Abstract
Objective
To report the results of liver transplantation in 31 Asian patients with chronic hepatitis B using lamivudine prophylaxis in an open-label study.
Summary Background Data
Chronic hepatitis B is a prevalent cause of end-stage liver disease in Asia, but the results of liver transplantation in these patients are poor.
Methods
Thirty-one Asian patients with chronic hepatitis B underwent liver transplantation using lamivudine prophylaxis (100 mg daily). Twenty-three (74%) patients had detectable serum hepatitis B envelope antigen (n = 18) or hepatitis B virus DNA (n = 11) before treatment, and seven had associated hepatocellular carcinoma. Lamivudine was continued indefinitely after transplantation, and hepatitis B immune globulin was not used.
Results
The actuarial patient and graft survival rates were 84% and 81%, respectively. Five patients died of causes unrelated to hepatitis B, and 26 patients were alive at a median follow-up of 16 months (range 6–47) after transplantation. One (3.8%) patient developed recurrent hepatitis B resulting from viral breakthrough at week 53 and survived after retransplantation using adefovir and hepatitis B immune globulin treatment. The remaining 25 surviving patients had no biochemical or histologic evidence of recurrent hepatitis, and serum hepatitis B virus DNA remained negative by polymerase chain reaction. In six patients, hepatitis B surface antigen (HBsAg) persisted or reappeared in serum. Among 19 patients who became negative for HBsAg from 5 to 431 days after transplantation, 13 developed anti-HBsAb that lasted a median of 6 months (range 1–21). None of the seven patients with hepatocellular carcinoma developed recurrent tumor.
Conclusions
Asian patients with chronic hepatitis B may achieve a good outcome after liver transplantation using lamivudine prophylaxis.
Hepatitis B virus (HBV) infection is endemic in Asia. Among more than 300 million people with chronic HBV infection worldwide, more than 75% are of Asian origin. 1 In Hong Kong, approximately 10% of the population test positive for hepatitis B surface antigen (HBsAg). 2 Previous studies have shown that in 66.3% of patients with cirrhosis 3 and more than 85% of those with hepatocellular carcinoma, 4 the disease was HBV-related, which makes chronic HBV infection the most important cause of end-stage liver disease in Hong Kong.
Orthotopic liver transplantation is the most effective treatment for end-stage liver disease. For patients with chronic HBV infection, however, there is a high risk of recurrent hepatitis that rapidly progresses to graft failure. 5 Although the use of long-term passive immunoprophylaxis using high-dose hepatitis B immune globulin (HBIG) has significantly reduced the incidence of recurrent graft infection and improved survival, 6,7 the use of HBIG requires repeated parenteral administration in a large amount, is costly, and is not completely effective. There is a paucity of information in the literature regarding the outcome of orthotopic liver transplantation in Asians with chronic hepatitis B infection. Occasional reports 8,9 have suggested a higher early death rate, a more virulent course of recurrence, and a poorer graft survival in Asian patients compared with non-Asians. Until recently, many transplant centers have been reluctant to consider Asians with chronic HBV infection for transplantation.
Lamivudine is an oral nucleoside analog that inhibits HBV replication regardless of immunosuppression. Studies of lamivudine treatment in Asian and non-Asian patients with chronic hepatitis B have demonstrated its safety and efficacy in suppressing HBV DNA synthesis and in controlling chronic hepatitis. 10–12 Promising results have also been reported in preliminary studies using lamivudine after transplantation as prophylaxis against HBV recurrence, 13 and as treatment of recurrent or de novo hepatitis B. 14,15 We undertook an open-label study to assess the efficacy and safety of lamivudine, without HBIG, for prophylaxis of reinfection after liver transplantation in Asian patients with chronic hepatitis B.
METHODS
From September 1995 to February 1999, 31 patients (Table 1) who were positive for HBsAg and had evidence of chronic hepatitis B infection underwent liver transplantation at Queen Mary Hospital, Hong Kong, using primary lamivudine prophylaxis in an open-label study. These represented 47% of the 66 liver transplants performed at our institute during this period. The protocol was approved by the Ethics Committee of the Faculty of Medicine, the University of Hong Kong, and informed consent was obtained from every patient. There were 28 men and 3 women with a median age at transplantation of 42 years (range 17–60). All patients were ethnic Chinese and all liver transplants were primary. Before transplantation, 12 patients required intensive care, 9 were in the hospital with complications of liver disease, and 10 were at home requiring regular medical care. Twenty-three patients had cirrhosis and eight had acute flare of chronic hepatitis B resulting in rapid deterioration of liver function. Seven patients had hepatocellular carcinoma: four had incidental tumors found in the explant (one tumor nodule, n = 2; two tumor nodules, n = 1; multiple tumor nodules, n = 1), each of which was 3 cm or less in diameter; each of two patients had two 2-cm asymptomatic tumors on pretransplant imaging; and one patient had a 2.5-cm tumor treated with transarterial chemoembolization for 30 months before transplantation. The grafts were from cadaveric donors in 17 patients and living donors in 14.
Table 1. DEMOGRAPHIC DATA AT TRANSPLANTATION
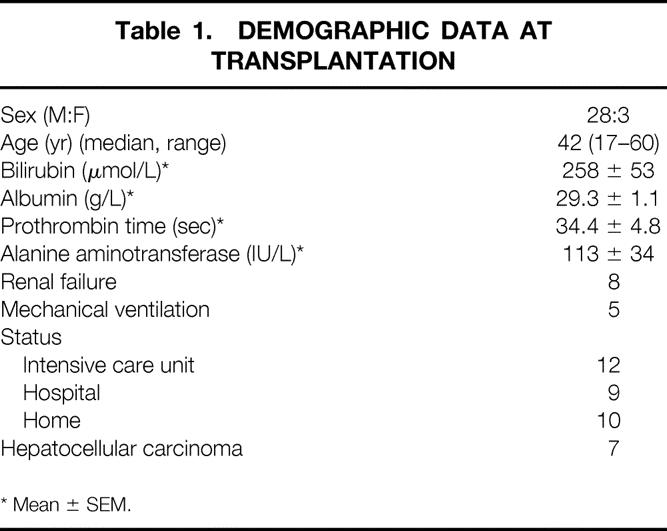
* Mean ± SEM.
Viral Serology and Lamivudine Treatment
All patients were positive for HBsAg (Auszyme Monoclonal EIA, Abbott Laboratories, Chicago, IL) in serum (Table 2). Before lamivudine treatment, 23 (74%) patients had evidence of active viral replication as shown by positive serum hepatitis B envelope antigen (HBeAg) (Axsym HBe 2 MEIA, Abbott Laboratories) or HBV DNA (>2.5 pg/mL) using branched-chain DNA (bDNA) assay (Quantiplex, Chiron Diagnostics, Emeryville, CA). Six were positive for both HBeAg and HBV DNA, 10 were positive for HBeAg and negative for HBV DNA, 5 were negative for HBeAg and positive for HBV DNA, and 2 were positive for HBeAg and unknown for HBV DNA. The median pretreatment serum HBV DNA concentration was 29 pg/mL (range 2.6–920) for the 11 patients with positive HBV DNA. No patient was coinfected with hepatitis C virus or delta virus.
Table 2. HEPATITIS B SEROLOGIC MARKERS
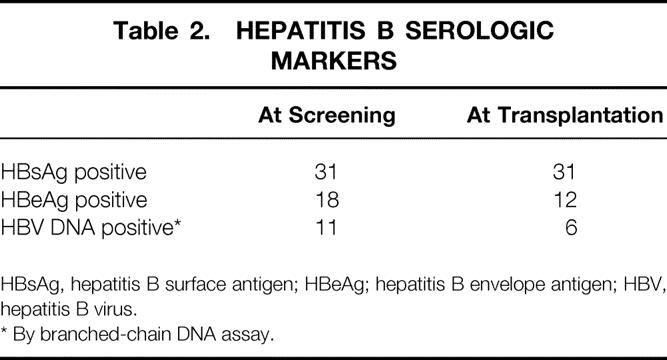
HBsAg, hepatitis B surface antigen; HBeAg; hepatitis B envelope antigen; HBV, hepatitis B virus.
* By branched-chain DNA assay.
All patients, irrespective of viral replication status, received oral lamivudine 100 mg daily after they had been accepted on the waiting list. Sixteen patients were treated with lamivudine for more than 4 weeks (median 95 days; range 54–613 days) before transplantation. Of these patients, five of eight HBeAg-positive patients and five of eight HBV DNA-positive patients were seroconverted before surgery. The remaining 15 patients underwent liver transplantation in an urgent or semiurgent situation within 4 weeks (median 3 days; range 1–16 days) of starting lamivudine treatment, a reflection of the late presentation and referral for transplantation as well as the feasibility of early transplantation from living donors. Only 1 of 10 HBeAg-positive patients and 0 of the 3 HBV DNA-positive patients were seroconverted. Neither the persistence of HBeAg nor that of HBV DNA in serum was considered a contraindication to liver transplant. Lamivudine prophylaxis was continued indefinitely after transplantation, and HBIG was not used.
Follow-Up
Immunosuppression consisted of a double regimen of cyclosporine with steroid (n = 11) or tacrolimus with steroid (n = 20). Azathioprine was not used. Intravenous hydrocortisone 1 g was given during surgery, followed by methylprednisolone 200 mg/day tapered to oral prednisolone 20 mg/day by day 7. The dosage of prednisolone was progressively reduced with the aim of complete withdrawal in 12 months for those receiving cyclosporine and 6 months for those receiving tacrolimus. On discharge, all patients were followed up weekly at the clinic and then at increasing intervals. Posttransplantation chemotherapy was not given to any of the seven patients with hepatocellular carcinoma. Standard laboratory tests for liver function, serum HBsAg/anti-HBsAb, and HBeAg/anti-HBeAb were measured at each visit. Serum HBV DNA was measured quantitatively every 3 months by bDNA assay. Negative samples were confirmed qualitatively by polymerase chain reaction (PCR) with nested primers directed at the surface and core gene regions of HBV. When the reappearance of HBV DNA in serum was documented, HBV DNA was amplified by PCR and direct cycle sequencing analysis was performed to detect mutations at the YMDD motif of the DNA-polymerase gene. Liver biopsies were performed according to clinical needs. The samples were examined for histologic evidence of recurrent hepatitis and stained immunohistochemically for HBsAg and hepatitis B core antigen (HBcAg). A routine liver biopsy was performed for all surviving patients in February 1999 and the presence of HBV DNA in liver tissue was tested using PCR. All follow-up information of the surviving patients was updated to August 31, 1999.
RESULTS
Clinical Outcome
Five patients, including two who were in the intensive care unit before transplantation, died of causes unrelated to HBV at 5 to 36 days after surgery. The causes of death were intracerebral hemorrhage, bacterial endocarditis, systemic candidiasis, and graft failure from hepatic venous outflow obstruction and from inadequate graft size in one patient each. None of these patients had detectable HBV DNA in serum, and four were HBsAg-negative at the time of death. There was no histologic evidence of recurrent hepatitis on liver biopsy or postmortem examination. The remaining 26 patients were alive at a median follow-up after transplantation of 16 months (range 6–47), and 22 had been followed up for more than 1 year. One (3.8%) patient developed recurrent hepatitis B at week 53 and survived after retransplantation. The actuarial patient and graft survival rates were 84% and 81%, respectively. None of the seven patients with hepatocellular carcinoma had evidence of recurrent tumor at a median follow-up of 28 months (range 10–47).
Immunosuppression and Graft Rejection
Ten (32.3%) patients had 13 episodes of acute cellular rejection that were treated by steroid pulse therapy (n = 5), conversion from cyclosporine to FK506 (n = 4), or increase in dosage of maintenance FK506 (n = 4). Steroid use was stopped in 22 of the 26 surviving patients a median of 11 months (range 6–40) after transplantation, and 4 were taking a minimal dose of prednisolone (2.5 mg/day).
Efficacy of Lamivudine and Viral Serology
There was no adverse effect attributed to lamivudine, and lamivudine therapy was continued in all survivors. Serum HBV DNA became negative by bDNA assay and by PCR in all HBV DNA-positive patients soon after transplantation. Reappearance of HBV DNA with recurrent hepatitis secondary to viral breakthrough and lamivudine resistance occurred in patient 15 53 weeks after transplantation. He tested negative for HBeAg and positive for HBV DNA (130 pg/mL) before lamivudine therapy, and HBV DNA became undetectable with lamivudine treatment before transplantation. After transplantation, serum HBsAg was negative and anti-HBsAb was positive from day 10. Serum anti-HBsAb became negative at week 37, and both HBsAg and HBV DNA became positive at week 53. Sequence analysis revealed a YIDD mutant at the YMDD motif of the DNA-polymerase gene of HBV. Liver biopsy was positive for HBsAg and HBcAg on immunostaining. His graft function deteriorated rapidly and retransplantation was performed at week 70 using a combination of adefovir and HBIG prophylaxis. He remained well with normal graft function 25 weeks after retransplantation with an anti-HBsAb titer of more than 100 IU/L and no detectable serum HBV DNA by PCR.
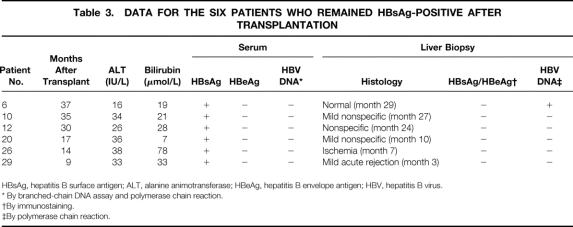
The remaining 25 survivors had no biochemical or histologic evidence of recurrent hepatitis B. Serum HBV DNA remained negative at the latest follow-up as measured by both bDNA assay and PCR in all 25 patients. Nineteen patients became negative for HBsAg from 5 to 431 days after transplantation and remained so at a median follow-up of 13 months (range 6–47). Thirteen of these 19 patients developed anti-HBsAb in their serum a median of 4 days (range 2–30) after transplantation, and this persisted a median of 6 months (range 1–21). The median serum alanine aminotransferase level was 25 IU/L (range 8–80) and the total bilirubin was 15 μmol/L (range 8–36). In six patients, however, HBsAg persisted or reappeared after a period of temporary clearance. Before lamivudine therapy, five of these six patients had evidence of active viral replication (positive for HbeAg and negative for HBV DNA, n = 4; positive for HBeAg, n = 1; HBV DNA unknown, n = 1). Two had received lamivudine therapy for more than 4 weeks before transplantation, and both cleared HBeAg before transplantation. The liver function tests, viral serology at the last follow-up, and the most recent liver biopsy studies for these six patients are shown in Table 3. Only patient 26 had notable abnormal liver function that was attributed to delayed portal vein thrombosis. Serum HBV DNA remained undetectable by PCR 10 to 36 months after transplantation.
Table 3. DATA FOR THE SIX PATIENTS WHO REMAINED HBsAg-POSITIVE AFTER TRANSPLANTATION
HBsAg, hepatitis B surface antigen; ALT, alanine animotransferase; HBeAg, hepatitis B envelope antigen; HBV, hepatitis B virus.
* By branched-chain DNA assay and polymerase chain reaction.
†By immunostaining.
‡By polymerase chain reaction.
Liver Biopsy
Apart from patient 15, who had retransplantation for recurrent hepatitis B, there was no histologic feature of recurrent hepatitis in the latest liver biopsy sample of the remaining 25 survivors, and immunostaining for HBsAg and HBcAg was negative in all. Patients 6 and 16, one positive and one negative for serum HBsAg after transplantation, had HBV DNA in liver tissue detectable by PCR.
DISCUSSION
Despite the existence of an effective hepatitis B vaccine for nearly 20 years, chronic hepatitis B remains the most important cause of end-stage liver disease in Asia, including Hong Kong. In this series, patients with chronic hepatitis B constituted nearly 50% of all those who underwent liver transplantation for end-stage liver disease at Queen Mary Hospital during the study period. The clinical characteristics of our patients reflect two main concerns with the poor outcome of liver transplantation in Asian patients with chronic hepatitis B: early death resulting from the advanced stage of the liver disease, and late graft loss resulting from recurrent hepatitis.
First, because of the late presentation and referral for transplantation, most of our patients had advanced liver disease, and 12 of 31 required intensive care before transplantation. Ho et al 16 showed that late referral and more advanced chronic liver disease probably accounted for the worse outcome of Asian patients with chronic hepatitis B after liver transplantation at Stanford University. In Asian countries, the extreme scarcity of cadaveric organ donors and the longer waiting time before transplantation aggravate this problem. Living-donor liver transplantation offers an option for these patients to receive earlier transplantation. 17 With the use of this technique in 45% of the patients in the present series, our early death rate of 16% was acceptable and was low compared with those of 30% to 40% reported previously. 9,16
Second, the presence of active HBV replication has been reported to predict a higher recurrence rate and a poor outcome. 18 Most Asian patients have hepatitis B infection acquired early from mother to newborn at birth or between close contacts in early childhood. 19 The immune tolerance to HBV after infection at an early stage of life results in a higher rate of chronicity, recurrent flares of activity, and hence a high viral load. Three quarters of our patients had evidence of active viral replication as shown by the presence of HBeAg or HBV DNA in serum before lamivudine treatment. Twelve patients were positive for HBeAg and six were positive for HBV DNA at the time of transplantation. Nonetheless, our results showed that lamivudine prophylaxis alone, without the need for additional HBIG therapy, is highly effective in preventing recurrent hepatitis B in this group of high-risk Asian patients. The graft survival rate of 81% at a median follow-up of 16 months after transplantation is comparable to that in patients who receive transplants for liver diseases unrelated to HBV. The incidence of recurrent hepatitis B resulting from viral breakthrough is low (3.8%). Twenty-five of the 26 survivors had no biochemical or histologic evidence of recurrent hepatitis and serum HBV DNA has remained negative by the highly sensitive PCR. Most of the patients cleared HBsAg, and some even developed anti-HBsAb in serum. This spontaneous appearance of anti-HBsAb was unexpected. It occurred without the need for additional HBIG therapy or any vaccine and despite continuing immunosuppressive therapy. The spontaneous development of immunity offered additional protection against the development of viral mutants and recurrent hepatitis, although this lasted for an average of 6 months only. The significance of the persistence or reappearance of HBsAg in the serum of six patients was not clear. Only one of these six had HBV DNA detected in liver tissue by PCR. Although liver transplantation removed the major source of HBV in the diseased liver, and lamivudine is highly effective in suppressing HBV replication, the persistence of HBV may continue in extrahepatic organs. Long-term follow-up study is required to determine the outcome of this “healthy HBsAg carrier” status after transplantation.
In a multicenter trial of lamivudine in compensated Chinese patients with chronic hepatitis B, lamivudine resistance resulting from mutation at the YMDD locus occurred in 14% of patients who were treated for 12 months. 12 Compared with our study population, these nontransplant patients had a higher viral load and hence a higher risk for viral mutation because all of them had serum HBV DNA levels of at least 5 pg/mL and the major source of HBV in the liver was not removed. Our viral breakthrough rate at a median follow-up of 16 months was 3.8%, which is less than the 10% recurrence rate reported in an early series using lamivudine as prophylaxis after liver transplantation. 13 The lower frequency in our population may be attributed to a low level of immunosuppression as a result of the deliberate use of fewer immunosuppressive drugs for maintenance and treatment of rejection. We used a double regimen of steroid and cyclosporine or tacrolimus, avoiding the use of azathioprine and rapidly tapering the steroid. Nonetheless, the incidence of rejection was low and rejection episodes were easily controlled. In addition, chemotherapy, which might further suppress the immune system, was not given to patients with hepatocellular carcinoma after transplantation. Immunosuppressive agents may increase HBV expression by directly stimulating viral replication or indirectly by suppressing the host immune response to the virus. Both azathioprine and steroid have been shown to enhance HBV replication, 20 which in turn increases the risk of viral mutation. Reducing the amount of immunosuppression may improve the host immune response to the virus and contribute to a lower incidence of recurrent hepatitis B. 21
Recurrent hepatitis resulting from the lamivudine-resistant mutant at the YMDD locus has been reported to run a relatively benign course, 22,23 but our only patient with recurrence had rapid graft loss requiring retransplantation. In addition, although the incidence of viral breakthrough was low in the present series, it is possible that more mutants may develop with longer follow-up. Combination therapy has been suggested to overcome this problem of drug resistance. Excellent results have been reported with the use of combination of lamivudine and HBIG as prophylaxis. 24 Our study has shown that lamivudine prophylaxis in patients with chronic hepatitis B can result in spontaneous development of anti-HBsAb in serum after transplantation. Hence, the need for additional HBIG for all patients is questionable, particularly in view of the disadvantages of high cost, potential mercury toxicity, and the need for parenteral administration associated with HBIG therapy. The use of multiple antiviral agents as prophylaxis is a more attractive strategy. In view of the low incidence of viral breakthrough in the present series, combination therapy may be necessary only for selected patients at risk.
The absence of recurrence in 15 patients who received lamivudine for only 1 to 16 days before transplantation raised the additional question as to the best timing for the initiation of lamivudine treatment. Although pretreatment with lamivudine is usually started while the patient is waiting for a liver graft, 13 it may be possible to initiate treatment only when a liver graft becomes available to reduce the risk of inducing resistance. Further studies are required to define the best prophylactic strategy to prevent recurrence after liver transplantation in patients with chronic hepatitis B. In the meantime, however, our results showed that with the use of lamivudine prophylaxis alone, Asian patients with chronic hepatitis B can achieve good results after liver transplantation.
Footnotes
Correspondence: Dr. C.M. Lo, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong. E-mail: chungmlo@hkucc.hku.hk
Accepted for publication July 12, 2000.
References
- 1.Maynard JE. Hepatitis B: global importance and need for control. Vaccine 1990; 8 (suppl): S18–20. [DOI] [PubMed] [Google Scholar]
- 2.Public Health Report No. 3. Viral Hepatitis & Liver Cancer and Unintentional Injuries in Children. Hong Kong: Department of Health; 1998.
- 3.Lam KC, Lai CL, Wu PC, et al. Etiological spectrum of liver cirrhosis in the Chinese. J Chronic Dis 1980; 33: 375–381. [DOI] [PubMed] [Google Scholar]
- 4.Lai CL, Lam KC, Wong KP, et al. Clinical features of hepatocellular carcinoma: review of 211 patients in Hong Kong. Cancer 1981; 47: 2746–2755. [DOI] [PubMed] [Google Scholar]
- 5.Todo S, Demetris AJ, Van Thiel D, et al. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology 1991; 13: 619–626. [PMC free article] [PubMed] [Google Scholar]
- 6.Terrault NA, Zhou S, Combs C, et al. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology 1996; 24: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 7.Sawyer RG, McGory RW, Gaffey MJ, et al. Improved clinical outcomes with liver transplantation for hepatitis B-induced chronic liver failure using passive immunization. Ann Surg 1998; 227: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs JM, Martin P, Munoz SJ, et al. Liver transplantation for chronic hepatitis B in Asian males. Transplant Proc 1993; 25: 1904–1906. [PubMed] [Google Scholar]
- 9.Jurim O, Martin P, Shaked A, et al. Liver transplantation for chronic hepatitis B in Asians. Transplantation 1994; 57: 1393–1395. [DOI] [PubMed] [Google Scholar]
- 10.Dienstag JL, Perrillo RP, Schiff ER, et al. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med 1995; 333: 1657–1661. [DOI] [PubMed] [Google Scholar]
- 11.Nevens F, Main J, Honkoop P, et al. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology 1997; 113: 1258–1263. [DOI] [PubMed] [Google Scholar]
- 12.Lai CL, Chien RN, Leung NWY, et al. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med 1998; 339: 61–68. [DOI] [PubMed] [Google Scholar]
- 13.Grellier L, Mutimer D, Ahmed M, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet 1996; 348: 1212–1215. [DOI] [PubMed] [Google Scholar]
- 14.Nery JR, Weppler D, Rodriguez M, et al. Efficacy of lamivudine in controlling hepatitis B virus recurrence after liver transplantation. Transplantation 1998; 65: 1615–1621. [DOI] [PubMed] [Google Scholar]
- 15.Perrillo R, Rakela J, Dienstag J, et al. Multicenter study of lamivudine therapy for hepatitis B after liver transplantation. Hepatology 1999; 29: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 16.Ho BM, So SK, Esquivel CO, et al. Liver transplantation in Asian patients with chronic hepatitis B. Hepatology 1997; 25: 223–225. [DOI] [PubMed] [Google Scholar]
- 17.Lo CM, Fan ST, Liu CL, et al. Applicability of living donor liver transplantation to high-urgency patients. Transplantation 1999; 67: 73–77. [DOI] [PubMed] [Google Scholar]
- 18.Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med 1993; 329: 1842–1847. [DOI] [PubMed] [Google Scholar]
- 19.Lok ASF, Lai CL, Wu PC, et al. Hepatitis B virus infection in Chinese families in Hong Kong. Am J Epidemiol 1987; 126: 492–499. [DOI] [PubMed] [Google Scholar]
- 20.McMillan JS, Shaw T, Angus PW, et al. Effect of immunosuppressive and antiviral agents on hepatitis B virus replication in vitro. Hepatology 1995; 22: 36–43. [PubMed] [Google Scholar]
- 21.Gish RG, Keeffe E, Lim J, et al. Survival after liver transplantation for chronic hepatitis B using reduced immunosuppression. J Hepatol 1995; 22: 257–262. [DOI] [PubMed] [Google Scholar]
- 22.Schalm SW. Clinical implications of lamivudine resistance by HBV. Lancet 1997; 349: 3–4. [DOI] [PubMed] [Google Scholar]
- 23.Bain VG, Kneteman NM, Ma MM, et al. Efficacy of lamivudine in chronic hepatitis B patients with active viral replication and decompensated cirrhosis undergoing liver transplantation. Transplantation 1996; 62: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 24.Markowitz JS, Martin P, Conrad AJ, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology 1998; 28: 585–589. [DOI] [PubMed] [Google Scholar]