Abstract
Objective
To determine whether thermal injury and sepsis cause an increase in bone marrow norepinephrine release and whether such a release influences bone marrow monocytopoiesis.
Summary Background Data
The authors previously demonstrated enhanced bone marrow monocytopoiesis after burn with sepsis. They also showed that physiologic stress and bacterial challenge without injury could lead to a dynamic release of norepinephrine from the bone marrow compartment. In this study, they sought to determine the potential cause-and-effect relationship of bone marrow norepinephrine release on increased monocytopoiesis after burn sepsis.
Methods
Norepinephrine release from bone marrow was determined by traditional pulse-chase methods. Tissue and bone marrow norepinephrine content was ablated by chemical sympathectomy with 6-hydroxydopamine treatment. Clonogenic potential in response to colony-stimulating factors was determined in total nucleated bone marrow cells. Dual color flow cytometry was used to document the distribution pattern of monocyte progenitors.
Results
Burn sepsis induced increased norepinephrine release in bone marrow, spleen, and heart. Colony-forming assays demonstrated an increase in responsive colonies, which was significantly attenuated when norepinephrine content was reduced in animals before burn sepsis. Flow cytometric analysis of early and late monocyte progenitors showed a significantly altered distribution profile of monocyte progenitors in norepinephrine-depleted mice compared with norepinephrine-intact mice. Abrogation of bone marrow norepinephrine content resulted in a 62% survival rate in burn septic mice compared with no survivors in norepinephrine-intact mice.
Conclusions
These data suggest that enhanced bone marrow norepinephrine release after burn sepsis may play a role in bone marrow monocytopoiesis, thus contributing to the sustenance of inflammation.
Profound release of sympathetic neurotransmitters and hormones through activation of the autonomic nervous system is the central feature of the traditional cognitive “fight or flight” stress response. 1–3 In addition to cognitive stress, clinical examples of traumatic injury and bacterial infection also provoke intense sympathetic responses that may have important consequences on the death and complication rates. 4–9 Sympathetic responses to thermal injury, bacterial infection, or a combined insult are likely to serve important compensatory functions in maintaining both cardiovascular and metabolic homeostasis. 10–14 The more important question regarding sympathetic activation in thermal injury involves its potential influence on immune function. Indeed, severe thermal injury results in increased susceptibility to infection, 15,16 and patients with severe burn trauma often display significant impairment in cell-mediated immunity involving defective neutrophil chemotaxis, phagocytosis, and superoxide production. 17–20 Patients with sepsis and systemic inflammatory response may also have monocytosis, 21,22 suggesting excessive cytokine production through increased circulating and tissue monocyte/macrophages. Because adrenergic mechanisms are reported to be involved in various host-defense functions, 23–25 we have begun to consider the possibility that sympathetic activation after thermal injury may in some way contribute to the immune components of the pathophysiology.
Investigations into mechanisms that could account for the immunosuppression of patients with severe burns have focused primarily on functional alterations in circulating and tissue leukocytes. 26–33 In contrast, our group has focused on the bone marrow responses that follow thermal injury with sepsis, because the bone marrow is a major source of immune cells both in the circulation and tissues. Using a murine model, we have demonstrated that severe thermal injury with sepsis results in a shift in myeloid commitment toward monocytopoiesis and away from granulocytopoiesis. 34,35 Several key factors suggest the possibility that the observed changes in myelopoiesis may be in part mediated by sympathetic nerve activation associated with thermal injury. First, adrenergic signaling has been shown by others to function in the regulation and control of hematopoiesis. 36,37 Maestroni and Conti 37 have shown the presence of adrenergic receptors on bone marrow immune cells and have also shown that adrenergic agonists stimulate lymphopoiesis while attenuating myelopoiesis. These findings are strengthened by work in different animal models showing that adrenergic agents can modulate lymphopoiesis and myelopoiesis. 38–41 A second important finding is the demonstration of sympathetic activation in the bone marrow compartment itself, where nerve-stimulated release of norepinephrine could reach high concentrations in close proximity to proliferating immune cells. We have recently reported the increase in bone marrow norepinephrine release in response to either cold exposure or bacteria 42 through the use of traditional pulse-chase experiments. More recently we have extended such measurements to our murine model of burn sepsis in preliminary experiments and showed increased bone marrow norepinephrine release in response to burn sepsis. 43
Taken together, these findings argue for a relation between sympathetic activation and alterations in the bone marrow production of immune cells that may lead to the development of opportunistic bacterial infection. Whereas experimental evidence suggests adrenergic regulation of myelopoiesis under normal conditions, evidence demonstrating such cause-and-effect relationships in injury states has never been examined. Therefore, we hypothesize that immune alterations induced by burn sepsis are mediated at least in part by sympathetic modulation of myelopoiesis in bone marrow consequent to the burn trauma.
We tested this premise of neural modulation of myeloid function in our murine model of thermal injury and infection by manipulating the peripheral stores of norepinephrine. Peripheral norepinephrine levels were reduced by using 6-hydroxydopamine (6-OHDA), and then animals were subjected to burn sepsis. Whereas bone marrow cells taken from mice subjected to burn sepsis and increased bone marrow norepinephrine release showed an increase in monocytopoietic potential, mice with reduced bone marrow norepinephrine stores did not. These results suggest that injury-induced sympathetic responses may have marked effects on bone marrow progenitor cells and may significantly alter leukocyte production after traumatic injury.
METHODS
Animals and Treatment Protocols
Adult male B6D2F1 mice (22–28 g, Jackson Laboratories, Bar Harbor, ME) were used in all experiments. Mice were housed in a central animal research facility that maintained an environment of controlled temperature, relative humidity, and a 12-hour light/dark cycle. The animals were maintained in the controlled environment for at least 1 week before being used in the experiments. All experimental protocols used in this study were approved by the Animal Care and Use Committee of Loyola University Medical Center.
Thermal injury was induced in mice essentially as described by Walker and Mason. 44 The animals were randomized into sham, burn, and burn sepsis groups and anesthetized with pentobarbital sodium (50 mg/kg), and the dorsal hair was removed with clippers. All mice were placed in a supine position in a Delrin template and burn and burn sepsis groups subjected to a 15% full-thickness dorsal scald by partial immersion into a 100°C water bath for 7 seconds. Higher-percentage burns resulted in unacceptable levels of death, severely limiting the usefulness of the model. Sham mice were immersed in room temperature water. All three groups were resuscitated with 2 mL intraperitoneal 0.9% NaCl. Mice in the burn sepsis group were inoculated with 1,000 colony-forming units (cfu) Pseudomonas aeruginosa (ATCC 19960, Rockville, MD) at the burn site immediately after the injury.
In a separate set of experiments, mice were treated with 100 mg/kg intraperitoneal 6-OHDA (Sigma Chemical Co., St. Louis, MO) in 0.5 mL saline containing 0.1% ascorbic acid as an antioxidant. Mice were divided into three groups and treated daily for 5 days consecutively and on day 7 were subjected to the thermal injury protocol described above. The extent of norepinephrine depletion was confirmed by determining norepinephrine levels in bone marrow, heart, and spleen, as described below.
NE and [3H] Norepinephrine Analysis
Hearts were homogenized in 1.0 mL cold 0.4 mol/L perchloric acid using a Polytron tissue homogenizer (Brinkman Instruments, Westbury, NY), and the supernatant was separated by centrifugation. The supernatant was then adjusted to pH 8.4 with 1 mol/L Tris buffer (pH 10) and mixed with activated acid-washed alumina. Alumina (J.T. Baker, Phillipsburg, NJ) had been purified according to the method of Anton and Sayer. 45 The alumina was washed with water, and norepinephrine was eluted with 0.2 mol/L acetic acid. Norepinephrine in the eluate was measured by electrochemical detection after high-pressure liquid chromatographic separation (BioAnalytical Systems, West Lafayette, IN). Recovery of norepinephrine from the alumina was routinely 85% efficient, and the samples were reported as uncorrected for recovery. Aliquots of the alumina eluates (0.1 mL) were mixed in 5.0 mL scintillation cocktail (Boi-Safe II; RPI, Mount Prospect, IL) and counted for [3H] in a scintillation counter (LS 6500, Beckman Instruments, Fullerton, CA). The specific activity of the [3H] norepinephrine (CPM/ng norepinephrine) was calculated as the quotient of [3H] norepinephrine in the tissue and the total tissue norepinephrine content.
Bone Marrow Cells
To assess bone marrow cell function, mice were killed at 72 hours after thermal injury. Total bone marrow from each femur pair was collected aseptically by eluting the medullary cavity with 3 mL Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal calf serum (FCS), penicillin (100 U/mL), and streptomycin (100 μg/mL) using a 1-mL syringe and a 25-gauge needle. An aliquot of the cell suspension was diluted in 3% acetic acid to lyse red blood cells, and the total nucleated cells were quantitated using a Neubauer hemocytometer.
Flow Cytometric Analysis
Murine bone marrow cells were labeled with fluorescent anti-ER-MP12 and anti-ER-MP20 antibodies 3 days after the initial injury and analyzed by dual color flow cytometry. After blocking the Fc receptors with a rat antimouse CD16/CD32 (FcγIII/II) antibody (1 μg/106 cells) (Pharmingen, San Diego, CA) for 5 minutes at 4°C, bone marrow cells (1 × 106 cells) were labeled with fluorescein isothiocyanate (FITC)-conjugated ER-MP20 (2 μL) and biotin-conjugated ER-MP12 (4 μL) (Accurate Chemical and Scientific Corp., Westbury, NY) for 30 minutes at 4°C. The cells were then washed three times with normal phosphate-buffered saline, resuspended in 100 μL phosphate-buffered saline with 0.1% bovine serum albumin (BSA), and incubated with streptavidin PE (5 μL) (Vector Laboratories, Burlingame, CA) for 30 minutes at 4°C. At the end of this time, the cells were washed three times with phosphate-buffered saline and then fixed with 1% paraformaldehyde before cytometric analysis.
Soft-Agar Clonogenic Assay
The clonogenic potential of the bone marrow cells was determined as previously described. 46 Total bone marrow cells (75,000 cells/well) were cultured in 1 mL McCoy’s medium containing 20% FCS, 0.3% Bacto agar (Difco Laboratories, Detroit, MI), penicillin (100 units/mL), and streptomycin (100 μg/mL). The cultures were stimulated with appropriate concentrations of either murine recombinant macrophage-colony stimulating factor (rM-CSF) or murine recombinant granulocyte macrophage-colony stimulating factor (rGM-CSF). The control cultures were incubated in the absence of any colony stimulating factor (CSF). All culture dishes in triplicate were incubated for 7 days at 37°C in a 10% CO2 atmosphere. At the end of this incubation time, colonies with greater than 50 cells were counted under a light microscope. Total number of cfu/femur was calculated from the total nucleated cell counts/femur and the number of colonies developing in culture. Statistical comparisons were made between norepinephrine-intact and norepinephrine-depleted preparations for sham, burn, and burn sepsis treatments using a group t test.
Norepinephrine Turnover Data Analysis
To determine norepinephrine turnover rates, specific activity of tissue norepinephrine after radiolabeled injection is plotted as a function of time on a semilogarithmic scale. The rate of decay of specific activity is a first-order function and is defined as a straight line with a negative slope, and decay lines are calculated by the method of least squares. 47,48 The rate constant represents the fraction of the norepinephrine pool replaced per unit time (h-1). Rate constants (k) are calculated from the slope of the logarithm of the specific activity versus time relationship (0.434 [k]=slope). Data are expressed as mean ± standard error of the mean.
Comparisons of the endogenous norepinephrine tissue content involved an independent analysis of variance. 49 For norepinephrine turnover experiments, differences between the slopes of the regression lines were tested with a Student t test, using the pooled standard error of sample regression. 49P < .05 was accepted as achieving statistical significance.
RESULTS
Sympathetic Activation
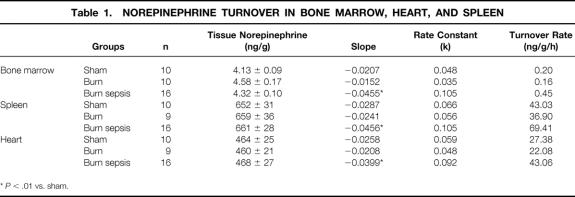
After burn sepsis, there was a significant increase in the norepinephrine release rate from bone marrow, spleen, and heart, but this did not occur in the burn or sham groups. Figure 1 displays the changing [3H] norepinephrine specific activity of bone marrow, spleen, and heart over a 7-hour period 3 days after burn sepsis compared with sham treatment. In all cases there was a statistically greater slope in the burn sepsis group, defining a greater rate of decay of labeled norepinephrine, presumably caused by greater efferent sympathetic nerve activity. 47,48 The slope of the [3H] norepinephrine decay line for all three tissues in the burn-only group, however, was similar to that of the sham group (Table 1). Because specific activity is a ratio of labeled norepinephrine to total norepinephrine pool, it is important to point out that tissue norepinephrine levels in all experiments did not change during the duration of the experiment (data not shown). Regression analysis of changes in the log of specific activity versus time resulted in a calculated straight line with a negative slope (R value ≥.7), which was treated as a first-order decay as previously defined 48 for calculation of rate constant and turnover rate.
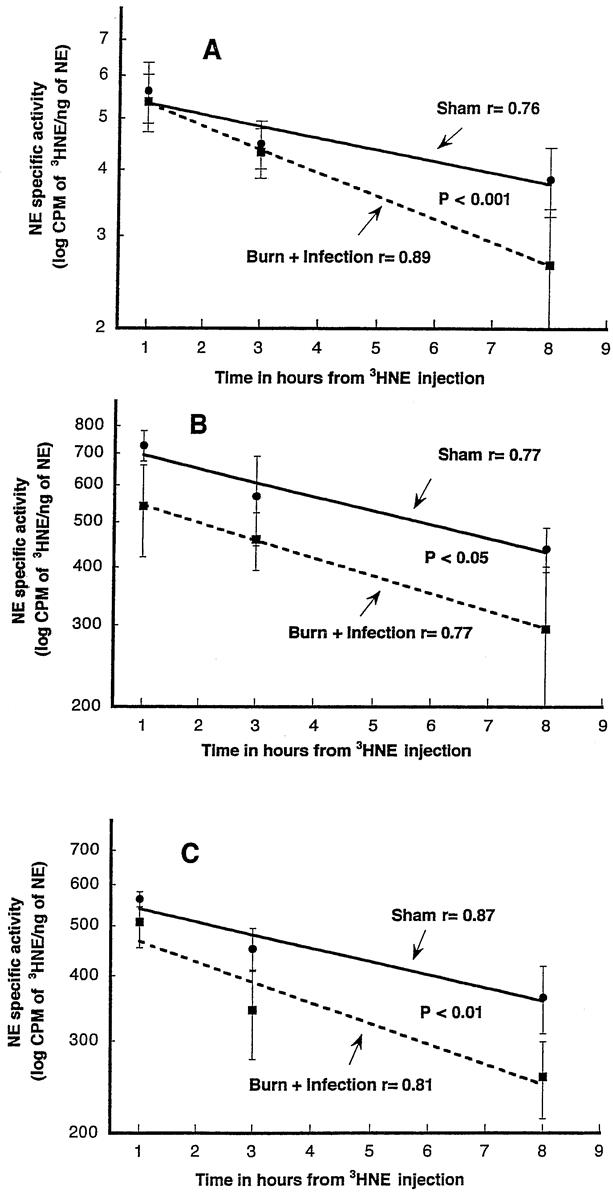
Figure 1. Effect of burn sepsis on the decay of bone marrow (A), spleen (B), and heart (C) [3H] norepinephrine (NE) specific activity. Mice were subjected to the burn sepsis protocol and 72 hours later were given 25 μCi [3H]NE intraperitoneally. One, 3, and 8 hours after radiotracer administration, tissues were taken for analysis. Each point represents the mean ± standard error for specific activity of organs from three to six mice. R values are the calculated least square regression coefficients using raw data points for each line. The slope of the burn sepsis decay line is significantly greater than for the sham group for bone marrow, spleen, and heart.
Table 1. NOREPINEPHRINE TURNOVER IN BONE MARROW, HEART, AND SPLEEN
*P < .01 vs. sham.
Additional information derived from the experimentally determined slopes and tissue level measurements is given in Table 1. Burn sepsis but not burn-alone or sham treatment resulted in increased norepinephrine turnover rates 3 days after injury. This occurred in bone marrow (2-fold increase) as well as heart and spleen tissues (1.6-fold increase for both). In addition, burn sepsis but not burn alone or sham treatment produced increased norepinephrine turnover at 24 hours after the same experimental paradigm (data not shown). Taken together, these results suggest that after burn sepsis there was continuous sympathetic activation with significant increases in norepinephrine release in bone marrow, spleen, and heart tissues.
Effects of Norepinephrine Reduction With 6-OHDA
Preliminary studies based on the work of others 50–53 suggested that a dose of 100 mg/kg was maximally effective in reducing tissue levels of norepinephrine in bone marrow, spleen, and heart. Tissue norepinephrine levels were determined during the same time course as that of the burn sepsis protocol to ensure that the 6-OHDA protocol was effective in maintaining reduced norepinephrine levels. Bone marrow norepinephrine was reduced by approximately 80%; heart and spleen were reduced by 92% to 93%. These reductions were maintained for the same duration as that of the experiment after burn (Table 2).
Table 2. TIME COURSE OF TISSUE NE LEVEL IN MICE GIVEN 6-OHDA
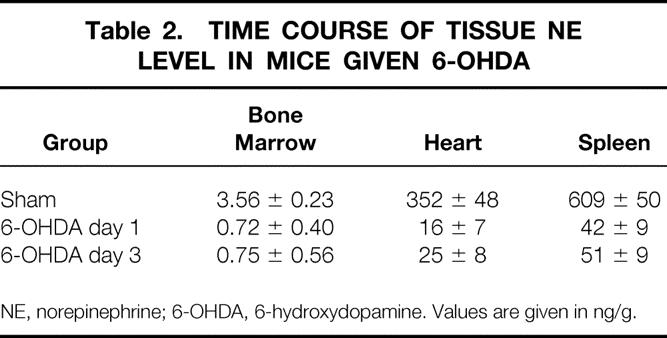
NE, norepinephrine; 6-OHDA, 6-hydroxydopamine. Values are given in ng/g.
Animals with reduced tissue norepinephrine levels as a result of 6-OHDA treatment were subjected to burn sepsis and then followed up for 12 days and compared with control animals given vehicle and subjected to the same protocol. Results (Fig. 2) show a death rate of 18% mortality at day 3 in norepinephrine-depleted mice compared with 44% in vehicle-treated mice. More dramatic, however, are the 10 of 16 total mice treated with 6-OHDA that survived burn sepsis after 7 days. The burn alone did not result in any deaths (data not shown). Norepinephrine-depleted mice surviving burn sepsis were monitored for 5 days beyond the time when all the vehicle-treated mice had died. This 62% survival rate with norepinephrine depletion, compared with no survivors beyond day 7 in the nontreated group, suggests that norepinephrine reductions in some way facilitate a positive outcome to burn sepsis.
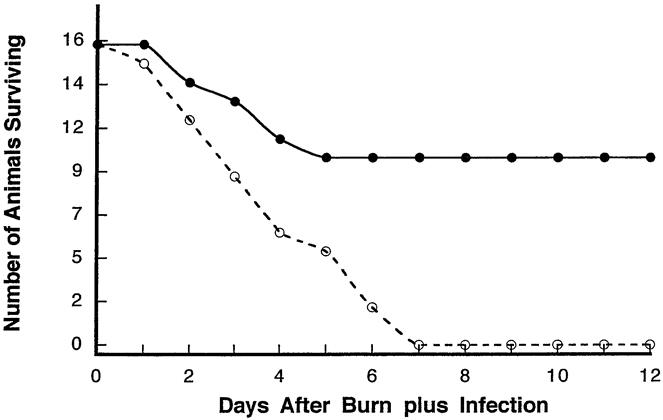
Figure 2. Effect of peripheral tissue norepinephrine depletion with 6-hydroxydopmaine compared with norepinephrine-intact mice on survival after burn sepsis (n = 16 per group).
Clonogenic Assay
To determine the effect of norepinephrine on bone marrow myeloid response after burn sepsis, the ability of bone marrow progenitors to form colonies in response to M-CSF and GM-CSF was tested in norepinephrine-depleted mice. Bone marrow cells were incubated in soft agar media with M-CSF or GM-CSF, and after 7 days the number of colonies (50 cells) that developed were counted. Colony formation response to M-CSF in bone marrow cells taken from sham injury (control) animals pretreated with 6-OHDA was greater (1,572 ± 121 colonies/femur) than in vehicle-treated animals (730 ± 71 colonies/femur) (Fig. 3). This was also the finding in response to GM-CSF, where 6-OHDA pretreatment followed by sham injury resulted in greater colonies (6,318 ± 627 colonies/femur) than vehicle treatment (4,040 ± 373 colonies/femur). Under these noninjury conditions, norepinephrine appears to inhibit basal myeloid colony-forming potential, as previously reported by Maestroni et al. 37,40,41 Colony formation in bone marrow cells removed from femurs after burn sepsis was significantly greater than in noninjured controls, demonstrating that injury with sepsis results in greater colony growth in vitro. However, pretreatment with 6-OHDA significantly reduced the colony formation responses to both M-CSF and GM-CSF compared with vehicle treatment in burn sepsis. For example, after burn sepsis in mice treated with 6-OHDA, there were 4,233 ± 365 colonies/femur formed in response to M-CSF and 8,514 ± 635 colonies/femur formed from cells taken from vehicle-treated mice. This same pattern of decreased colony formation with 6-OHDA pretreatment in burn sepsis was also seen with GM-CSF stimulation of bone marrow cells.
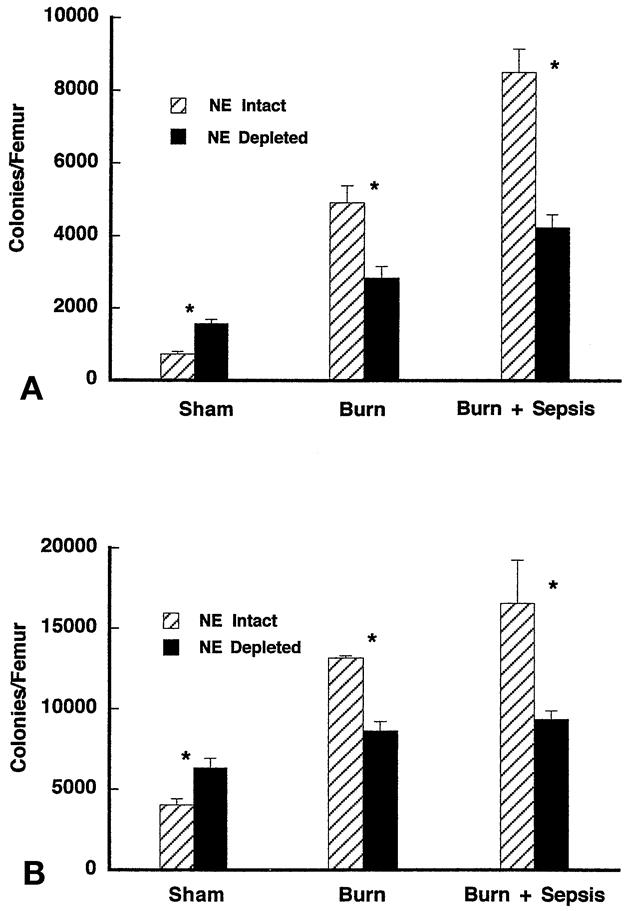
Figure 3. Soft-agar clonogenic assay for determination of specific growth factor-responsive bone marrow progenitors 72 hours after sham, burn, and burn sepsis in norepinephrine (NE)-intact and NE-depleted mice. Bone marrow cells (7.5 × 104 cells/mL) were incubated in RPMI media containing 0.3% agar in the presence of either (A) rM-CSF (10 ng/mL) or (B) rGM-CSF (10 ng/mL). Colonies (>50 cells) were counted with a light microscope after 7 to 10 days of incubation. Total nucleated bone marrow cell counts were used to determine the number of growth factor-responsive progenitors per femur. All assays were done in triplicate. Data represent mean ± standard error of the mean; n = 5;P < .05 for comparison of bars in each pair.
Colony formation in cells taken from bone marrow of animals subjected to burn alone were in general less than that in cultures from burn sepsis but more than in uninjured controls. Further, norepinephrine depletion with 6-OHDA significantly reduced colony formation in response to M-CSF (2,850 ± 315 colonies/femur) compared with vehicle treatment (4,921 ± 468 colonies/femur) in cells from mice subjected to burn alone. Similar results involving norepinephrine depletion were also observed for colony formation responses to GM-CSF.
Changes in clonogenic potential rely on a constant number of total bone marrow cells. This is consistent with our observation that there were no differences in total bone marrow cellularity between the animals treated with 6-OHDA and vehicle for any experimental group (data not shown). Therefore, these results suggest that norepinephrine enhances the response of bone marrow progenitors to develop as monocytes after injury both with and without sepsis; this is in contrast to norepinephrine’s action in noninjured conditions, where it appears to inhibit monocyte colony formation.
Flow Cytometry
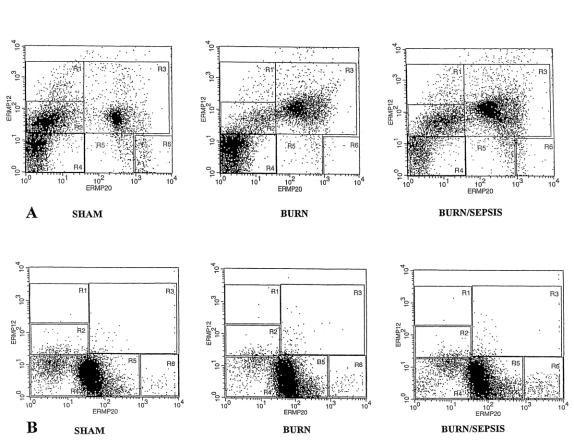
The influence of norepinephrine on bone marrow monocyte production was also assessed after burn with and without sepsis by determining the presence of developmental cell surface markers ER-MP12 and 20 using flow cytometry. Although ER-MP12 is an antibody to early monocyte progenitors and represents predominantly colony forming unit-macrophage (CFU-M), progressively more ER-MP20 antigen is expressed from the CFU-M stage onward, but it disappears after the monocytic stage. 54 By following the distribution pattern of the expression of these two antigens on the bone marrow cells, the phenotypic separation and identification of bone marrow monocyte precursors have been demonstrated. 55 The histogram of the flow cytometric data (Fig. 4) provides a visual separation of monocytic lineage into five distinct developmental compartments, in order of increasing maturity: R1 = ER-MP12High/20Negative; R2 = ER-MP12Medium/20Negative; R3 = ER-MP12Medium/20Medium; R4 = nonstain; R5 = ER-MP12Negative/20Medium; R6 = ER-MP12Negative/20High. Thus, compartment R2 of the histogram contains the most immature monocyte progenitors that primarily stain with ER-MP12 antibodies, and compartment R3 contains the intermediate developmental stage of monocyte progenitors, representing cells that stain with both ER-MP12 and 20. Cells in compartments 5 and 6 stain primarily for ER-MP20 and represent the most mature progenitors.
Figure 4. Dual color flow cytometric analysis for the expression of ER-MP12 and 20 antigens on whole nucleated bone marrow cells after sham, burn, and burn sepsis 72 hours after injury in norepinephrine-intact animals (A) versus norepinephrine-depleted animals (B). High expression of ER-MP12 represents blastic forms and early committed monocyte progenitors; high expression of ER-MP20 represents more mature promonocytes and monocytes. X and Y axes represent fluorescent intensity in log scale.
Figure 4 A displays the results of animals with intact norepinephrine stores and suggests that under control (sham) conditions, there was a substantial population of the ER-MP12Medium/20Negative monocyte progenitors (R2 compartment, 31.31% of total fluorescent cells [TF]). Immature monocyte progenitors (R2) decreased with both burn (9.76% TF) and burn sepsis (21.58% TF). The intermediate-stage compartment (R3) had a substantial population in the sham group (21.9% TF); however, the intermediate cells increased with burn (31.4% TF) and even more so with burn sepsis (51.02% TF).
Results with norepinephrine-depleted animals subjected to the same protocols are displayed in Figure 4 B and present an entirely different distribution pattern of ER-MP12- and 20-stained cells. The norepinephrine-depleted sham group had very few cells in the immature (R2, 2.02% TF) and intermediate (R3, 0.68% TF) compartment, with most cells staining for the ER-MP20 marker lineage (R5 + R6 = 60.38% TF), indicating a dramatic increase in the mature monocyte progenitor cells. Burn in norepinephrine-depleted animals resulted in further increases in mature monocyte progenitors (R5 + R6 = 81.61% TF), similar to that for burn sepsis in norepinephrine-depleted mice (R5 + R6 = 78.51% TF). The dramatic differences observed with norepinephrine depletion for ER-MP12 and 20 marker distribution under control conditions and in response to burn both with and without sepsis suggest that monocyte maturation pathways may be greatly influenced by the presence of norepinephrine.
DISCUSSION
Our results provide the first evidence of a modulation of bone marrow myelopoiesis by sympathetic activation after burn sepsis. After burn sepsis, norepinephrine release in bone marrow was markedly increased and the monocytopoietic potential of bone marrow cells was greatly elevated. Reduction of tissue norepinephrine stores before burn significantly attenuated the elevated monocytopoietic potential of bone marrow cells and dramatically increased the survival rate of injured septic animals from 0% to 60%. Such reductions in monocytopoietic potential and improved survival are consistent with the widely accepted concept that the increased availability and activation of monocyte/macrophage after burn sepsis promote systemic inflammatory responses, sepsis, and multiple organ failure. Linking the increases in bone marrow norepinephrine release and bone marrow monocytopoiesis defines a previously unknown role for norepinephrine in the pathophysiology of injury and sepsis. Recognition of the relation between sympathetic nerves and the regulation of myelopoietic function under injury conditions is the most significant aspect of our study and sheds new light on the importance of sympathetic activation in bone marrow myelopoiesis.
The pathophysiologic role of autonomic sympathetic nerves is often approached by using pharmacologic agents to reduce neurotransmitter levels and then repeating the paradigm to examine the absence of the nerves on the physiologic variables. 6-OHDA was used to deplete peripheral norepinephrine selectively in this study as well as in many other studies because it does not cross the blood–brain barrier. 56 This agent acts by selectively entering and destroying norepinephrine-containing nerve terminals in adult animals at low doses (100 mg/kg) 57–59; at higher doses it is more generally toxic. 60 Norepinephrine concentrations in various tissues of mice treated with 100 mg/kg, including lymphoid organs, decrease to less that 10% of controls, and these findings are consistent with our results. 61,62 Because only nerve terminals are destroyed, with cell soma remaining intact, tissue norepinephrine content returns in weeks with regrowth of the nerve terminals and vesicles containing norepinephrine. Our protocol caused a near-total reduction in norepinephrine as measured in heart and spleen (93–94%) but less of a reduction in bone marrow (80%). In neither instance, however, was there a significant return of norepinephrine levels during any part of our experimental protocol. The limited total blood flow to the relatively small bone marrow compartment, as well as the isolated nature of the compartment, may be significant in the 20% of control norepinephrine levels being insensitive to 6-OHDA. Preliminary studies using higher doses did not result in any further reduction in bone marrow norepinephrine levels and in fact caused death in several animals. Considering the significant effect of the 80% reduction on the myelopoietic potential, further norepinephrine reductions would probably not have changed the outcome.
The potential for 6-OHDA to have nonspecific effects has been addressed by several independent studies, but the results in each instance show the absence of any effects except those on the peripheral adrenergic nerve terminal. 51–53,62–64 In brief, these reports examined the consequences of greatly increased 6-OHDA in the extracellular fluid by selectively blocking 6-OHDA uptake into peripheral adrenergic nerve terminals using desipramine, a selective blocker of norepinephrine and thus 6-OHDA uptake. These in vivo studies exaggerated extracellular exposure to 6-OHDA and thus would be expected to exaggerate any nonspecific effects that might occur in the absence of uptake blockers. These studies reported a variety of functional variables in tissues from 6-OHDA animals pretreated with desipramine that were indistinguishable from those of control animals not treated with 6-OHDA. The results of these studies confirmed that 6-OHDA has a selective depleting effect on adrenergic nerve terminals and suggested to us that the results are due to the singular effect of the absence of norepinephrine in bone marrow. However, considering the global effect of peripheral norepinephrine depletion by 6-OHDA, one cannot rule out other effects of norepinephrine depletion that also may contribute to the observed positive outcome. Such effects may include an alteration in systemic immune response and rheology.
Adrenergic stimulation of myelopoiesis was previously reported in a series of papers by Maestroni et al 37,38,41,42 suggesting an inhibitory influence of adrenergic stimulation on myelopoiesis, based on early observations of myelopoietic protection during radiation therapy. 39,40 Collectively, this work implies that under normal noninjury conditions, myelopoiesis is inhibited by alpha-adrenergic stimulation; this is consistent with the findings of the present report as seen with sham (control) group results. Under these noninjured conditions, the clonogenic response to M-CSF and GM-CSF is increased when bone marrow norepinephrine is withdrawn through the 80% reduction in tissue level. This control state of adrenergic inhibition of myelopoiesis appears to be reversed as a consequence of thermal injury in that norepinephrine reduction with thermal injury is associated with decreased clonogenic responses (see Fig. 3).
Fluorescence-activated cell sorter (FACS) analysis indicated markedly different patterns in monocyte progenitor cell populations from norepinephrine-intact versus nor-epinephrine-depleted animals. Although these displays represent a single point in time, they do suggest differences in the sequence of cell development of monocytes as a result of norepinephrine. First, the dramatic cell reduction of early and intermediate monocyte progenitors (R3 and R2) in the sham group suggests a reduction in cells of this development stage under normal nonstressed conditions. Second, burn sepsis in norepinephrine-intact mice results in increased numbers of intermediate progenitor stage (R3) cells compared with sham treatment, but such increases are absent in mice with reduced norepinephrine content (R3). Taken together, these changes in monocyte development markers after norepinephrine depletion in the sham and burn sepsis groups suggest that norepinephrine in some way mediates the kinetics of monocyte maturation. Further kinetic analysis of monocyte development under these conditions will require real-time dynamic changes in cell populations.
Myelopoiesis in the bone marrow environment involves highly coordinated changes in cell signaling to achieve appropriate proliferation and differentiation. Information as to how this process may be altered as a consequence of injury states and release of sympathetic neurotransmitter norepinephrine is lacking, but the results of reports involving other cell and organ systems support the concept that adrenergic stimulation can influence both proliferation and differentiation. For example, adrenergic mechanisms have been shown to modulate differentiation or proliferation in osteoclasts, 65 fibroblasts, 66 oligodendrocytes, hepatocytes, lymphocytes, and cancer cell lines. 67
The murine animal model in the present study involves essential clinical features of burn sepsis, but there may be important limitations. Our animal model involved immediate infection after burn, whereas burn patients typically develop septic complications in the second week of their hospital stay. This does not, however, alter our belief that our results provide important new information linking increased sympathetic activation in bone marrow and increased myelopoietic potential after burn sepsis. In understanding the pathophysiology of injury states, our results dictate a new conceptual framework in which to consider immune responses that occur in response to injury. This new frame of reference implies that immune responses after injury should be considered in regard to the potential action of sympathetic neurotransmitters on cellular events, including the bone marrow compartment.
Footnotes
Supported by MH53562 (S.B.J.), GM56424 (R.S.), and GM42577 (R.L.G.).
Correspondence: Stephen B. Jones, PhD, Burn and Shock Trauma Institute, Loyola University Medical Center, Building 110, Room 4219, 2160 S. First Ave., Maywood, IL 60153. E-mail: sjones@luc.edu
Accepted for publication May 19, 2000.
References
- 1.Lewis GP. Physiological mechanisms controlling secretory activity of adrenal medulla. In: Greep RO, Astwood EB. Handbook of Physiology. Section 7: Endocrinology; vol VI: Adrenal Gland. Washington DC: American Physiological Society; 1975:309–319.
- 2.Douglas WW. Secretomotor control of adrenal medullary secretion: synaptic, membrane and ionic events in stimulus-secretion coupling. In: Greep RO, Astwood EB. Handbook of Physiology. Section 7: Endocrinology; vol VI: Adrenal Gland. Washington DC: American Physiological Society; 1975:367–388.
- 3.Richter SD, Schurmeyer TH, Shedlowski M, et al. Time kinetics of the endocrine response to acute psychological stress. J Clin Endocrinol Metabol 1996; 81: 1956–1960. [DOI] [PubMed] [Google Scholar]
- 4.Wilmore DW, Long JM, Mason AD, et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg 1974; 180: 653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crum RL, Hansbrough DW, Shackford SR, et al. Cardiovascular and neurohumoral responses following burn injury. Arch Surg 1990; 125: 1065–1069. [DOI] [PubMed] [Google Scholar]
- 6.Goodall MCC, Stone C, Hayes BW. Urinary output of adrenaline and noradrenaline in severe thermal burns. Ann Surg 1957; 145: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedict CR, Grahame-Smith DG. Plasma noradrenaline and adrenaline concentrations and dopamine-β-hydroxylase activity in patients with shock due to septicaemia, trauma and haemorrhage. Q J Med [new series] 1978; 67: 1–20. [PubMed] [Google Scholar]
- 8.Frayn KN, Little RA, Maycock PF, et al. The relationship of plasma catecholamines to acute metabolic and hormonal responses to injury in man. Circ Shock 1985; 16: 229–249. [PubMed] [Google Scholar]
- 9.Davies CL, Newman RJ, Molyneux SG, et al. The relationship between plasma catecholamines and severity on injury in man. J Trauma 1984; 24: 99–105. [DOI] [PubMed] [Google Scholar]
- 10.Abboud FM, Thames MD. Interaction of cardiovascular reflexes in circulatory control. In: Shepherd JT, Abboud FM, eds. Handbook of Physiology. Section 2: The Cardiovascular System; vol III: Peripheral Circulation and Organ Blood Flow, part 2. Bethesda, MD: American Physiological Society; 1983:675–753.
- 11.Levy MN, Martin PJ. Neural control of the heart. In: Berne RM, ed. Handbook of Physiology. Section 2: The Cardiovascular System; vol I: The Heart. Bethesda, MD: American Physiological Society; 1979:581–620.
- 12.Felig P, Bergman M. The endocrine pancrease: diabetes mellitus. In: Felig P, Baxter JD, Frohman LA, eds. Endocrinology and Metabolism. New York: McGraw-Hill; 1995: 1107–1250.
- 13.Wolf RR, Durkot MJ. Evaluation of the role of the sympathetic nervous system in the response of substrate kinetics and oxidation to burn injury. Circ Shock 1982; 9: 395–406. [PubMed] [Google Scholar]
- 14.Wolf RR, Shaw JHF. Glucose and FFA kinetics in sepsis: role of glucagon and sympathetic nervous system activity. Am J Physiol 1985; 248 (Endocrinol. Metab. 11):E236–243. [DOI] [PubMed] [Google Scholar]
- 15.Stratta RJ, Glenn DW. Immune parameters in burned patients: effect and therapeutic interventions. J Trauma 1986; 26: 7–17. [DOI] [PubMed] [Google Scholar]
- 16.Polk HC, George CD, Wellhausen SR, et al. A systematic study of host defense processes in badly injured patients. Ann Surg 1986; 204: 282–299. [PMC free article] [PubMed] [Google Scholar]
- 17.Sartorelli KH, Silver JM, Gamelli RL. The effect of granulocyte colony-stimulating factor (G-CSF) upon burn-induced defective neutrophil chemotaxis. J Trauma 1991; 31: 523–530. [DOI] [PubMed] [Google Scholar]
- 18.Solomkin JS. Neutrophil disorders in burn injury: complement, cytokines and organ injury. J Trauma 1990; 30 (supp):S80–85. [DOI] [PubMed] [Google Scholar]
- 19.Warden GD, Mason AD Jr, Pruitt BA Jr. Evaluation of leukocyte chemotaxis in vitro in thermally injured patients. J Clin Invest 1974; 45: 1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duque RE, Phan SH, Hudson JL, et al. Functional defects in phagocytic cells following thermal injury. Am J Pathol 1985; 118: 166–121. [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson V, Hansbrough J, Buerk C, et al. Regulation of granulopoiesis following severe thermal injury. J Trauma 1983; 23: 19–24. [DOI] [PubMed] [Google Scholar]
- 22.Volenec FJ, Wood GW, Mani MM, et al. Mononuclear cell analysis of peripheral blood from burn patients. J Trauma 1979; 19: 86–93. [DOI] [PubMed] [Google Scholar]
- 23.Besedovsky HO, Del Rey A. Immune-neuro-endocrine interactions. Facts and hypotheses. Endocr Rev 1996; 17: 64–102. [DOI] [PubMed] [Google Scholar]
- 24.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsivness. Ann Rev Pharmacol Toxicol 1995; 35: 417–448. [DOI] [PubMed] [Google Scholar]
- 25.Madden KS, Felten SY, Felten DL, et al. Sympathetic nervous system modulation of the immune system. II. Induction of lymphocyte proliferation and mirgration in vivo by chemical sympathectomy. J Neuroimmunol 1994; 49: 67–75. [DOI] [PubMed] [Google Scholar]
- 26.Miller-Graziano CL, Fink M, Wu JY, et al. Mechanisms of altered monocyte PGE2 production in severely injured patients. Arch Surg 1988; 123: 293–299. [DOI] [PubMed] [Google Scholar]
- 27.Miller C, Szabo G, Griffey K. Elevated IL-6 production by immunosuppressed trauma patients’ monocytes. J Leukoc Biol 1989; 46: 323. [Google Scholar]
- 28.Faist E. The mechanisms of host defense dysfunction following shock and trauma. Curr Top Microbiol Immunol 1996; 216: 259–274. [DOI] [PubMed] [Google Scholar]
- 29.Miller C, Baker CC. Changes in lymphocyte activity after thermal injury. J Clin Invest 1979; 63: 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faist E, Schinkel C, Zimmer S, et al. Inadequate interleukin-2 synthesis and interleukin-2 messenger expression following thermal and mechanical trauma in humans is caused by defective transmembrane signaling. J Trauma 1993; 34: 846–854. [DOI] [PubMed] [Google Scholar]
- 31.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock 1996; 6: S27–38. [PubMed] [Google Scholar]
- 32.Miller-Graziano CL, Szab G, Kodys K, et al. Aberrations in post-trauma monocyte subpopulation: role in septic shock syndrome. J Trauma 1990; 30: S86–S97. [PubMed] [Google Scholar]
- 33.Miller-Graziano CL, Zhu D, Kodys K. Differential induction of human monocyte transforming growth factor beta-1 production and its regulation by IL-4. J Clin Immunol 1994; 14: 61–72. [DOI] [PubMed] [Google Scholar]
- 34.Shoup M, Weisenberger JM, Wang JL, et al. Mechanisms of neutropenia involving myeloid maturation arrest in burn sepsis. Ann Surg 1998; 228: 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santangelo S, Gamelli RL, Shankar R. Myeloid commitment shifts toward monocytopoiesis following thermal injury and sepsis. Ann Surg 2000; 233: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maestroni GJM. Adrenergic regulation of haematopoiesis. Pharm Res 1995; 32: 249–252. [DOI] [PubMed] [Google Scholar]
- 37.Maestroni GJM, Conti A. Modulation of hematopoiesis via α1-adrenergic receptors on bone marrow cells. Exp Hematol 1994; 22: 313–320. [PubMed] [Google Scholar]
- 38.Dresch C, Minc J, Poirier O, et al. Effect of beta-adrenergic agonists and beta-blocking agents on hemopoiesis in human bone marrow. Biomedicine 1981; 34: 93–98. [PubMed] [Google Scholar]
- 39.Dresch C, Minc J, Mary JY. In vivo protection of normal mouse hematopoiesis by a β2 blocking agent during S-phase chemotherapy. Cancer Res 1984, 44: 493–497. [PubMed] [Google Scholar]
- 40.Togni M, Maestroni GJM. Hematopoietic rescue in mice via α-adrenoceptors on bone marrow B-cell precursors. Int J Oncol 1996; 9: 313–318. [DOI] [PubMed] [Google Scholar]
- 41.Maestroni GJM, Conti A, Pederinis E. Effect of adrenergic agents on hematopoiesis after syngenic bone marrow transplantation in mice. Blood 1992; 80: 1178–1182. [PubMed] [Google Scholar]
- 42.Tang Y, Shankar R, Gamelli R, et al. Dynamic norepinephrine alterations in bone marrow: evidence of functional innervation. J Neuroimmunol 1999; 96: 182–189. [DOI] [PubMed] [Google Scholar]
- 43.Tang Y, Shankar R, Santangelo S, et al. Norepinephrine release is increased in bone marrow following thermal injury. Shock 1999; 11 (suppl 1): 6. [Google Scholar]
- 44.Walker HL, Mason AD Jr. A standard animal burn. J Trauma 1968; 8: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 45.Anton AH, Sayer DF. Study of the factors affecting the aluminium oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther 1962; 138: 360–375. [PubMed] [Google Scholar]
- 46.Cooper S, Broxmeyer HE. Clonogenic methods in vitro for the enumeration of granulocyte-macrophage progenitor cells (CU-GM) in human bone marrow and mouse bone marrow and spleen. J Tiss Cult Meth 1991; 13: 77–82. [Google Scholar]
- 47.Brodie BB, Costa E, Dlabac A, et al. Application of steady-state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther 1966; 154: 493–498. [PubMed] [Google Scholar]
- 48.Neff NH, Tozer TN, Hammer W, et al. Application of steady-state kinetics to the uptake and decline of [3H]NE in the rat heart. J Pharmacol Exp Ther 1968; 160: 48–52. [PubMed] [Google Scholar]
- 49.Snedecor S, Cochron WG. Statistical Methods. Ames, Iowa: Iowa State University Press; 1971: 258–296.
- 50.Kohm AP, Sanders VM. Suppression of antigen-specific Th2 cell-dependent IgM and IgG1 production following norepinephrine depletion in vivo. J Immunol 1999; 162: 5299–5308. [PubMed] [Google Scholar]
- 51.Madden KS, Felten SY, Felten DL, et al. Sympathetic neural modulation of the immune system I. Depression of T-cell immunity in vivo and in vitro following chemical sympathectomy. Brain Behav Immun 1989; 3: 72–89. [DOI] [PubMed] [Google Scholar]
- 52.Lyte M, Bailey MT. Neuroendocrine-bacterial interactions in a neurotoxin-inducedmodel of trauma. J Surg Res 1997; 70: 195–201. [DOI] [PubMed] [Google Scholar]
- 53.Tsao C-W, Cheng J-T, Shen C-L, Lin Y-S. 6-hydroxydopamine induces thymocyte apoptosis in mice. J Neuroimmunol 1996; 65: 91–95. [DOI] [PubMed] [Google Scholar]
- 54.Leenen PJM, Melis M, Slieker WAT, et al. Murine macrophage precursor characterization II. Monoclonal antibodies against macrophage precursor antigen. J Immunol 1990; 20: 27–34. [DOI] [PubMed] [Google Scholar]
- 55.De Bruijn MFTR, Slieker WAT, van der Loo JCM, et al. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol 1994; 24: 279–284. [DOI] [PubMed] [Google Scholar]
- 56.Utretsky NJ, Iverson LL. Effects of 6-hydroxydopamine on noradrenaline-containing neurones in the rat brain. Nature 1969; 221: 557–559. [DOI] [PubMed] [Google Scholar]
- 57.Kostrzewa RM, Jacobwitz DM. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev 1974; 26: 199–288. [PubMed] [Google Scholar]
- 58.Laverty R, Sharman DF, Vogt M. Action of 2,4,5,-trihydroxyphenylethylamine on the storage and release of noradrenaline. Br J Pharamcol 1965; 24: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Porter CC, Totaro JA, Stone CA. Effects of 6-hydroxydopamine and some other compounds on the concentration of norepinephrine in the hearts of mice. J Pharmacol Exp Ther 1963; 140: 308–316. [PubMed] [Google Scholar]
- 60.Schor NFT. Adjunctive use of Thiofos (WR2721) with free radical-generating chemotherapeutic agents in mice: new caveats for therapy. Cancer Res 1987; 47: 5411–5414. [PubMed] [Google Scholar]
- 61.Williams JM, Peterson RG, Shea PA, et al. Sympathetic innervation of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res Bull 1981; 6: 83–94. [DOI] [PubMed] [Google Scholar]
- 62.Felten DL, Livnat S, Felten SY, et al. Sympathetic innervation of lymph nodes in mice. Brain Res Bull 1984; 13: 693–699. [DOI] [PubMed] [Google Scholar]
- 63.Ackerman KD, Madden KS, Livnat S, et al. Neonatal sympathetic denervation alters the development of in vitro spleen cell proliferation and differentiation. Brain Behav Immun 1991; 5: 235–261. [DOI] [PubMed] [Google Scholar]
- 64.Tiegs G, Bang R, Neuhuber WL. Requirement of peptidergic sensory innervation for disease activity in murine models of immune hepatitis and protection by β-adrenergic stimulation. J Neuroimmunol 1999; 96: 131–143. [DOI] [PubMed] [Google Scholar]
- 65.Frediani U, Becherini L, Lasagni L, et al. Catecholamines modulate growth and differentiation of human preosteoclastic cells. Osteoporosis Int 1996; 6: 14–21. [DOI] [PubMed] [Google Scholar]
- 66.Saito T, Tazawa K, Yokoyama Y, et al. Surgical stress inhibits the growth of fibroblasts through the elevation of plasma catecholamine and cortisol concentrations. Surg Today 1997; 27: 627–631. [DOI] [PubMed] [Google Scholar]
- 67.Bertin B, Strosberg AD, Marullo S. Activation of a beta 2-adrenergic receptor/Gs alpha fusion protein elicits a desensitization-resistant cAMP signal capable of inhibiting proliferation of two cancer cell lines. Receptors Channels 1997; 5: 41–51. [PubMed] [Google Scholar]