Abstract
Objective
To evaluate the association of clinicopathologic factors and prognostic value with the expression of cyclooxygenase 1 and 2 in patients with gastric adenocarcinoma.
Summary Background Data
Epidemiologic studies have indicated that nonsteroidal antiinflammatory drugs reduce the risk of colon cancer by as much as 40% and also decrease the risk of gastric cancer. Recently, gastric cancer was found to express constitutive cyclooxygenase 1 and inducible cyclooxygenase 2 isoenzymes. Nonsteroidal antiinflammatories, which may function as cyclooxygenase inhibitors, inhibited the growth of gastric cancer cells. These two isoenzymes’ expressions associated with traditional clinicopathologic factors have not been fully evaluated, and their prognostic value for determining survival in patients remains to be clarified.
Methods
Seventy-one specimens resected from patients with gastric adenocarcinoma were investigated by immunohistochemical stain against cyclooxygenase 1 and 2. The 71 specimens were divided into stain-positive and stain-negative groups. Correlations between cyclooxygenase 1 and 2 expression, various clinicopathologic factors *including vascular invasion and Helicobacter pylori infection), and prognosis were studied.
Results
The cyclooxygenase 2-positive group was significantly correlated with vascular invasion and H. pylori infection by univariate and multivariate analysis. In patients with cyclooxygenase 2-positive cancer, the prognosis was significantly poorer than in those with cyclooxygenase 2-negative cancer. However, multivariate analysis showed that vascular invasion, serosal invasion, and lymph node metastasis were independent prognostic factors for patients with gastric cancer, but cyclooxygenase 2 expression was not. There was no significant correlation between cyclooxygenase 1 expression and clinicopathologic factors and prognosis.
Conclusions
Upregulated cyclooxygenase 2 expression was associated with H. pylori infection in gastric cancer and was also strongly correlated with positive vascular invasion, which was an independent prognostic factor for poorer survival in this study. The usefulness of cyclooxygenase 2 inhibitors in the prevention or treatment of gastric cancer remains undetermined but deserves further investigation.
Gastric cancer is a major cause of death throughout the world 1 and is the fourth most common malignancy in Taiwanese men and the sixth in women. 2 Gastric cancer recurred in 39% of patients who had undergone curative resection, 3 and many chemotherapeutic drugs have failed to provide complete reduction of gastric cancer in the patients. 4
Recent epidemiologic studies have shown that chronic intake of nonsteroidal antiinflammatory drugs (NSAIDs), which function as cyclooxygenase inhibitors, reduced the incidence of colon cancer and polyps by as much as 40% to 50%. 5,6 In a large prospective study, the use of aspirin was also associated with a reduced risk of gastric and esophageal cancer. 7,8
The best-known target of NSAIDs is cyclooxygenase, the key enzyme in the conversion of arachidonic acid to prostanoids. There are two isoforms, cyclooxygenase 1 and 2. 9,10 Cyclooxygenase 1 is considered to be a housekeeping gene and is thought to be related to the cytoprotection of gastric mucosa. Cyclooxygenase 2 is an inducible intermediate-early gene, and its roles have been connected to inflammation and carcinogenesis. 11 A recent study showed that expression of cyclooxygenase 1 and 2 was detected in human gastric cancer. 12 Approximately 70% of gastric cancer specimens expressed cyclooxygenase 2 protein in the study by Murata et al. 13 Therefore, it is interesting to correlate clinicopathologic factors and prognosis of gastric cancer with cyclooxygenase 1 and 2 protein expression, and this may be helpful in the prevention and therapy of gastric cancer.
METHODS
Resected specimens from 71 patients with gastric cancer who underwent curative gastrectomy at our institution from 1995 to 1996 were studied. The patients comprised 48 men and 23 women (age range 35–81 years, average age 62.3). Thirty-eight specimens were obtained from the antrum, 18 from the body, and 14 from the cardia. Normal (nontumor) specimens were collected from accompanying stomach tissues, which were apart from gastric cancer in each case. Paraffin sections of gastric cancer were submitted for cyclooxygenase 1 and 2 immunohistochemical staining.
Immunohistochemical Staining
Tissue immunohistochemical staining was performed with monoclonal anticyclooxygenase 2 antibody and polyclonal anticyclooxygenase 1 antibody (Cayman Chemical Co., Ann Arbor, MI) and the avidin–biotin peroxidase complex kit (Zymed, San Francisco, CA). Resected gastric tissues were fixed in 10% buffered formalin. Specimens were embedded in paraffin, serially sectioned onto microscope slides at a thickness of 4 to 5 μm, and then deparaffinized. The slides were immersed for 45 minutes in 0.3% peroxide in methanol to deplete the activity of endogenous peroxidase. Nonspecific binding sites were saturated with 0.3% bovine serum albumin. Normal goat serum was diluted to 1:66.7 in phosphate-buffered saline for 20 minutes. Primary antibodies against cyclooxygenase 1, cyclooxygenase 2, or control normal rabbit serum (Vector) were used at a dilution of 1:40 and applied to tissue sections and incubated in a humidified chamber at room temperature for 30 minutes. The sections were then washed with phosphate-buffered saline for 10 minutes, and biotinylated goat antirabbit IgG (Vector, Burlingame, CA) was applied to the tissue sections and incubated at room temperature for 30 minutes. After being washed with phosphate-buffered saline for 10 minutes, these tissues were incubated with avidin DH-biotinylated peroxidase (Vector) for 45 minutes. The color was developed by immersing sections in freshly prepared diamino-benzidine for 2 to 5 minutes. The sections were counterstained with 0.5% light green (Sigma, St. Louis, MO). The normal gastric mucosa was used as a positive control, and the primary antibody replaced by the normal serum was used as a negative control. For the results, negative immunoreactivity implied that staining was absent throughout the specimen. Positive immunoreactivity of cancer cells implied that brownish granular staining was present primarily in cytoplasm and the perinuclear area of the cell, and the background staining was light green. Specificity was determined by preadsorption of anticyclooxygenase 1 or 2 with the cyclooxygenase 1 or 2 synthetic polypeptide (1 mg/mL) before staining.
Clinicopathologic factors including age, sex, gross types of tumors (Borrmann classification), histologic types of tumors (Lauren classification), depth of tumor invasion, status of lymph node metastasis, vascular invasion, and Helicobacter pylori infection documented with histologic findings were reviewed in the database. Vascular invasion was considered to be definite only when tumor cells and red blood cells were noted together in an endothelium-lined vascular space or when tumor cells were found in an endothelium-lined vascular space with a definite smooth muscle layer. The tissue was considered positive for H. pylori if faintly blue-staining, curved bacilli were seen in the mucus of crypts just adjacent to the tumor using hematoxylin and eosin staining. The follow-up period ranged from 3 to 40 months after surgery.
Statistics
The Fisher exact probability test and the chi-square test were used for the statistical analysis that related the expression of cyclooxygenase 1 and 2 proteins and the traditional clinicopathologic factors. A logistic regression model was used to test the correlation between cyclooxygenase 2 protein expression and clinicopathologic factors by multivariate analysis, and was tested by the Wald chi-square test. Survival rates were calculated using the Kaplan-Meier method and analyzed by the log-rank test. The influence of each variable on survival was assessed by the Cox proportional hazard regression model. P < .05 was considered significant.
RESULTS
Immunostaining appeared to be localized in the cytosolic and perinuclear regions in cyclooxygenase-positive cancer cells, consistent with endoplasmic reticulum localization of cyclooxygenase enzymes. In general, cyclooxygenase 1 was expressed in normal gastric epithelial cells and cyclooxygenase 2 was not (Fig. 1). Cyclooxygenase 1 and 2 protein was positively stained in 61% (43/71) and 68% (48/71) of the gastric cancer specimens, respectively.
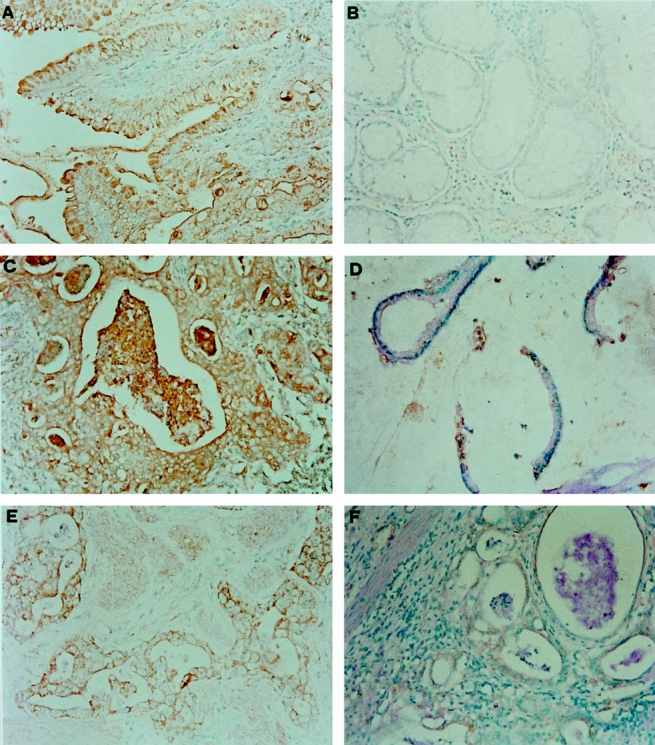
Figure 1. In normal gastric epithelial cells, immunostaining was positive for cyclooxygenase 1 (A) and negative for cyclooxygenase 2 (B). Representative gastric cancer tissues were positively (C) or negatively (D) immunostained with anticyclooxygenase 1 antibodies. Gastric cancer tissues were positively (E) or negatively (F) immunostained with anticyclooxygenase 2 antibodies.
Correlations between the expression of cyclooxygenase 1 and 2 protein and the clinicopathologic factors are shown in Tables 1 and 2. There was no correlation between cyclooxygenase 1 expression and clinicopathologic factors. A significant correlation was found between cyclooxygenase 2 protein expression and cancer vascular invasion (P = .028) and H. pylori infection (P = .011) by univariate analysis. Multivariate analysis also showed a significant correlation between cyclooxygenase 2 protein expression and vascular invasion (P = .0296) and H. pylori infection (P = .0277) (Table 3).
Table 1. CYCLOOXYGENASE 1 EXPRESSION AND CLINICOPATHOLOGIC FACTORS: UNIVARIATE ANALYSIS
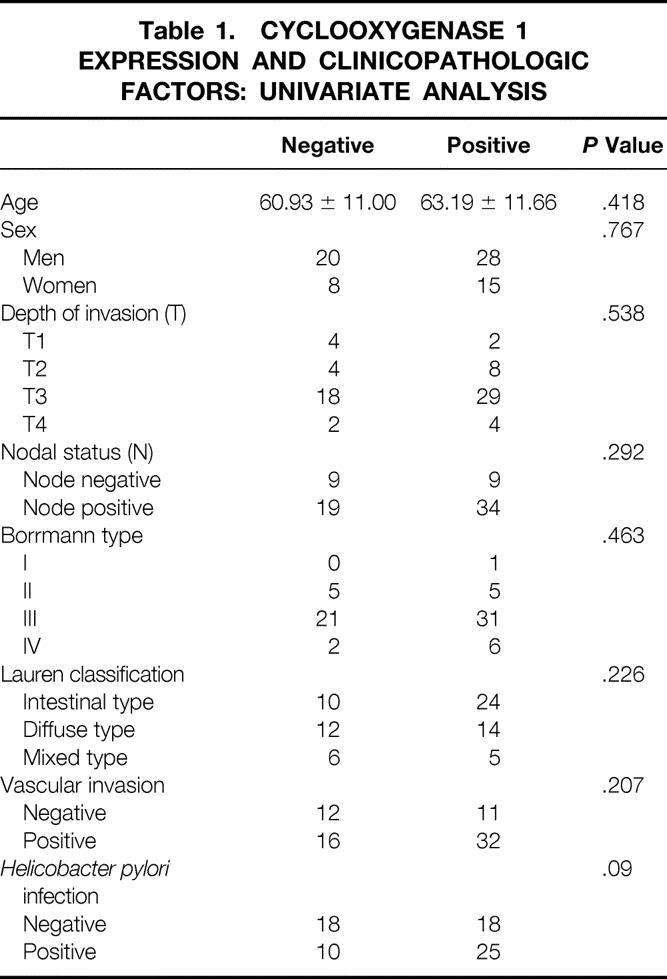
Table 2. CYCLOOXYGENASE 2 EXPRESSION AND CLINICOPATHOLOGIC FACTORS: UNIVARIATE ANALYSIS
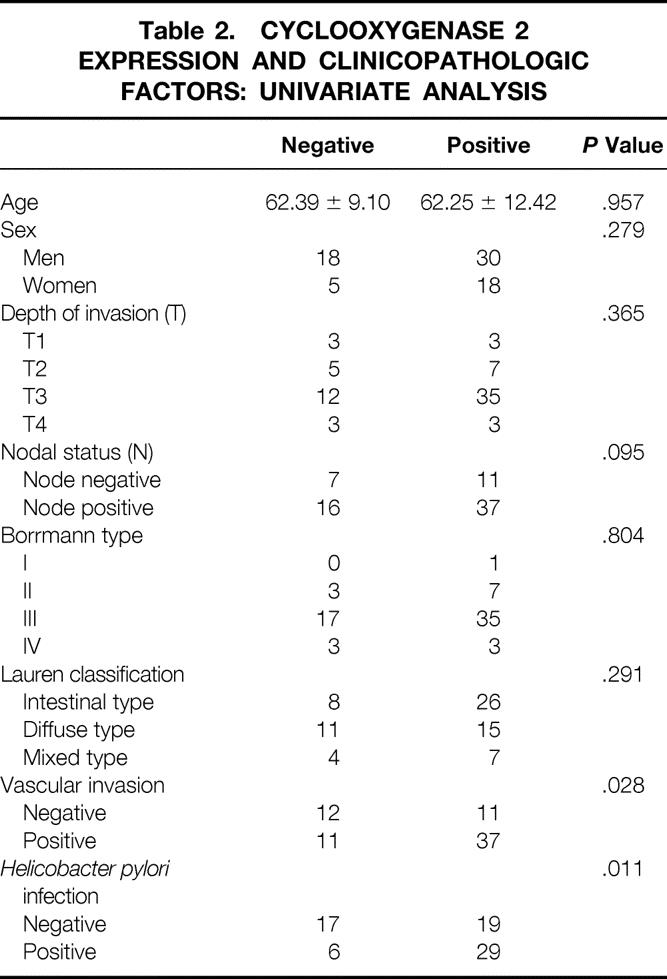
Table 3. CYCLOOXYGENASE 2 EXPRESSION AND CLINICOPATHOLOGIC FACTORS: MULTIVARIATE ANALYSIS
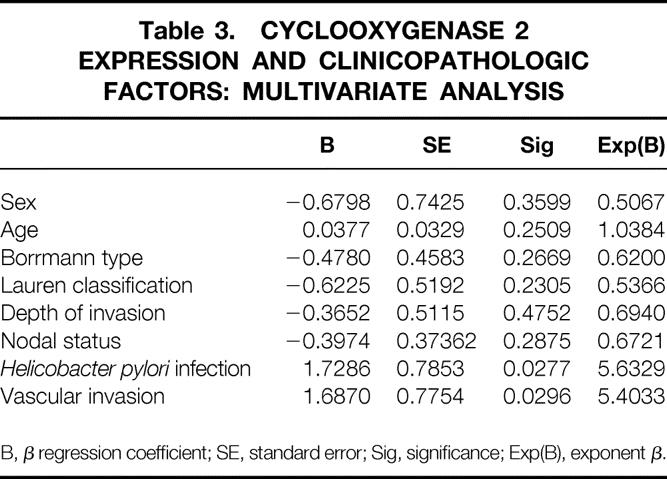
B, β regression coefficient; SE, standard error; Sig, significance; Exp(B), exponent β.
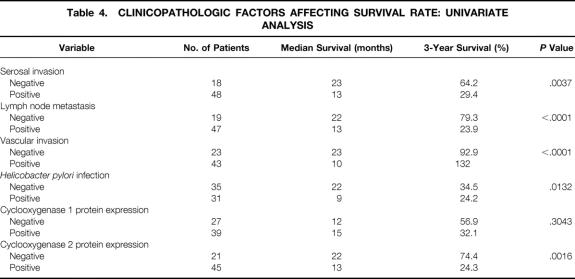
Univariate analysis showed that five variables were significant adverse prognostic factors for survival after curative resection of gastric cancer: serosal invasion, lymph node metastasis, vascular invasion, H. pylori infection, and cyclooxygenase 2 protein expression. Cyclooxygenase 1 protein expression was found to have no prognostic value (Table 4). Multivariate analysis showed that serosal invasion, lymph node metastasis, and vascular invasion were independent prognostic factors. Cyclooxygenase 2 protein expression and H. pylori infection were not independent prognostic factors (Table 5).
Table 4. CLINICOPATHOLOGIC FACTORS AFFECTING SURVIVAL RATE: UNIVARIATE ANALYSIS
Table 5. CLINICOPATHOLOGIC FACTORS AFFECTING SURVIVAL RATE: MULTIVARIATE ANALYSIS
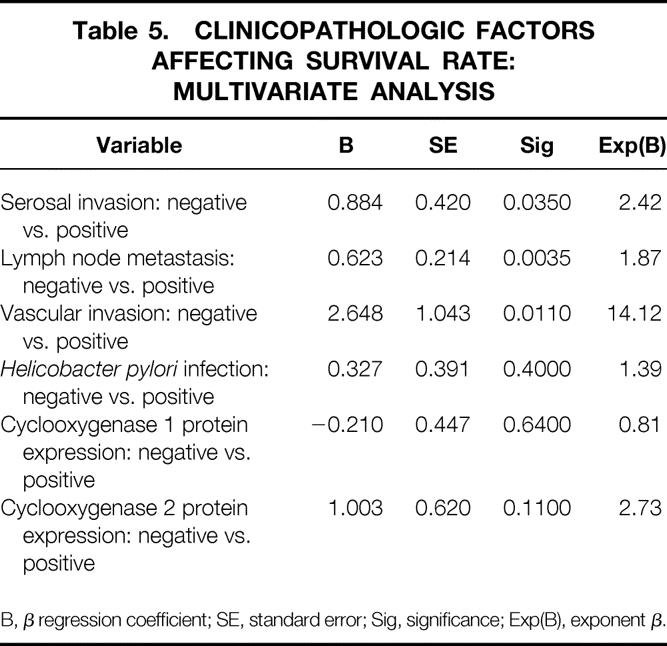
B, β regression coefficient; SE, standard error; Sig, significance; Exp(B), exponent β.
DISCUSSION
Our results showed that cyclooxygenase 1 protein expression was not correlated with traditional clinicopathologic factors and prognostic value in gastric cancer. Cyclooxygenase 2 protein expression was not associated with histologic differentiation (intestinal type or diffuse type of gastric cancer), gross morphology (Borrmann classification), depth of tumor invasion, or lymph node metastasis. However, it was significantly correlated with vascular invasion of cancer and H. pylori infection. On univariate analysis, the prognosis of patients with cyclooxygenase 2-positive cancer was poorer than that of those with cyclooxygenase 2-negative cancer, but it was not an independent prognostic indicator. In addition to invasion depth and lymph node metastasis, vascular invasion of cancer was an independent prognostic factor.
Vascular invasion has been reported as an independent prognostic factor in gastric cancer. 14–19 Cyclooxygenase 2 expression of gastric cancer was significantly correlated with vascular invasion in this study. Cancer cells with cyclooxygenase 2 overexpression had metastatic potential in vitro because the enzyme enhanced metalloproteinase 2 activity, which could break down the basement membrane, resulting in vascular invasion and metastasis. 20,21 However, cyclooxygenase 2 expression of cancer has been associated with angiogenesis, 22,23 making the vascular invasion step easier to accomplish. 24 These factors may explain why elevated cyclooxygenase 2 expression in gastric cancer was associated with vascular invasion in this study. However, elevated cyclooxygenase 2 expression was not an independent prognostic factor, possibly reflecting the clinical and molecular complexities in gastric cancer; elevated cyclooxygenase 2 expression in gastric cancer may be associated with metastasis and progression but not with survival. A recent study showed that cyclooxygenase 2 could regulate angiogenesis by modulating the production of angiogenic factors in colon cancer cells, a process that could be inhibited by NSAIDs. 25 The effect of NSAIDs on gastric cancer after curative resection remains unknown and needs further investigation with solid clinical trials.
Infection with H. pylori is recognized as the primary cause of peptic ulcers and their recurrence. Compelling evidence has also been found linking H. pylori infection to gastric cancer and its premalignant lesion. 26–29 The possible mechanisms of H. pylori-induced carcinogenesis may be chronic epithelial injury and inflammation caused by the ammonia that is produced by the bacteria in the gastric microenvironment. 30,31H. pylori induces inflammatory cells to produce reactive oxygen metabolites that may damage DNA and promote carcinogenesis, 32,33 and it also increases the proliferative activity of epithelial cells. 34 We found that cyclooxygenase 2 expression of gastric cancer was significantly correlated with H. pylori infection; this result was consistent with a recent in vitro study showing that H. pylori upregulated cyclooxygenase 2 mRNA expression and prostaglandin E2 synthesis in a gastric cancer cell line. 35 Therefore, H. pylori-induced gastric carcinogenesis may be associated with the elevated expression of cyclooxygenase 2 in gastric epithelium.
Whether the eradication of H. pylori can prevent gastric cancer remains contradictory. Some reports have described regression of preneoplastic changes, such as atrophy or reversal of intestinal metaplasia, with H. pylori eradication. 36–39 Uemura et al 40 reported that eradication of H. pylori was effective in secondary prevention of tumor recurrence in patients with early gastric cancer who underwent endoscopic resection. In contrast, other investigators found that H. pylori eradication had no effect or an effect that was not sustained. 41–44 Cyclooxygenase 2 overexpression leads to phenotypic changes in intestinal epithelial cells that could enhance their tumorigenic potential. 45 However, cyclooxygenase 2 expression remained and was not eliminated in gastric epithelium after successful eradication of H. pylori. 46 This may suggest that combined treatment of cyclooxygenase 2 inhibitors and H. pylori infection may be more useful in eliminating H. pylori-induced cyclooxygenase 2 expression than either anti-H. pylori antibiotics or cyclooxygenase 2 inhibitors alone. This issue may deserve further study.
In conclusion, upregulated cyclooxygenase 2 expression was associated with H. pylori infection in gastric cancer and was also strongly correlated with positive vascular invasion, which was an independent prognostic factor for poorer survival in this study. Cyclooxygenase 2 may represent a possible therapeutic target in patients with gastric cancer, but the utility of cyclooxygenase 2 inhibitors in the prevention or treatment of gastric cancer remains undetermined and deserves further investigation.
Footnotes
Supported by a grant (NSC89-2314-B-002-218) from National Science Council, Taipei, Taiwan.
Correspondence: King-Jen Chang, MD, PhD, Department of Surgery, National Taiwan University Hospital, No. 7, Chung-Shan S. Road, Taipei, Taiwan.
Accepted for publication May 3, 2000.
References
- 1.Coleman MP, Esteve J, Damiecki P, et al. Trends in Cancer Incidence and Mortality. Lyon, France: IARC Scientific Publications No. 121; 1993:193–224. [DOI] [PubMed]
- 2.Cancer Incidence in Taiwan. Taipei: Taiwanese Cancer Registry; 1998.
- 3.Wanebo HJ, Kennedy BJ, Chemiel J, et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 1993; 218: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelsen DP. Adjuvant and neoadjuvant therapy for gastric cancer. Semin Oncol 1996; 23: 379–387. [PubMed] [Google Scholar]
- 5.Smalley W, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol 1997; 39: 1–20. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Egan KM, Hunter DJ, et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med 1995; 333: 609–614. [DOI] [PubMed] [Google Scholar]
- 7.Thun MJ. Aspirin, NSAIDs, digestive tract cancers. Cancer Metastasis Rev 1994; 13: 269–277. [DOI] [PubMed] [Google Scholar]
- 8.Farrow DC, Vaughan TL, Hansten PD, et al. Use of aspirin and other nonsteroidal anti-inflammatory drug and risk of esophageal and gastric cancer. Cancer Epidemiol Biomark Prev 1998; 7: 97–102. [PubMed] [Google Scholar]
- 9.Eberhart CE, DuBois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology 1995; 109: 285–301. [DOI] [PubMed] [Google Scholar]
- 10.Smith WL, DeWitt DL. Biochemistry of prostaglandin endoperoxide H synthase-1 and synthase-2 and their differential susceptibility to nonsteroidal anti-inflammatory drugs. Semin Nephrol 1995; 15: 179–194. [PubMed] [Google Scholar]
- 11.Crofford LJ. COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol 1997; 24 (suppl 49): 15–19. [PubMed] [Google Scholar]
- 12.Ristmaki A, Honkanen N, Jankala H, et al. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997; 57: 1276–1280. [PubMed] [Google Scholar]
- 13.Murata H, Kawano S, Tsuji S, et al. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol 1999; 94: 451–455. [DOI] [PubMed] [Google Scholar]
- 14.Okada M, Kojima S, Murakami M, et al. Human gastric carcinoma: prognosis in relation to macroscopic and microscopic features of the primary tumor. J Natl Cancer Inst 1983; 71: 275–279. [PubMed] [Google Scholar]
- 15.Ribeiro MM, Seoxas M, Sobrinho-Simoes M. Prognosis in gastric carcinoma. The preeminence of staging and futility of histological classification. Dig Dis Pathol 1988; 1: 51–68. [Google Scholar]
- 16.Korenaga D, Haraguchi M, Okamura T, et al. DNA ploidy and tumor invasion in human gastric cancer. Arch Surg 1989; 14: 314–318. [DOI] [PubMed] [Google Scholar]
- 17.Gabbert HE, Meier S, Gerharz CD, Hommel G. Incidence and prognostic significance of vascular invasion in 529 gastric cancer patients. Int J Cancer 1991; 49: 203–207. [DOI] [PubMed] [Google Scholar]
- 18.Setala LP, Kosma VM, Marin S, et al. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer 1996; 74: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maehara Y, Tomoda M, Hasuda S, et al. Prognostic value of p53 protein expression for patients with gastric cancer–a multivariate analysis. Br J Cancer 1999; 79: 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsujii M, Kawano S, DuBois N. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 1997; 94: 3336–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reich R, Martin GR. Identification of arachidonic acid pathways required for the invasive and metastatic activity of malignant tumor cells. Prostaglandins 1996; 51: 1–17. [DOI] [PubMed] [Google Scholar]
- 22.Sawaoka H, Kawano S, Tsuji M, et al. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol 1998; 274: G1061–1067. [DOI] [PubMed] [Google Scholar]
- 23.Levy GN. Prostaglandin H synthases, nonsteroidal anti-inflammatory drugs, and colon cancer. FASEB J 1997; 11: 234–247. [PubMed] [Google Scholar]
- 24.Nagyl JA, Brown LF, Senger DR, et al. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta 1989; 948: 305–326. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell WJ, Kelleher D, Keating JJ, et al. Enhanced secretion of prostaglandin E2 by tissue-fixed macrophages in colon carcinoma. Digestion 1990; 47: 160–166. [DOI] [PubMed] [Google Scholar]
- 26.Hansson LE, Engstrand L, Nyren O, et al. Helicobacter pylori infection: independent risk indicator of gastric adenocarcinoma. Gastroenterology 1993; 105: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 27.Guarner J, Mohar A, Parsonnet J, Helperin D. The association of Helicobacter pylori with gastric cancer and preneoplastic gastric lesions in Chiapas, Mexico. Cancer 1993; 71: 297–301. [DOI] [PubMed] [Google Scholar]
- 28.Hu PJ, Mitchell HM, Li YY, et al. Association of Helicobacter pylori with gastric cancer and observations on the detection of this bacterium in gastric cancer cases. Am J Gastroenterol 1994; 89: 1806–1810. [PubMed] [Google Scholar]
- 29.Endo S, Ohkusa T, Saito Y, et al. Detection of Helicobacter pylori infection in early-stage gastric cancer. Cancer 1995; 75: 2203–2208. [DOI] [PubMed] [Google Scholar]
- 30.Mobley, HL, Cortesia MJ, Rosenthal LAE, Jones BD. Characterization of urease from Campylobacter pylori from gastric biopsies. Am J Clin Pathol 1988; 26: 831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsujii M, Kawano S, Tsuji S, et al. Ammonia: a possible promotor in Helicobacterpylori-related gastric carcinogenesis. Cancer Lett 1992; 65: 15–18. [DOI] [PubMed] [Google Scholar]
- 32.Baik SC, Youn HS, Chung MH, et al. Increased oxidation DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res 1996; 56: 1279–1282. [PubMed] [Google Scholar]
- 33.Mannick EE, Bravo LE, Zarama G, et al. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobactor pylori gastritis: effect of antibioitics and antioxidants. Cancer Res 1996; 56: 3238–3243. [PubMed] [Google Scholar]
- 34.Peek RM Jr, Moss SF, Tham KT, et al. Helicobacter pylori cagAt strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst 1997; 89: 863–868. [DOI] [PubMed] [Google Scholar]
- 35.Romano M, Ricci V, Memoli A, et al. Helicobacter pylori upregulates cyclooxygenase =2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem 1998; 273: 28560–28563. [DOI] [PubMed] [Google Scholar]
- 36.Recavarren-Arce S, Leon-Barua R, Cok J, et al. Helicobacter pylori and progressive gastric pathology that predisposes to gastric cancer. Scand J Gastroenterol 1991; 181 (suppl): 51–57. [DOI] [PubMed] [Google Scholar]
- 37.Borody TJ, Andrews P, Jankiewicz E, et al. Apparent reversal of early gastric mucosa atrophy after triple therapy of Helicobacter pylori. Am J Gastroenterol 1993; 88: 1266–1268. [PubMed] [Google Scholar]
- 38.Hack HM, Fennerty MB, Sampliner R, et al. Reversal of intestinal metaplasia after treatment of H. pylori infection. Gastroenterology 1994; 106: A87. [Google Scholar]
- 39.Genta RM, Lew GM, Graham DY. Change in gastric mucosa following eradication of H. pylori. Modern Pathol 1993; 6: 281–289. [PubMed] [Google Scholar]
- 40.Uemura N, Mukai T, Okamoto S, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomark Prevent 1997; 6: 639–642. [PubMed] [Google Scholar]
- 41.Jankiewicz K, Louw JA, Marks IN. Long-term histological consequences of suppression/eradication of Helicobacter pylori in antral mucosa. Euro J Gastroenterol Hepatol 1993; 5: 701–705. [Google Scholar]
- 42.Witteman EM, Mravunac M, Becx MJCM, et al. Improvement of gastric inflammation and resolution of epithelial damage one year after eradication of Helicobacter pylori. J Clin Pathol 1995; 48: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borody TJ, Clark IW, Andrews P, et al. Eradication of Helicobacter pylori may not reverse severe gastric dysplasia. Am J Gastroenterol 1995; 90: 498–499. [PubMed] [Google Scholar]
- 44.Sung JY, Lin SR, Ching JYL, et al. Effects of curing Helicobacter pylori infection on precancerous gastric lesions: one-year follow-up of a prospective randomized study in China. Gastroenterology 1998; 114: A296. [Google Scholar]
- 45.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995; 83: 493–501. [DOI] [PubMed] [Google Scholar]
- 46.McCarthy CJ, Crofford LJ, Greenson J, et al. Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pylori infection. Am J Gastroenterol 1999; 94: 1218–1223. [DOI] [PubMed] [Google Scholar]