Abstract
Objective
To review the outcome of resection of the suprarenal or infrarenal inferior vena cava (IVC) and possible indications for prosthetic replacement.
Summary Background Data
Involvement of the IVC has long been considered a limiting factor for curative surgery for advanced tumors because the surgical risks are high and the long-term prognosis is poor. Prosthetic replacement of the IVC is controversial.
Methods
The authors retrospectively reviewed a 7-year series of 14 patients who underwent en bloc resection including a circumferential segment of the IVC. The tumor was malignant in 12 patients and benign in 2. The resected segment of the IVC was located above the kidneys in eight patients and below in six. Resection was performed without extracorporeal circulation in all patients.
Results
In all but one patient, IVC resection was associated with multivisceral resection, including extended nephrectomy (n = 8), major hepatic resection (n = 3), digestive resection (n = 3), and infrarenal aortic replacement (n = 2). Prosthetic replacement of the IVC was performed in eight patients cases and was more common after resection of a suprarenal (6/8) than an infrarenal segment of the IVC (2/6). One patient died of multiorgan failure. Major complications occurred in 29% of patients. Symptomatic complications of prosthetic replacement occurred in one patient (acute postoperative thrombosis, successfully treated by surgical disobstruction). Graft-related infection was not observed. Marked symptoms of venous obstruction developed in three of the six patients who did not undergo venous replacement. In patients undergoing surgery for malignant disease, the estimated median survival was 37 months and the actuarial survival rate was 67% at 1 year.
Conclusion
Multivisceral resection including a segment of IVC is justified to achieve complete extirpation in selected patients with extensive abdominal tumors. Prosthetic replacement of the IVC may be required, particularly in cases of suprarenal resection. It is a safe procedure with a low complication rate and good functional results.
Clinical conditions requiring resection of the inferior vena cava (IVC) are rare. The main indications are traumatic or iatrogenic injury, chronic postthrombotic or membranous occlusion, and malignancy, including renal cell carcinoma, 1 Wilms tumor, 2 leiomyosarcoma, 3 adrenal tumor, 4 hepatic carcinoma, 5 and retroperitoneal metastatic lymph nodes from testicular carcinoma. 6 Involvement of large vessels has been considered evidence of advanced disease and a contraindication for resection of abdominal tumors. Indeed, the surgical risks are high and the long-term survival rate is poor. Nevertheless, en bloc resection including not only IVC resection but also major hepatectomy has been proposed in selected patients to achieve tumor extirpation. 7–11 Caval replacement after IVC resection is controversial. 3,10 Prosthetic venous grafts have been used successfully for only three decades. 12 Experimental and clinical findings indicate that expanded polytetrafluoroethylene (ePTFE) is the best replacement material. 13
Forging an opinion on the utility of IVC resection and the indication for replacement is difficult because, with the possible exception of cases involving renal cell carcinoma, 1,14–17 most publications on the subject have been sporadic single-case reports. Only three institutional series including 6 to 10 patients have been published by the Mayo Clinic in Minnesota, 8 Princess Grace Hospital in Monaco, 10 and the UCLA Medical Center in California. 18 The aim of this study was to describe the outcome of IVC resection in a series of 14 patients with extensive abdominal tumors and to discuss our policy for IVC replacement.
METHODS
Between January 1992 and December 1998, 14 en bloc resections including a circumferential segment of the IVC were carried out in two collaborating surgical departments of the University of Marseille Medical School to achieve extirpation of extensive malignant or benign abdominal tumors. Procedures involving lateral IVC resection, transcaval removal of tumor thrombus, or cardiopulmonary bypass were not included. The records of these patients were retrospectively reviewed. Follow-up data were available in all patients for periods of 1 to 79 months up to December 31, 1999 (median 17 months). Three patients (patients 2, 5, and 10) were described in previous reports. 11,19
Patients
Table 1 shows the main characteristics of the 14 patients in this series. There were eight men and six women with a median age of 56 years (range 30–77). The indication for IVC resection was primary caval malignancy in two patients, invasion by adjacent primary or recurrent malignancy in six and two patients respectively, and invasion by retroperitoneal lymph node metastasis in two patients. In the remaining two patients, the indication was benign tumor involvement. One patient had two synchronous malignancies (Fig. 1). Patients with recurrent malignancy or metastatic lymph nodes were considered candidates for surgery if recurrence occurred more than 1 year after the primary therapy (1, 5, 6, and 7 years) and there was no distant metastasis. Five patients had undergone previous abdominal surgery for the same disease. In one patient the IVC had been clipped 5 years before resection for iliofemoral thrombosis. Previous nonsurgical treatment included chemotherapy in one patient and immunotherapy in another. No patient had undergone radiation therapy.
Table 1. PATIENT DATA
IVC, inferior vena cava.
* Patient with partial or complete involvement of the confluence of the renal vein.
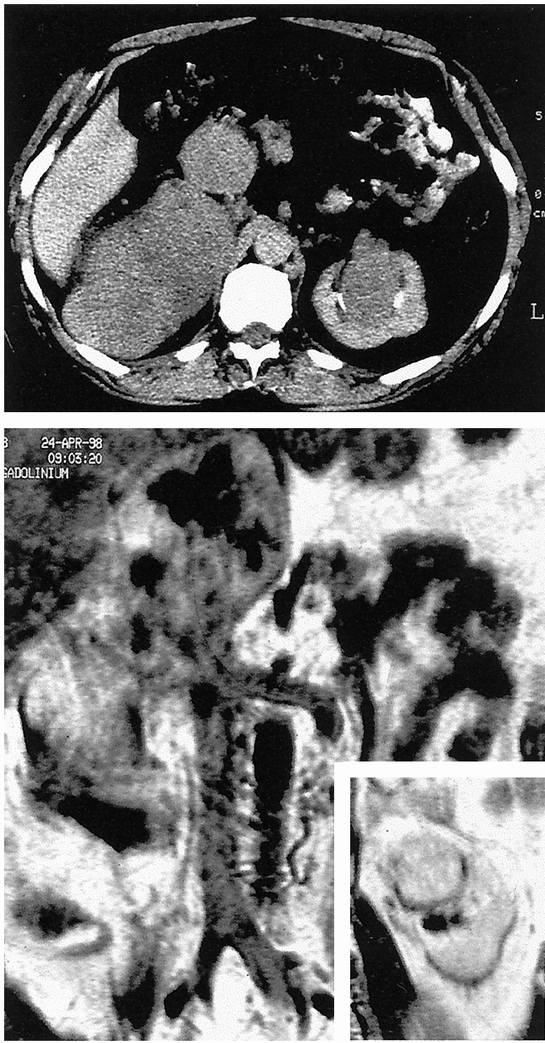
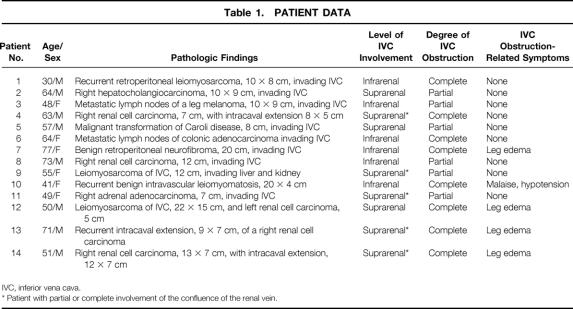
Figure 1. Patient 12. Abdominal computed tomography scan (top) and nuclear magnetic resonance imaging (bottom) showing a large right retroperitoneal mass involving the retrohepatic inferior vena cava and pushing the right kidney forward (not shown), and a synchronous mass in the sinus of the left kidney. In this difficult case, the patient was informed of the possible need for bilateral nephrectomy, but conservation of the right kidney turned out to be possible.
Four of the 14 patients had lower extremity edema and one had malaise and hypotension from impaired venous return. 19 Tumoral involvement and IVC invasion were assessed by ultrasonography, computed tomography scanning, or magnetic resonance imaging in all patients. Preoperative assessment of the upper limit of intravascular involvement was determined by these methods or by cavography, which also allowed study of the collateral circulation, or by transesophageal echocardiography to rule out atrial extension. Extraabdominal metastasis was assessed by chest roentgenography in all patients. Caval involvement was identified before surgery in all but one patient (patient 5). Involvement of the IVC was classified as infrarenal (n = 6) or suprarenal (n = 8) with or without involvement of the renal venous confluence. Obstruction was complete in eight patients and partial in six. In two patients, preoperative imaging revealed encasement of the aorta.
Surgical Techniques
Surgery was performed without extracorporeal circulation through an abdominal incision in 12 patients and through a right thoracoabdominal incision in 2 (patients 4 and 14). After intraperitoneal inspection, the IVC and major tributaries were controlled by placing vessel loops above and below the tumor. If necessary, the liver was released and the suprahepatic or intrapericardial IVC was controlled. Caval resection was undertaken in patients who had a primary tumor of the vessel wall, invasion of more than half the circumference of the vessel wall by an adjacent tumor, and massive intraluminal tumor growth suspected to be adherent to the vessel wall (Fig. 2). Complete gross tumor resection was achieved in all but one patient (patient 5), who had a suspicious residual lesion in the liver remnant. Liver resections were performed after either total hepatic vascular exclusion or sequential cross-clamping of first the portal triad (Pringle maneuver) and then the IVC below the remaining hepatic veins, allowing revascularization of the liver. 11 The latter procedure was also used in patients requiring retrohepatic IVC resection without hepatectomy, in which case cross-clamping of the portal triad started on separation of the caudate lobe from the IVC and ended after control of the IVC below the hepatic veins. The extent of liver resection was classified according to Couinaud’s anatomical segmentation. 20 As described elsewhere, 21 suprarenal resection was performed without renal protection.
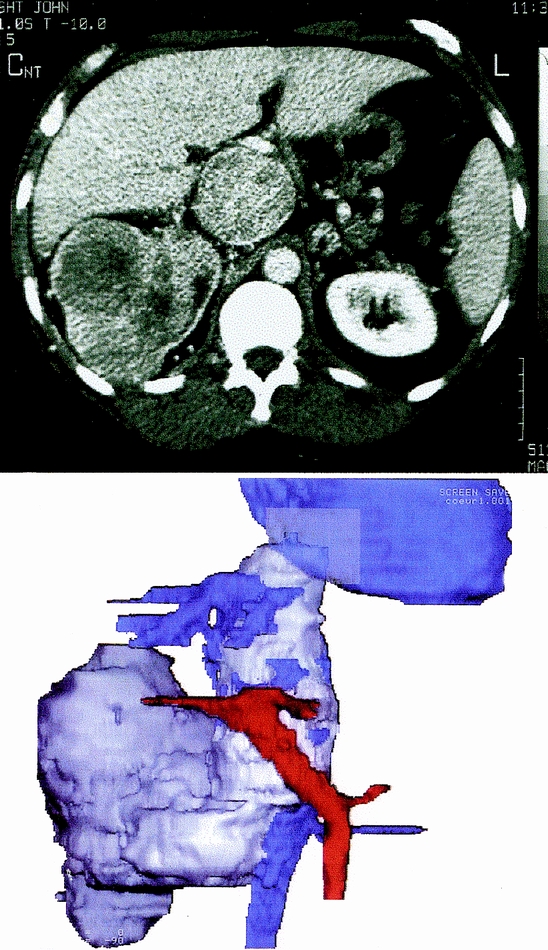
Figure 2. Patient 14. Abdominal contrast-enhanced computed tomography scan (top) and three-dimensional reconstruction (bottom) showing a tumor in the right kidney with massive intracaval extension. Note the upper extremity of the tumor thrombus extending above the ostia of the hepatic veins. This case illustrates the limit of surgery without cardiopulmonary bypass.
All IVC replacements were made using ePTFE prosthetic grafts (a ringed reinforced tube 20 mm in diameter for suprarenal replacement and a bifurcated prosthesis for infrarenal replacement). In two patients, an arteriovenous fistula was performed between either the remaining left renal vessels (patient 12) or the right renal artery and homolateral gonadic vein (patient 14). Routine suction drainage of the subphrenic and periprosthetic spaces was associated when possible with omental interposition between the graft and resected viscera. As recommended, prophylactic antibiotics 22 were administered in all patients, and anticoagulation with subcutaneous or intravenous heparin was performed after surgery until discharge. In patients who underwent prosthetic replacement, warfarin therapy was started before discharge and continued indefinitely. Graft patency was not systematically assessed.
RESULTS
Surgical Data
The procedures performed are summarized in Figure 3. Surgical data are given in Table 2. In all but one patient, IVC resection was associated with multivisceral resection, including extended nephrectomy in eight patients, major hepatic resection in three, digestive resection in three, and infrarenal aortic replacement using bifurcated Dacron grafts in two. The mean duration of surgery was 360 minutes (range 225–570). Median intraoperative transfusion volume was 5 units packed red blood cells (range 0–10 units).
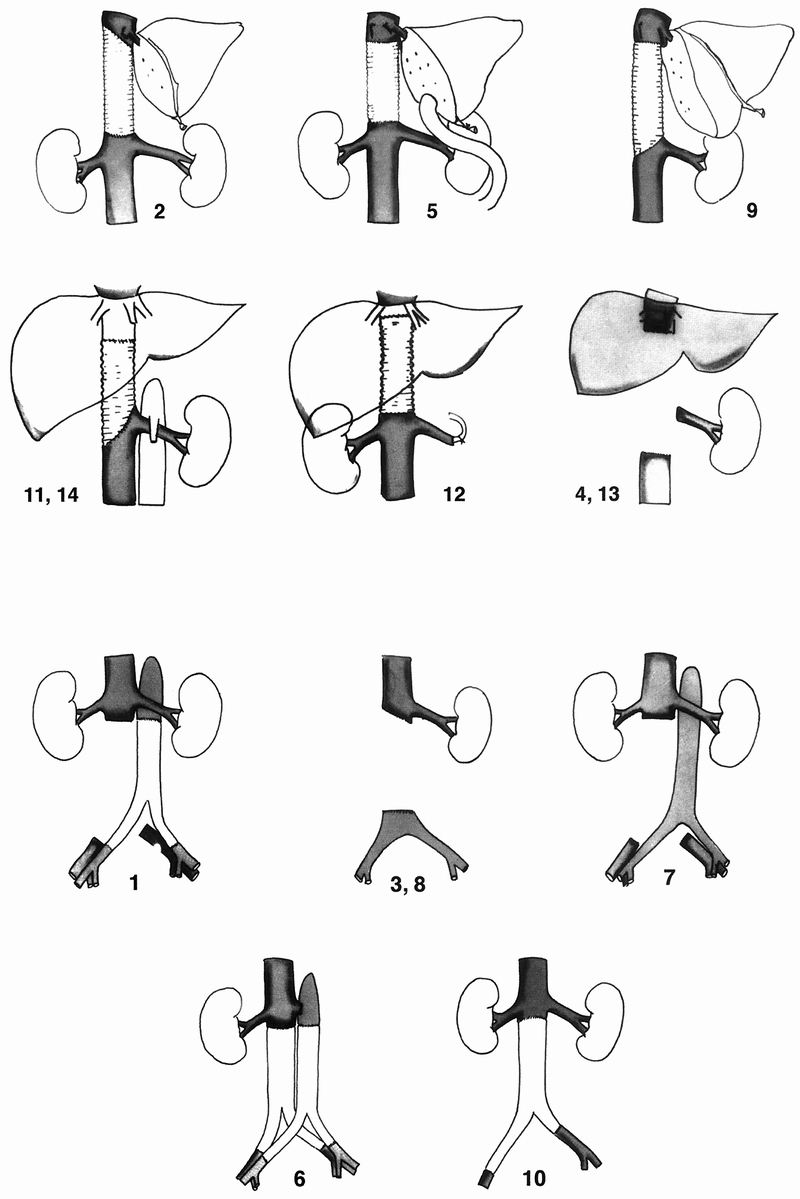
Figure 3. (A) The procedure performed in each case of suprarenal inferior vena cava resection with or without venous replacement. Numbers are the same as in tables. (B) The procedure performed in each case of infrarenal inferior vena cava resection without (top) or with (bottom) venous replacement.
Table 2. SURGICAL PROCEDURES AND OUTCOME
ERN, extended right nephrectomy; ELN, extended left nephrectomy.
* With adjunction of an arteriovenous fistula.
† Due to upper limb thrombosis after a humeral fracture.
Prosthetic replacement of the IVC was not systematically performed. The criteria for replacement were as follows: level of resected segment (more likely for suprarenal than infrarenal segments), degree of IVC obstruction (more likely for partial than complete obstruction), and resulting findings (i.e., symptoms, collateral circulation, and intraoperative hemodynamic status after tumor and IVC resection). Replacement was performed in six of eight patients undergoing suprarenal IVC resection (75%). The indication was partial obstruction with no collateral circulation in four patients and complete obstruction with preoperative swelling of the lower extremities in two patients. Replacement was not performed in the other two patients with complete suprarenal obstruction and prior (patient 13) or concomitant (patient 4) right nephrectomy; the left renal vein was ligated, assuming that collateral venous outflow was sufficient. Replacement was performed in two of the six patients undergoing infrarenal IVC resection (33%). The indication was resection of the iliac venous confluence and suppression of preexisting collaterals in both. Replacement was not performed in the remaining four patients (two with partial and two with complete infrarenal obstruction).
Early Outcome
Patient 8 died on the 16th postoperative day of cytomegalovirus-related pneumopathy resulting in multiple organ failure. Four patients had major complications. Patient 6 had a left subphrenic abscess, successfully treated by percutaneous drainage. Patient 9 had recurrent pleural effusion. Patient 10 had acute arterial thrombosis of the right lower limb, treated by surgical embolectomy. Patient 11 had acute iliocavoprosthetic thrombosis, manifested as major edema of the lower body, on the second postoperative day. This patient was treated by surgical thrombectomy and required three additional interventions to control intraperitoneal bleeding.
The median postoperative hospital stay was 21 days (range, 10–49). There was no evidence of graft-related infection during the hospital stay or since discharge.
Venous Sequelae
Marked symptoms of venous obstruction developed in three of the six patients who did not undergo prosthetic replacement. In patient 4, vascular imaging performed during the third postoperative month showed complete iliocaval thrombosis. Patient 8’s postoperative status prevented vascular imaging until death. Patient 13 had persistent leg edema for 4 months after surgery. This patient had had invalidating preoperative leg edema and was ineligible for prosthetic reconstruction because of complete thrombotic obstruction below the intracaval tumor.
Venous obstruction was also observed in two of the eight patients who underwent prosthetic replacement. In patient 6, asymptomatic unilateral iliac graft thrombosis was incidentally discovered by routine angiography during the third postoperative month. Patient 11 remained asymptomatic with a patent graft 8 months after postoperative thrombectomy.
Late Outcome
Tumor recurrence was observed within 2 to 33 months (median, 16) in 7 of the 12 patients who underwent surgery for malignant disease. Two of these patients underwent surgical resection for hepatic recurrence. Patient 2 underwent segmentectomy (segment 2) at 43 months and remained asymptomatic until death 69 months after the initial procedure. Patient 11 underwent right hepatectomy at 8 months and died after surgery of mesenteric infarction. The other five patients with recurrent malignancy were treated by chemotherapy. Estimated median survival in patients with malignancy was 37 months. The estimated actuarial survival rate was 67% at 1 year and 56% at 3 years.
DISCUSSION
This series describing 14 patients who underwent IVC resection for neoplasms with or without prosthetic replacement is the largest to date. Unlike three previously published series, 8,10,18 our series includes patients with a variety of tumoral conditions at different levels of the IVC. Our results indicate that prosthetic replacement of the IVC after resection of abdominal neoplasms can be performed safely and avoids venous sequelae. These findings justify surgical treatment to achieve extirpation in selected patients with primary caval neoplasms or large abdominal tumors with caval involvement, even when reconstruction of the aorta, IVC, or digestive tract is required. 3,6,23
In most patients, circumferential resection of the IVC is necessary to be curative. Lateral resection should not be performed in patients with primary caval tumors with extraluminal extension. 3 In patients with renal cell carcinoma with extensive intraluminal involvement, open thrombectomy alone or patch resection of the IVC around the origin of the renal vein carries the risk of late recurrence from the venous wall, as in patient 13 in this series and in previous reports. 10,17,24–26 Procedures can usually be performed through an abdominal or thoracoabdominal incision without extracorporeal circulation. Venovenous bypass, which was required in all patients older than 50 at the Mayo Clinic, 8 was never needed in our experience, despite the inclusion of 10 patients older than 50. In agreement with previous authors, 1,10,15,27 we think that cardiopulmonary bypass is unnecessary unless the tumor extends significantly into the atrium. Alternatively, various clamping techniques can be used depending on hemodynamic conditions to control blood flow in the IVC and its tributaries, especially in patients requiring major hepatic resection. 10,11
Aggressive management of advanced abdominal tumors can produce long-term survival. The 5-year actuarial survival rate after curative resection exceeds 50% for renal cell carcinoma with caval extension 16,17 and 28% for primary leiomyosarcoma. 28 In the present series, estimated median survival was 37 months for patients with malignant disease. In comparison, median survival without resection is 1 month for patients with primary leiomyosarcoma. 28 Similarly, survival is less than 1 year for patients with renal cell carcinoma with caval extension treated by nephrectomy alone. 14
Replacement after IVC resection is controversial. In patients with complete IVC obstruction, collateral circulation usually provides sufficient venous drainage so that obstructive symptoms are uncommon. 1,18,28,29 However, the problem is different in patients treated surgically for extensive neoplasms. Wide retroperitoneal resection including a segment of the IVC disrupts preexisting venous channels and thus can reduce collateral venous return. 10,18 A review of the literature 28 showed that documented or suspected lower limb venous thrombosis occurred after radical resection of IVC leiomyosarcoma in 22 of 82 patients (27.5%). Our experience confirms previous evidence 8,10,18 that IVC replacement is useful in most patients. Indeed, early or late postoperative symptoms of venous obstruction were observed in two patients who could have benefited from reconstruction (patients 4 and 8), whereas preoperative lower limb swelling disappeared after surgery with reconstruction in two patients (patients 12 and 14).
Another risk of IVC ligation is renal failure. In patients with complete suprarenal obstruction who undergo IVC resection including the renal confluence and right kidney, ligation of the left renal vein is possible because multiple pathways are available for renal venous outflow. 1,10 We did this in two patients (patients 4 and 13) with no impact on renal function, despite marked symptoms of venous obstruction (see above). However, renal vein ligation can be deleterious. In a four-patient series, Huguet et al 10 reported one case of intraoperative anuria requiring anastomosis of the renal vein to the caval graft, and one postoperative death resulting from renal failure. In patients with partial obstruction of the suprarenal IVC and poorly developed collateral circulation, the risk of renal failure after IVC ligation is much greater. As a predictive test, it has been suggested to measure venous pressure in the unaffected proximal renal vein after caval clamping and perform IVC replacement when pressure exceeds 40 mmHg. 30 We did not use this test but rather took into consideration preoperative renal function and hemodynamic status during intraoperative venous occlusion. In patient 2, the decision for replacement was made after completing IVC ligation because hypotension and oliguria persisted despite adequate vascular loading. These symptoms resolved immediately after reconstruction (Fig. 4). This experience has probably led us to perform IVC replacement more frequently, in agreement with the results of other recent reports involving replacement of the suprarenal 8,10 and even the infrarenal IVC. 18
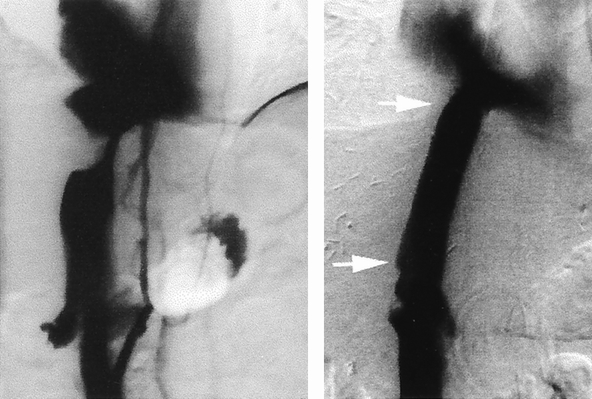
Figure 4. Patient 2. Preoperative cavography (left) showing a tight stenosis of the upper part of the retrohepatic inferior vena cava resulting from carcinoma of the right hepatic lobe. Note the backflow into the renal veins and one collateral lumbar vein. Despite this drainage, extended right hepatectomy with inferior vena cava resection and ligation led to intraoperative oliguria and hypotension, which were reversed by venous reconstruction. Cavography on the postoperative day 14 (right) showed a patent prosthesis. The extremities of the graft are designated by the white arrows.
The most widely used prosthesis for IVC replacement is the ringed reinforced ePTFE graft because it provides the best results, given the length of the missing segment and the need for strength to resist compression in the abdomen. Indeed, graft collapse may be a factor in thrombosis. 12,13,18 Like several other authors, 8,10 we prefer a 20-mm-diameter graft for best congruency with the native vessel. Some have recommended smaller grafts (14–16 mm) for infrarenal replacement to increase blood velocity. 18 Although good long-term patency has been reported without anticoagulation, 18 we routinely administer anticoagulation therapy to all patients undergoing IVC replacement. Another concern with the use of synthetic grafts is infection. 8,23 We observed no infection related to intestinal and urinary tract resection or to biliary leak after liver resection. As stated in previous reports, 8,10,18 omental interposition between the graft and resected viscera may have contributed to this result. Prosthetic IVC replacement appears safe, even though one major complication related to acute thrombosis of the prosthesis was observed. In fact, we interpret the poor tolerance of postoperative obstruction in this patient as proof that reconstruction was justified. In an effort to prevent such complications by enhancing blood flow and pressure within the graft, we used vessels remaining in the surgical field to perform arteriovenous fistula 3,8,12 in two subsequent patients. Because no adverse effect on cardiac function was observed after 20 and 13 months, these fistulas have not yet been removed.
In conclusion, multivisceral resection including the IVC is a technically demanding procedure but can be useful to allow extension of potentially curable resection. Prosthetic caval replacement can be performed easily and has a low complication rate. We advocate more widespread use of reconstruction to improve the quality of survival after IVC resection.
Acknowledgments
The authors thank Gilles Houvenaeghel, MD, and Philippe Piquet, MD, for their assistance in the management of two patients in this series.
Footnotes
Correspondence: Prof. Y. Patrice Le Treut, Service de Chirurgie, Générale et Transplantation Hépatique, Hôpital de la Conception, 147 Boulevard Baille, 13385 Marseille Cedex 5, France. E-mail: yletreut@ap-hm.fr
Accepted for publication August 15, 2000.
References
- 1.Kearney GP, Bedford Waters W, Klein LA, et al. Results of inferior vena cava resection for renal cell carcinoma. J Urol 1981; 125: 769–773. [DOI] [PubMed] [Google Scholar]
- 2.Sarti L. Total prosthetic transplantation of the inferior vena cava, with venous drainage restoration of the one remaining kidney on the graft, successfully performed on a child with Wilms’ tumor. Surgery 1970; 67: 851–855. [PubMed] [Google Scholar]
- 3.Kieffer E, Berrod JL, Chomette G. Primary tumors of the inferior vena cava. In: Surgery of Veins. Bergan JJ, Yao JST, eds. New York: 1985:423–443.
- 4.Brabrand K, Soreide JA. Adrenal cortical carcinoma with invasion into the inferior vena cava. Br J Surg 1987; 74: 598–599. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Koep LJ, Weil R, et al. Right trisegmentectomy for hepatic neoplasms. Surg Gynecol Obstet 1980; 150: 208–214. [PMC free article] [PubMed] [Google Scholar]
- 6.Beck SD, Lalka SG. Long-term results after inferior vena caval resection during retroperitoneal lymphadenectomy for metastatic germ cell cancer. J Vasc Surg 1998; 28: 808–814. [DOI] [PubMed] [Google Scholar]
- 7.Iwatsuki S, Todo S, Starzl TE. Right trisegmentectomy with a synthetic vena cava graft. Arch Surg 1988; 123: 1021–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bower TC, Nagorney DM, Toomey BJ, et al. Vena cava replacement for malignant disease: is there a role? Ann Vasc Surg 1993; 7: 51–62. [DOI] [PubMed] [Google Scholar]
- 9.O’Malley KJ, Stuart RC, McEntee GP. Combined resection of the inferior vena cava and extended right hepatectomy for leiomyosarcoma of the retrohepatic cava. Br J Surg 1994; 81: 845–846. [DOI] [PubMed] [Google Scholar]
- 10.Huguet C, Ferri M, Gavelli A. Resection of the suprarenal inferior vena cava. The role of prosthetic replacement. Arch Surg 1995; 130: 793–797. [DOI] [PubMed] [Google Scholar]
- 11.Le Treut YP, Giudici P, Betances L, et al. Résection et remplacement de la veine cave supra-rénale au cours des hépatectomies partielles. Presse Med 1995; 24: 169–174. [PubMed] [Google Scholar]
- 12.Gloviczki P, Pairolero PC, Cherry KJ, et al. Reconstruction of the vena cava and of its primary tributaries: a preliminary report. J Vasc Surg 1990; 11: 373–381. [DOI] [PubMed] [Google Scholar]
- 13.Robinson RJ, Peigh PS, Fiore AC, et al. Venous prostheses: improved patency with external stents. J Surg Res 1984; 36: 306–311. [DOI] [PubMed] [Google Scholar]
- 14.Sosa ER, Muecke EC, Vaughan DE, et al. Renal cell carcinoma extending into the inferior vena cava: the prognostic significance of the level of vena caval involvement. J Urol 1984; 132: 1097–1100. [DOI] [PubMed] [Google Scholar]
- 15.Bintz M, Cogbill TH, Klein AS. Surgical treatment of renal cell carcinoma involving the inferior vena cava. J Vasc Surg 1987; 6: 566–571. [PubMed] [Google Scholar]
- 16.Suggs WD, Smith RB, Dodson TF, et al. Renal cell carcinoma with inferior vena caval involvement. J Vasc Surg 1991; 14: 413–418. [DOI] [PubMed] [Google Scholar]
- 17.Babu SC, Mianoni T, Shah PM, et al. Malignant renal tumor with extension to the inferior vena cava. Am J Surg 1998; 176: 137–139. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar R, Eilber FR, Gelabert HA, et al. Prosthetic replacement of the inferior vena cava for malignancy. J Vasc Surg 1998; 28: 75–81. [DOI] [PubMed] [Google Scholar]
- 19.Bertrand P, Amabile P, Hardwigsen J, et al. Intravenous leiomyomatosis with caval involvement: report of a case with radical resection and venous replacement. Arch Surg 1998; 133: 460–462. [DOI] [PubMed] [Google Scholar]
- 20.Couinaud C. Le foie, études anatomiques et chirurgicales. Paris: Masson et Cie; 1957.
- 21.Maraan BM, Taber RE. The effects of inferior vena caval ligation on cardiac output: an experimental study. Surgery 1968; 63: 966–969. [PubMed] [Google Scholar]
- 22.Martin C, Pourriat JL. The French Society of Anesthesia and Resuscitation. Recommendations for the practice of antibiotic prophylaxis in surgery. Current status 1999. Chirurgie 1999; 124: 441–447. [PubMed] [Google Scholar]
- 23.Kraybill WG, Callery MP, Heiken JP, et al. Radical resection of tumors of the inferior vena cava with vascular reconstruction and kidney autotransplantation. Surgery 1997; 121: 31–36. [DOI] [PubMed] [Google Scholar]
- 24.Vekemans KM, Schroder FH. Prosthetic replacement of the inferior vena cava in renal cell carcinoma surgery. Eur Urol 1991; 19: 262–266. [DOI] [PubMed] [Google Scholar]
- 25.Barone GW, Kahn MB, Cook JM, et al. Recurrent intracaval renal cell carcinoma: the role of intravascular ultrasonography. J Vasc Surg 1991; 13: 506–509. [PubMed] [Google Scholar]
- 26.Okada Y, Kumada K, Habuchi T, et al. Total replacement of the suprarenal inferior vena cava with an expanded polytetrafluoroethylene tube graft in 2 patients with tumor thrombi from renal cell carcinoma. J Urol 1989; 141: 111–114. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal JT, Colonna JO, Drinkwater DC. Leiomyosarcoma of the vena cava with atrial extension: long-term survival following resection and caval replacement without circulatory arrest. Urology 1995; 46: 876–878. [DOI] [PubMed] [Google Scholar]
- 28.Mingoli A, Feldhaus RJ, Cavallaro A, et al. Leiomyosarcoma of the inferior vena cava: analysis and search of world literature on 141 patients and report of three new cases. J Vasc Surg 1991; 14: 688–699. [DOI] [PubMed] [Google Scholar]
- 29.Duckett JJ, Lifland JH, Peters PC. Resection of the inferior vena cava for adjacent malignant diseases. Surg Gynecol Obstet 1973; 136: 711–716. [PubMed] [Google Scholar]
- 30.Clayman RJ, Gonzalez R, Fraley EE. Renal cancer invading the inferior vena cava: clinical review and anatomical approach. J Urol 1980; 123: 157–163. [DOI] [PubMed] [Google Scholar]