Abstract
Objective
To determine benefits of conservative versus surgical treatment in patients with necrotizing pancreatitis.
Summary Background Data
Infection of pancreatic necrosis is the most important risk factor contributing to death in severe acute pancreatitis, and it is generally accepted that infected pancreatic necrosis should be managed surgically. In contrast, the management of sterile pancreatic necrosis accompanied by organ failure is controversial. Recent clinical experience has provided evidence that conservative management of sterile pancreatic necrosis including early antibiotic administration seems promising.
Methods
A prospective single-center trial evaluated the role of nonsurgical management including early antibiotic treatment in patients with necrotizing pancreatitis. Pancreatic infection, if confirmed by fine-needle aspiration, was considered an indication for surgery, whereas patients without signs of pancreatic infection were treated without surgery.
Results
Between January 1994 and June 1999, 204 consecutive patients with acute pancreatitis were recruited. Eighty-six (42%) had necrotizing disease, of whom 57 (66%) had sterile and 29 (34%) infected necrosis. Patients with infected necrosis had more organ failures and a greater extent of necrosis compared with those with sterile necrosis. When early antibiotic treatment was used in all patients with necrotizing pancreatitis (imipenem/cilastatin), the characteristics of pancreatic infection changed to predominantly gram-positive and fungal infections. Fine-needle aspiration showed a sensitivity of 96% for detecting pancreatic infection. The death rate was 1.8% (1/56) in patients with sterile necrosis managed without surgery versus 24% (7/29) in patients with infected necrosis (P <.01). Two patients whose infected necrosis could not be diagnosed in a timely fashion died while receiving nonsurgical treatment. Thus, an intent-to-treat analysis (nonsurgical vs. surgical treatment) revealed a death rate of 5% (3/58) with conservative management versus 21% (6/28) with surgery.
Conclusions
These results support nonsurgical management, including early antibiotic treatment, in patients with sterile pancreatic necrosis. Patients with infected necrosis still represent a high-risk group in severe acute pancreatitis, and for them surgical treatment seems preferable.
Necrotizing pancreatitis (NP) represents the severe form of human acute pancreatitis. 1,2 The past decade has seen a considerable increase in our understanding and management of NP. The natural course of severe acute pancreatitis progresses in two phases. The first 14 days are characterized by the systemic inflammatory response syndrome resulting from the release of inflammatory mediators. 3,4 In patients with NP, organ failure is common and often occurs in the absence of infection. 5 In addition to organ dysfunction, general derangements include hypovolemia, a hyperdynamic circulatory regulation, 6 fluid loss from the intravascular space, and increased capillary permeability. 7 The second phase, beginning approximately 2 weeks after the onset of the disease, is dominated by sepsis-related complications resulting from infection of pancreatic necrosis. 8,9 This is associated with multiple systemic complications, such as pulmonary, renal, and cardiovascular failure. In the natural course of the disease, infection of pancreatic necrosis occurs in 40% to 70% of patients, and it has become the most important risk factor of death from NP. 10–12 Of patients who die of acute pancreatitis, more than two thirds of deaths are due to late septic organ complications. 13–15
Antibiotic treatment with agents penetrating well into the pancreas 16 has been shown to prevent infection in severe acute pancreatitis and to lower the death rate. 17–22 Still, the general treatment principles in NP and particularly the role of surgery are controversial issues. During the 1980s, according to our experience, 60% to 70% of patients with NP were treated surgically. 23 In 1991, Bradley and Allen 24 introduced the concept of nonsurgical management of sterile necrosis, applying early antibiotic treatment. In 11 consecutive patients managed accordingly, there were no deaths. In the same study, 27 patients with infected necrosis were treated surgically using open packing, yielding a death rate of 15%.
The promising concept of using infection as the main parameter of surgical decision making has so far not been adopted generally. 25,26 Therefore, in a prospective single-center trial, we evaluated the role of nonsurgical treatment in patients with sterile necrosis and surgical treatment in patients with infected necrosis. In addition, we studied the effect of early antibiotic treatment on the further course of NP and its influence on the evolution and microbiologic characteristics of the pancreatic infection.
PATIENTS AND METHODS
Between January 1994 and June 1999, 204 consecutive patients with acute pancreatitis were recruited prospectively from the Department of Visceral and Transplantation Surgery at the University of Bern. Inclusion criteria were an elevation of serum amylase to more than three times the upper normal limit and a typical clinical picture, 27 including abdominal pain. 28 Patient stratification according to disease severity was performed as previously published. 29 NP was defined as the appearance of pancreatic or extrapancreatic necrosis 30 on contrast-enhanced computed tomography (CT) and a serum C-reactive protein value of more than 150 mg/L. CT was performed within 48 to 96 hours of admission and was repeated weekly in patients whose clinical condition did not improve. On admission all patients were treated medically according to generally accepted principles consisting of withholding oral intake, providing pain relief, and restoring fluid and electrolyte losses intravenously. If vomiting had been a prominent part of the clinical picture, a nasogastric tube was inserted. A proton pump inhibitor was given to prevent stress ulcers, and low-molecular-weight heparin (3,000 U/day) was given to prevent thrombosis. Pain treatment included the use of peridural anesthesia (n = 99) or intravenous narcotic analgesics. Clinical severity staging of acute pancreatitis was carried out using the Ranson prognostic signs and the APACHE II scoring system. Initially, patients were cared for in an intermediate care unit or, in case of organ failure, in the intensive care unit. In patients with NP, antibiotic treatment was given less than 24 hours after CT findings of necrosis. We used imipenem/cilastatin (3 × 500 mg/day, intravenously) for 14 days according to the study protocol.
Endoscopic retrograde cholangiography was performed in cases of biliary etiology, as proven by transcutaneous ultrasonography and laboratory findings of cholestasis such as elevated levels of serum aspartate aminotransferase, alkaline phosphatase, or bilirubin. Papillotomy was performed if stones or sludge were present in the common bile duct.
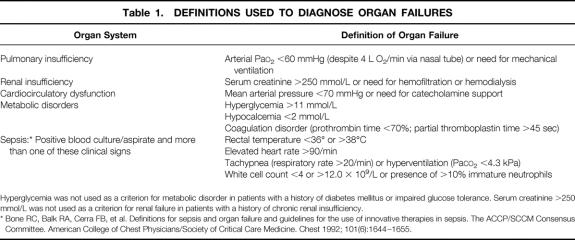
In patients with NP, CT-guided fine-needle aspiration (FNA) with consecutive Gram stain and bacteriologic culture was carried out if infection of necrosis was clinically suspected. The indication for FNA was defined during the course of NP in the following instances:
Newly developed signs of metabolic disorders and deterioration of organ failures of lung, kidney, or the cardiocirculatory system (for the definition of organ failure, see Table 1)
Newly developed increase in blood leukocytes or fever (>38.5°C) after initial response to conservative treatment.
Table 1. DEFINITIONS USED TO DIAGNOSE ORGAN FAILURES
Hyperglycemia was not used as a criterion for metabolic disorder in patients with a history of diabetes mellitus or impaired glucose tolerance. Serum creatinine >250 mmol/L was not used as a criterion for renal failure in patients with a history of chronic renal insufficiency.
* Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101(6):1644–1655.
Infection of pancreatic necrosis as proven by FNA was regarded as an indication for surgical treatment and resulted in surgical intervention (necrosectomy and continuous postoperative lavage of the necrotic cavities) within 24 hours. During surgery, at least two smears for bacteriologic culture were taken from the pancreatic necrosis and from the adjacent necrotic cavities in the retroperitoneum. Postoperative antibiotic treatment, including antimycotic treatment, was adapted according to the bacteriologic findings and microbiologic testing of resistance.
A complete follow-up was carried out (mean follow-up time 35 months) after hospital discharge to determine late consequences of edematous pancreatitis and sterile and infected pancreatic necrosis.
Data were analyzed using the Mann-Whitney or chi-square test where appropriate. P < .05 was considered significant.
RESULTS
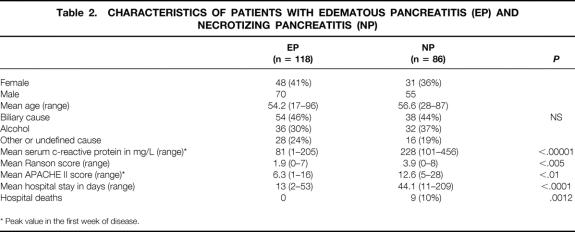
Patient characteristics are summarized in Table 2. There were 204 patients in the study, 125 (61%) men and 79 (39%) women. Mean age was 55.1 years (range 17–96). The cause was biliary in 92 (45%) patients, alcohol overindulgence in 68 (33%), and other or undefined in 44 (22%). According to CT and C-reactive protein findings, there were 118 (58%) and 86 (42%) patients with edematous pancreatitis and NP, respectively.
Table 2. CHARACTERISTICS OF PATIENTS WITH EDEMATOUS PANCREATITIS (EP) AND NECROTIZING PANCREATITIS (NP)
* Peak value in the first week of disease.
Edematous Pancreatitis
In 118 patients, pancreatic necrosis was ruled out by CT findings and C-reactive protein serum levels. Fifty-six patients stayed for a mean of 2.3 days (range 1–7) in intermediate care. No patient with edematous pancreatitis had multiple organ failure, but in 12 patients (10%) single organ failure was diagnosed (1 renal, 4 pulmonary insufficiency, 3 cardiocirculatory insufficiency, 4 metabolic disorders). Endoscopic retrograde cholangiopancreatography was performed early (in the first 24–48 hours) in 54 patients with biliary etiology, and papillotomy and stone retrieval were performed in 38 of those patients. In addition, endoscopic retrograde cholangiopancreatography was carried out before hospital discharge in 31 patients with an unknown cause and to exclude tumor-induced acute pancreatitis. Laparoscopic cholecystectomy was done in 35 patients a median of 8.6 days after admission (range 2–19), and open cholecystectomy was performed in 19 patients a median of 9 days after admission (range 2–19). There were no hospital deaths, and the mean hospital stay was 13 days (range 2–53).
Follow-Up
Hospital readmission occurred in seven patients (6%) after a mean of 8.3 months for the following reasons: recurrent acute pancreatitis (n = 4), duodenal obstruction (n = 1), and pancreatic pseudocyst (n = 1).
Necrotizing Pancreatitis
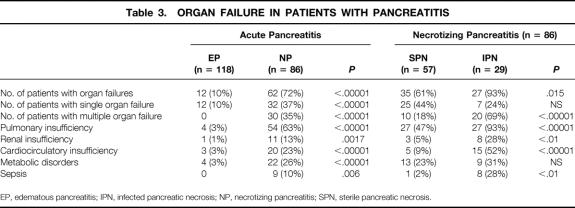
Eighty-six patients were staged as having NP based on CT findings and C-reactive protein levels. The mean Ranson score was 3.9 (range 0–8) and APACHE II scoring was 12.6 (range 5–28) (P < .01 vs. edematous pancreatitis) (see Table 2). Endoscopic retrograde cholangiography was carried out in 58 patients, and papillotomy and stone removal were performed in 20 of those patients. Again, endoscopy was performed in the first 24 to 48 hours in 38 patients with biliary etiology and later in 20 patients with a cause other than gallstones. Single and multiple organ failure occurred in 32 and 30 patients, respectively (P < .00001 vs. edematous pancreatitis) (Table 3).
Table 3. ORGAN FAILURE IN PATIENTS WITH PANCREATITIS
EP, edematous pancreatitis; IPN, infected pancreatic necrosis; NP, necrotizing pancreatitis; SPN, sterile pancreatic necrosis.
Sterile Necrosis
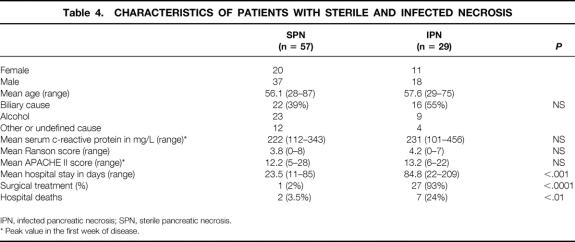
Data on gender, age, cause, C-reactive protein levels, Ranson score, APACHE II score, length of hospital stay, and hospital death rate are given in Table 4. The severity, based on the Ranson and APACHE II scores in the first week of the disease, was similar between sterile and infected necrosis. However, necrosis was considerably more extended in patients with infected necrosis (Table 5).
Table 4. CHARACTERISTICS OF PATIENTS WITH STERILE AND INFECTED NECROSIS
IPN, infected pancreatic necrosis; SPN, sterile pancreatic necrosis.
* Peak value in the first week of disease.
Table 5. MAXIMUM EXTENT OF NECROSIS ACCORDING TO COMPUTED TOMOGRAPHY FINDINGS
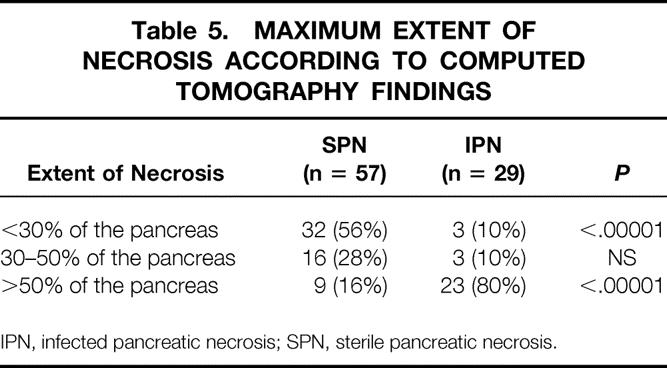
IPN, infected pancreatic necrosis; SPN, sterile pancreatic necrosis.
In the group with sterile necrosis, 56/57 patients were managed conservatively. In a woman with chronic lymphatic leukemia and sterile necrosis and early multiple organ failure (renal, pulmonary, cardiocirculatory, and metabolic), the study protocol was violated despite a negative FNA because of severe, rapid deterioration while receiving intensive care treatment. Surgical necrosectomy was carried out on day 11 after onset of symptoms. Intraoperative cultures were negative. After surgery, renal function and respiratory parameters improved. On day 18 an infection with methicillin-resistant Staphylococcus aureus and Candida albicans occurred, and the patient died on day 25 of septic multiple organ failure. Of the 56 patients managed conservatively, one patient with severe chronic alcohol disease died on day 13 of severe respiratory distress syndrome, not responding to resuscitation. Autopsy revealed massive necrosis retroperitoneally and severe diffuse lung injury. The death rate in patients with sterile necrosis managed without surgery was therefore 1.8% (1/56).
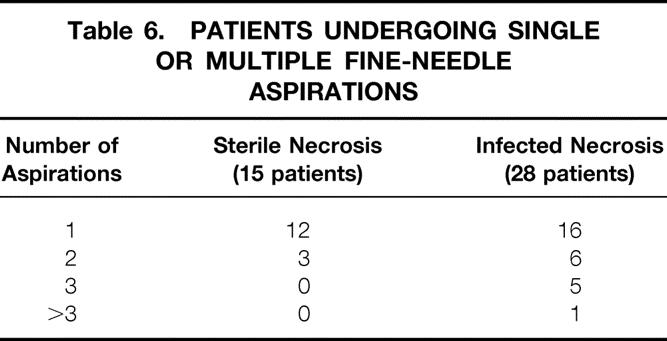
Fifteen patients (15/57; 26%) fulfilled the criteria for FNA. In these patients, 18 FNAs were performed, showing negative Gram stain as well as negative culture results (Table 6). The first FNA in these patients was performed on day 12 (range 4–22) after onset of symptoms.
Table 6. PATIENTS UNDERGOING SINGLE OR MULTIPLE FINE-NEEDLE ASPIRATIONS
Follow-Up
During the study period, 11 patients (19%) were readmitted to the hospital after a mean of 6.9 months for various pancreatitis-related complications such as recurrent pancreatitis (n = 4), pancreatic pseudocyst (n = 4), duodenal obstruction (n = 2), and splenic vein thrombosis (n = 1). One patient initially classified as and treated for sterile necrosis was now found to suffer from infected necrosis. This patient was readmitted after being at home for 14 days and subsequently underwent necrosectomy and postoperative lavage of the retroperitoneal cavity. The patient recovered after the second hospital admission. In four patients a pseudocyst was treated with a cystojejunostomy after a mean of 4.25 months (range 2–6). In none of them was the pseudocyst infected.
Infected Necrosis
FNA Results
In 27 patients with NP, preoperative CT-guided FNA revealed infected pancreatic necrosis. The first FNA was performed a mean of 17 days (range 3–48) after onset of symptoms. In one patient with infected necrosis, contrast-enhanced CT demonstrated air in the necrotic areas, indicating infection. This patient underwent surgery without a prior FNA. In another patient who subsequently died, FNA was negative despite pancreatic infection, and the patient was managed conservatively. In 26 patients both preoperative FNA and intraoperative culture results were available. Of eight monocultures diagnosed in the preoperative FNA, seven were confirmed by intraoperative cultures. In the eighth, intraoperative culture results showed a polymicrobial infection. The result of 18 polymicrobial infections found by FNA was confirmed in 17 by intraoperative cultures. In the remaining one, intraoperative culture revealed gram-negative infection only. Thus, 92% (24/26) of the FNA findings were correct with regard to the microbiologic differentiation. Overall, 27 of 28 patients (96%) were correctly diagnosed as having infected necrosis.
Surgical Treatment
A total of 27 patients with infected necrosis underwent necrosectomy and subsequent continuous lavage of the necrotic cavity by means of double-lumen drainage tubes. 23 Treatment parameters of these patients are outlined in Table 7 and bacteriologic results are summarized in Table 8. There were 18 patients with polymicrobial and 10 patients with monomicrobial infection. Different fungus species were found in eight patients (29%), or 17% of all positive cultures. In one patient with rapidly progressing multiple organ failure, infection was proven by FNA on day 15, but the patient died that same day before surgery was performed. Another patient who had three FNAs with negative results responded to intensive care unit treatment and conservative management. Back on a regular ward, he suddenly was found dead in his bed. Autopsy revealed multiple septic pulmonary emboli and infected pancreatic necrosis. Thus, only 27 of 29 patients with infected pancreatic necrosis underwent surgery.
Table 7. TREATMENT RESULTS IN PATIENTS WITH INFECTED NECROSIS AND SURGICAL TREATMENT (n = 27)
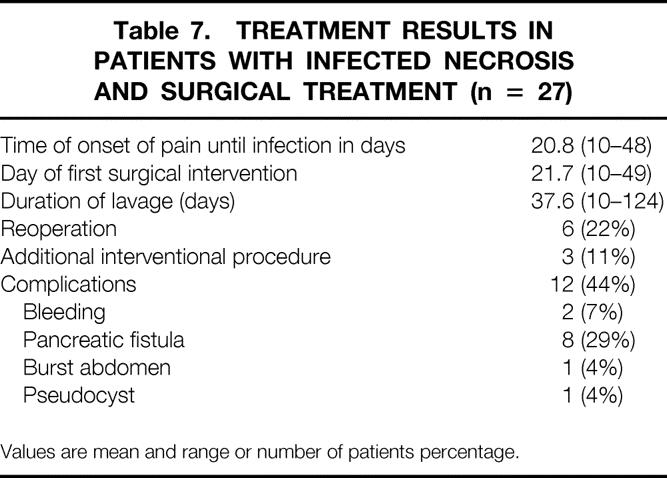
Values are mean and range or number of patients percentage.
Table 8. BACTERIOLOGIC FINDINGS IN 28 PATIENTS WITH INFECTED NECROSIS
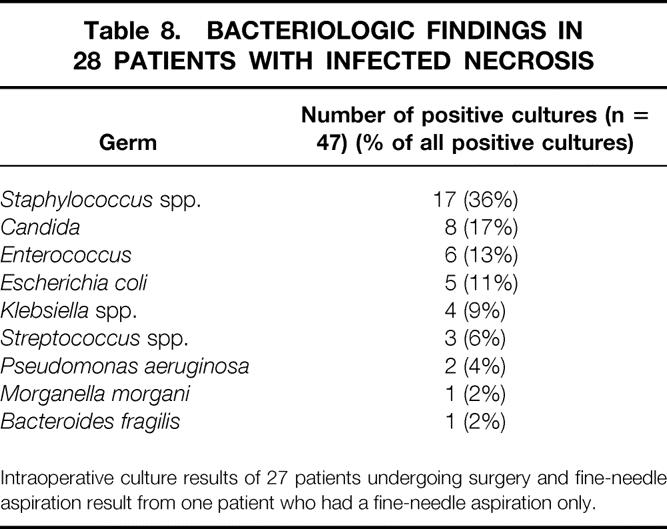
Intraoperative culture results of 27 patients undergoing surgery and fine-needle aspiration result from one patient who had a fine-needle aspiration only.
The death rate in patients with infected necrosis was 24% (7/29). Causes of death were nonresponding multiple organ failure (n = 4), myocardial infarction/insufficiency (n = 2), and multiple septic pulmonary emboli (n = 1).
Follow-Up
During the study period, seven patients (32%) were readmitted to the hospital after a mean of 4 months (range 1.5–6) for various pancreatitis-related complications: persistent pancreatic fistula (n = 3), recurrent pancreatitis (n = 1), pancreatic pseudocyst (n = 1), pancreatic abscess (n = 1), and chronic pain (n = 1). The patient with the abscess was successfully treated by a percutaneous drainage procedure. Two of the patients readmitted because of a fistula were managed conservatively; one patient was treated surgically. The pancreatic pseudocyst was surgically drained with a cystojejunostomy. All seven patients recovered and were discharged home.
Intent-to-Treat Analysis
Analysis of the patients in an intent-to-treat manner (nonsurgical vs. surgical treatment) showed that 3/58 (5%) died while receiving conservative management (1 patient with sterile necrosis, 2 patients with infected necrosis) and 6/28 (21%) died after surgery (5 with infected necrosis, 1 with sterile necrosis) (P = .05). This intent-to-treat analysis seems necessary because in the patients whose NP was managed without surgery, two died in whom infection of necrosis was not diagnosed in a timely fashion.
DISCUSSION
The concept of conservative treatment of severe acute pancreatitis originates from several sources. First, we learned that an episode of severe acute pancreatitis progresses in two phases. The first 10 to 14 days are characterized by a systemic inflammatory response syndrome maintained by the release of various inflammatory mediators. 3,4 The production of such mediators in large amounts may lead to distant organ failure, and surgery does not seem to be the appropriate intervention at that stage of the disease. Second, intensive care treatment has improved significantly during the past decade, and patients with severe acute pancreatitis are best managed there. 31 Third, septic complications in the second phase of the disease can be reduced and delayed by using appropriate antibiotics. 16,21,22,32 Fourth, one previous study provided evidence that conservative management is feasible in patients with sterile necrosis. 24
Our study demonstrates and confirms that conservative treatment of sterile necrosis using early antibiotics is safe and effective. Of 56 patients with sterile NP managed without surgery, 1 patient died of severe respiratory distress syndrome. Recent prospective controlled trials have demonstrated that the prevalence of pancreatic infection in NP could be lowered significantly to 10% to 43% if antibiotics with proven efficacy in acute pancreatitis were given early. 17,19,33 Our trial supports this by showing an infection rate of 34% in NP, which is lower than the prevalence data of up to 70% from the 1980s observed in patients not receiving antibiotic treatment prophylactically. 8,34,35 Pancreatic infection is regarded as the main risk factor for death in patients with acute pancreatitis, 9 and preventing this risk factor seems to represent a major step forward in the management of NP. This statement is further supported by the data from several contemporary randomized studies that have been subjected to meta-analysis, showing an improvement in outcome associated with antibiotic treatment. 21,22,32 Consequently, a significant body of clinicians use antibiotic prophylaxis in the initial treatment of patients with predicted severe disease. 36 Ruling out infection in NP and thereby preventing surgical measures, which are still standard approaches for infected necrosis, might reduce hospital expenses considerably in the future. Data from other groups argue for surgical treatment in sterile necrosis, particularly in patients with ongoing multiple organ failure or local complications such as an inflammatory enlargement of the pancreas causing obstruction of the gastrointestinal tract or common bile duct. 25,26 Our trial demonstrates that 33 of 35 patients with sterile necrosis and organ failure responded well to intensive care treatment including mechanical ventilation, catecholamine treatment for cardiocirculatory failure, and hemodialysis for renal failure. However, in our series one patient with early multiple organ failure not responding to intensive care died after surgical necrosectomy for sterile necrosis. It can be presumed that if antibiotic treatment is successful in preventing infection, surgery in NP might not be able to achieve a better goal than conservative treatment. Thus, in the event of rapidly progressing multiple organ failure in sterile necrosis, surgical measures might not overcome this problem, just as conservative treatment does not in the rare cases of so-called “fulminant pancreatitis.”37
In our study, severity staging showed no differences between patients with sterile and infected necrosis. This finding may be explained by the fact that Ranson and APACHE II scores were obtained during the first week of the disease process. The Ranson criteria, by definition, are recorded in the initial 48 hours. The APACHE II score was calculated daily and the maximum score in the first week was taken for statistical analysis. However, infection of peripancreatic or pancreatic necrosis was diagnosed after a mean of 21 days. It seems likely that this is why patients with infected necrosis, despite the initially documented equal severity of disease, stayed considerably longer in the hospital and had more organ failures that occurred after the first week in the hospital. Others have reported similar results, 1 but some have not. 5
The reason for the difference in the prevalence of organ failure, especially in the subgroup of patients with sterile necrosis, might arise from different criteria for classifying patients and defining organ failure. Also, in patients with infected necrosis, contrast-enhanced CT revealed more patients with extended necrosis compared with those with sterile necrosis. It cannot be determined from our trial whether our result (i.e., more extensive necrosis in the patients with infected necrosis) is the cause of the occurrence of infection or whether infection is the cause of the extended necrosis. Several authors consider extended necrosis (i.e., necrosis of >50% of the pancreas) to be a risk factor for infection. 2,23,38,39 A recent paper demonstrated that in patients with sterile necrosis, the extent of necrosis correlated with the frequency of organ failure, whereas infected necrosis was associated with organ failure irrespective of the extent of necrosis, 1 supporting the concept that infection is the major determinant for the outcome.
The present death rate of 10% in patients with NP closely parallels the one described by Branum et al, 40 who described 50 patients with NP treated with surgery; 12% of them died. Similar to the results of Bradley and Allen, 24 we found a higher death rate in patients with infected necrosis. However, in two patients with infected necrosis who ultimately died, the infection was not diagnosed in time and therefore no surgery was performed. In an intent-to-treat analysis, these deaths were shifted to the patients with sterile necrosis, thereby rendering insignificant the difference in death rate between patients with infected and sterile necrosis.
In this series patients with sterile necrosis were discharged home after a mean of 23.5 days; only three patients stayed longer than 2 months (see Table 4). None of these three was readmitted to the hospital for persistent pain or pancreatitis. According to a recent report, 41 it might be possible that return to work in these patients could be hastened by performing surgery at 4 weeks if the patient remains symptomatic.
A second major finding of our trial was that early antibiotic treatment of NP changes the spectrum of bacteria in patients in whom infection develops. Bacterial translocation from the gut has been demonstrated to be the main cause of infection in NP. 42–45 Therefore, the bacterial spectrum of infection has been described as primarily gram-negative and in part anaerobic. After we administered antibiotics with a dominant efficacy against gram-negative germs and anaerobes (imipenem/cilastatin), the bacteria in more than half of our patients with pancreatic infection were found to be gram-positive. In addition, we found eight patients (29%) infected with fungus. In a series of 57 patients with NP, Grewe et al 46 described 7 (12%) with fungal infection. These patients were treated with a mean of four different antibiotics for a mean of 23 days. Such findings raise the question of the duration of primary antibiotic treatment (14 days in our trial) and of the need to use agents effective against gram-positive bacteria and fungi concomitantly or consecutively. In addition, it is likely that these “secondary” infections do not originate in the gut but rather are hospital-acquired, entering the pancreas by means of hematogenous routes from venous or urinary catheters, tracheal tubes, and so forth. This argument of nosocomial infection is supported by the time when infection occurred in our trial (later than 20 days); in earlier studies, approximately 40% of infections with gram-negative germs occurred within 2 weeks after admission. 8,17,19,47 The question of additional primary or secondary anti–gram-positive and antifungal treatments in NP must be addressed in future clinical trials.
Surgery remains the gold standard in the treatment of infected pancreatic necrosis, 48 and necrosectomy and continuous closed lavage was successful in 67% of our patients with infected necrosis. However, 22% needed a secondary intervention and 11% an additional interventional procedure, and the complication rate was 44%. Fernandez-del Castillo et al41 recently described 64 patients treated surgically and found a single surgical procedure to be sufficient in 69%. In the present study, infection of necrotic tissue occurred a mean of 21 days after onset of the disease, and surgery was performed during the 24 hours after its diagnosis. More than 3 weeks after the onset of the disease, the demarcation between viable and necrotic tissue is easier to assess than earlier in the disease process. This approach decreases the bleeding risk and minimizes the surgery-related loss of vital tissue leading to surgery-induced endocrine and exocrine pancreatic insufficiency. 49 The same policy of delaying surgery until demarcation of pancreatic necrosis is far advanced has been reported by others. 41
Recently, considerable interest has been generated in the nonsurgical management of infected necrosis using truly conservative or interventional measures. Single-center reports in so far small patient groups have demonstrated that even in infected necrosis, some patients recover with nonsurgical management. 50 It is important to differentiate between infected necrosis and pancreatic abscess, a condition that has been shown to respond to interventional treatment. 51 Nevertheless, there might be patients with infected necrosis without multiple organ failure who would respond to nonsurgical measures including antibiotics, and future trials need to define this population. To extend this argument, at least from a theoretical point of view it might be possible that in our group of patients classified as having sterile necrosis, some might have been infected and did not undergo FNA because of a silent clinical course, responding well to conservative management. Indeed, one patient originally classified as having sterile necrosis was readmitted to the hospital with infected necrosis 14 days after the primary discharge.
In summary, the data from our study support the concept of using conservative treatment for sterile NP versus surgery for infected necrosis, an approach that could improve the clinical success rate in NP in terms of the death rate of the past.
Footnotes
Correspondence: Markus W. Büchler, MD, Dept. of Visceral and Transplantation Surgery, Inselspital, University of Bern, CH-3010 Bern, Switzerland.
E-mail: markus.buechler@insel.ch
Accepted for publication February 23, 2000.
References
- 1.Isenmann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br J Surg 1999; 86: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 2.Reber HA, McFadden DW. Indications for surgery in necrotizing pancreatitis. West J Med 1993; 159 (6): 704–707. [PMC free article] [PubMed] [Google Scholar]
- 3.Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg 1998; 175 (1): 76–83. [DOI] [PubMed] [Google Scholar]
- 4.Gloor B, Reber HA. Effects of cytokines, and other inflammatory mediators on human acute pancreatitis. J Int Care Med 1998; 13 (6): 305–312. [Google Scholar]
- 5.Tenner S, Sica G, Hughes M, et al. Relationship of necrosis to organ failure in severe acute pancreatitis. Gastroenterology 1997; 113 (3): 899–903. [DOI] [PubMed] [Google Scholar]
- 6.Beger HG, Bittner R, Buchler M, et al. Hemodynamic data pattern in patients with acute pancreatitis. Gastroenterology 1986; 90 (1): 74–79. [DOI] [PubMed] [Google Scholar]
- 7.Knoefel WT, Kollias N, Warshaw AL, et al. Pancreatic microcirculatory changes in experimental pancreatitis of graded severity in the rat. Surgery 1994; 116 (5): 904–913. [PubMed] [Google Scholar]
- 8.Beger HG, Bittner R, Block S, Buchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology 1986; 91 (2): 433–438. [DOI] [PubMed] [Google Scholar]
- 9.Uhl W, Schrag HJ, Wheatley AM, Büchler MW. The role of infection in acute pancreatitis. Dig Surg 1994; 11: 214–219. [Google Scholar]
- 10.Gerzof SG, Banks PA, Robbins AH, et al. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology 1987; 93 (6): 1315–1320. [DOI] [PubMed] [Google Scholar]
- 11.Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg 1997; 21 (2): 130–135. [DOI] [PubMed] [Google Scholar]
- 12.Bassi C, Falconi M, Girelli F, et al. Microbiological findings in severe pancreatitis. Surg Res Comm 1989; 5: 1–4. [Google Scholar]
- 13.Bradley EL. Antibiotics in acute pancreatitis. Current status and future directions. Am J Surg 1989; 158 (5): 472–477. [DOI] [PubMed] [Google Scholar]
- 14.Buchler M, Uhl W, Beger HG. Complications of acute pancreatitis and their management. Curr Opin Gen Surg 1993; 282–286. [PubMed] [Google Scholar]
- 15.Schmid SW, Uhl W, Friess H, et al. The role of infection in acute pancreatitis. Gut 1999; 45 (2): 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchler M, Malfertheiner P, Friess H, et al. Human pancreatic tissue concentration of bactericidal antibiotics. Gastroenterology 1992; 103 (6): 1902–1908. [DOI] [PubMed] [Google Scholar]
- 17.Luiten EJ, Hop WC, Lange JF, Bruining HA. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg 1995; 222 (1): 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pederzoli P, Bassi C, Vesentini S, Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet 1993; 176 (5): 480–483. [PubMed] [Google Scholar]
- 19.Sainio V, Kemppainen E, Puolakkainen P, et al. Early antibiotic treatment in acute necrotising pancreatitis. Lancet 1995; 346 (8976): 663–667. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz M, Isenmann R, Meyer H, Beger HG. [Antibiotic use in necrotizing pancreatitis. Results of a controlled study]. Dtsch Med Wochenschr 1997; 122 (12): 356–361. [DOI] [PubMed] [Google Scholar]
- 21.Powell J, Miles R, Siriwardena A. Antibiotic prophylaxis in the initial management of severe acute pancreatitis. Br J Surg 1998; 85: 582–587. [DOI] [PubMed] [Google Scholar]
- 22.Golub R, Siddiqi F, Pohl D. Role of antibiotics in acute pancreatitis: a meta-analysis. J Gastrointest Surg 1998; 2: 496–503. [DOI] [PubMed] [Google Scholar]
- 23.Beger HG, Buchler M, Bittner R, et al. Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br J Surg 1988; 75 (3): 207–212. [DOI] [PubMed] [Google Scholar]
- 24.Bradley EL, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg 1991; 161 (1): 19–25. [DOI] [PubMed] [Google Scholar]
- 25.Rau B, Pralle U, Uhl W, et al. Management of sterile necrosis in instances of severe acute pancreatitis. J Am Coll Surg 1995; 181 (4): 279–288. [PubMed] [Google Scholar]
- 26.Rattner DW, Legermate DA, Lee MJ, et al. Surgical debridement of symptomatic pancreatic necrosis is beneficial irrespective of infection. Am J Surg 1992; 163: 105–110. [DOI] [PubMed] [Google Scholar]
- 27.Levitt MD, Eckfeldt JH. Diagnosis of acute pancreatitis. In: Go VLW, Dimagno EP, Gardner JD, et al, eds. The Pancreas: Biology, Pathobiology, and Disease. New York: Raven Press; 1993: 613–635.
- 28.Uhl W, Buchler MW, Malfertheiner P, et al. A randomized, double-blind, multicenter trial of octreotide in moderate to severe acute pancreatitis. Gut 1999; 45: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchler M, Malfertheiner P, Uhl W, et al. Gabexate mesylate in human acute pancreatitis. Gastroenterology 1993; 104: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 30.Sakorafas GH, Tsiotos GG, Sarr MG. Extrapancreatic necrotizing pancreatitis with viable pancreas: a previously underappreciated entity. J Am Coll Surg 1999; 188 (6): 643–648. [DOI] [PubMed] [Google Scholar]
- 31.Sigurdsson GH. Intensive care management of acute pancreatitis. Dig Surg 1994; 11: 231–241. [Google Scholar]
- 32.Wyncoll D. The management of severe acute necrotizing pancreatitis: an evidence-based review of the literature. Intensive Care Med 1999; 25: 146–156. [DOI] [PubMed] [Google Scholar]
- 33.Bassi C, Falconi M, Talamini G, et al. Controlled clinical trial of pefloxacin versus imipenem in severe acute pancreatitis. Gastroenterology 1998; 115: 1513–1517. [DOI] [PubMed] [Google Scholar]
- 34.Rattner DW, Warshaw AL. Surgical intervention in acute pancreatitis. Crit Care Med 1988; 16 (1): 89–95. [DOI] [PubMed] [Google Scholar]
- 35.Frey CF, Bradley EL, Beger HG. Progress in acute pancreatitis. Surg Gynecol Obstet 1988; 167 (4): 282–286. [PubMed] [Google Scholar]
- 36.Powell JJ, Campbell E, Johnson CD, Siriwardena AK. Survey of antibiotic prophylaxis in acute pancreatitis in the UK and Ireland. Br J Surg 1999; 86 (3): 320–322. [DOI] [PubMed] [Google Scholar]
- 37.Farthmann EH, Lausen M, Schoffel U. Indications for surgical treatment of acute pancreatitis. Hepatogastroenterology 1993; 40 (6): 556–562. [PubMed] [Google Scholar]
- 38.Reber HA, Truman HS. Surgical intervention in necrotizing pancreatitis. Gastroenterology 1986; 91: 479–481. [DOI] [PubMed] [Google Scholar]
- 39.McFadden DW, Reber HA. Indications for surgery in severe acute pancreatitis. Int J Pancreatol 1994; 15 (2): 83–90. [DOI] [PubMed] [Google Scholar]
- 40.Branum G, Galloway J, Hirchowitz W, et al. Pancreatic necrosis: results of necrosectomy, packing, and ultimate closure over drains. Ann Surg 1998; 227: 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandez-del Castillo C, Rattner DW, Makary MA, et al. Débridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg 1998; 228: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moody FG, Haley-Russell D, Muncy DM. Intestinal transit and bacterial translocation in obstructive pancreatitis. Dig Dis Sci 1995; 40: 1798–1804. [DOI] [PubMed] [Google Scholar]
- 43.Runkel NS, Moody FG, Smith GS, et al. The role of the gut in the development of sepsis in acute pancreatitis. J Surg Res 1991; 51 (1): 18–23. [DOI] [PubMed] [Google Scholar]
- 44.Widdison AL, Karanjia ND, Reber HA. Routes of spread of pathogens into the pancreas in a feline model of acute pancreatitis. Gut 1994; 35: 1306–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widdison AL, Alvarez C, Chang YB, et al. Sources of pancreatic pathogens in acute pancreatitis in cats. Pancreas 1994; 9: 536–541. [DOI] [PubMed] [Google Scholar]
- 46.Grewe M, Tsiotos GG, Luque de-Leon E, Sarr MG. Fungal infection in acute necrotizing pancreatitis. J Am Coll Surg 1999; 188: 408–414. [DOI] [PubMed] [Google Scholar]
- 47.Beger HG. Surgical management of necrotizing pancreatitis. Surg Clin North Am 1989; 69: 529–549. [DOI] [PubMed] [Google Scholar]
- 48.Gloor B, Uhl W, Büchler MW. Changing concepts in the surgical management of acute pancreatitis. Baillière’s Clin Gastroenterol 1999; 13: 303–315. [DOI] [PubMed] [Google Scholar]
- 49.Aultman DF, Bilton BD, Zibari GB, et al. Nonoperative therapy for acute necrotizing pancreatitis. Am Surg 1997; 63: 1114–1117. [PubMed] [Google Scholar]
- 50.Freeny PC, Hauptmann E, Althaus SJ, et al. Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. Am J Roentgenol 1998; 170 (4): 969–975. [DOI] [PubMed] [Google Scholar]
- 51.Bradley EL. A clinically based classification system for acute pancreatitis. Arch Surg 1993; 128: 586–590. [DOI] [PubMed] [Google Scholar]