Abstract
Objective
To evaluate the quality of life (QoL) in patients undergoing anterior resection (AR) or abdominoperineal extirpation (APE) for rectal cancer in a sample of patients recruited from a field trial.
Summary Background Data
Abdominoperineal resection has been reported to put patients at higher risk of disruption to QoL than sphincter-preserving surgery.
Methods
Fifty patients treated with AR and 23 patients treated with APE were prospectively followed up. All patients were treated in curative attempt and were disease-free throughout the study. QoL was assessed before surgery and 6 to 9 and 12 to 15 months after surgery.
Results
Multivariate analysis of variance and subsequent post hoc comparisons revealed a main effect for time (role function, emotional function, body image, future perspective, and micturition-related problems) and group in favor of APE (sleeping problems, constipation, diarrhea), and a time-by-group interaction (role function). No significant results were obtained for the remaining scores, but patients undergoing APE consistently had more favorable QoL scores than those undergoing AR. Multivariate analysis and post hoc comparisons revealed a particularly poor QoL for patients undergoing low AR. They had a significantly lower total QoL, role function, social function, body image, and future perspective, and more gastrointestinal and defecation-related symptoms than patients undergoing high AR.
Conclusion
Patients undergoing APE do not have a poorer QoL than patients undergoing AR. Patients undergoing low AR have a lower QoL than those undergoing APE. Attention should be paid to QoL concerns expressed by patients undergoing low AR.
It is the state of the art that whenever feasible, rectal cancer should be treated with sphincter-preserving surgical techniques. A vast body of literature suggests that patients who have a colostomy have a worse quality of life (QoL) than those without a stoma. 1–11 However, any interpretation of the seemingly unequivocal results on this issue must take into account the major drawbacks of many of the investigations focusing on QoL aspects in patients with rectal cancer. A main problem is related to general disagreement among QoL researchers as to which method and instrument are most appropriate for accrual of QoL information. An abundance of techniques can be used to collect QoL data, including structured, semistructured, or nonstructured interviews, standardized and nonstandardized questionnaires, and ad hoc questions. This incompatibility in data accrual has resulted in a broad range of inventories used to assess QoL in patients with colorectal cancer and has also contributed to a body of inconsistent findings. In an excellent survey on QoL aspects in patients treated for cancer of the rectum, Camilleri-Brennan and Steele 10 described the current data situation in the field and concluded that “the methodological shortcomings of previous work must be rectified if quality of life studies are to have relevance inpatient management.” Of 54 papers on the subject published in English, only 14 could be identified as prospective trials. The remaining 40 papers were either cross-sectional or retrospective in design. Only three studies were dedicated to a comprehensive assessment of QoL using well-established questionnaires with proven psychometric properties. 12–14 The remaining studies were found to be limited to isolated symptoms, such as sexual dysfunction and defecation-related or urologic symptoms. Most of the studies used unvalidated questionnaires. Sample sizes were typically less than 25 patients, and follow-up periods ranged from 1 month after surgery to 30 years. Therefore, despite the flood of data on the subject, the present situation of research into QoL in patients with rectal cancer is unsatisfactory.
The present study was set up to clarify the inconsistencies that have arisen in the past years of studying QoL aspects in patients with rectal cancer by implementing solid methodology. It was part of a prospective investigation to evaluate the process- and outcome-oriented quality of oncologic care in a region in Germany. Guideline-oriented measures and data on complications, death rates, survival, and QoL aspects were used as critical endpoints. In concert with the overwhelming evidence in the literature that patients who undergo sphincter-preserving techniques have a better QoL than those who undergo rectal amputation, the aim of this study was to evaluate this finding in the present sample of patients. A major goal was to determine which aspects of QoL might be compromised the most after either anterior resection (AR) or abdominoperineal extirpation (APE). To provide precise information to clinicians who care for these patients, we tried to attain as much methodologic precision as possible in a clinical setting. Consistently, a clearly defined subgroup of patients undergoing either APE or AR for rectal cancer was chosen and prospectively followed up for 15 months regarding QoL.
METHODS
Patients
From a convenience sample, a group of 23 patients undergoing APE and a group of 50 undergoing AR were selected for the investigation. Inclusion criteria were as follows: the patient’s surgery had to have been undertaken with a curative attempt (R0 resection), the patient had to be free of recurrence throughout the study period, and both clinical and QoL data had to be available at each point of assessment. In this article we do not report on the clinical data; instead, we focus on the QoL findings.
Instruments
Questionnaires were used to collect clinical and QoL data. Clinical information included tumor stage, surgical technique, and tumor recurrence. QoL data were obtained using the European Organization for Research and Treatment of Cancer (EORTC) QLQ C30 questionnaire (version 1.0, EORTC Study Group on quality of life, Brussels, Belgium) and the complementary colorectal module CR 38. These are well-established tools for the assessment of QoL in patients with cancer; both have been proven reliable and valid. 15,16 For almost two decades, the QLQ C30 instrument has been used to measure QoL in various samples of patients with cancer. 2,17–20
Procedure
The study was prospective and comprised three points of assessment. The first assessment took place shortly after the diagnosis but before treatment started, the second 6 to 9 months after surgery, and the third 12 to 15 months after surgery. Eligible patients were approached for the first assessment when in the hospital awaiting the initiation of treatment. They were informed of the goals of the study and the method of data collection and were invited to participate in the study, but also assured that their refusal would not jeopardize their treatment at the hospital. Informed consent was obtained from every patient who participated in the study. The patients were then administered the QoL questionnaire and their clinical data were recorded. The second and third assessments were carried out in the outpatient department when the patients received routine follow-up care. They were given a QoL questionnaire that they could complete on the spot or fill out at home and return to the hospital; self-addressed stamped envelopes were provided. At each assessment, relevant clinical data were obtained by the treating physician.
Analysis
The QoL questionnaires at the three assessment points were analyzed using the statistical package SPSS for Windows, release 8.0. Only data from patients with three consecutive assessments were included. In concert with the EORTC QLQ C30 scoring manual, QoL forms with more than 50% missing items per subscale were excluded from the analysis. Descriptive statistics and multivariate testing were used. Demographic and clinical data were calculated using descriptive statistics. QoL information was processed using multivariate analysis of variance, followed by individual post hoc comparisons for the two groups and each assessment point. Because QoL information was collected at three assessment points (time) and in two groups of patients (group), multiple analysis was used in a time-by-group design. As a level of significance, α = 5% was accepted.
RESULTS
The Fisher exact test showed no difference in the proportion of male and female participants in the two study groups (P = .07). The APE group comprised 18 men (78.3%) and 5 women (17.9%), the AR group 27 men (54%) and 23 women (46%). There was no significant difference in age (t test, P = .76, df = 71). The mean age was 61.44 years in the AR group and 62.17 years in the APE group. A chi-square test on the distribution of tumor stages in the two groups was not significant (Pearson chi-square = 0.99, df = 2). In the APE group, seven patients (30.4%) were diagnosed with tumors staged UICC T1, eight patients (34.8%) T2, and eight (34.8%) T3. In the AR group, 15 patients (30%) were found to have a tumor staged T1, 17 (34%) T2, and 18 (36%) T3. The proportion of APE and AR patients in the study sample roughly corresponded to the respective proportion in the total population of patients with rectal cancer. Twenty-three patients (31.5%) in the study underwent APE and 50 patients (68.5%) underwent AR. Before surgery, 18 patients (36%) in the AR group and 13 patients (56.5%) in the APE group received neoadjuvant treatment (Fisher exact test, P = .13). After surgery, a comparable number of 25 AR patients (50%) and 11 APE patients (47.8%) received adjuvant treatment (Fisher exact test, P = 1.00). Next, the level of anastomosis was evaluated in patients undergoing AR. Fifteen patients (30%) had a level of anastomosis extending up to 5 cm from the anocutaneous line; in 35 patients (70%) it was higher. A Fisher exact test on the percentage of postoperative complications among AR and APE patients was not significant (P = .16). Eleven patients (22%) in the AR group and 9 (39.1%) in the APE group had a complicated recovery.
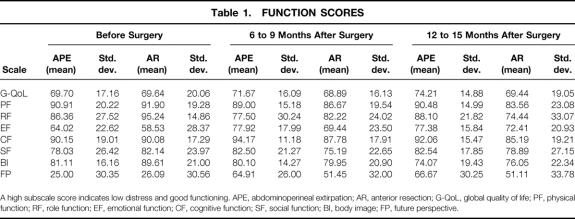
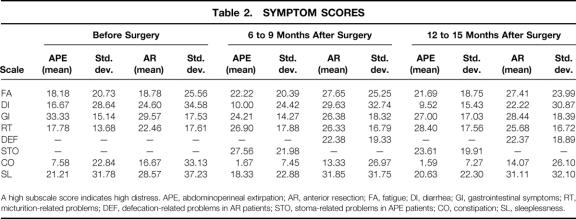
Multiple analysis of variance and subsequent post hoc comparisons were calculated for the QoL data. Possible subscale scores on each of the function and symptoms scales can range from 0 to 100. A high score on a functional subscale indicates good functioning and little restriction in QoL; conversely, a high score on the symptoms scales symbolizes severe distress. Table 1 shows the means and standard deviations of the QLQ C30 and CR 38 functional scales for each group at each assessment. Table 2 lists the respective scores for the QLQ C30 and CR 38 symptoms scales. Analysis of the QoL questionnaire yielded mostly nonsignificant results for the groups at the first, second, and third assessment points. However, as shown in Tables 1 and 2, on most of the scales the APE patients had superior, although not significantly better, scores than the AR patients.
Table 1. FUNCTION SCORES
A high subscale score indicates low distress and good functioning. APE, abdominoperineal extirpation; AR, anterior resection; G-QoL, global quality of life; PF, physical function; RF, role function; EF, emotional function; CF, cognitive function; SF, social function; BI, body image; FP, future perspective.
Table 2. SYMPTOM SCORES
A high subscale score indicates high distress. APE, abdominoperineal extirpation; AR, anterior resection; FA, fatigue; DI, diarrhea; GI, gastrointestinal symptoms; RT, micturition-related problems; DEF, defecation-related problems in AR patients; STO, stoma-related problems in APE patients; CO, constipation; SL, sleeplessness.
Multiple analysis of variance produced a main effect on the factor “time” for the variables role function (F = 3.04, df = 2, P = .50), emotional functioning (F = 6.25, df = 2, P = .002), body image (F = 3.49, df = 2, P = .03), and future perspective (F = 20.57, df = 2, P = .00). Both patient groups showed a decrease in role function from the first to the second assessment and a decrease in body image from the second to the third assessment, but there was a consistent increase in emotional well-being and future perspective across the time of the study (Tables 1 and 3). Post hoc comparisons revealed a marginally significant difference for micturition-related problems (P = .05), which increased for both groups from the first to the second assessment. Multiple analysis revealed a main effect on the factor “group” for the variables constipation (F = 8.23, df = 1, P = .01) and diarrhea (F = 8.67, df = 1, P = .004). On both variables, APE patients had significantly better scores than AR patients. Post hoc comparisons showed a significant difference for sleeplessness (P = .03): AR patients reported significantly more sleep disturbances than APE patients. Finally, a marginal time-by-group interaction resulted for the variable role function (F = 3.09, df = 2, P = .05). AR patients reported a constant decrease in role function, and APE patients had some postoperative decrease at the second assessment but an improvement at the third.
Table 3. SIGNIFICANT RESULTS, APE, AND AR
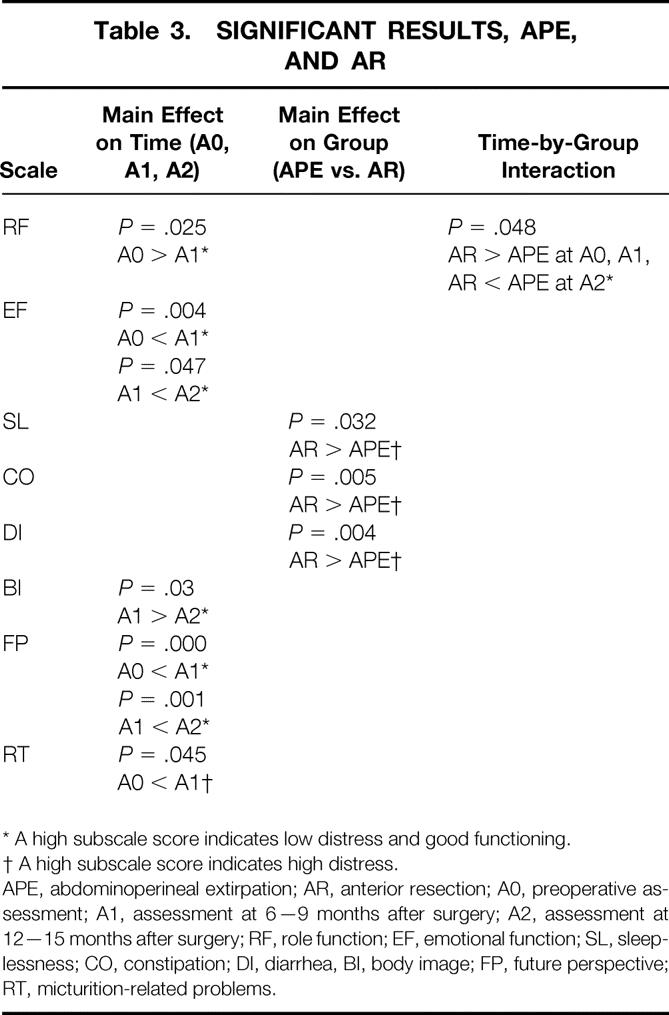
* A high subscale score indicates low distress and good functioning.
† A high subscale score indicates high distress.
APE, abdominoperineal extirpation; AR, anterior resection; A0, preoperative assessment; A1, assessment at 6—9 months after surgery; A2, assessment at 12—15 months after surgery; RF, role function; EF, emotional function; SL, sleeplessness; CO, constipation; DI, diarrhea, BI, body image; FP, future perspective; RT, micturition-related problems.
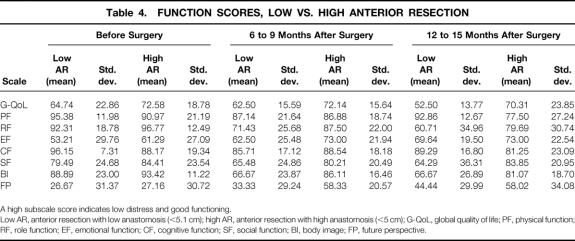
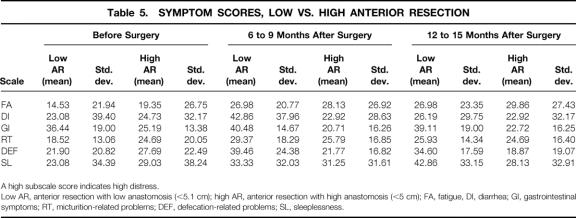
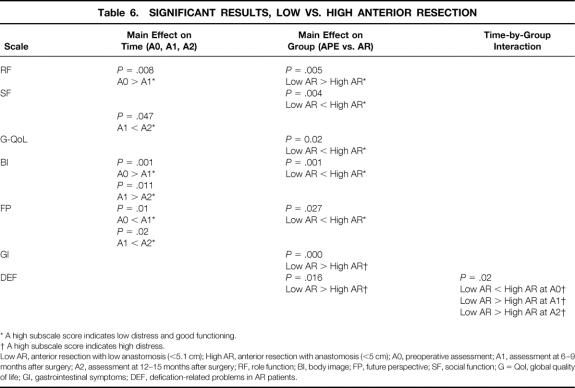
To evaluate whether the level of anastomosis affected the AR patients’ assessment of their postoperative QoL, AR patients were divided into those with anastomoses up to 5 cm from the anocutaneous line and those with higher levels of anastomosis. Multiple analysis of variance and post hoc comparisons were calculated for the 15 low AR patients and the 35 high AR patients, resulting in a main effect on the factor “time” for the variables role function (F = 9.50, df = 2, P = .00), emotional function (F = 3.24, df = 2, P = .04), body image (F = 9.02, df = 2, P = .00), and future perspective (F = 6.51, df = 2, P = .002), a main effect on the factor “group” for the variables role function (F = 8.31, df = 1, P = .005), social function (F = 8.40, df = 1, P = .004), global QoL (F = 5.54, df = 1, P = .02), body image (F = 12.59, df = 1, P = .001), future perspective (F = 5.03, df = 1, P = .03), gastrointestinal symptoms (F = 27.17, df = 1, P = .00), and defecation-related symptoms (F = 6.02, df = 1, P = .02), and a time-by-group interaction for the variable defecation-related symptoms (F = 4.03, df = 2, P = .02) (Tables 4, 5, and 6). Post hoc comparisons showed a postoperative decrease for role function in both groups at the second (P = .008) and third assessments (P = .001); the low AR patients had significantly lower scores than the high AR patients (P = .005). Similar decreases in both groups across time were obtained for body image (P = .001 and P = .01) and future perspective (P = .01 and P = .02). On both variables, the high AR patients had significantly better scores than the low AR patients (body image, P = .001; future perspective, P = .027). The low AR patients reported significantly better social functioning (P = .004) and global QoL (P = .02) than the high AR patients and tended to have better emotional functioning at the second (P = .07) and third assessments (P = .07). Post hoc contrasts revealed significantly more gastrointestinal symptoms for the low AR patients than the high AR ones (P = .000) as well as more defecation-related problems (P = .016). The time-by-group interaction (P = .02) showed that at the first assessment, the low AR patients had fewer defecation-related symptoms than the high AR ones, but at the second and third assessments they had significantly more (see Tables 4, 5, and 6).
Table 4. FUNCTION SCORES, LOW VS. HIGH ANTERIOR RESECTION
A high subscale score indicates low distress and good functioning.
Low AR, anterior resection with low anastomosis (<5.1 cm); high AR, anterior resection with high anastomosis (<5 cm); G-QoL, global quality of life; PF, physical function; RF, role function; EF, emotional function; CF, cognitive function; SF, social function; BI, body image; FP, future perspective.
Table 5. SYMPTOM SCORES, LOW VS. HIGH ANTERIOR RESECTION
A high subscale score indicates high distress.
Low AR, anterior resection with low anastomosis (<5.1 cm); high AR, anterior resection with high anastomosis (<5 cm); FA, fatigue, DI, diarrhea; GI, gastrointestinal symptoms; RT, micturition-related problems; DEF, defecation-related problems; SL, sleeplessness.
Table 6. SIGNIFICANT RESULTS, LOW VS. HIGH ANTERIOR RESECTION
* A high subscale score indicates low distress and good functioning.
† A high subscale score indicates high distress.
Low AR, anterior resection with low anastomosis (<5.1 cm); High AR, anterior resection with anastomosis (<5 cm); A0, preoperative assessment; A1, assessment at 6–9 months after surgery; A2, assessment at 12–15 months after surgery; RF, role function; BI, body image; FP, future perspective; SF, social function; G = Qol, global quality of life; GI, gastrointestinal symptoms; DEF, defication-related problems in AR patients.
DISCUSSION
This study assessed QoL issues prospectively in patients undergoing APE or AR for rectal cancer. Unexpectedly, the APE patients showed a consistent tendency toward a better QoL than the AR patients, especially those undergoing low AR, on most of the scales in the EORTC QLQ 30 and CR 38 questionnaire. Although the differences between the two groups were mostly nonsignificant, APE patients tended to exhibit superior physical, emotional, cognitive, and social function and reported less fatigue, gastrointestinal symptoms, sleeplessness, constipation, and diarrhea. Only for the subscale on body image problems did APE patients score slightly less favorably.
The formulation of a precisely defined patient group in this investigation prevented the results from being distorted by clinical confounding factors (e.g., tumor spread, tumor localization). However, the application of austere inclusion criteria in a study bears the risk of producing a nonrepresentative sample. For instance, a selection of patients with all tumor stages who show no evidence of tumor recurrence throughout a study period of 15 months might exemplify a subgroup of patients strongly determined to fight their cancer. The literature on the prominent concept of “fighting spirit” has revealed that patients with a fighting attitude may survive longer than patients who stoically accept their fate. 21–25 Further, the strict research design limited the number of patients eligible for the study, which produced a small sample. Small group size, however, complicates interpretation of the study findings. The small sample size is a critical point in our investigation, calling for cautious conclusions at this stage. Nonetheless, because high psychometric quality can be assured only when austere inclusion criteria are adopted, we chose this rigorous research design for the present study. Replication of our results is warranted.
In line with numerous studies in the literature showing that patients with rectal cancer undergoing colostomy report body image disruption, disfigurement, embarrassment, and secondary social isolation, 6,7,9,26,27 across time our group of APE patients showed a consistent decrease in body image scores. However, the AR patients exhibited a similar degree of deterioration in body image scores. Patients undergoing low AR expressed the most pronounced problems with body image after surgery. Although body image problems in the APE patients may well be a result of their difficulty in accepting the colostomy, the AR patients’ deterioration in body image may stem from a postoperative increase in defecation-related problems. In particular, patients undergoing low AR frequently report problems with continence and controlling their stools, 10,28 which is well in line with the results found in our patients after low AR.
An unanticipated finding was a consistent picture of a superior QoL among APE patients versus AR patients. Although mostly nonsignificant, the consistency in these trends was startling and deserves further explanation.
First, our finding, although it does not conform with most of the evidence in the literature, is not entirely novel. Koller et al, 29 discussing various options of incorporating QoL information in daily clinical work, contrasted the QoL profiles of a group of 9 patients undergoing APE and 11 undergoing AR. They found a better QoL on all the EORTC QLQ 30 scales in the APE patients versus the AR group.
Second, when attempting to embed our results in the body of psychological concepts and theories, Festinger’s 30 theory of cognitive dissonance immediately comes to mind. This theory focuses on how beliefs and behavior can change attitudes. It posits that inconsistent cognitions cause an emotional state of dissonance and uneasiness in a person. According to Festinger, there is a general drive in people to reduce dissonance, which is attained by increasing consistency among the dissonant cognitions. Research has shown that the most prominent strategy to increase consistency is by harmonizing the inconsistent cognitions or behavior. Usually, this requires a change in some attitude-relevant cognitions. 31 Translating this concept to the area of rectal cancer, it would predict that although patients do not like undergoing mutilating surgery, they see the need to do so because they want to survive. Therefore, they decide to undergo the surgery by their own free will. Festinger’s theory predicts that these are contradictory cognitions, causing cognitive dissonance to arise. Striving for cognitive consonance, patients are likely to reevaluate their life with a permanent colostomy by judging the situation as overly positive. 32
A different interpretation of our results focuses on the patients’ expectations of surgery. Patients are likely to draw on their preoperative expectations of how a colostomy will inhibit their postoperative life. Because of preconceived negative notions about colostomy in the community, before surgery many patients are extremely worried about the possible debilitating consequences of the stoma on their future life. After surgery, many of them realize that having a colostomy does not restrict them as much as they had anticipated. In this light, their QoL may appear to them as better than expected. 33
Yet another approach moves away from the perspective of the unexpectedly positive evaluation of QoL among APE patients toward the unexpectedly poor appraisal of QoL in AR patients. Recently there has been a growing body of evidence that AR patients have negative outcomes, such as weakened sphincter control or excessive defecation. 1,5 In particular, patients undergoing low AR are at risk of negative sequelae. Our analysis of patients undergoing low versus high AR clearly reveals that patients with low anastomoses have more severe impairment of QoL than those with high AR and those undergoing APE. Patients with low AR reported a significantly poorer global QoL, role function, social function, body image, and future perspective and had significantly more gastrointestinal and defecation-related symptoms. Usually, patients awaiting a sphincter-preserving procedure confidently approach surgery because they are convinced the procedure will free them from their bowel problems. They feel disillusioned when their continence is compromised after surgery, 33 and feelings of embarrassment and shame about their partial incontinence or frequent bowel movements arouse disappointment and frustration. The APE patients’ reports of superior QoL could in fact be a result of the AR patients’ comparatively lower satisfaction with their postoperative QoL.
In conclusion, based on the results of earlier studies of QoL of patients undergoing AR versus APE and our own findings, we deduce that patients undergoing APE do have some restriction in their postoperative QoL, such as body image. However, equally important is the fact that patients undergoing sphincter-preserving surgery have some postoperative deterioration in QoL as well. Their postoperative bowel problems can be severely disruptive to their social life.
Our results should be used as an impetus to reevaluate the most appropriate treatment option for each individual patient. In the past decades, tremendous progress in the development of novel surgical techniques for the treatment of rectal cancer was made. Sphincter-preserving approaches are now used more frequently, which undoubtedly is a favorable development. However, we must not underestimate the cost of sphincter-preserving techniques on a patient’s QoL. As much as we earlier attended to possible restrictions in QoL after AR, which triggered the development of new sphincter-preserving treatment approaches, 28 we should now critically evaluate the possible deleterious consequences of AR on QoL. The ultimate goal should be to determine which patient benefits most from which type of surgery, given his or her life circumstances.
Footnotes
Correspondence: Prof. Dr. Peter M. Schlag, Klinik für Chirurgie und Chirurgische Onkologie, Robert-Rössle-Klinik, Universitätsklinikum Charité, Lindenberger Weg 80, D-13125 Berlin.
Accepted for publication June 15, 2000.
References
- 1.Sprangers MAG, Taal BG, Aaronson NK, et al. Quality of life in colorectal cancer: stoma vs. nonstoma patients. Dis Colon Rectum 1995; 38: 361–369. [DOI] [PubMed] [Google Scholar]
- 2.Zieren HU, Jacobi CA, Zieren J, et al. Lebensqualitätserfassung nach Resektion colorektaler Carcinome. Chirurgie 1996; 67: 703–709. [PubMed] [Google Scholar]
- 3.Yeager ES, Van Heerden JA. Sexual dysfunction following proctocolectomy and abdominoperineal resection. Ann Surg 1980; 191: 169–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Küchenhoff J, Wirsching M, Drüner HU, et al. Coping with a stoma: a comparative study of patients with rectal carcinoma or inflammatory bowel diseases. Psychother Psychosom 1981; 36: 98–104. [DOI] [PubMed] [Google Scholar]
- 5.Williams NS, Price R, Johnston D. The long-term effect of sphincter-preserving operations for rectal carcinoma on the function of the anal sphincters in man. Br J Surg 1980; 67: 203–208. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald LD, Anderson HR. Stigma in patients with rectal cancer: a community study. J Epidemiol Community Health 1984; 38: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mac Donald LD, Anderson HR. The health of rectal cancer patients in the community. Eur J Surg Oncol 1985; 11: 235–241. [PubMed] [Google Scholar]
- 8.Santangelo ML, Romano G, Sassaroli C. Sexual function after resection for rectal cancer. Am J Surg 1987; 154: 502–504. [DOI] [PubMed] [Google Scholar]
- 9.Grundmann R, Said S, Krinke S. Lebensqualität nach Rektumresektion und -exstirpation. Ein Vergleich mit Hilfe verschiedener Meβparameter. Deutsch Med Wochens 1989; 114; 12:453–457. [DOI] [PubMed]
- 10.Camilleri-Brennan J, Steele RJC. Quality of life after treatment for rectal cancer. Br J Surg 1998; 85: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 11.Schaube J, Scharf P, Herz R. Lebensqualität nach karzinombedingter Rektumexstirpation. Deutsch Med Wochens 1996; 121: 153–158. [DOI] [PubMed] [Google Scholar]
- 12.Whynes DK, Neilson AR, Robinson MHE, et al. Colorectal cancer screening and quality of life. Qual Life Res 1994; 3: 191–198. [DOI] [PubMed] [Google Scholar]
- 13.Whynes DK, Neilson AR. Symptoms before and after surgery for colorectal cancer. Qual Life Res 1997; 6: 61–66. [DOI] [PubMed] [Google Scholar]
- 14.Hallböök O, Hass U, Wänström A, et al. Quality of life measurement after rectal excision for cancer. Comparison between straight and colonic J-pouch anastomosis. Scand J Gastroenterol 1997; 32: 490–493. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson NK, Cull A, Kaasa S, et al. The European Organization for Research and Treatment of Cancer (EORTC) modular approach to quality of life assessment in oncology. Int J Ment Health 1994; 23 (2): 75–96. [Google Scholar]
- 16.Sprangers MAG, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR 38). Eur J Cancer 1999; 35: 238–247. [DOI] [PubMed] [Google Scholar]
- 17.Koller M, Kussmann J, Lorenz W, et al. Symptom reporting in cancer patients. The role of negative affect and experienced social stigma. Cancer 1996; 77: 983–995. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsson L, Hallberg IR, Lovén L. Experiences of daily life and life quality in men with prostate cancer. An explorative study. Part I. Eur J Cancer Care 1997; 6: 108–116. [DOI] [PubMed] [Google Scholar]
- 19.Coates A, Porszolt F, Osoba A. Quality of life in oncology practice: prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer 1997; 33: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 20.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire H&N 35. J Clin Oncol 1999; 17: 1008–1019. [DOI] [PubMed] [Google Scholar]
- 21.Cella DF, Tulsky DS. Measuring quality of life today: methodological aspects. Oncology 1990; 4 (5): 29–39. [PubMed] [Google Scholar]
- 22.Greer S, Morris T, Pettingale KW. Psychological response to breast cancer: effect on outcome. Lancet 1979; ii: 785–787. [DOI] [PubMed] [Google Scholar]
- 23.Pettingale KW, Morris T, Greer S, et al. Mental attitudes to cancer: an additional prognostic factor. Lancet 1985; i: 750. [DOI] [PubMed] [Google Scholar]
- 24.Temoshok L. Personality, coping style, emotion and cancer: towards an integrative model. Cancer Surv 1987; 6: 545–567. [PubMed] [Google Scholar]
- 25.Fawzi FI, Fawzi NW, Hyn CS, et al. Malignant melanoma: effects of an early structured psychiatric intervention, coping and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry 1993; 50:681–689. Chirurgie. [DOI] [PubMed]
- 26.Keltikangas-Järvinen L, Loven E, Möller C. Psychic factors determining the long-term adaptation of colostomy and ileostomy patients. Psychother Psychosom 1984; 41: 153–159. [DOI] [PubMed] [Google Scholar]
- 27.Kirkpatrick JR. The stoma patient and his return to society. Front Radiat Ther Oncol 1980; 14: 20–25. [DOI] [PubMed] [Google Scholar]
- 28.Williams NS, Johnston D. The quality of life after rectal excision for low rectal cancer. Br J Surg 1983; 70: 460–462. [DOI] [PubMed] [Google Scholar]
- 29.Koller M, Kuβmann J, Lorenz W, et al. Die Messung von Lebensqualität in der chirurgischen Tumornachsorge. Methoden, Probleme und Einstazmöglichkeiten. [PubMed]
- 30.Festinger LA. A Theory of Cognitive Dissonance. Palo Alto, CA: Stanford University Press; 1957.
- 31.Fiske ST, Taylor SE. Cognitive approaches to attitudes. In: Fiske ST, Taylor SE. Social Cognition. New York: McGraw-Hill; 1991: 462–509.
- 32.Bernhard J, Hürny CH. Gastrointestinal cancer. In: Holland JC, ed. Psycho-Oncology. New York: Oxford University Press; 1998: 324–339.
- 33.Wan GJ, Counte MA, Cella DF. The influence of personal expectations on cancer patients’ reports of health-related quality of life. Psycho-Oncology 1997; 6: 1–11. [DOI] [PubMed] [Google Scholar]