Abstract
Objective
To analyze the authors’ experience with sentinel lymph node biopsy (SLNB) and the subsequent incidence and pattern of recurrence in patients with positive and negative nodes.
Summary Background Data
Lymphatic mapping with SLNB has become widely accepted in the management of patients with melanoma who are at risk for occult regional lymph node metastases. Because this procedure is relatively new, the pattern of recurrence after SLNB is not yet clear.
Methods
All patients with primary cutaneous melanoma who underwent SLNB from 1991 through 1998 were identified from a prospective single-institution melanoma database.
Results
Three hundred fifty-seven consecutive patients with localized primary cutaneous melanoma who underwent SLNB were identified. The sentinel node was identified in 332 patients (93%) and was positive in 56 (17%). Fourteen percent of patients had developed a recurrence at a median follow-up of 24 months. The median time to recurrence was 13 months. The 3-year relapse-free survival rates for patients with positive and negative nodes were 56% and 75%, respectively. SLN status was the most important predictor of disease recurrence. The site of first recurrence in patients with negative and positive nodes was more commonly locoregional than distant. Reexamination of the SLN in 11 patients with negative nodes with initial nodal and in-transit recurrence showed evidence of metastases in 7 (64%).
Conclusions
Patients with positive sentinel nodes have a significantly increased risk for recurrence. The early pattern of first recurrence for patients with negative and positive results is characterized by a preponderance of locoregional sites, similar to that reported in previous series of elective lymph node dissection. These data underscore the need for careful pathologic analysis of the SLN as well as a careful, directed locoregional physical examination in the follow-up of these patients.
In 1999, an estimated 44,200 cases of melanoma were diagnosed in the United States alone, and the incidence of this malignancy continues to increase. 1 There is controversy over the optimal surgical management of patients with disease clinically limited to the primary cutaneous lesion. The main point of contention continues to be the management of the regional nodal basins that drain the site of the primary lesion in patients at risk for nodal metastases.
Several retrospective series suggested a survival benefit for patients undergoing elective lymph node dissection (ELND). 2–6 In these studies, the benefit appeared to be limited to patients with intermediate-thickness melanomas. The results of prospective randomized trials have been mixed, although significant differences exist in their scope and design. The largest and most recent trial, the Intergroup Melanoma Surgical Trial, included patients with 1- to 4-mm primary cutaneous melanomas. Lymphoscintigraphy was performed on all patients with truncal lesions. In this trial, there was no increase in survival for the ELND group as a whole. Subset analysis demonstrated an improvement in survival in patients with 1- to 2-mm primary lesions (when analyzed by actual treatment received). Patients younger than 60 years with nonulcerated lesions between 1 and 2 mm derived the most benefit in this trial. For patients with primary tumors 1 to 3 mm thick, the subsequent incidence of clinical nodal metastases in the observation arm of the trial was significantly greater than the incidence found on pathologic review of the ELND specimens (17.7% vs. 11.3%). This suggested that relevant positive nodes might have been missed in the routine pathologic processing of ELND specimens.
Sentinel lymph node mapping and biopsy (SLNB) has been shown to be highly accurate in staging nodal basins at risk for regional metastases in patients with primary cutaneous melanoma. In the experience reported by Morton et al 7 in 1992 where nonsentinel nodes (non-SLN) from immediate backup dissections were subjected to routine examination and immunohistochemical (IHC) analysis, the SLN was positive in 38 of 40 patients found to have any positive node (SLN or non-SLN). In this study the SLN reflected the status of the nodal basins in 192 of 194 patients with clinical stage 1 disease. Similar rates of accuracy have been reported by other groups. 8,9
Given the short time since the initial description of this technique, only a few reports exist on the prognostic significance of SLN status. In addition, the pattern of initial recurrence in patients undergoing SLNB has not been well described.
In this report we present a large, consecutive single-institution experience with SLNB for primary cutaneous melanoma. The patterns of early recurrence for patients with positive and negative SLNs are described, and a review of the current literature is presented.
METHODS
Patients
From May 1991 through December 1998, 357 patients with primary cutaneous melanoma underwent attempted SLN mapping and biopsy at Memorial Sloan-Kettering Cancer Center. All patients had histologically confirmed melanoma with clinically negative regional lymph nodes. Patient data, including clinical characteristics, pathologic findings, and follow-up, were entered prospectively into the melanoma database.
Postoperative follow-up included physical examination every 3 to 4 months for the first year, every 3 to 6 months for the second year, and every 6 to 12 months thereafter. Chest radiographs were performed every 6 to 12 months during the first 2 to 3 years. Serum lactate dehydrogenase levels and a complete blood count were also obtained during the first 2 to 3 years of follow-up. Further investigations, including computed tomography and positron emission tomography scanning, were performed on a selective basis.
Mapping Technique
Immediately before wide excision of the primary, 1 to 3 mL isosulfan blue dye (Lymphazurin 1%; Hirsch Industries, Inc., Richmond, VA) was injected intradermally around the primary or biopsy site. Lymphoscintigraphy was performed on 319 of 357 patients (89%) with the intradermal injection of 99mTc-sulfur colloid (400 μCi) on the day of surgery. Although not performed before 1995, lymphoscintigraphy was routinely performed in all patients since. The SLN was defined as a lymph node found to be stained with the blue dye or with a count greater than 10% of the hottest SLN. Each SLN was removed and submitted separately for analysis. Frozen-section analysis of the SLN was performed in most patients, although that is not our current practice. All patients underwent wide local excision of the primary melanoma or reexcision of the previous biopsy site to achieve margins consistent with standards based on tumor thickness. All patients with metastases in the SLN or SLNs underwent a complete lymph node dissection of the affected nodal basin either at the same setting, if the frozen section was positive, or within 2 to 3 weeks when detected on review of the permanent sections. A planned simultaneous elective lymph node dissection was performed in 35 patients during the early period of our experience to confirm the efficacy of the SLNB technique.
Conventional histologic examination (hematoxylin and eosin [H&E] staining) was routinely applied to bisected SLNs and non-SLNs. Beginning in late 1997, SLNs were routinely subjected to H&E staining of serial sectioned specimens and IHC staining (S-100, HMB-45) in the event that the initial H&E staining of the bisected specimen did not reveal evidence of metastatic disease. Examination of lymphadenectomy specimens and non-SLNs harvested during SLNB were analyzed by conventional H&E staining of bisected lymph node specimens.
Statistical Analysis
Association between factors was determined by chi-square analysis for categorical variables. Means testing continuous variables was performed with the Student t test. Disease recurrence and survival curves were constructed using the Kaplan-Meier product-limit method and were analyzed by the log-rank procedure. Multiple covariate analyses of disease-free and disease-specific survival were performed using the Cox proportional hazards regression model incorporating factors that on univariate analysis were statistically significant (P < .05) or demonstrated a trend toward significance. Tumor thickness and age were treated as continuous variables for both univariate and multiple covariate analyses.
RESULTS
Patients
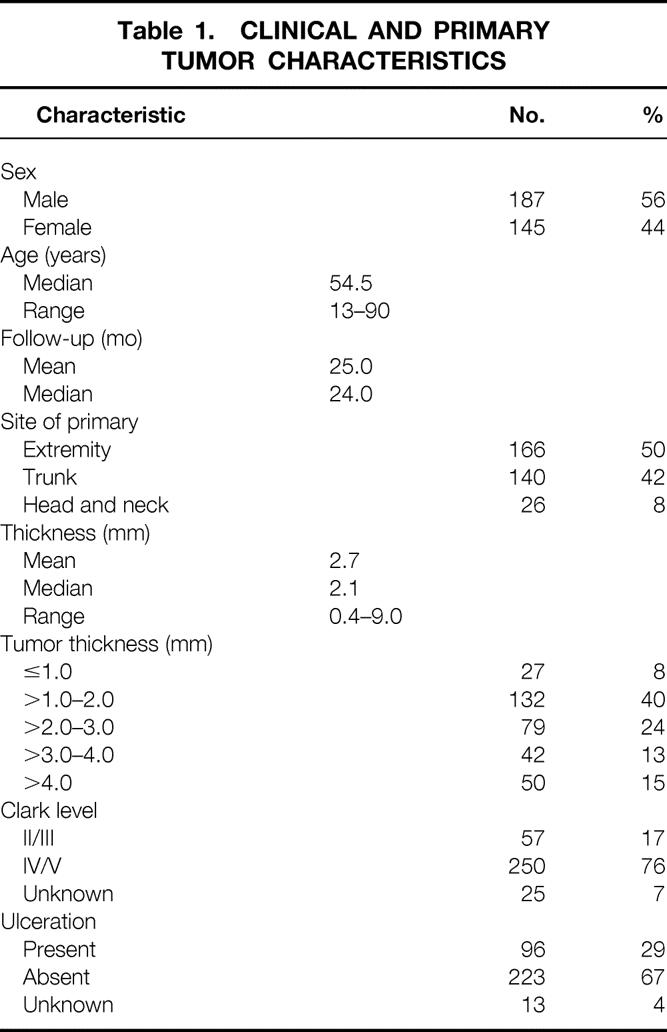
From May 1991 through December 1998, 357 patients with primary cutaneous melanoma underwent attempted SLN mapping and biopsy at Memorial Sloan-Kettering Cancer Center. Most of these patients (79%) were accrued in the last 3 years of the study. SLNB was successful in 332 of the 357 patients (93%) and the results were positive for metastases in 56 (17%). Twenty-four patients with negative nodes underwent a planned confirmatory ELND and were excluded from the recurrence analyses. The clinical and pathologic characteristics of the 332 patients who underwent successful SLNB are presented in Table 1. The median age was 55 years, and 56% were men. Truncal primaries were present in 135 patients (42%); extremity and head and neck were the primary sites in 155 (48%) and 32 (10%), respectively. The median tumor thickness was 2.1 mm. Eighty-four percent of the primary lesions were between 0.75 and 4.0 mm. Thick (>4.0 mm) primary tumors were present in 15% of the patients. A Clark level IV or V depth of invasion was seen in 250 (81%) of the 307 primary tumors where data were available. Ulceration was present in 92 of the 322 (29%) primary lesions.
Table 1. CLINICAL AND PRIMARY TUMOR CHARACTERISTICS
Results of Lymphatic Mapping
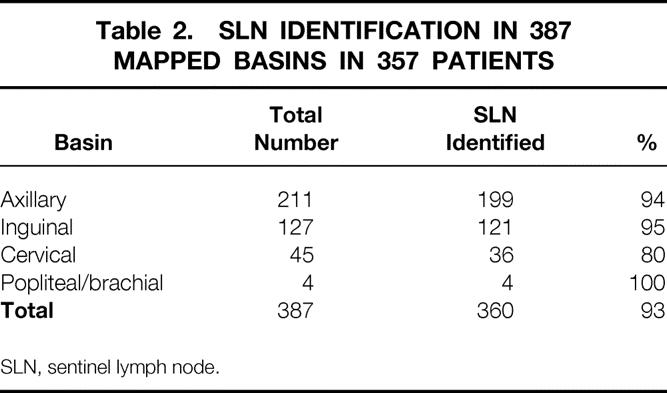
A total of 387 nodal basins were mapped in the 357 patients. An SLN was identified in 360 of the 387 mapped nodal basins (95%) in 332 of 357 patients (93%). Lymphoscintigraphy was performed in 319 of the 357 patients. An SLN was identified in 303 of 319 patients who underwent preoperative lymphoscintigraphy (96%). Lymphoscintigraphy failed to identify a draining nodal basin in 5 of the 319 patients. Of the 38 patients not undergoing lymphoscintigraphy early in our experience, 27 had primary tumors in the extremity. In patients undergoing preoperative lymphoscintigraphy, a single nodal basin was mapped in 290 patients (91%), two nodal basins were mapped in 27 patients (8%), and three basins were mapped in two patients (1%). Axillary, inguinal, and cervical nodal basins represented 54%, 33%, and 12% of successfully mapped basins (Table 2). The mean and median numbers of SLNs harvested per basin were 2.2 and 2.0, respectively. One SLN was harvested from 42% of basins, two SLNs were harvested from 28%, three were harvested from 10%, and more than three were harvested from 13%.
Table 2. SLN IDENTIFICATION IN 387 MAPPED BASINS IN 357 PATIENTS
SLN, sentinel lymph node.
Histologic Results
Our current method for pathologic review of the SLN involves serial sectioning with IHC staining if the initial H&E staining of the bisected node does not reveal metastatic disease. This method was used in 123 of the 332 patients with successful mapping. In the remaining 209, analyses of the SLNs were performed by conventional H&E staining of the bisected specimens. Retrospective serial sectioning, IHC, or molecular analysis for tyrosinase mRNA was performed in selected patients with negative nodes who had a recurrence.
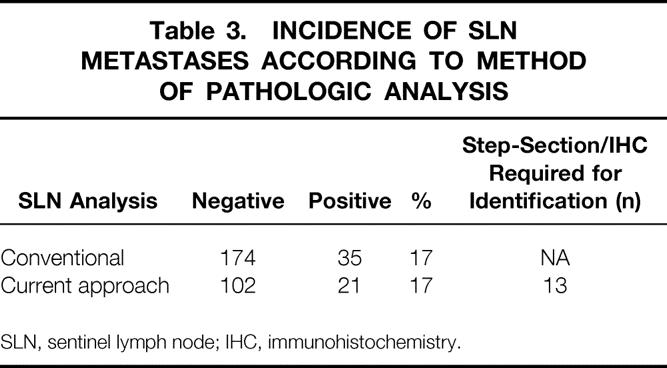
The incidence of positive nodes detected with our current approach of SLN analysis, compared with when we were performing only H&E staining on bisected specimens, is listed in Table 3. Using our current approach of step-sectioning with H&E and IHC staining in instances where the H&E staining of the bisected SLN does not demonstrate metastases (January 1998 to December 1998), SLN metastases were detected in 21 of 123 patients (17%). Thirteen of the 21 identified were not appreciated on the initial H&E staining and required either step-sectioning or IHC for identification. During the initial experience where only H&E staining of the bisected SLN specimen was performed (May 1991to July 1997), the detection rate was also 17%. During these two periods, the proportions of T stages, ulceration, primary tumor location, gender, and age were similar (data not shown).
Table 3. INCIDENCE OF SLN METASTASES ACCORDING TO METHOD OF PATHOLOGIC ANALYSIS
SLN, sentinel lymph node; IHC, immunohistochemistry.
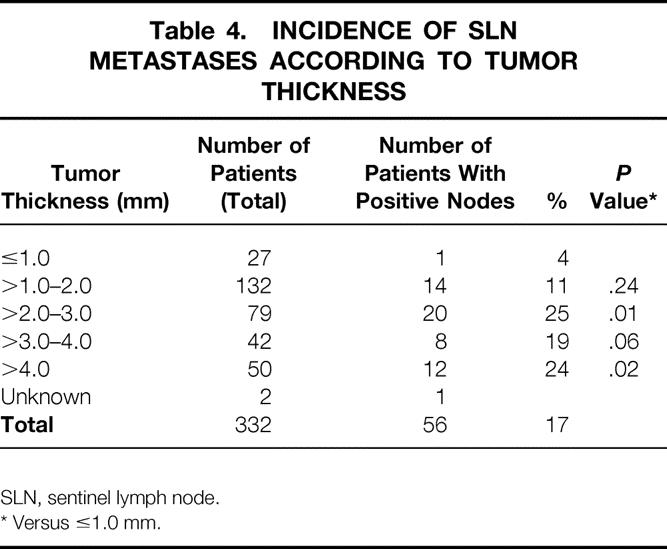
Metastases were detected during or after surgery in 56 patients. The incidence of metastasis in the SLN was significantly associated with the tumor thickness (Table 4). For patients with tumor thicknesses of 1.0 mm or less, the incidence was 4%. This was less frequent than the incidence for tumors 1.0 to 2.0 mm thick (11%, P = .03), 2.0 to 3.0 mm thick (25%, P = .01), 3.0 to 4.0 mm thick (19%, P = .06), and more than 4.0 mm thick (24%, P = .02). The mean tumor thickness in patients with an SLN metastasis was 3.4 mm, compared with 2.5 mm in patients without evidence of metastases (P < .01).
Table 4. INCIDENCE OF SLN METASTASES ACCORDING TO TUMOR THICKNESS
SLN, sentinel lymph node.
* Versus ≤1.0 mm.
There was no significant difference in the mean age or gender pattern in comparing patients with a positive or negative SLN (Table 5). In addition, the percentage of patients with a Clark IV or V primary tumor was not significantly greater, nor was the proportion of patients with axially located primary tumors. The presence of ulceration in the primary tumor was not associated with an increased incidence of SLN metastases. Of 59 basins (in 58 patients) found to have a positive node, only 6 (10%) were found to have a positive non-SLN in the subsequent lymphadenectomy specimen. Only one patient with a positive non-SLN had a tumor thickness (1.4 mm) of less than 2.5 mm. Of 13 basins where the SLN was identified only through step-sectioning with H&E or IHC staining, only one patient was found to have a positive non-SLN.
Table 5. FACTORS ASSOCIATED WITH SLN POSITIVITY
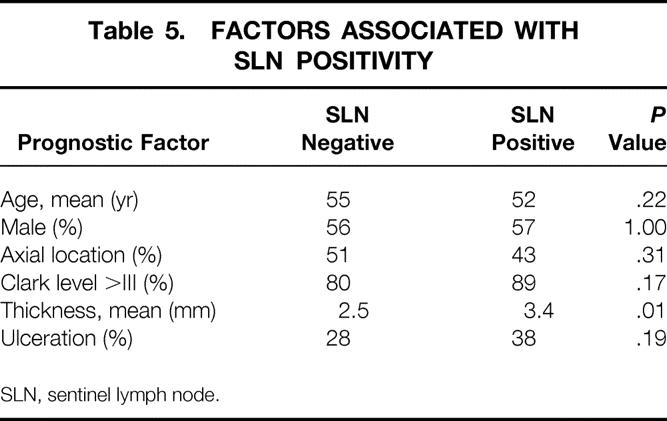
SLN, sentinel lymph node.
Predictors of Recurrence
Twenty-four of the 332 patients undergoing successful SLN mapping also underwent a confirmatory ELND in the presence of a negative SLN and were thus excluded from the following analysis of recurrence. Fifty-seven of the remaining 308 patients with successful SLNB had a recurrence at an overall median follow-up of 24 months. The median time to recurrence was 13 months. In patients with no evidence of metastases in the SLN, the overall incidence of recurrence was 14%, compared with 40% in those with SLN metastases. The rate of relapse-free survival at 3 years was 75% versus 58%, respectively (Fig. 1, P < .01). SLN status for the entire group was the most important predictor of disease-free survival on univariate analysis compared with other patient and tumor characteristics commonly associated with prognosis (Table 6). SLN status, tumor thickness (continuous variable), and age were the only factors on univariate and multivariate analyses to be statistically significant predictors of disease-free survival.
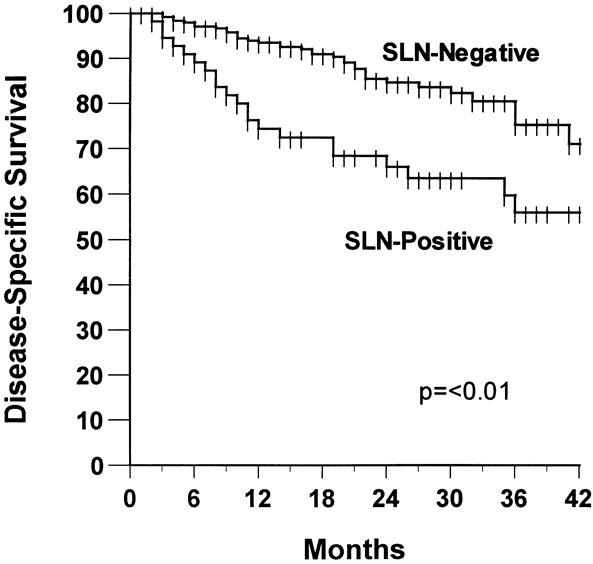
Figure 1. Relapse-free survival according to sentinel lymph node (SLN) status.
Table 6. PROGNOSTIC FACTORS INFLUENCING RELAPSE-FREE SURVIVAL
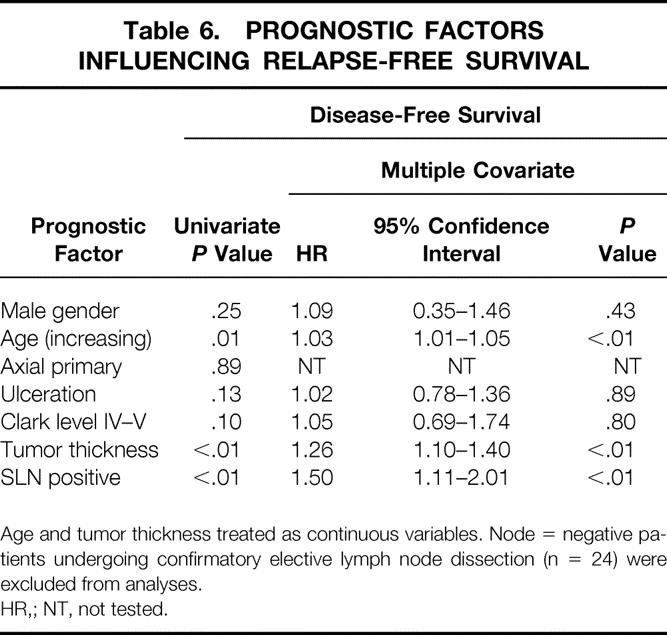
Age and tumor thickness treated as continuous variables. Node = negative patients undergoing confirmatory elective lymph node dissection (n = 24) were excluded from analyses.
HR,; NT, not tested.
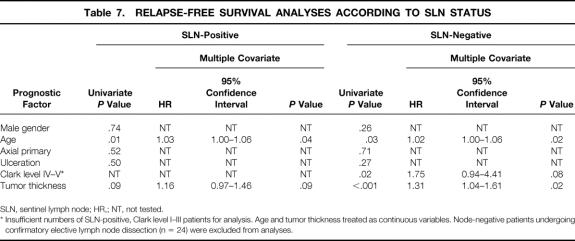
When stratified according to SLN status, univariate and multivariate analyses revealed that tumor thickness and age were significant prognostic factors of relapse-free survival in patients with a negative SLN (Table 7). In contrast, for patients with a positive SLN, tumor thickness did not provide additional prognostic information. Although when analyzed as a continuous variable, increasing age was a significant negative prognostic factor of disease-specific survival, the hazard ratio of 1.03 (95% confidence interval 1.00–1.06) indicates that the effect of this variable is minimal.
Table 7. RELAPSE-FREE SURVIVAL ANALYSES ACCORDING TO SLN STATUS
SLN, sentinel lymph node; HR,; NT, not tested.
* Insufficient numbers of SLN-positive, Clark level I–III patients for analysis. Age and tumor thickness treated as continuous variables. Node-negative patients undergoing confirmatory elective lymph node dissection (n = 24) were excluded from analyses.
Pattern of Recurrence
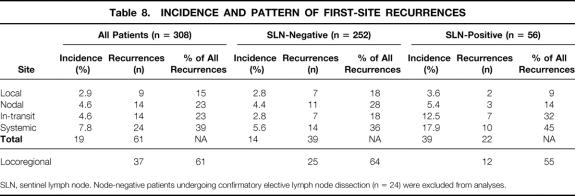
The sites of first recurrence according to SLN status are listed in Table 8. One patient known to have died of his disease was lost to follow-up, and consequently the site of the first recurrence could not be ascertained. In the remaining 56 patients, first-site recurrences were categorized as local, in-transit, nodal (same basin), or systemic (including distant nodal). Local recurrences were defined as recurrences occurring within 2 cm of the prior surgical scar. Recurrences between this boundary and the draining nodal basin were defined as in-transit recurrences. In 5 of the 56 patients (8.9%), first-site recurrences synchronously (two patients with systemic as one of the sites) manifested in more than one site; as a result, 61 first-site recurrences are reported for the 56 patients (see Table 8). Of the 308 patients with SLN identification, 9 patients (2.9%) developed a local recurrence. In-transit, nodal, and systemic first-site recurrences occurred in 4.5%, 4.5%, and 7.8%, respectively. In patients with a positive SLN, the incidence of local, in-transit, nodal, and systemic recurrences was 3.5%, 12.5%, 5.3%, and 17.8%, respectively. Locoregional recurrences constituted 61% of all first-site recurrences; distant recurrences accounted for 39%. The percentage of locoregional recurrences was not statistically different between patients with positive nodes (55%) and negative nodes (64%).
Table 8. INCIDENCE AND PATTERN OF FIRST-SITE RECURRENCES
SLN, sentinel lymph node. Node-negative patients undergoing confirmatory elective lymph node dissection (n = 24) were excluded from analyses.
Reevaluation of SLN Status in Patients With Negative Nodes With Recurrence
Of the original population of 332 patients undergoing successful SLNB, 67 patients had a recurrence. The original assessment of 53 of these patients involved routine H&E staining of a single section; the remainder were examined according to our current SLN protocol. A reevaluation of the negative SLNs in 11 patients with nodal, 7 in-transit, 3 or combined nodal and in-transit 1 recurrence as a component of their first recurrence was performed by either serial sectioning with H&E/IHC staining or attempted detection of tyrosine mRNA by polymerase chain reaction analysis (Table 9). Of these 11 patients, evidence of micrometastatic disease was found in 7 (64%). Six of the seven patients with an isolated nodal first-site recurrence had micrometastatic disease (86%). One of three patients with in-transit recurrence in the absence of nodal recurrence had a positive result on reevaluation. Nodal recurrences occurred in 7 of 150 patients with negative nodes whose initial pathologic examination was by H&E staining only (5%) and in 4 of 102 patients with negative nodes where the initial examination was with serial sectioning and IHC (4%, not significant).
Table 9. REEVALUATION OF THE NEGATIVE SLN IN PATIENTS WITH NODAL AND IN-TRANSIT RECURRENCE
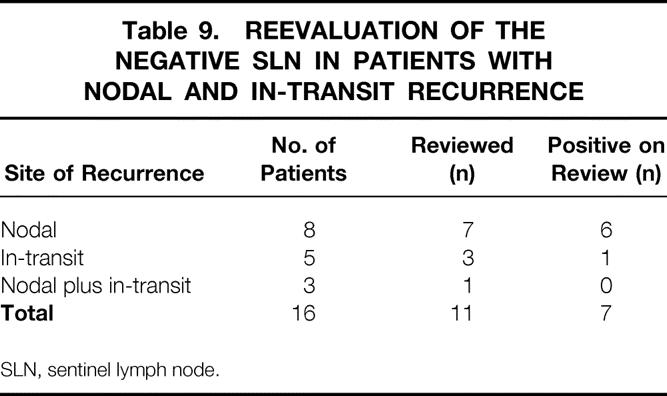
SLN, sentinel lymph node.
DISCUSSION
Despite the reports of a survival benefit from ELND in several large retrospective studies 2,3,6,10 and a recent randomized trial in patients with intermediate-thickness primary cutaneous melanoma, 11 there remains considerable debate as to the optimal management of the regional nodes. The opponents of routine ELND cite the significant complications produced by ELND, 11 as well as data from other negative retrospective and prospective randomized trials. 12–14 In support of ELND, the most recent update of the WHO #14 Trial of truncal melanoma, although an overall negative trial, showed that patients with occult nodal metastases undergoing ELND had a better survival than patients who subsequently developed clinical nodal metastases requiring therapeutic lymph node dissection (TLND). 12 The introduction of intraoperative lymphatic mapping and SLNB by Morton et al 7 introduced a new dimension into this debate when it became clear that a less morbid procedure could identify patients with occult metastases and thereby allow a more selective application of lymph node dissection. Without significant evidence for its therapeutic benefit, this technique was rapidly adopted in the care of patients with intermediate and thick primary cutaneous melanoma, as a means of both pathologically staging the draining nodal basin or basins and identifying patients who might derive a therapeutic benefit from selective lymph node dissection. The ability to identify patients at significantly greater risk for recurrence became more compelling when a positive randomized trial of adjuvant interferon alfa-2B in the management of high-risk patients was reported in 1996. 15
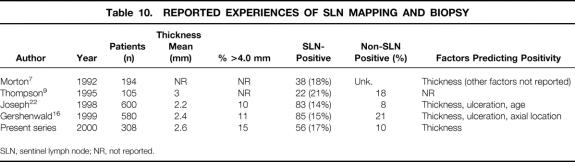
The technique of SLN mapping and biopsy has been shown by several centers to be an accurate method of staging clinically negative nodal basins in patients with primary cutaneous melanoma. 7,9,16–18 Several consistent features of SLN mapping and biopsy have emerged that are further substantiated in this present series (Table 10). In mature experiences, the SLN identification rate exceeds 95%, especially when both blue dye and radiocolloid are used. SLN status is directly related to tumor thickness, with positive rates of approximately 10% and 25% to 40% for tumor thicknesses less than 1.5 mm and greater than 4.0 mm, respectively. Other prognostic factors, including age and ulceration, have variably been shown to predict SLN metastases. Most patients with SLN metastases have no demonstrable metastases to the non-SLNs on completion lymphadenectomy. Non-SLN metastases are rarely demonstrated in patients whose primary tumor is less than 1.5 mm thick, suggesting that SLNB (i.e., no selective lymph node dissection) might be sufficient in this population. In the few studies that have addressed disease-free survival, SLN status is the most significant predictor of disease recurrence compared with other commonly listed prognostic factors (thickness, ulceration, Clark level, age, sex, tumor location). 16–18 In our series, disease recurred in 35 of 252 (14%) patients with negative nodes at a median follow-up of 23 months. The median time to recurrence in these patients was 16.2 months. In patients with positive nodes, the incidence of recurrence at a median follow-up of 27 months was 39%. The median time to recurrence in patients with positive nodes was 10.7 months.
Table 10. REPORTED EXPERIENCES OF SLN MAPPING AND BIOPSY
SLN, sentinel lymph node; NR, not reported.
In patients without SLN metastases, tumor thickness was a significant predictor of disease-free survival (see Table 7). Age and Clark level, although predictive on univariate analyses, were not significant on multivariate analysis. As reported by Gershenwald et al, 16 we found no additional prognostic significance for thickness in patients with positive nodes. Although follow-up is short, these studies together have suggested that recurrence in patients with thin and intermediate-thickness melanomas who have negative nodes appears to be uncommon and predicted by tumor thickness and possibly ulceration, Clark level, and age. In addition to being uncommon, recurrence occurs later in patients with positive nodes than in those with negative nodes.
Although the technical details and prognostic benefit of SLN mapping and biopsy have been well described, the pattern of recurrence is not as clearly defined. As this procedure has become more widely adopted, large single-institutional series are becoming mature enough to comment on this issue. Recent reports have addressed the pattern of recurrence in patients with negative nodes 17,19 and in patients with negative and positive nodes. 20 The present series documents the pattern of recurrence in patients with negative and positive nodes in a large institutional series. A review of the literature on recurrence patterns after ELND and SLNB is provided in an attempt to understand the early experience of the latter technique as it relates to disease recurrence.
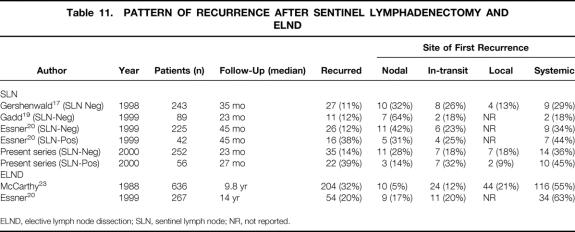
Systemic recurrence (n = 14) was the most common site of first recurrence in patients with negative nodes (see Table 8), followed by recurrence in the previously mapped regional draining basin (n = 11). Locoregional recurrences (local, in-transit, and nodal) collectively represented 64% of first-site recurrences in these patients, emphasizing the importance of physical examination in follow-up. The incidence of recurrence was much greater in the patients with positive nodes (40% vs. 14%), although the distribution between locoregional and systemic was not statistically different. In-transit recurrences constituted a larger proportion of the first-site recurrences in the patients with positive nodes (32%) versus those with negative nodes (18%). In contrast, nodal recurrences accounted for 28% of first-site recurrences in patients with negative nodes versus 14% in those with positive nodes. This probably reflects the nodal control achieved through subsequent complete lymphadenectomy. This difference may also be due to defining a false-negative result as a negative result on an SLN pathologic examination in patients with SLN metastases. Reexamination of the SLN in patients with negative nodes and nodal recurrences suggests that these failures most often represent pathologic false-negative events (see Table 9). These data strongly suggest that nodal recurrence in patients found to have negative nodes by rigorous histologic or molecular evaluation is extremely uncommon. Because the incidence of nodal recurrence in patients with negative nodes whose SLNs were initially examined by H&E staining only was no different than that in those examined with step-sectioning and IHC, it is not clear that these will be prospectively picked up in the future. These data are consistent with the findings of other studies (Table 11) that have reexamined negative SLNs in patients with recurrence. 17,18,20 It is not clear why in our experience the proportion of patients found to have positive nodes did not increase after the introduction of step-sectioning and IHC into our regular pattern of histologic analysis. The fact that 13 of 21 patients found to have positive nodes in the era of step-sectioning and IHC required these studies to demonstrate their micrometastases suggests that in our experience, these additional studies were additive. As previously stated, there were no differences in patient or tumor characteristics when comparing patients whose nodes were analyzed by a single H&E-stained section only with those who had step-sectioning and IHC in the event of a negative initial single H&E-stained section. Our overall detection rate was not markedly different from that reported by Essner et al 20 and Gershenwald et al. 17 In these studies, step-sectioning, IHC, or both were incorporated into the routine pathologic analysis at an earlier stage in their SLN experience. Whether our inability to increase the detection rate of SLN metastases with step-sectioning and IHC represents missed micrometastases is not clear, because the follow-up is short for the patients analyzed with this approach.
Table 11. PATTERN OF RECURRENCE AFTER SENTINEL LYMPHADENECTOMY AND ELND
ELND, elective lymph node dissection; SLN, sentinel lymph node; NR, not reported.
The pattern of recurrence in the present series appears to be similar to that described by Gershenwald et al 17 in patients with negative nodes, where at a median follow-up of 35 months, nodal, in-transit, local, and systemic recurrences accounted for 32%, 26%, 13%, and 29% of the first-site recurrences, respectively. The pattern of first-site recurrence in patients after ELND was well described by McCarthy et al in 1988. In a population of 636 patients undergoing ELND for primary, clinically node-negative cutaneous melanoma, 204 (32%) patients had recurrence. The median follow-up was 9.8 years. Distant (systemic) recurrences accounted for 55% of all first-site recurrences in this mature study. Nodal, in-transit, and local recurrences represented 5%, 12%, and 21% of the first-site recurrences, respectively. The greater proportion of systemic first-site recurrences could reflect the appearance of systemic first-site recurrences during the later part of the study. This notion is supported by Soong et al, 21 who in 1998 reported on the changing pattern of first-site recurrence as a function of the disease-free interval. These authors catalogued the first-site recurrence in 1,085 patients with localized melanoma (percentage ELND unknown). Of the recurrences in the first 2 years, only 16% were distant; 55% were regional (nodal and in-transit). With an increasing disease-free interval, the percentage of new distant first-site recurrences increased: 37% of first-site recurrences occurring between 2 and 5 years were distant.
Although there appears to be a relatively increased proportion of locoregional recurrence in patients undergoing SLN mapping and biopsy compared with the reported experiences of ELND, the data from Soong et al 21 and Essner et al 20 would suggest that this is just a snapshot at a point in time where locoregional recurrences are more prevalent. The data from our series and those of both Gershenwald et al 16,17 and Essner et al 20 also suggest that nodal recurrences can be minimized through a more detailed analysis of the SLN.
In conclusion, SLN mapping and biopsy is a very accurate method of staging the draining nodal basins in patients with primary cutaneous melanoma. SLN status is the most significant prognostic factor known in determining the risk of subsequent recurrence. Careful evaluation of the SLN with step-sectioning and IHC is important in identifying these patients at risk for recurrence; minimal-volume disease may be missed by single H&E-stained section analysis. Nodal recurrence in the presence of a negative SLN after rigorous pathologic analysis with step-sectioning and IHC is uncommon. The early pattern of first recurrence for patients with negative and positive nodes is characterized by a preponderance of locoregional sites; first recurrence occurs earlier in the latter group. The pattern of recurrence at this point in follow-up does not appear to differ from the reported ELND experience. This also underscores the need for careful physical examination in the follow-up of patients with primary cutaneous melanoma.
Long-term follow-up from the large institutional studies previously reported and the results of current national trials of SLNB will be critical in defining the ultimate outcome in patients undergoing SLNB. At this point, recurrence patterns appear similar to that seen in patients undergoing ELND.
Footnotes
Correspondence: Bryan M. Clary, MD, Box 3247, DUMC, Durham, NC 27710. E-mail: clary001@mc.duke.edu
Accepted for publication July 12, 2000.
References
- 1.Landis S, Murray T, Bolden S, et al. Cancer statistics 1999. CA Cancer J Clin 1999; 49: 8–31. [DOI] [PubMed] [Google Scholar]
- 2.Drepper H, Kohler C, Bastian B, et al. Benefit of elective lymph node dissection in subgroups of melanoma patients. Cancer 1993; 72: 741–749. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy W, Shaw H, Milton G. Efficacy of elective lymph node dissection in 2,347 patients with clinical stage I malignant melanoma. Surg Gynecol Obstet 1985; 161: 575–580. [PubMed] [Google Scholar]
- 4.Rompel R, Garbe C, Buttner P, et al. Elective lymph node dissection in primary malignant melanoma: a matched-pair analysis. Melanoma Res 1995; 5: 189–194. [DOI] [PubMed] [Google Scholar]
- 5.Wanebo H, Woodruff J, Fortner J. Malignant melanoma of the extremities: a clinicopathologic study using levels of invasion (microstage). Cancer 1975; 35: 666–676. [DOI] [PubMed] [Google Scholar]
- 6.Reintgen D, Cox E, McCarty KJ, et al. Efficacy of elective lymph node dissection in patients with intermediate-thickness primary melanoma. Ann Surg 1983; 198: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton D, Wen D, Wong J, et al. Technical details of intraoperative lymphatic mapping for early-stage melanoma. Arch Surg 1992; 127: 392–399. [DOI] [PubMed] [Google Scholar]
- 8.Reintgen D, Cruse C, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg 1994; 220: 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson J, McCarthy W, Bosch C, et al. Sentinel lymph node status as an indicator of the presence of mestastatic melanoma in regional lymph nodes. Melanoma Res 1995; 5: 255–260. [DOI] [PubMed] [Google Scholar]
- 10.Balch C, Soong S, Milton G, et al. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg 1982; 196: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balch C, Soong S, Bartolucci A, et al. Efficacy of an elective regional lymph node dissection of 1- to 4-mm-thick melanomas for patients 60 years of age and younger. Ann Surg 1996; 224: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cascinelli N, Morabito A, Santinami M, et al. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. Lancet 1998; 351: 793–796. [DOI] [PubMed] [Google Scholar]
- 13.Sim F, Taylor W, Pritchard D, et al. Lymphadenectomy in the management of stage I malignant melanoma: a prospective randomized study. Mayo Clin Proc 1986; 61: 697–705. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi U, Cascinelli N, Adamus J, et al. Primary cutaneous melanoma 2 mm or less in thickness: results of a randomized study comparing wide with narrow surgical excision: a preliminary report. N Engl J Med 1988; 318: 1159. [DOI] [PubMed] [Google Scholar]
- 15.Kirkwood J, Strawderman M, Ernstoff M, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutabneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996; 14: 7–17. [DOI] [PubMed] [Google Scholar]
- 16.Gershenwald J, Thompson W, Mansfield P, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol 1999; 17: 976–983. [DOI] [PubMed] [Google Scholar]
- 17.Gershenwald J, Colome M, Lee J, et al. Patterns of recurrence following a negative sentinel lymph node biopsy in 243 patients with stage I or II melanoma. J Clin Oncol 1998; 16: 225–2260. [DOI] [PubMed] [Google Scholar]
- 18.Gadd M, Coit D. Recurrence patterns and outcome in 1019 patients undergoing axillary or inguinal lymphadenectomy for melanoma. Arch Surg 1992; 127: 1412–1416. [DOI] [PubMed] [Google Scholar]
- 19.Gadd M, Cosimi A, Yu J, et al. Outcome of patients with melanoma and histologically negative sentinel lymph nodes. Arch Surg 1999; 134: 381–387. [DOI] [PubMed] [Google Scholar]
- 20.Essner R, Conforti A, Kelley M, et al. Efficacy of lymphatic mapping, sentinel lymphadenectomy, and selective complete lymph node dissection as a therapeutic procedure for early-stage melanoma. Ann Surg Oncol 1999; 6: 442–449. [DOI] [PubMed] [Google Scholar]
- 21.Soong S, Harrison R, McCarthy W, et al. Factors affecting survival following local, regional, or distant recurrence from localized melanoma. J Surg Oncol 1998; 67: 228–233. [DOI] [PubMed] [Google Scholar]
- 22.Joseph E, Brobeil A, Glass F, et al. Results of complete lymph node dissection in 83 melanoma patients with positive sentinal nodes. Ann Surg Oncol 1998; 5 (2): 119–125. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy W, Shaw H, Thompson J, et al. Time and frequency of recurrence of cutaneous stage I malignant melanoma with guidelines for follow-up study. Surgery, Gynecology and Obstetrics 1988; 166: 497–502. [PubMed] [Google Scholar]