive approaches to PEH may be associated with a lower complication rate, a shorter hospital stay, and faster recovery.
Methods
From July 1995 to February 2000, 100 patients (median age 68) underwent laparoscopic repair of a giant PEH. Follow-up included heartburn scores and quality of life measurements using the SF-12 physical component and mental component summary scores.
Results
There were 8 type II hernias, 85 type III, and 7 type IV. Sac removal, crural repair, and antireflux procedures were performed (72 Nissen, 27 Collis-Nissen). The 30-day death rate was zero; there was one surgery-related death at 5 months from a perioperative stroke. Intraoperative complications included pneumothorax, esophageal perforation, and gastric perforation. There were three conversions to open surgery. Major postoperative complications included stroke, myocardial infarction, pulmonary emboli, adult respiratory distress syndrome, and repeat operations (two for abscess and one each for hematoma, repair leak, and recurrent hernia). Median length of stay was 2 days. Median follow-up at 12 months revealed resumption of proton pump inhibitors in 10 patients and one repeat operation for recurrence. The mean heartburn score was 2.3 (0, best; 45, worst); the satisfaction score was 91%; physical and mental component summary scores were 49 and 54, respectively (normal, 50).
Conclusion
This report represents the largest series to date of laparoscopic repair of giant PEH. In the authors’ center with extensive experience in minimally invasive surgery, laparoscopic repair of giant PEH was successfully performed in 97% of patients, with a minimal complication rate, a 2-day length of stay, and good intermediate results.
Gastroesophageal reflux disease (GERD) affects millions of Americans: up to 11% of the U.S. population reports daily symptoms of heartburn. 1 One of the common associations of GERD is the presence of a hiatal hernia. The incidence of hiatal hernia in the general population is approximately 5 per 1,000, but 95% of these are small, sliding type I hernias that are rarely associated with serious complications. 2 The remaining 5% can be classified as giant paraesophageal hernias (PEHs) and are associated with significant complications. 3
Without surgical intervention, giant PEHs are associated with progression of symptoms in up to 45% of patients. 4 In a classic report of nonsurgical observation of a group of minimally symptomatic patients with giant PEH, 26% died of catastrophic complications including torsion, gangrene, perforation, and massive hemorrhage. 5 In the subset of patients who develop gastric volvulus, the death rate can be as high as 100%. 6,7 Given the significant complications that can occur, giant PEH should be electively repaired. When repair is performed electively, the death rate is less than 1% to 2% in most series. 6,8–10
Traditionally, repair of giant PEH has been performed through an open laparotomy or thoracotomy. This population of patients is often elderly, with comorbidities, which has led to concern over surgical referral. With the advent of laparoscopy, giant PEHs are now being approached with minimally invasive techniques. Less invasive procedures may decrease the amount of postoperative pain and the perioperative complication rate and shorten recovery time. Recently, a few series have reported that laparoscopic repair of PEH is technically feasible, effective, and safe. 11–13 Most of these reports did not give the details of the size of the hernia, which can greatly affect the technical difficulty of the repair. Our previous work in this field showed a favorable short-term outcome when comparing laparoscopic with open repair of PEH, but it also did not specifically address giant PEH. 14 In the current study, we present our experience of 100 consecutive laparoscopic repairs of giant PEH that had at least one third of the stomach located intrathoracically.
METHODS
Patient Selection
A retrospective review of the University of Pittsburgh tertiary care hospitals’ patient database and the patient medical records identified 100 patients who underwent elective repair of a giant PEH between July 1995 and February 2000. Surgical consent was obtained from all patients after the risks, benefits, and alternatives to the procedure were explained. Giant PEH was defined as having at least one third of the stomach herniated into the chest (Fig. 1). This criterion was applied in the evaluation of the surgical reports and radiographic studies. The percentage of the stomach herniated into the thoracic cavity on barium esophagram was assessed by a single radiologist (W.C.). Preoperative evaluation included upper endoscopy, a barium esophagram, 24-hour esophageal pH study, and esophageal manometry. Patients were contacted after surgery to assess their quality of life. Global quality of life was measured using the SF-12, a generic measure of health status that was developed and extensively validated by the Medical Outcomes Trust. 15 Raw scores were entered into an outcomes analyzer software program (Assist Technology, Scottsdale, AZ) from which physical component summary (PCS) and mental component summary (MCS) scores were generated. These scores were compared with U.S. normal values. Disease-specific heartburn scores were measured using the Gastroesophageal Reflux Health-Related Quality-of-Life scale (GERD-HRQOL). This instrument consists of nine items and produces scores from 0 (best score, no heartburn) to 45 (worst score, most severe heartburn). Although this instrument was not administered before surgery in this study, baseline scores have previously been reported to be approximately 28 before surgery for PEH. 16 The GERD-HRQOL also contains an additional item that addresses satisfaction (1 = satisfied, 2 = neutral, 3 = dissatisfied).
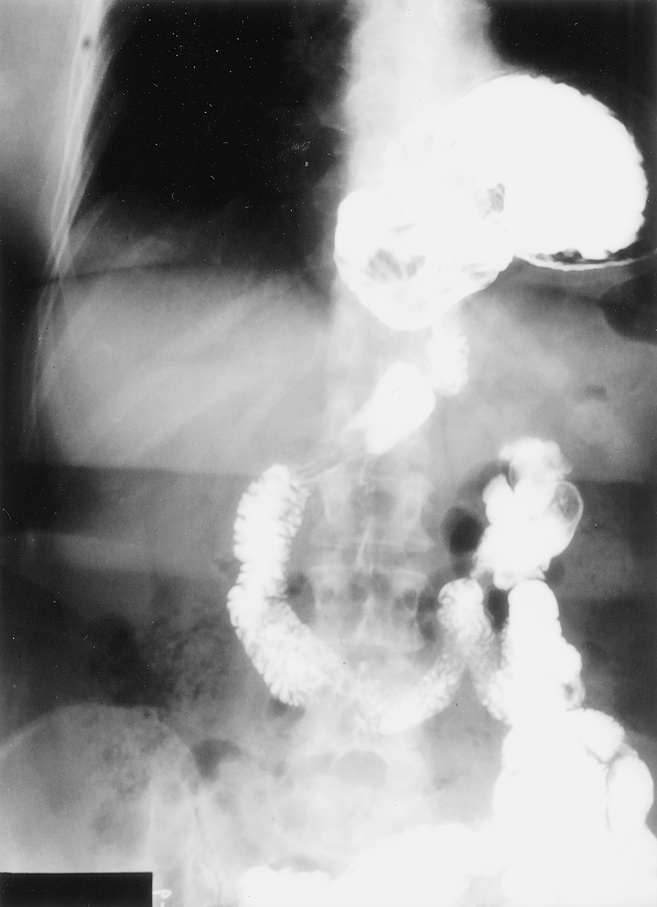
Figure 1. Preoperative barium esophagram demonstrating a giant paraesophageal hernia.
Surgical Technique
The patient is placed in the supine position with the surgeon on the right side and the assistant on the left. Pneumatic stockings are applied before the procedure to minimize the risk of deep vein thrombosis. Subcutaneous heparin is given twice daily because we have found compliance problems with the consistent application of the stockings after surgery. Four 5-mm and one 10- to 15-mm laparoscopic ports (Versaport, United States Surgical Corp. [USSC], Norwalk, CT) are placed in the upper abdomen (Fig. 2). The left lateral segment of the liver is retracted anteriorly with a 5-mm flexible retractor (Snowden Pencer, Genzyme, Tucker, GA) and secured to a stationary holding device (Mediflex, Islanda, NY). After exposure, the herniated stomach is reduced into the abdomen using atraumatic graspers (Snowden Pencer) in a hand-over-hand fashion (Fig. 3). Dissection is started by dividing the gastrosplenic ligament beginning just lateral to the midportion of the greater curvature of the stomach using ultrasonic dissection with the harmonic scalpel (Ethicon, Cincinnati, OH) or the ultrasonic shears (USSC). The short gastric vessels are divided along with the posterior alveolar attachments to the fundus. Once the greater curvature is completely mobilized, the gastrohepatic ligament is divided and the right crus of the diaphragm exposed. This is continued to expose the joining of the right and left crura at the retroesophageal space. The hernia sac is excised beginning sharply at the diaphragmatic hiatus and then bluntly dissected from the intrathoracic cavity, with care taken to identify and spare the vagal nerves (Figs. 4, 5). Pleural tears are carefully watched for; if they occur, they may require a pigtail thoracostomy. The surgeon and the anesthesiologist must communicate closely during this procedure because changes in blood pressure or inspiratory pressures may indicate a tension pneumothorax. After reduction of the sac, it is excised and removed through the 10- to 15-mm trocar site. In some patients with extensive adhesions of the hernia sac, a lighted bougie may facilitate identification of the esophageal wall and vagal nerves.
Figure 2. Laparoscopic port sites.
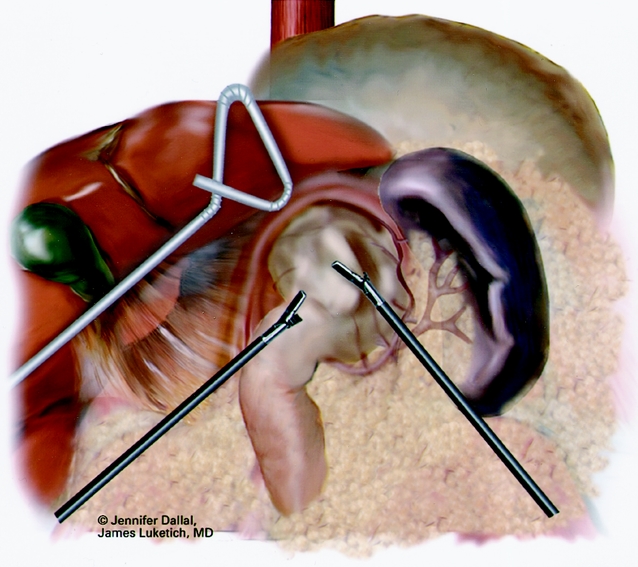
Figure 3. Laparoscopic “hand-over-hand” reduction of herniated stomach.
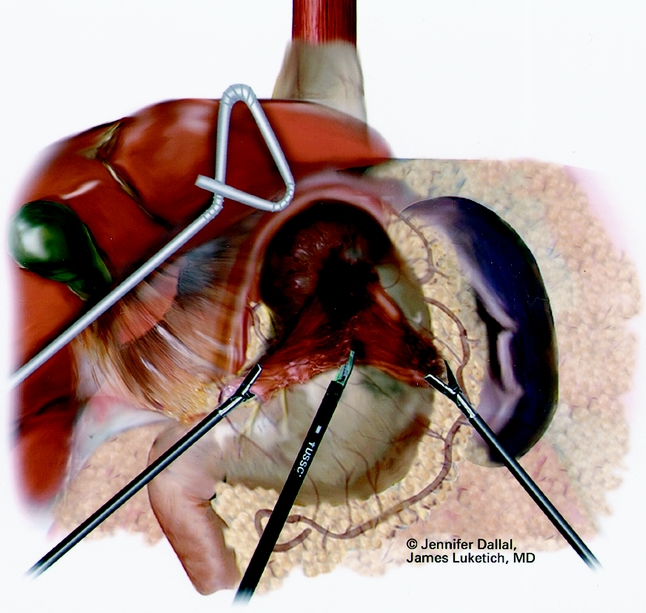
Figure 4. Laparoscopic sac and fat pad dissection.
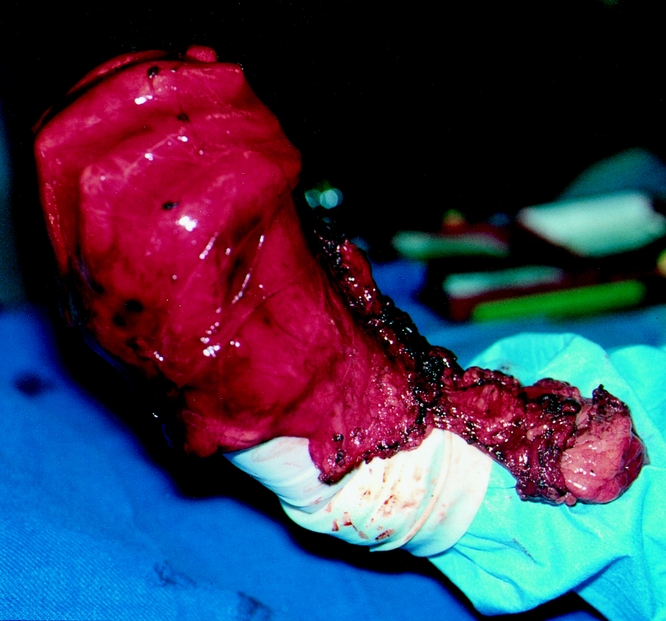
Figure 5. Laparoscopically resected gastroesophageal fat pad with hiatal hernia sac.
In our initial experience, a Collis gastroplasty was not routinely performed. As experience was gained, careful identification of the gastroesophageal junction after fat pad excision frequently revealed a shortened esophagus in the setting of a giant PEH. Briefly, the esophageal fat pad is carefully and completely mobilized laterally, sweeping the anterior vagus to the right of the esophagus. The distal esophagus is then mobilized at the level of the diaphragmatic hiatus circumferentially to determine whether esophageal shortening is present. Our practice is to assess for tension by pulling on the stomach caudally once the gastroesophageal junction has been mobilized. If the esophagogastric junction does not remain below the diaphragmatic hiatus with an adequate segment of intraabdominal esophagus, a Collis gastroplasty is added before fundoplication.
A Maloney esophageal bougie is placed into the stomach along the lesser curve. The diameter of the bougie is determined based on preoperative history, patient size, and results of esophageal manometry. Assuming adequate esophageal motility, we typically use a 50F bougie. A large tapered needle attached to a #2 suture is straightened and tied to the point of the anvil of the EEA stapler, and the needle is passed through the stomach from posterior to anterior adjacent to the bougie (Fig. 6). The needle serves as a guide for the anvil of the EEA stapler, which is pulled gently through the posterior and anterior stomach walls adjacent to the bougie. Judicious application of the electrocautery facilitates passage of the anvil tip. The EEA stapler is then inserted into the abdomen through the port site lying just to the right of the midline and is attached to the anvil. The fired EEA stapler creates a circular defect in the stomach wall that allows completion of the gastroplasty segment with the endo-GIA stapler (Fig. 7). The endo-GIA II (USSC) is fired in a cranial direction, snugly against the bougie to create at least 3 cm of tension-free intraabdominal neoesophagus. Staple lines are carefully examined for potential leaks.
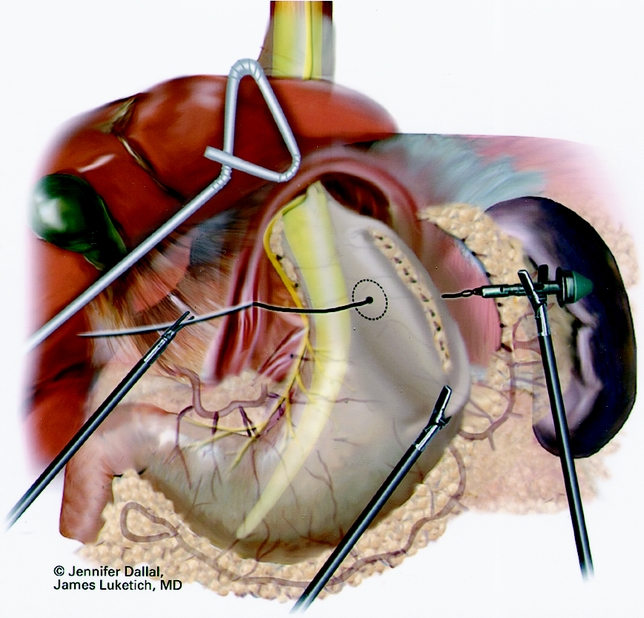
Figure 6. Anvil positioning for EEA stapler.
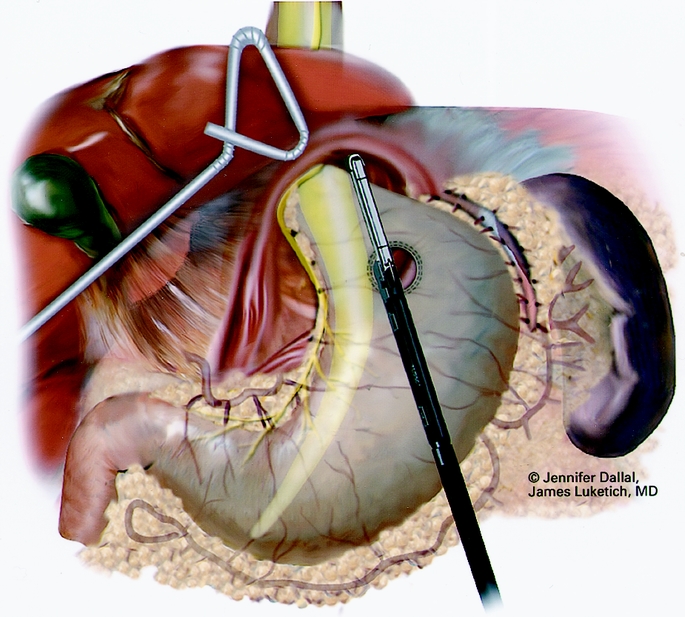
Figure 7. Creation of neoesophagus with endoscopic stapler.
The neoesophagus is wrapped with the mobilized gastric fundus in a 2- to 3-cm floppy Nissen fundoplication (Fig. 8). Care is taken to cover the point of overlap of the EEA circular staple line and the Endo-GIA II line with the gastric wrap. After the fundoplication, the bougie is removed and a nasogastric tube positioned in the stomach under direct visualization. The crura are reapproximated posteriorly to complete the surgical procedure (Fig. 9). The hiatal hernia defect is closed primarily by approximating the crura below the esophagus with interrupted 0 braided polyester suture (Surgidac; USSC) using the Endostitch (USSC), a laparoscopic suturing device. In most cases the crura are approximated primarily without excess tension. In unusual cases of an excessively large defect, a path of Gore-Tex (W.L. Gore, Flagstaff, AZ) is used to reinforce the closure. A gastrotomy or a gastropexy is not routinely performed. Before closing, endoscopy is routinely performed with intraluminal insufflation to rule out esophageal or gastric leaks. A nasogastric tube is placed under laparoscopic guidance. The decision to convert to an open procedure is at the discretion of the operating surgeon.
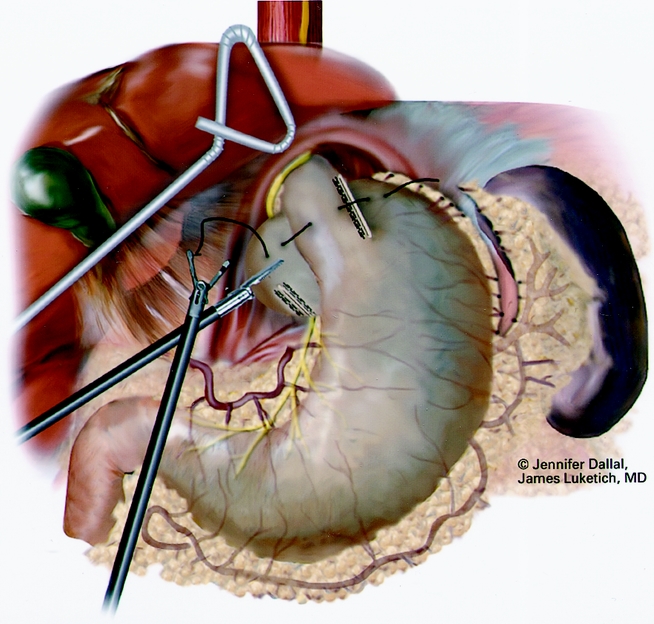
Figure 8. Suturing of 360° wrap around Collis segment.
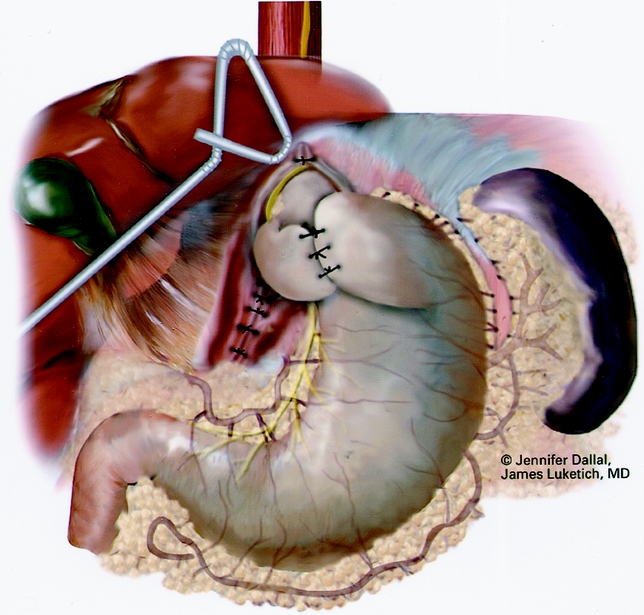
Figure 9. Completed crural repair and Nissen fundoplication.
After surgery, the nasogastric tube is removed on postoperative day 1 and a barium swallow is obtained to evaluate the repair and verify the absence of a leak. If no leak is present, clear liquids are started. If a clear liquid diet is tolerated, patients are discharged home on postoperative day 2. Dietary advancement to a soft diet and a regular diet occurs over the subsequent 2 to 3 weeks.
RESULTS
The outcomes of 100 consecutive laparoscopic repairs of giant PEH from July 1995 to February 2000 were included. All patients undergoing elective repair of a giant PEH during this period were included. Demographics and symptoms are listed in Table 1. The most common symptoms included typical symptoms of heartburn (55%), postprandial abdominal pain (31%), nausea (28%), and either regurgitation or vomiting (37%). The hernia types included 8 type II, 85 type III, and 7 type IV. All patients underwent preoperative contrast studies to characterize their PEH, and outside reports or surgeon’s review demonstrated at least one third of the stomach in the chest. To confirm the degree of herniated stomach present according to the surgeon’s assessment at surgery and endoscopy, 62 preoperative contrast studies were retrieved and reviewed by an independent radiologist. All 62 had at least one third of the stomach in the chest, with a mean percentage of intrathoracic stomach of 58%.
Table 1. DEMOGRAPHICS AND PREOPERATIVE SYMPTOMS

The median surgical time was 3.67 hours (range 2.0–11.5). The median length of stay was 2 days. Complete sac removal and crural repair was performed in all patients. The crural repair was primary in 96 patients; 4 had a mesh repair. All but one of the patients underwent an antireflux procedure. There were 72 Nissen procedures and 27 Collis-Nissen fundoplications. There were no emergent conversions to an open procedure. There were three conversions to open procedures (two cases of severe adhesions, inability to reduce the stomach safely laparoscopically in one patient). Intraoperative complications included pneumothorax requiring a chest tube (n = 4), esophageal perforation (n = 5), and gastric perforation (n = 3). The perforations were small and easily repaired laparoscopically. On postoperative day 1, a barium esophagram was performed to evaluate the repair and rule out leaks (Fig. 10).
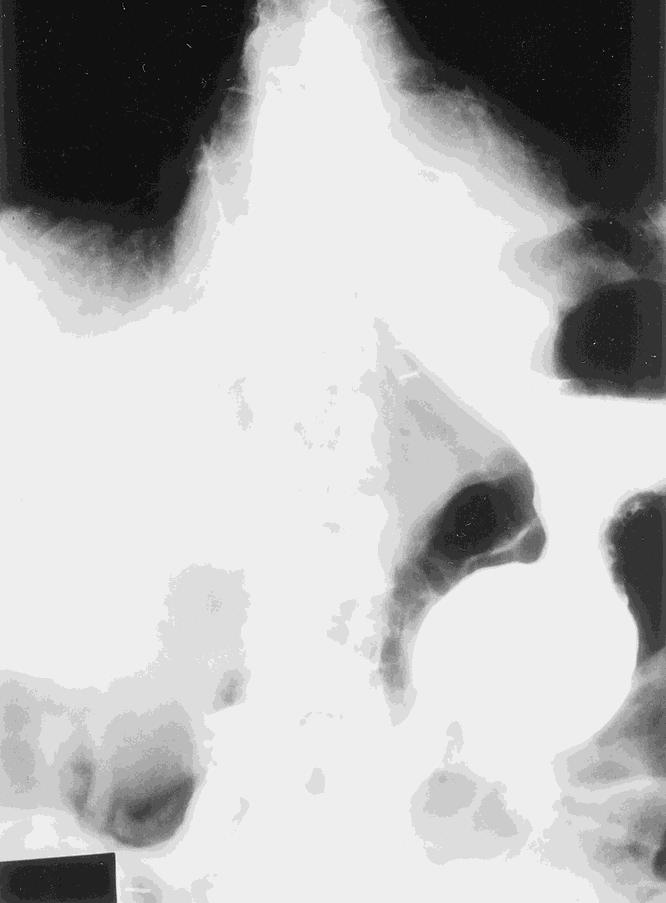
Figure 10. Postoperative barium esophagram.
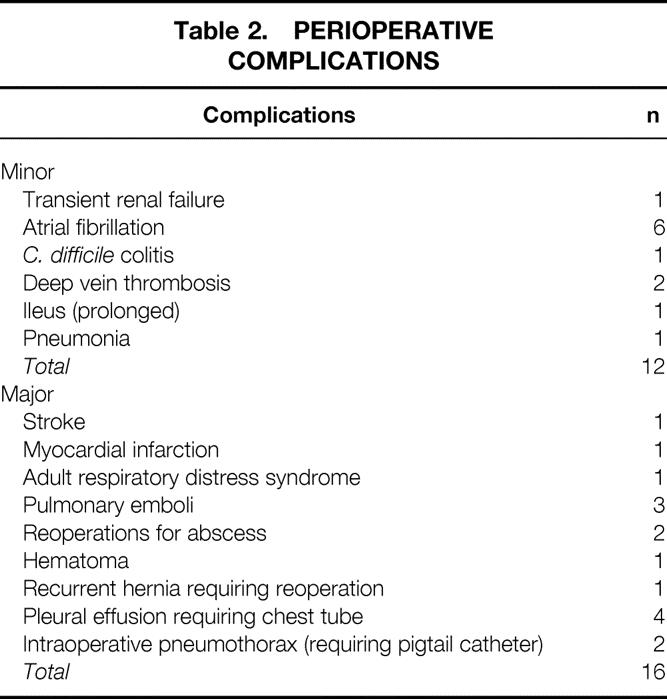
The 30-day death rate was zero. Major perioperative complications included stroke (n = 1), myocardial infarction (n = 1), adult respiratory distress syndrome (n = 1), pulmonary emboli (n = 3), reoperations for abscess (n = 2), hematoma (n = 1), recurrent hernia requiring reoperation (n = 1), and pleural effusion requiring a chest tube (n = 4). The patient who had a stroke did not make a full recovery and died 5 months later, yielding an overall surgical death rate of 1%. One of the abscesses was related to a leak from the Collis stapled gastroplasty and was managed by an open reoperation, with a 10-day hospital stay and complete recovery. In the other patient, an inadvertent small gastric perforation appeared to be the source. This patient had a long septic course (107-day hospital stay) with ultimate complete recovery. Minor perioperative complications occurred in 12% of the patients (Table 2) .
Table 2. PERIOPERATIVE COMPLICATIONS
Median follow-up was 12 months (range 2–48 months). Follow-up was complete in 90 patients. At the time of follow-up, four patients had died. This is not unexpected because PEH tends to occur in the elderly, a group who often have many premorbid conditions.
The mean follow-up score for the GERD-HRQOL was 2.3 (possible range, 0 [best] to 45 [worst]). A satisfaction survey found only 9% of patients dissatisfied with the outcome of their surgery. The mean physical component score of the SF-12 was 49, and the mental component score was 54. These scores were not significantly different from the mean score for age-matched controls without GERD in the United States, which is 50.
DISCUSSION
Once a patient has developed a symptomatic giant PEH, surgical intervention should be considered. Multiple retrospective reports have documented a high complication rate with prolonged medical management. In one report, progression of symptoms occurred in 45% of medically managed patients 4; in another report, life-threatening complications occurred in more than 25% of patients who did not undergo surgery. 5 Once a complication, such as obstruction, strangulation, or perforation, occurs, the surgical death rate ranges from 16% to 50%. Elective open surgical series have consistently shown a low death rate (1–2%), but there is still reluctance on the part of patients and referring doctors to consider elective open surgical repair because of the associated comorbidities and potential for complications and prolonged recovery.
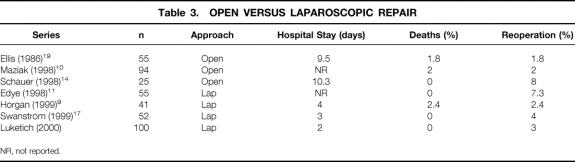
Laparoscopic approaches have the potential to lower the surgical complication rate and encourage more consistent referral of patients for elective repair with an earlier return to normal activity levels. Several initial reports of laparoscopic repair showed it was a technically feasible and safe procedure in centers with extensive experience in laparoscopic surgery (Table 3). Horgan et al 9 reported that laparoscopic repair of PEH was a technically difficult procedure but effective, with a mean hospital stay of 4 days and a single surgical death among 41 patients. In another study, Edye et al 11 reported on 55 patients undergoing laparoscopic repair of PEH and again found it a safe and technically feasible procedure, with no reported surgical deaths and acceptable outcomes, although details of hospital stay were not reported. Swanstrom et al, 17 in a report on 52 patients undergoing laparoscopic repair of PEH, found a mean surgical time of 4 hours, a hospital stay of 3 days, no surgical deaths, and good outcomes.
Table 3. OPEN VERSUS LAPAROSCOPIC REPAIR
NR, not reported.
As our experience with giant PEH has increased, we have more frequently recognized an association with shortening of the esophagus. This remains a controversial point, and the incidence of acquired shortening of the esophagus in association with GERD is unknown and questioned by some surgeons. There is less controversy as to the existence of shortening of the esophagus in association with giant PEH, and some authors report up to a 100% incidence in this setting. Altorki et al 18 evaluated 52 patients with giant PEH and reported that in 77% the gastroesophageal junction was in the mediastinum. Severe esophagitis and transmural fibrosis as a cause of esophageal shortening were not observed. The authors postulated that cephalad migration of the gastroesophageal junction with subluxation of its attachments allowed the longitudinal muscle layer of the esophagus to shorten, resulting in “pseudoshortening.” In that series, extensive esophageal mobilization without the addition of a Collis lengthening procedure resulted in good clinical results in 90% of patients.
Maziak et al 10 reported the gastroesophageal junction was located above the diaphragmatic hiatus is 91 of 94 patients with a giant PEH. In this group, esophageal manometry was used to measure the distance between the upper and lower esophageal sphincter. The average distance was only 15.4 cm, consistent with significant esophageal shortening (the normal distance is 20.4 cm). In that large series, a Collis gastroplasty was added in 80% of patients for esophageal shortening, with a 93% success rate at a median follow-up of 6 years. In the current series, we identified esophageal shortening in 27% of patients and performed 27 Collis gastroplasties. However, in our more recent experience the incidence approached 90%, similar to the results found by Maziak et al. In contrast, Ellis et al 19 reported that of 55 surgical procedures performed for PEH, only two patients were identified with a shortened esophagus.
The current study confirms the technical feasibility and safety of elective laparoscopic repair in 100 consecutive patients with giant PEH. The 30-day death rate was zero; the hospital stay was 2 days. There was one delayed death from complications of a perioperative stroke, yielding an overall death rate of 1%. At 3 weeks, patients returned to the clinic for routine assessment (Fig. 11). Follow-up at 1 year showed that 91% of patients were satisfied with the surgery. Specialized outcome measures were also favorable at short-term follow-up: the GERD-HRQOL score was 2.3 on a scale of 0 to 45. 16 The preoperative GERD-HRQOL score for patients with PEH is reported to be approximately 28. 16 The SF-12 showed a median physical component score of 49 and a mental component score of 55; these both are near the normal score of 50. Our surgical time averaged close to 4 hours, similar to the results of other laparoscopic reports and longer than most comparable open procedures, which are generally less than 3 hours. 14 However, the shorter hospital stay and more rapid return to preoperative activity levels may justify the increase in surgical time and the associated costs of specialized laparoscopic equipment. As experience was gained, the laparoscopic surgical time declined to close to 3 hours. Long-term outcomes will need to be reviewed to determine the durability of the repair.
Figure 11. Abdominal port sites 3 weeks after surgery.
This study does not specifically address the ongoing debate over the necessity of adding an antireflux procedure after repair of a giant PEH. Most of our patients had a history of GERD symptoms, as has been found in other series 9,10,14,17 but refuted by some authors. 19 In addition to historical features, we found that most of our patients had a type III hernia, which is believed to be a continuum in the natural history of longstanding type I hernias, which are classically associated with GERD. As such, we chose to include an antireflux procedure in all our patients, and we obtained a satisfactory improvement in the postoperative quality of life and GERD-HRQOL scores.
The laparoscopic approach to the reduction of giant PEH is feasible, safe, and effective in centers with extensive experience in minimally invasive esophageal surgery. It also appears to offer the benefit of a shorter hospital stay and a quicker recovery. Long-term follow-up is ongoing and will be required to confirm our good short-term findings. As other centers gain experience with minimally invasive surgery, this approach will become more widespread.
Discussion
Dr. John G. Hunter (Atlanta, Georgia): This well-studied and beautifully presented paper focuses our attention on one of the most difficult laparoscopic operations, which is repair of the massive paraesophageal hernia. I would like to focus my comments on two issues: the need for repair in the high-risk asymptomatic patient and the need for Collis gastroplasty as an adjunct to repair.
Last week at the SAG meeting, one of our residents compared outcomes in 120 patients with paraesophageal hernia to 880 with GERD undergoing laparoscopic repair. The paraesophageal hernia patients were 15 years older, the operation took an hour longer, the morbidity was ten times higher, and the only mortalities in 1000 patients were three elderly patients with paraesophageal hernia. Whether the access is laparoscopic or open, this is not a benign procedure in a high-risk population. With this background, we question the recommendation that asymptomatic patients with paraesophageal hernia should undergo repair. As far as I could tell, this indication stands on a single report from Mr. Belsey in 1967 that catastrophic complications occurred in 29% of asymptomatic patients observed, a finding that has never been reproduced.
Dr. Luketich, do you recommend that elderly or infirm asymptomatic patients with normal hematocrits and normal endoscopies undergo laparoscopic repair? Or is there a role for observation of these patients? Perhaps to help answer this question, how many emergency procedures were required for strangulated paraesophageal hernias at your hospital during the time of this study?
One of the truly puzzling differences between abdominal and thoracic surgeons is their views of esophageal length. Abdominal surgeons believe that most esophagi can be mobilized to allow the lower esophageal sphincter to comfortably lie below the diaphragm, but thoracic surgeons find the need to perform Collis gastroplasty in the majority of patients with massive hernias.
We have reported the need to perform Collis gastroplasty in 4% of all patients and 20% of those with massive paraesophageal hernias. Since the introduction of Collis at Pitt, how frequently has it been necessary? What criteria are applied to determine the need for a Collis gastroplasty? Is there a difference in frequency of application between abdominal and thoracic surgeons? Since we added laparoscopic Collis gastroplasty to our repertoire, we have seen a decreasing frequency of hiatal hernia recurrence. Do you think this explains your low recurrence rate?
Presenter Dr. James D. Luketich (Pittsburgh, Pennsylvania): Certainly the issue of esophageal length is controversial. Have we developed specific criteria that we can prospectively apply to our patients that will allow us to perhaps preoperatively identify who will need a Collis? No. We use a combination of the preoperative study and the intraoperative findings and a very careful dissection of the fat pad off the GE junction to facilitate assessment of esophageal length.
During the operation we think there is a tendency laparoscopically to overestimate the intraabdominal length of esophagus. Pneumoperitoneum elevates the diaphragm, a rigid bougie pushes the esophagus into the abdomen, and downward tension from a Penrose drain encircling the esophagus may all contribute to a false sense of security of the length of the intraabdominal segment of esophagus. All three of those maneuvers can lead to an overestimate of the intraabdominal length of esophagus.
We have not been able to prospectively identify criteria that can be universally applied. Assessment of esophageal length requires a careful consideration of a number of criteria which must be evaluated both preoperatively and intraoperatively.
Once esophageal shortening is recognized, the Collis technique adds about 20 minutes to the laparoscopic operation. There is a potential for staple line leaks postoperatively; this should be watched for carefully.
In terms of the number of patients that are truly asymptomatic with a giant paraesophageal hernia, I think that this represents only a small percent. Certainly Mr. Belsey stressed in his original description that asymptomatic patients could develop serious complications.
In our experience, we don’t see very many patients that on careful questioning are truly asymptomatic. Sometimes the patient is denying symptoms but the spouse recalls that after meals, severe postprandial symptoms exist. Some of these patients have simply learned to live with this discomfort over such long periods of time that they don’t really recall what it was like to have a normal stomach located in the abdomen.
Are there truly asymptomatic patients? If a patient has severe comorbidities and is a poor operative risk and feels perfectly fine and with no evidence of impending torsion or periodic severe symptoms, you could make an argument for selective nonoperative management. And we do so in rare cases.
Dr. Tom R. Demeester (Los Angeles, California): Dr. Luketich and his colleagues have demonstrated, as have others, that paraesophageal hernia can be corrected through a limited-access approach. The emphasis of excision of the hernia sac is appropriate, as this has been found to be the key to the dissection. Unique in their experience is the addition of a Collis gastroplasty in one quarter of their patients. This may be the explanation for their apparent high success, since some of these patients clearly have a short esophagus, and, if not managed appropriately, can be a cause for recurrence.
My main concern with Dr. Luketich’s report is that a high percentage of these patients are asymptomatic and surgery is performed to avoid the catastrophic complication of distention and torsion of the hernia leading to ischemia, perforation, or hemorrhage. Consequently, the use of symptoms may not be the best outcome measured. Rather a better outcome measure would be freedom from recurrent herniation. Our experience with the repair of 41 PEH has shown that the freedom from recurrent herniation is the poorest following the laparoscopic approach and best following the open approach, particularly after a transthoracic approach (J Am Coll Surg 2000;190:553–560). Most of the recurrent herniation occurs after the first year of follow-up and is asymptomatic. Our explanation for this observation is that transthoracic mobilization of the esophagus effectively released the tension on the repair in those patients with a short esophagus. Perhaps Dr. Luketich’s use of the gastroplasty accomplished the same task. A second explanation may be that the wide hiatus commonly seen in these patients is difficult to close laparoscopically and leads to breakdown and reherniation. Consequently, many surgeons are placing some form of a patch over their hiatal closure. The long-term result of the patch technique is still uncertain. In contrast to the laparoscopic technique, the open transthoracic approach gives excellent exposure for closure of the hiatus.
I have the following questions for Dr. Luketich. First, could you comment on your indication for a Collis gastroplasty in these patients? Second, since most reherniations are asymptomatic, are you obtaining follow-up UGI studies to evaluate your repairs? Third, have you done a subanalysis between patients who had and did not have a Collis gastroplasty?
Dr. Luketich: I would agree with you that there are hernia recurrences out there that are, at least at an early stage, totally clinically asymptomatic. We do perform a barium esophagogram on day 1, at 3 weeks postop, and at 1 year to carefully look for radiographic recurrences. In our limited experience with the recurrences that do occur radiographically, ultimately some of these patients have become symptomatic. I think this is a very good point, and we are continuing to evaluate long-term outcomes.
Vagal nerve injuries: currently we are collecting prospective intraoperative data forms that must be filled out right after the operation by the data coordinator in the OR. We found when we retrospectively reviewed these 100 cases, vagal anatomy wasn’t detailed consistently in the operative note. Prospectively collecting this data in the operating room should clarify this issue.
I would suggest that at least 5% of cases involve some degree of vagal injury to one or both of the main trunks. There are cases when the vagus is partly adherent to the sac, and it can be quite difficult to assure that you have freed it up, especially anteriorly. Also, as you pull the fat pad from the posterior esophagus, there is a tendency to pull the posterior vagus up and inadvertently injure it. We did not have to do any pyloroplasties in follow-up on these patients, so we suspect that vagal injuries that did occur did not result in any major or long-term clinical sequelae. We have observed some cases of delayed gastric emptying or prolonged problems with distress that could be attributed to postvagotomy syndrome.
In terms of the Collis versus non-Collis, we think this is very important. We are currently prospectively collecting this data. We are not randomizing since we feel strongly that if you definitely recognize short esophagus, it wouldn’t be ethically justified to randomize to a nonlengthening procedure.
There is some difference in philosophy even within our group in terms of diagnosing esophageal shortening, and we are attempting to sort that out. We are collecting data prospectively and would like to create a predictive index that would allow us to perhaps make a more objective identification of patients who require a lengthening procedure.
Dr. Philip E. Donahue (Chicago, Illinois): I too enjoyed Dr. Luketich’s emphasis on hernia sac excision, especially the portion of the sac to the right of the esophagus, since this is very difficult to discern before that left portion has been dissected.
I would like you to expand a little bit upon the role of the modern therapeutic armamentarium, especially proton pump inhibitors. Do you think the incidence of esophageal shortening is less in patients treated after 1990 than it was in earlier cohorts? Dr. DeMeester has already emphasized the controversy about esophageal length, and you have already described what you think about that.
My second question relates to your approach in patients with postoperative paraesophageal hernia; that is, after previous fundoplication or other operations which disrupt esophageal ligament. Do you think there is a role for laparoscopic exploration in such patients? Does the absence of the sac imply a great risk of very, very dense adhesions high above the diaphragm which can’t be approached via the laparoscopic route?
Thirdly, how often have you had to employ a counterincision in the diaphragm to get the stomach out of the chest when the hiatal opening is relatively small?
Dr. Luketich: In terms of the incidence or the changing incidence of acquired shortening of the esophagus in the era of proton pump inhibitors, we have no data on this. The incidence appears to have changed in Pittsburgh since Dr. Griff Pearson visited a couple of years ago. Perhaps we were just educated. I think that as we learn more about it and look closer, we are recognizing esophageal shortening more frequently. I think some of the recurrences in the laparoscopic era are perhaps due to unrecognized shortening.
Again I will stress that the laparoscopic pneumoperitoneum raises the diaphragm and gives a false sense of the intraabdominal segment. Most surgeons today use a bougie; this pushes the esophagus down by a couple centimeters. Also, tension on the Penrose drain wrapped around the esophagus may lead to errors in assessment of intraabdominal length. These three features of a laparoscopic operation may lead to a false sense of the intraabdominal length that will remain tension-free in the abdomen once the surgeon is done with the repair. So I am not sure if the incidence has truly changed with PPIs; we think it is important to look carefully for that esophageal shortening.
In terms of postop recurrences following a previous hiatal hernia repair, I think it is reasonable to approach these laparoscopically but to have a low threshold for converting to open if you don’t feel you can accomplish the redo surgery safely laparoscopically. Our experience has been that many redos can be done laparoscopically. It partly depends on what was done at the first operation.
In terms of having to open to actually reduce the stomach, in our series there were 3 out of 100 that we converted. All of the conversions were due to adhesions around the stomach or failure to safely remove the sac. The conversions did occur in our first 50. Have we just gotten better at releasing adhesions and dissecting out the sac, or have we just been lucky in our second 50 and perhaps we haven’t come up against a really tough one? I think there should be a low threshold to open if you cannot safely reduce the stomach and excise the sac completely.
Dr. F. Griffith Pearson (Toronto, Ontario, Canada): I would emphasize what other discussants have stated—that these giant intrathoracic stomachs are difficult repairs, even for very experienced surgeons using open techniques. In his manuscript, Dr. Luketich acknowledges that there is a significant learning curve associated with these more complex repairs using laparoscopic approaches. However, with experience, he now obtains very favorable outcomes, no mortality, and small morbidity, in a large number of elderly patients. Importantly, he has undoubtedly reduced length of stay and thus costs in these cases.
He has stated that his observations are similar to ours in that most of these giant hernias are simply late-stage, garden-variety sliding hernias, not the rare type II paraesophageal hernia, in which the esophagogastric junction lies in its normal abdominal location. This sliding category has important implications for management. Many of these patients have suffered longstanding reflux esophagitis, maybe in the distant past and poorly remembered by these elderly individuals. But like ourselves, Dr. Luketich has recognized the common associated problem of acquired short esophagus in selected cases and is adding—and I gather from your manuscript with increasing frequency—a lengthening gastroplasty to reduce tension on the repair.
We reported our experience in 1998 with 94 consecutive massive paraesophageal surgical repairs seen over a 26-year period. Ninety-one of the 94 were sliding hernias, as evidenced by the preoperative finding at radiology and endoscopy with the EG junction located in the mediastinum. The intrathoracic position of the EG junction was confirmed at operation. We added a gastroplasty in 82 of the 94 cases. With a mean follow-up of just under 10 years, only two patients have had a recurrent hernia and reoperation.
I believe that it is failure to recognize even subtle degrees of acquired shortening that has resulted in a higher incidence of recurrent hernia and reoperation in patients managed by a standard repair. [Slide] I have summarized here the results in the largest of the reported series using open techniques and laparoscopic techniques. You will see that where a gastroplasty was not added, where a lengthening procedure was not added (e.g., F. H. Ellis’ group, managed by open repair), a very high 11% recurrence rate and 8% reoperation rate. Dr. Mark Allen at the Mayo Clinic and our group in Toronto added gastroplasty liberally and had very low recurrence and reoperation rates using laparoscopic repairs. Hinder, no gastroplasty, reports a high recurrence and reoperation rate. R. Trus Hunter and Swanson, with limited application, have kind of intermediate-range recurrence and reoperation. And Luketich, with that very favorable 1% rate.
My question—just what is used to define the presence or absence of shortening?—has been asked. And Dr. Luketich has answered it.
Dr. Luketich: The gold standard has been set by the open operation, in particular Dr. Pearson’s results. It remains to be seen if we can achieve and maintain these excellent results with the laparoscopic approach as long-term follow-up is reported in the future.
Dr. Carlos A. Pellegrini (Seattle, Washington): Today with the techniques that we can use to mobilize the esophagus all the way to the thoracic inlet, I think that the thoracic approach would have less application than it used to have. The mobilization of the esophagus, in my opinion, cannot be a reason to do a thoracoscopic or thoracotomy approach. The question is, have you had a need to close any of the hernias with mesh? If so, have you put the mesh around the hiatus or through a relaxing incision in the diaphragm?
Dr. Luketich: Out of the 100 cases that were presented today, 96 were repaired primarily and 4 patients required a mesh Gore-Tex repair. We have used the relaxing incision in two patients, and in two we fashioned Gore-Tex mesh in a semicircular pattern to allow a tension-free repair of the defect.
Footnotes
Correspondence: James D. Luketich, MD, Section of Thoracic Surgery, UPMC Presbyterian, Suite C800, 200 Lothrop St., Pittsburgh, PA 15213.
Presented at the 120th Annual Meeting of the American Surgical Association, April 6–8, 2000, The Marriott Hotel, Philadelphia, Pennsylvania.
E-mail: LuketichJD@MSX.UPMC.edu
Accepted for publication April 2000.
References
- 1.Duranceau A, Jamieson GG. Hiatal hernia and gastroesophageal reflux. In: Sabiston DCJ, Lyerly HK, eds. Textbook of surgery. Philadelphia: WB Saunders; 1997: 767–783.
- 2.MacArthur KE. Hernias and volvulus of the gastrointestinal tract. In: Feldman M, Scharschmidt BF, Sleisenger MH, Klein S, eds. Sleisenger & Fordtran’s gastrointestinal and liver disease. Philadelphia: WB Saunders; 1998: 318–327.
- 3.Haas O, Rat P, Christophe M, Friedman S, Favre JP. Surgical results of intrathoracic gastric volvulus complicating hiatal hernia. Br J Surg 1990; 77: 1379–1381. [DOI] [PubMed] [Google Scholar]
- 4.Treacy PJ, Jamieson GG. An approach to the management of para-oesophageal hiatus hernias. Aust NZ J Surg 1987; 57: 813–817. [DOI] [PubMed] [Google Scholar]
- 5.Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia: long-term results with 1,030 patients. J Thorac Cardiovasc Surg 1967; 53: 33–54. [PubMed] [Google Scholar]
- 6.Ozdemir IA, Burke WA, Ikins PM. Paraesophageal hernia: a life-threatening disease. Ann Thorac Surg 1973; 16: 547–554. [DOI] [PubMed] [Google Scholar]
- 7.Hill LD. Incarcerated paraesophageal hernia: a surgical emergency. Am J Surg 1973; 126: 286–291. [DOI] [PubMed] [Google Scholar]
- 8.Williamson WA, Ellis FH Jr, Streitz JM Jr, Shahian DM. Paraesophageal hiatal hernia: is an antireflux procedure necessary? Ann Thorac Surg 1993; 56: 447–451. [DOI] [PubMed] [Google Scholar]
- 9.Horgan S, Eubanks TR, Jacobsen G, Omelanczuk P, Pellegrini CA. Repair of paraesophageal hernias. Am J Surg 1999; 177: 354–358. [DOI] [PubMed] [Google Scholar]
- 10.Maziak DE, Todd TR, Pearson FG. Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg 1998; 115: 53–60. [DOI] [PubMed] [Google Scholar]
- 11.Edye M, Salky B, Posner A, Fierer A. Sac excision is essential to adequate laparoscopic repair of paraesophageal hernia. Surg Endosc 1998; 12: 1259–1263. [DOI] [PubMed] [Google Scholar]
- 12.Gantert WA, Patti MG, Arcerito M, et al. Laparoscopic repair of paraesophageal hiatal hernias. J Am Coll Surg 1998; 186: 428–432. [DOI] [PubMed] [Google Scholar]
- 13.Paul MG, DeRosa RP, Petrucci PE, Palmer ML, Danovitch SH. Laparoscopic tension-free repair of large paraesophageal hernias. Surg Endosc 1997; 11: 303–307. [DOI] [PubMed] [Google Scholar]
- 14.Schauer PR, Ikramuddin S, McLaughlin RH, et al. Comparison of laparoscopic versus open repair of paraesophageal hernia. Am J Surg 1998; 176: 659–665. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Kosinski M, Keller SD, Lincoln RI. How to score the SF-12 physical and mental summary scales. In: 1998 SF-12. Lincoln, RI: Quality Metric Incorporated; 1998.
- 16.Velanovich V, Vallance SR, Gusz JR, Tapia FV, Harkabus MA. Quality of life scale for gastroesophageal reflux disease. J Am Coll Surg 1996; 183: 217–224. [PubMed] [Google Scholar]
- 17.Swanstrom LL, Jobe BA, Kinzie LR, Horvath KD. Esophageal motility and outcomes following laparoscopic paraesophageal hernia repair and fundoplication. Am J Surg 1999; 177: 359–363. [DOI] [PubMed] [Google Scholar]
- 18.Altorki NK, Yankelevitz D, Skinner DB. Massive hiatal hernias: the anatomic basis of repair. J Thorac Cardiovasc Surg 1998; 115: 828–35. [DOI] [PubMed] [Google Scholar]
- 19.Ellis FH Jr, Crozier RE, Shea JA. Paraesophageal hiatus hernia. Arch Surg 1986; 121: 416–420. [DOI] [PubMed] [Google Scholar]