Abstract
Objective
To determine the optimal site and frequency for vancomycin-resistant enterococci (VRE) surveillance to minimize the number of days of VRE colonization before identification and subsequent isolation.
Summary Background Data
The increasing prevalence of VRE and the limited therapeutic options for its treatment demand early identification of colonization to prevent transmission.
Methods
The authors conducted a 3-month prospective observational study in medical and surgical intensive care unit (ICU) patients with a stay of 3 days or more. Oropharyngeal and rectal swabs, tracheal and gastric aspirates, and urine specimens were cultured for VRE on admission to the ICU and twice weekly until discharge.
Results
Of 117 evaluable patients, 23 (20%) were colonized by VRE. Twelve patients (10%) had VRE infection. Of nine patients who developed infections after ICU admission, eight were colonized before infection. The rectum was the first site of colonization in 92% of patients, and positive rectal cultures preceded 89% of infections acquired in the ICU. This was supported by strain delineations using pulsed-field gel electrophoresis. Twice-weekly rectal surveillance alone identified 93% of the maximal estimated VRE-related patient-days; weekly or admission-only surveillance was less effective. As a test for future VRE infection, rectal surveillance culture twice weekly had a negative predictive value of 99%, a positive predictive value of 44%, and a relative risk for infection of 34.
Conclusions
Twice-weekly rectal VRE surveillance of critically ill patients is an effective strategy for early identification of colonized patients at increased risk for VRE transmission, infection, and death.
First identified in 1986, vancomycin-resistant enterococcus (VRE) has rapidly become one of the leading causes of nosocomial infection in U.S. hospitals. 1–4 A recent 12-month survey of 49 hospitals in the United States identified 419 enterococcal bacteremias and reported that 17% were caused by vancomycin-resistant strains. 5 The prevalence of VRE colonization varies widely among hospitals. The highest rates, up to 53%, have been reported in the large teaching hospitals in the northeastern states. 6–8 Once colonized, a person has a 5- to 10-fold increased risk of developing infection. 9 These infections lead to an increased death rate and higher costs. 9,10
Targeted infection control interventions aimed at reducing VRE colonization depend largely on identifying colonized patients to allow isolation and cohorting. 11–13 VRE surveillance strategies have not been tested to determine the optimal frequency or anatomical sites used for VRE surveillance cultures. Accordingly, we sought to identify an efficient strategy for VRE surveillance that would minimize the time to identification of colonization, and therefore the time to effective patient isolation and cohorting, and provide an early warning of possible future VRE infection.
METHODS
Design
We conducted a prospective VRE surveillance study in the 16-bed surgical intensive care unit (SICU) and the 12-bed medical intensive care unit of the Johns Hopkins Hospital. The hospital is a 1,000-bed, tertiary care hospital that serves the greater Baltimore area and has a large national and international referral population.
During a 3-month period, June through August 1996, we prospectively enrolled all patients admitted to the SICU and MICU whose expected length of stay on one of these units was at least 3 days based on the diagnosis on admission to the study unit. Subjects were eligible for analysis if they had a VRE culture performed and met the 3-day length of unit stay criterion or died before unit discharge (regardless of length of unit stay). The Institutional Review Board of the Johns Hopkins Medical Institutions approved this study.
Swabs or specimens for surveillance cultures were obtained from the oropharynx, rectum, urine, gastric aspirate, and endo- or nasotracheal aspirates of all eligible patients on admission to and discharge from the unit and twice weekly (Mondays and Thursdays) while on the unit. Age, sex, hospital ward or unit, severity of illness (Acute Physiology and Chronic Health Evaluation [APACHE] II score), recent medical history, and details of the unit admission were recorded on enrollment into the study. Throughout the unit stay, vital signs, clinical events, antimicrobial medications administered, and microbiologic and other laboratory data were collected in standardized case report forms.
An events committee of at least three physician investigators, trained in either infectious diseases or critical care medicine, determined whether patients met the following endpoint definitions.
Colonization was defined as VRE isolated from any surveillance or clinical culture. Urinary colonization was defined as 100,000 or fewer colony-forming units (cfu) in a clean-catch sample or 10,000 cfu or fewer in a catheterized sample. Incident VRE colonization was preceded by a negative culture for VRE at the same surveillance site. Colonization was prevalent if the first culture at a given site grew VRE.
Urinary tract infections were defined by the presence of higher colony counts than colonization (>100,000 cfu [clean catch] or >10,000 cfu [catheter]) and clinical signs or symptoms of infection. Bacteremia was defined as VRE cultured in blood or on an intravascular catheter tip with 15 cfu or more. Wound infections (after primary closure) and abscesses were defined by a positive culture of VRE in the presence of purulent drainage. We defined a VRE infection as incident if the infection was not present at the time of unit admission but developed after admission to the unit. Infections that were clinically apparent at the time of unit admission were designated as prevalent infections.
Microbiology
Specimens for VRE surveillance culture were transported to the microbiology laboratory within 1 hour of sample collection and plated on trypticase soy agar (BBL, Cockeysville, MD) supplemented with 20 μg/mL vancomycin to select for vancomycin-resistant strains of enterococci. After overnight incubation at 37°C, organisms having a colonial morphology consistent with Enterococcus species were further speciated. 14 We chose several colonies with similar colonial morphology for antibiotic susceptibility testing, which was performed by agar dilution in Mueller-Hinton II Agar (BBL). Susceptibility testing and interpretation of minimum inhibitory concentrations (MIC) were performed according to National Committee for Clinical Laboratory Standards guidelines. 15,16 The breakpoint for vancomycin resistance was 16 μg/mL. VRE species from isolates shown to be from a pure, not mixed, culture of VRE were saved at −70°C in trypticase soy broth with 5% glycerol. Presumptive VRE identification was reported at 24 hours based on growth in the presence of 20 μg/mL, stain, catalase, and rapid L = pyroglutamic acid β = naphthylamide (PYR) test. Strain delineation was determined by pulsed-field gel electrophoresis of SmaI restriction digests of genomic DNA with a Biorad Genepath System (Biorad Laboratories, Hercules, CA) according to standard methods. 17 Electrophoresis was performed with a 1% agarose gel in 0.5× TBE (0.045 mol/L Tris-Borate, pH 8.3, 0.0012 mol/L ethylene diamine tetraacetic acid [EDTA]) for 24 hours at 14°C with a ramped pulse time from 5 to 30 seconds. A single colony was picked from a plate that had been subcultured from the −70°C frozen isolate. Specimens selected for analysis included the first VRE colonizing isolate and the first VRE infection defining pairs in VRE-infected patients. The criteria for band pattern interpretation established by Tenover et al 18 were applied to this analysis as follows: no band differences, identical; one to three band differences, epidemiologically related; and four to six band differences, not highly related but may be more distantly related.
Data Management and Analysis
Data were analyzed using SPSS (Version 7.5, Chicago, IL). Categorical variables were compared using chi-square and the Fisher exact tests. The Mann-Whitney test was used to compare nonparametric variables. Because patients from the SICU and MICU were not different in mean APACHE II scores, age, gender, or length of stay, all patients were combined as a single ICU for the analysis. The VRE colonization point prevalence (number of patients with VRE per number of patients cultured) was calculated for the study units on each of the 24 surveillance days throughout the 3-month study and reported as a median of these surveillance days.
We calculated VRE days to provide a quantitative method for comparison of our twice-weekly surveillance strategy with estimates of more and less frequent surveillance strategies. VRE days were defined as the number of days a patient remained in the study unit after VRE colonization. We described four categories of VRE patient-days, in order of surveillance culture frequency: maximum estimated, observed, estimates of less frequent surveillance methods, and no surveillance. The “maximum estimate of VRE days” was an estimate of the results of a daily surveillance culture strategy by assuming the first day of colonization was the midpoint between the last negative and first positive VRE culture. The “observed VRE days” was based on the unadjusted dates of VRE colonization identified with twice-weekly surveillance. Estimates of “less than twice-weekly” rectal surveillance were made by calculating VRE days after removing certain surveillance cultures from the database of twice-weekly cultures: admission, Monday, or Thursday surveillance cultures were removed, the first VRE colonization day was recalculated, and the VRE days was determined. A “no surveillance” strategy used the VRE infection date as the VRE identification date. The marginal increase in VRE days detected was defined as the difference between VRE days detected by any given surveillance strategy and no surveillance, divided by the difference between the maximum estimated VRE days and no surveillance strategy.
Sensitivity, specificity, and positive and negative predictive values were calculated for various combinations of surveillance site cultures. Only cultures that were actually taken were included in this analysis. Cultures that were missed or not possible (because of the absence of nasogastric or endo- or nasotracheal tubes) were not included in these site-specific analyses. In these calculations, VRE infection at any time while in the study unit was the clinical condition against which the diagnostic test, the strategy of twice-weekly VRE surveillance cultures throughout unit admission, was evaluated. A positive test was defined as a VRE-positive culture at a specified surveillance site at any time during the ICU stay; if VRE infection occurred, the VRE-positive culture must have preceded VRE infection by at least 1 day, consistent with our microbiologic procedures. A negative test was defined in two different situations. In the first, a negative test was defined when all surveillance cultures throughout the unit stay were negative for VRE. If a positive VRE culture followed a diagnosis of VRE infection, we considered that test negative because a positive surveillance culture after infection has no predictive value for infection. A second scenario was chosen to provide a more clinically relevant, 1-week predictive value of the surveillance culture strategy. In this second analysis, a negative test was defined by each individual surveillance culture negative for VRE rather than the composite of all surveillance cultures throughout the unit admission. Each surveillance culture was then evaluated for its ability to predict VRE infection in the next week.
RESULTS
Enrollment Characteristics
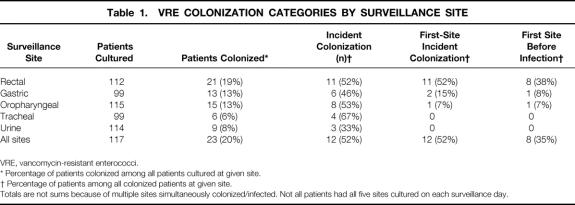
One hundred thirty-nine patients were enrolled in the study and 117 (84%) were eligible for analysis, 75 in the SICU and 42 in the MICU. The remaining 22 patients did not meet eligibility criteria because they were discharged from the unit in less than 3 days. Fourteen patients had multiple admissions to the units: nine patients had two admissions, four patients had three admissions, and one patient had four admissions. The median number of surveillance cultures for each patient was three (interquartile range [IQR], 2–5). Rectal, oropharyngeal, and urine surveillance cultures were collected as scheduled 96% to 98% of the time in the 117 eligible patients (Table 1). Because nasogastric and endo- or nasotracheal tubes were present based on clinical necessity, cultures of these sites were limited to 85% of the scheduled surveillance dates.
Table 1. VRE COLONIZATION CATEGORIES BY SURVEILLANCE SITE
VRE, vancomycin-resistant enterococci.
* Percentage of patients colonized among all patients cultured at given site.
† Percentage of patients among all colonized patients at given site.
Totals are not sums because of multiple sites simultaneously colonized/infected. Not all patients had all five sites cultured on each surveillance day.
Colonization
Colonization was identified in 23 of 117 (20%) patients (Fig. 1). The median VRE colonization point prevalence during the study period was 20% (IQR, 14–31%). Vancomycin-resistant Enterococcus faecium was the first organism cultured in all instances; vancomycin-resistant Enterococcus faecalis was also cultured in three patients. The prevalence rate of VRE colonization on admission to the study units was 9% (11/117). The prevalence was not statistically different when the patient’s previous location was considered (exact P = 1.0): admission from home (10%), transfer from another hospital (9%), and transfer from a nonstudy nursing unit in our hospital (12%).
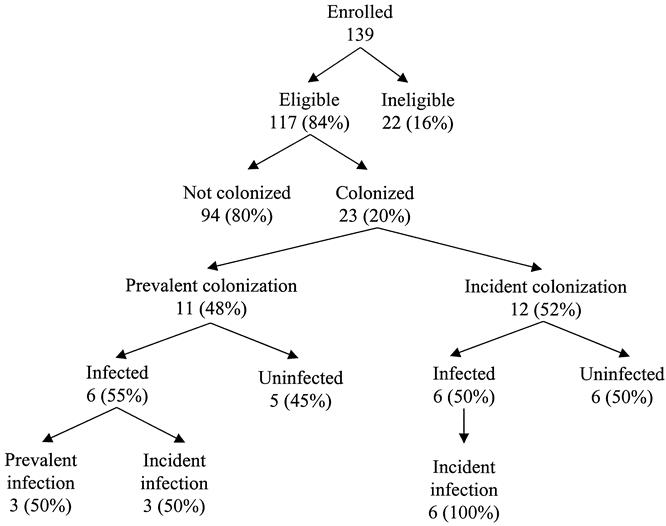
Figure 1. Schematic of vancomycin-resistant enterococci events. The denominator for the percentages is the number in the branch immediately above the percentage.
Twelve of 23 (52%) VRE-colonized patients became colonized with VRE after admission to the intensive care unit (see Table 1); in these, the time from study unit admission to colonization was 7.5 days (IQR, 4–12). The rectum was the most common positive surveillance site: it was positive in 21 of 23 (91%) VRE-colonized patients. Neither of the two patients without rectal colonization (one with gastric colonization and one with oropharyngeal and urine colonization) developed VRE infection. Among the 12 incident VRE colonizations, the rectum was the first positive surveillance site in 92% (11/12).
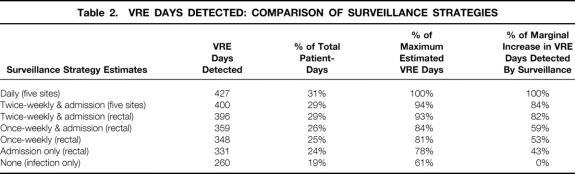
Twice-weekly VRE surveillance at all five sites identified 400 VRE days (Table 2). This represented 29% of the 1,382 patient days in the study. The sensitivity of twice-weekly rectal-only surveillance cultures was 99% (396/400) of the VRE days identified with all five sites and 93% of the maximum estimate of VRE days (427). When the no surveillance strategy was used, 61% of VRE days were identified by clinical cultures. The marginal increase (vs. no surveillance) of an admission plus twice-weekly rectal surveillance strategy represented 82% of the maximum estimated VRE days detectable. This decreased to 59% for an admission plus weekly (Monday or Thursday) rectal surveillance strategy.
Table 2. VRE DAYS DETECTED: COMPARISON OF SURVEILLANCE STRATEGIES
Infection
Twelve study patients developed VRE infections for a VRE infection prevalence rate of 10%. Nine of these were incident infections (75%), having occurred a median of 14 days after ICU admission (IQR, 9–15) and 8 days after VRE colonization (IQR, 5–9). E. faecium was the species cultured in all cases of VRE infection. Seven of the 12 (58%) VRE-infected patients had multiple sites of VRE infection (two to five). Bacteremia was the most common infection, present in eight (67%) of the VRE-infected patients. Most bacteremias (75%), however, followed infection at other sites, most commonly urine (3/8) and wound (3/8) infections, which were the first sites infected in 42% and 33% of VRE-infected patients, respectively. The death rate of patients colonized by VRE was 45.7%, although only 22.2% of patients infected by VRE were assessed as having death primarily attributable to VRE infection.
Colonization Predicting Infection
Incident VRE infection followed colonization in 9 of 20 colonized patients, for an attack rate of 45% (see Table 1). (The three prevalent VRE infections were excluded because there were no colonization data before those infections.) The rectum was colonized before infection in 89% (8/9) of the incident VRE infections; in the single remaining infection, infection and rectal colonization occurred on the same day. Eight of nine first rectal isolate/first infection isolate pairs were genetically identical by pulsed-field gel electrophoresis analysis having all bands in common (Fig. 2). In the single exception, the rectal isolate was not closely related to the abdominal infection isolate: the pair differed by more than three bands. The median time from rectal colonization to infection was 8.5 days (range 2–13, n = 8). All but the gastric site (median 9 days; n = 2) had shorter times to infection after colonization.
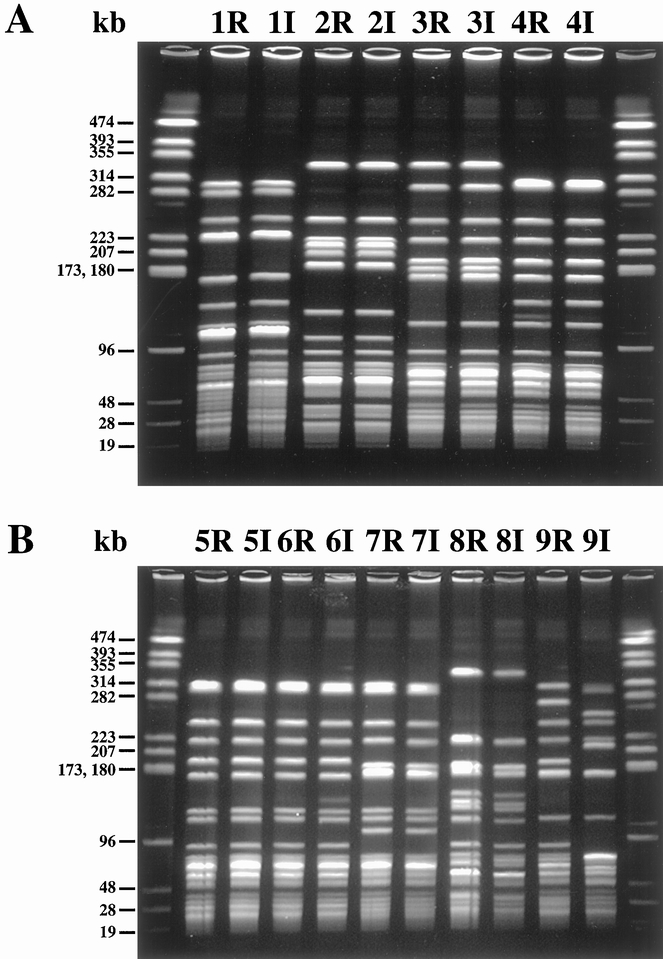
Figure 2. Pulsed-field gel electrophoresis (PFGE) of colonization/infection pairs. PFGE of SmaI DNA digests of paired vancomycin-resistant enterococci isolates from nine patients with infection acquired in the intensive care unit. First colonizing rectal isolates are designated 1R to 9R. The corresponding first-infected site isolate from the same patient is designated 1I to 9I. End lanes contain NotI-digested Enterococcus faecalis OGIRF molecular weight marker. Band sizes are shown to the left in kilobase (kb) pairs. Patients 1 to 4 are in panel A, patients 5 to 9 in panel B.
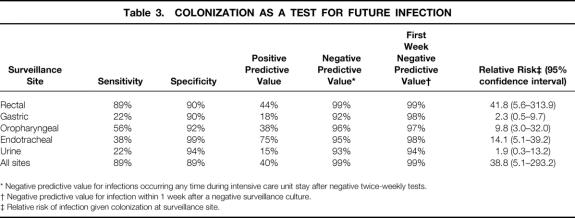
To evaluate the twice-weekly VRE surveillance strategy as an indicator for future VRE infection, positive and negative predictive values were determined (Table 3). If only the rectal site were considered, the negative predictive value was 99% and the positive predictive value was 44%. A patient was 42 times more likely to develop a VRE infection once VRE was isolated on a rectal surveillance swab (relative risk 41.8, 95% confidence interval, 5.6–313.9). A negative test in this scenario required that all surveillance cultures throughout the ICU stay be considered together, which is not possible until the end of the unit stay. Accordingly, to determine a more clinically useful statistic, we assessed the value of the rectal surveillance culture as a test to predict VRE infection in the next week.
Table 3. COLONIZATION AS A TEST FOR FUTURE INFECTION
* Negative predictive value for infections occurring any time during intensive care unit stay after negative twice-weekly tests.
† Negative predictive value for infection within 1 week after a negative surveillance culture.
‡ Relative risk of infection given colonization at surveillance site.
Using this time-limited criterion, the negative predictive value remained high, between 98% and 99%, for each surveillance date during the ICU stay (data shown for the initial surveillance culture in Table 3). All other single-site strategies, when compared with the rectal site, were far less sensitive, although the specificity was similar. The use of multiple surveillance sites did not improve either the negative or the positive predictive value when compared with rectal surveillance alone.
To assess whether less frequent rectal surveillance of ICU patients predicted VRE infection, the sensitivity, specificity, and positive and negative predictive values were recalculated using less frequent intervals. Using day of admission plus once a week (Monday or Thursday; results were the same) samples thereafter, these values decreased slightly (sensitivity 78%, specificity 90%, positive predictive value 41%, negative predictive value 98%) compared with twice-weekly sampling (see Table 3). The median time from colonization to infection decreased slightly (8.5 vs. 6 days) with weekly sampling. In addition, dropping the admission culture would have had no effect on positive or negative predictive values but would have further decreased the time from colonization to infection. The negative predictive value for infection in the week after any surveillance culture, using admission and weekly surveillance, remained between 98% and 99% for every week of the study. When sampling was decreased in frequency to every 2 weeks after an admission culture, the value of the test declined further (sensitivity 56%, specificity 90%, positive predictive value 33%, and negative predictive value 96%). The negative predictive value for infection in a 2-week interval after a surveillance culture dropped slightly to 98% for the first 2-week interval after admission but declined to 88% in the second 2-week period.
Cost Estimates
The cost of a single VRE surveillance culture in our study was estimated to be $22.50 based on the cost of the culture itself on selective media, culture swabs, and nursing time. An admission plus twice-weekly rectal-only VRE surveillance strategy, therefore, would cost $67.50 for the median of three cultures we observed in our patients (who had a week-long median length of stay). In a multivariate analysis of VRE infection costs, which controlled for APACHE scores and other clinical covariates, we estimated the incremental cost of a VRE infection to be $22,880. 19
DISCUSSION
We found that twice-weekly rectal surveillance was slightly superior to less frequent surveillance strategies in the identification of VRE colonization and the prediction of VRE infection. Using additional surveillance sites did not offer additional benefit in either category, despite considerably more effort and expense.
We discovered that 20% of high-risk patients were colonized with VRE in our institution. This represented 29% of patient-days in this study. The twice-weekly rectal surveillance strategy identified 93% of the maximum estimate of VRE days, far more than the 61% identified by no surveillance strategy. The rectal-only strategy missed only 4 VRE days compared with surveillance of all five sites. The 4-day difference represents a minimal decrease in VRE identification but would halve the microbiology costs compared with surveillance plans that culture only two sites.
The marginal benefit of VRE days identified by active surveillance varied greatly with surveillance frequency. The twice-weekly plus admission strategy identified 82% (136/167) of the VRE days identified by surveillance. This rate dropped significantly to 59% (99/167) with weekly plus admission surveillance. This less frequent surveillance reduced surveillance costs an estimated 33% (dropping one of three [median] cultures our patients received with twice-weekly surveillance). An admission-only or weekly-only (without admission) surveillance strategy, compared with twice weekly, would have reduced VRE days detected by 39% or 29%, respectively, with a cost reduction of 67%.
To determine the acceptability of later identification of VRE colonization associated with an admission plus weekly rectal surveillance strategy compared with a more frequent twice-weekly surveillance strategy, one must consider both the risk of person-to-person VRE colonization avoided by earlier identification and isolation of VRE-colonized patients and the future risk for VRE infection. This study did not assess the risk of person-to-person colonization, but the attack rate in our study was 45%. Our estimates may be applicable only to high-prevalence situations. The cost of a surveillance–identification–isolation strategy relative to the cost of infections averted should be used to determine the threshold VRE prevalence for instituting routine surveillance.
Austin et al 12 estimated that infection control strategies reduce the basic reproductive rate of VRE by nearly 3 secondary colonizations per index colonization, from 3 to 4 without infection control measures to 0.7 with infection control measures. If one conservatively assumes that identification of VRE-colonized patients with subsequent effective infection control measures (improved handwashing, isolation, cohorting 11,13) accounts for only half of the roughly three VRE colonizations estimated to be the infection control impact 12 and that a twice-weekly rectal surveillance strategy accounts for one third of all VRE colonizations identified (as indicated by our data), then the addition of admission plus twice-weekly VRE surveillance compared with no surveillance could account for one sixth of the infection control impact. This translates to one colonization prevented per two colonizations identified. Given our 45% attack rate, this represents one VRE infection potentially prevented per four colonizations identified. Because our colonization rate was 1 in 5 patients (20%), 20 patients would require surveillance to prevent one VRE infection. Even allowing for a severalfold error in the foregoing assumptions in this cost speculation, the marginal cost of surveillance for 20 patients ($1,350) compares favorably with the cost of a single VRE infection avoided ($28,220). 19
Rectal colonization preceded infection in nearly all incident VRE infections in this study (89%). Pulsed-field gel electrophoresis analysis proved that in eight of nine cases, the first rectal isolate was genotypically identical to the infection-defining isolate. This is consistent with a report by Beezhold et al, 20 who found that VRE colonization occurred in all patients with VRE bacteremia. Further, a positive rectal culture preceded infection more frequently and earlier than cultures from any other surveillance site, a median of 8.5 days before infection. This strategy of rectal VRE surveillance, therefore, could provide early identification of the patients at highest risk for VRE infection—namely, those with rectal VRE colonization, among whom nearly all future risk for VRE infection lies.
An effective VRE surveillance program could significantly improve the efficiency of presumptive VRE treatments that may evolve. Throughout our study, rectal VRE surveillance had a 98% to 99% negative predictive value for excluding a VRE infection in the next week. The twice-weekly surveillance strategy had a positive predictive value of 44%. Thus, results of twice-weekly rectal VRE surveillance might prove useful in the selection of patients for presumptive treatment strategies to avoid unnecessarily treating patients at almost no risk for future VRE infection. Decreasing the frequency of surveillance cultures to once a week only slightly diminished the positive and negative predictive values of the strategy compared with twice-weekly surveillance. Every-other-week surveillance was significantly less effective.
In conclusion, twice-weekly rectal surveillance for VRE is an effective method for identifying critically ill patients at both low risk for VRE infection (high negative predictive value, 98%) and significant relative risk for future infection (43, 95% confidence interval 6–324). A twice-weekly surveillance strategy identifies nearly all VRE colonization days (93%) and could optimize targeted infection control interventions that depend on identification of VRE colonization. Given our high attack rate of VRE infection after colonization (45%) and the high VRE-attributable death rate (37%) reported by others, rectal surveillance for VRE colonization identifies patients at a significantly increased risk of death associated with VRE. 21 The clinical value of early identification of VRE colonization remains to be proven in conjunction with other VRE prevention and control strategies.
Acknowledgments
The authors thank the many helpful personnel in the intensive care units, the Department of Hospital Epidemiology and Infection Control, and the microbiology laboratory, without whose contributions this work would not have been possible.
Footnotes
Supported in part by an unrestricted educational grant from Pfizer, Inc.
Correspondence: Craig W. Hendrix, MD, Division of Clinical Pharmacology, Harvey 502, 600 N. Wolfe St., Baltimore, MD 212987. E-mail: chendrix@jhmi.edu
Accepted for publication July 18, 2000.
References
- 1.Leclerq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med 1988; 319: 157–161. [DOI] [PubMed] [Google Scholar]
- 2.Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infections. Am J Med 1991; (suppl 3B):S72—75. [DOI] [PubMed]
- 3.Centers for Disease Control & Prevention. Nosocomial enterococci resistant to vancomycin, United States, 1989–1993. MMWR 1993; 42: 597–599. [PubMed] [Google Scholar]
- 4.Mainous MR, Lipsett PA, O’Brien M. Enterococcal bacteremia in the surgical intensive care unit. Does vancomycin resistance affect mortality? The Johns Hopkins SICU Study Group. Arch Surg 1997; 132: 76–81. [DOI] [PubMed] [Google Scholar]
- 5.Jones RN, Marshall SA, Pfaller MA, et al. Nosocomial enterococcal blood stream infections in the SCOPE Program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. Diagn Microbiol Infect Dis 1997; 29: 95–102. [DOI] [PubMed] [Google Scholar]
- 6.Quale J, Landman D, Atwood E, et al. Experience with a hospital-wide outbreak of vancomycin-resistant enterococci. Am J Infect Control 1996; 24: 372–379. [DOI] [PubMed] [Google Scholar]
- 7.Morris JG Jr, Shay DK, Hebden JN, et al. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med 1995; 123: 250–259. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein JW, Roe M, Towns M, et al. Resistant enterococci: a prospective study of prevalence, incidence, and factors associated with colonization in a university hospital. Infect Control Hosp Epidemiol 1996; 17: 36–41. [DOI] [PubMed] [Google Scholar]
- 9.Montecalvo MA, Shay DK, Gedris C, et al. A semiquantitative analysis of the fecal flora of patients with vancomycin-resistant enterococci: colonized patients pose an infection control risk. Clin Infect Dis 1997; 25: 929–930. [DOI] [PubMed] [Google Scholar]
- 10.Gillis G, Eng-Chong M, Henseleit S, et al. Financial impact of an outbreak of vancomycin-resistant enterococcus (VRE) colonization and infection in an inpatient/ambulatory dialysis program [abstract]. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, Sept. 28–Oct. 1, 1997;37.
- 11.Montecalvo MA, Jarvis WR, Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med 1999; 131: 269–272. [DOI] [PubMed] [Google Scholar]
- 12.Austin DJ, Bonten MJ, Weinstein RA, et al. Vancomycin-resistant enterococci in intensive-care hospital settings: transmission dynamics, persistence, and the impact of infection control programs. Proc Natl Acad Sci USA 1999; 96: 6908–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jochimsen EM, Fish L, Manning K, et al. Control of vancomycin-resistant enterococci at a community hospital: efficacy of patient and staff cohorting. Infect Control Hosp Epidemiol 1999; 20: 106–109. [DOI] [PubMed] [Google Scholar]
- 14.Facklam RR, Collins MD. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol 1989; 27: 731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Methods for Dilution Antimicrobial Susceptibility Tests for Bac-teria That Grow Aerobically: Approved Standard, 4th ed, vol 17(2). NCCLS publication no. M7-A4. Wayne, PA: National Committee for Clinical Laboratory Standards; 1997.
- 16.Performance Standards for Antimicrobial Susceptibility Testing; Ninth Informational Supplement: Approved Standard, 4th ed, vol 19(1). NCCLS publication no. M100-S9. Wayne, PA: National Committee for Clinical Laboratory Standards; 1997.
- 17.Miranda AG, Singh KV, Murray BE. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J Clin Microbiol 1991; 29: 2752–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995; 33: 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelz R, Hendrix C, Swoboda S, et al. Incremental costs associated with ICU enterococcal infection. 19th Annual Meeting of the Surgical Infection Society, Seattle, WA, May 1999; 59:12.
- 20.Beezhold DW, Slaughter S, Hayden MK, et al. Skin colonization with vancomycin-resistant enterococci among hospitalized patients with bacteremia. Clin Infect Dis 1997; 24: 704–706. [DOI] [PubMed] [Google Scholar]
- 21.Edmond MB, Ober JF, Weinbaum DL, et al. Vancomycin-resistant Enterococcusfaecium bacteremia: risk factors for infection. Clin Infect Dis 1995; 20: 1126–1133. [DOI] [PubMed] [Google Scholar]