Abstract
Objective
To evaluate intrahepatic vascular and biliary anatomy of the left lateral segment (LLS) as applied to living-donor and split-liver transplantation.
Summary Background Data
Living-donor and split-liver transplantation are innovative surgical techniques that have expanded the donor pool. Fundamental to the application of these techniques is an understanding of intrahepatic vascular and biliary anatomy.
Methods
Pathologic data obtained from cadaveric liver corrosion casts and liver dissections were clinically correlated with the anatomical findings obtained during split-liver, living-donor, and reduced-liver transplants.
Results
The anatomical relation of the left bile duct system with respect to the left portal venous system was constant, with the left bile duct superior to the extrahepatic transverse portion of the left portal vein. Four specific patterns of left biliary anatomy and three patterns of left hepatic venous drainage were identified and described.
Conclusions
Although highly variable, the biliary and hepatic venous anatomy of the LLS can be broadly categorized into distinct patterns. The identification of the LLS duct origin lateral to the umbilical fissure in segment 4 in 50% of cast specimens is significant in the performance of split-liver and living-donor transplantation, because dissection of the graft pedicle at the level of the round ligament will result in separate ducts from segments 2 and 3 in most patients, with the further possibility of an anterior segment 4 duct. A connective tissue bile duct plate, which can be clinically identified, is described to guide dissection of the segment 2 and 3 biliary radicles.
Reduced-organ liver transplantation (RLT) involves the surgical creation of a liver graft from a living-donor, 1–3 the reduction of a larger cadaveric graft, 4,5 or the division of an adult cadaveric liver during split-liver transplantation. 6–9 The use of RLT techniques has increased the supply of pediatric donor organs and reduced pretransplant complications and death, 2,10,11 but the use of RLT involves unique surgical considerations and predisposes to specific complications. 3,12–15 Surgical considerations in the performance of RLT include adequacy of the transplanted liver volume to meet the recipient’s metabolic needs; anatomical positioning of the highly variable grafts to optimize vascular inflow, venous outflow, and biliary drainage; and use of microsurgical techniques to perform complex biliary and vascular reconstructions. 12,16–18
In our 7 years of experience performing RLT, we have found that biliary complications occur in approximately 15% of RLT grafts 19,20; other centers have reported similar figures. 21,22 We propose that this relatively high incidence of biliary complications is not due to inherent technical failures but rather to incomplete understanding of the surgical anatomy of the left bile duct system as applied to the creation of a left lateral segment (LLS) graft, and a lack of accurate noninvasive techniques before and during surgery to assess intrahepatic biliary and vascular drainage territories. The aim of this clinicopathologic study was to investigate the anatomy of the human left bile duct system as applied to RLT through ex vivo analysis of human liver casts and the correlation of these data with in vivo anatomical data collected during the performance of 34 living-donor, 12 reduced-cadaveric liver, and 2 split-liver transplantations.
METHODS
Human Liver Corrosion Casts and Cadaveric Dissections
The creation of human liver casts by injection of acrylic resin followed by caustic tissue corrosion has been previously described. 23 In the present study, 40 human cadaver livers were surgically removed at autopsy (prior research consent granted) with preservation of the hilar vascular pedicle, the inferior vena cava to the confluence of the renal veins, and the superior vena cava to the origin of the right atrium. The origins of the hepatic artery, portal vein, and common bile duct were identified and the cystic duct was isolated and suture-ligated. The inferior vena cava was prepared by ligation of the adrenal, phrenic, and lumbar veins, with double suture-ligation of the superior margin of the inferior vena cava. The inferior margin of the inferior vena cava, portal vein, hepatic artery, and common bile duct were then cannulated with polyethylene tubing and the liver was gently perfused with physiologic saline to flush the organ. After suture-ligation of identified leaks and drainage of the saline solution, acrylic resin was infused (Jet Acrylic Resin; Lang Dental, Chicago, IL; 1:1 concentration) into the inferior vena cava, portal vein, hepatic artery, and common bile duct. The resins were color-coded to assist in identification of structures. After infusion, the acrylic resin was polymerized for 1 hour at room temperature (15°C) before digestion of the hepatic parenchyma. Digestion was achieved by whole-organ immersion in 20 L 1.8 mol/L NaOH solution with aluminum shavings for 24 hours at 15°C. After digestion, the casts were removed, thoroughly washed with water, and carefully cleaned to remove any residual organic material before examination.
The accuracy of our technique in the duplication of intrahepatic anatomy has been previously verified. 23 Measurements performed before and after parenchymal digestion consistently revealed a decrease of no more than 5% in total organ anteroposterior and lateral dimensions.
Human liver casts were examined for the relation of Couinaud segment 2, 3, and 4 bile ducts and vascular structures to the umbilical fissure and a plane that transects segment 4 located 1 cm lateral to the umbilical fissure. Identification of the umbilical fissure was determined by an imaginary line extending from the middle of the umbilical portion of the portal vein to the left hepatic vein at a point 5 mm from its outlet to the inferior vena cava. This line defines a plane that passes through the center of the umbilical fissure (personal communication, R. Uflacker, 1997).
The union of segment 2 and 3 bile ducts to form the LLS bile duct was compared with this plane defining the umbilical fissure. The LLS duct is the structure formed by the union of biliary ducts from segments 2 and 3; the left hepatic duct is the union of the LLS duct and the duct or ducts of segment 4. The occurrence of a segment 4 duct crossing the plane of the umbilical fissure was also noted.
Cadaveric dissections were performed on 20 autopsy specimens (previous consent given for organs to be used for research purposes). The dissections followed the planes used in RLT to mimic these surgical procedures, and the analyses were performed as described above.
Patients and Data Collection
We performed a retrospective review of all pertinent clinical, surgical, and pathologic data on children undergoing RLT at the University of California San Francisco from June 1990 through August 1997. During this period, 99 children underwent orthotopic liver transplantation. Of this group, 27 boys and 22 girls, representing 49% of our pediatric transplant population, were identified as recipients of RLTs: 34 living-donor, 12 reduced-liver, 2 split-liver, and 1 auxiliary orthotopic liver transplantations. We previously used reduced-adult cadaveric liver transplantation for children awaiting transplantation but have replaced this procedure with ex vivo split-liver transplantation as described by Broelsch et al. 6 The distribution of the 49 grafts were Couinaud segments 2 and 3, n = 45; 1 through 4, n = 3; and 2, 3, and 4, n = 1. RLT recipient indications for transplantation, United Network for Organ Sharing status, and demographic data were those typically encountered at a regional pediatric referral center (Table 1).
Table 1. RECIPIENT STUDY POPULATION
Surgical Techniques
Reduced-organ grafting is based on the segmental hepatic anatomy classically described by Couinaud, 24,25 with the surgical techniques well defined. 26–29 Briefly, creation of an LLS graft (segments 2 and 3) is achieved by hilar dissection at the base of the round ligament with isolation of the left hepatic artery, left portal vein branch, and left bile duct branch, followed by parenchymal transection immediately lateral to the falciform ligament. 6 Dissection of the left bile duct in living- and split-liver donation requires a compromise between protecting the common hepatic duct in the donor and optimizing the caliber and length of the duct in the recipient. Arterial anastomoses are performed using interrupted 7-O nonabsorbable suture; venous anastomoses are performed using running absorbable 7-O suture with intraoperative magnification (hepatic artery ≥6×, portal vein and suprahepatic and infrahepatic vena cava ≥2.5×). Biliary anastomoses are performed by end-to-side hepatojejunostomy between the LLS duct from segments 2 and 3 and the jejunum without an internal stent using single-layer, interrupted 7-O absorbable suture and at least 4× magnification.
Since April 1994, we have incorporated routine planned exploration with protocol open liver biopsy of pediatric RLT recipients on posttransplant day 7 as a way to avert posttransplant complications resulting from technical problems. 19 Previous data from our group demonstrated that routine application of this technique reduces posttransplant complications and the hospital stay of patients found to have technical complications without significantly increasing the hospital stay of children who undergo a negative laparotomy. 19
RESULTS
Pathologic Analysis: Left Duct Biliary Anatomy
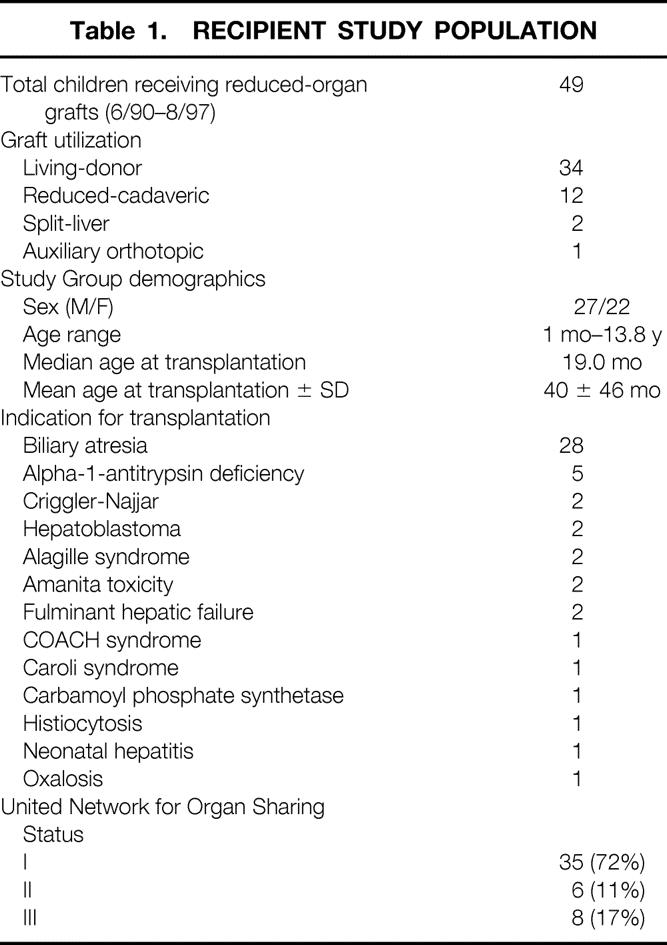
Analysis of hepatic casts and cadaveric dissections revealed four distinct patterns of left bile duct anatomy (Fig. 1).
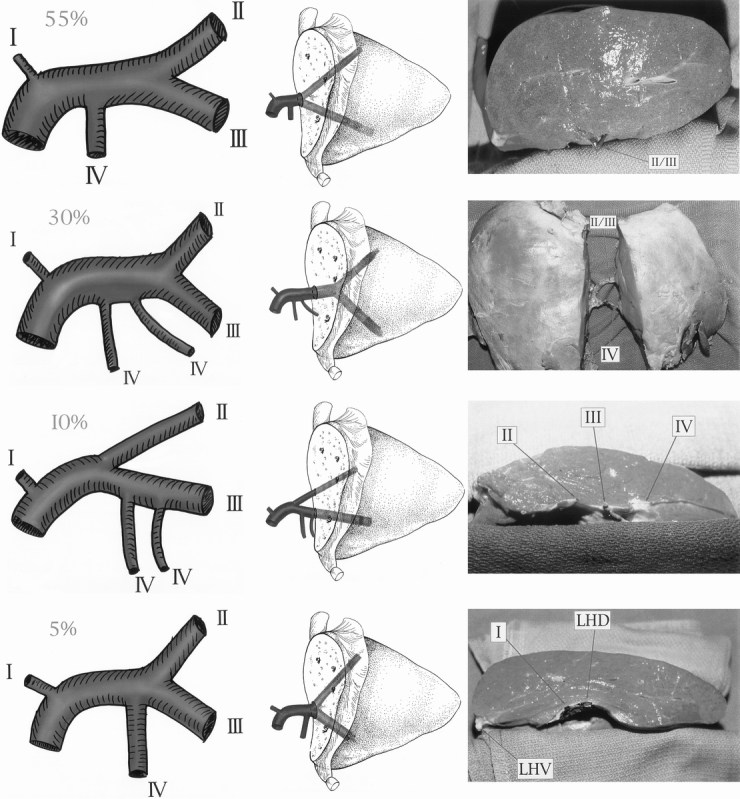
Figure 1. The four biliary variants of the left lateral segment. Each variant is depicted in cartoon form (left panels), in relation to the left lateral segment graft (center panels), and by photograph (right panels). LHD, left hepatic duct; LHV, left hepatic vein.
The most common pattern was the union of segment 2 and 3 ducts to form the LLS duct close to the umbilical fissure (see Fig. 1A). The incidence of this pattern was 55%. The union of segment 2 and 3 ducts was at the umbilical fissure in 5% of specimens, to the right of the umbilical fissure in 50% of specimens, and to the left of the umbilical fissure in 45% of specimens with this specific anatomical pattern. The LLS duct was then joined by a single segment 4 duct between the umbilical fissure and the hilum to form the left hepatic duct.
The second most common anatomical pattern identified was creation of the LLS duct close to the umbilical fissure with two parallel ducts from segment 4 joining to form the left hepatic duct (see Fig. 1B). Typically, one segment 4 duct is on the umbilical portion of the left portal vein, and one is close to the union of the right hepatic duct. The incidence of this pattern was 30%.
The third biliary pattern identified was a single segment 3 duct that receives the duct from segment 4 and joins segment 2 close to the hepatic hilum (see Fig. 1C). In this variant, an LLS duct is absent. This pattern was identified in 10% of specimens.
The fourth biliary pattern was defined by segment 2 and 3 ducts joining immediately to the right of the umbilical fissure to form a short LLS duct that receives the segment 4 duct just after crossing the umbilical fissure to form the left hepatic duct (see Fig. 1D). The incidence of this pattern was 5%. Notably, in 10% of specimens, at least one distinct duct from the right liver was observed to cross Cantlie’s line and join the left duct system.
Transection of the liver through segment 4 in a plane 1 cm to the right of the umbilical fissure yielded a single LLS duct from segment 2 and 3 in 90% of specimens.
Relation of the Left Biliary Duct System to the Left Portal Vein
The relation of the left bile duct to the portal vein and hepatic artery was analyzed. The relation of the left hepatic artery with respect to the left portal vein branch has been previously described, 24,25 but few anatomical descriptions of left biliary anatomy with respect to the portal vein exist. We determined that the orientation of the left biliary duct system as superior to the transverse portion of the left portal vein was constant. The left hepatic duct remained anterosuperior in 35%, posterosuperior in 35%, and exactly midline on the portal vein in 20% of specimens; in 10% of variants where there is a late union to form the left hepatic duct, the segment 2 duct remained posterosuperior while the segment 3 and 4 ducts coursed anterosuperior to join just before the hilum. The orientation in a specific cast was preserved throughout the extrahepatic course of the portal vein and maintained as the left portal vein branch penetrated the hepatic parenchyma at the umbilical fissure.
Violation of the Umbilical Fissure by Segment 4 Biliary Radicles
As noted in previous reports from our center and others, biliary complications, specifically missed biliary radicles, occur in as many as 15% of RLT recipients. 19–22 The observance of missed biliary radicles may represent tributaries from segment 2 and 3 ducts or branches originating from segment 4. In 30% of specimens, biliary radicles originating from segment 4 were identified as crossing the umbilical fissure. The most common biliary pattern to exhibit segment 4 radicles that crossed the umbilical fissure was an LLS duct that receives two segment 4 ducts with a left branch close to the fissure. When segment 4 biliary radicles were observed to cross the umbilical fissure, they were consistently located anterior to the left portal vein and the left lateral bile duct (Fig. 2).
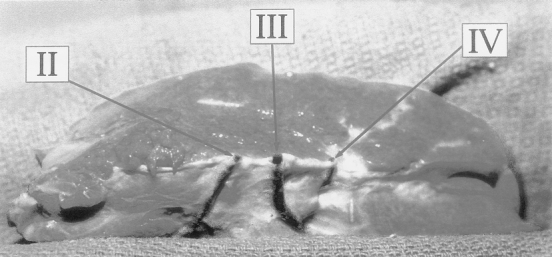
Figure 2. Cadaveric dissection depicting segment 4 biliary radicles crossing the umbilical fissure to drain the anterior aspect of segment 3. Segment 4 radicles are located anterior to the principal duct of segment 3 outside the connective tissue sheath and may be the source of posttransplant biliary leaks if not identified and ligated.
Anatomy of the Left Hepatic Vein
Analysis of the hepatic venous anatomy of the LLS revealed three distinct anatomical variants of the left hepatic vein (Fig. 3).
Figure 3. The three variants of the left hepatic vein.
The most common variant, observed in 73% of specimens, was a union of segment 2 and 3 veins to form a principal left hepatic vein at the umbilical fissure (see Fig. 3A). Notably, this variant would receive significant tributaries draining the posterior aspects of segment 4 as it approached the inferior vena cava.
The second most commonly observed pattern was separate large veins, each draining an individual segment, that united to form the left hepatic vein at the level of the inferior vena cava (see Fig. 3B). In this pattern, each venous channel received tributaries from the posterior aspects of segment 4 before uniting just before the inferior vena cava. This was observed in 14% of specimens.
The third anatomical variant, identified in 13% of specimens, was a union of segment 2 and 3 draining veins in the parenchyma of the graft to form the left hepatic vein medial to the umbilical fissure (see Fig. 3C). In this variant, the left hepatic vein is a large single vessel that directly empties into the inferior vena cava without receiving significant tributaries from segment 4.
Clinical Analysis: Identification of a Left Bile Duct Plate
To correlate our anatomical findings with in vivo clinical data, we undertook a retrospective review of 34 living-donor, 12 reduced-liver, 2 split-liver, and 1 auxiliary orthotopic liver transplantations performed at our center from July 1992 through May 1997 to determine the incidence of clinical identification of a transverse connective tissue plate at the conjunction of the ducts of segments 2 and 3. Duct anatomy was determined in situ after blind peripheral transection of the transverse plate at the level of the round ligament. Since December 1995, the transverse connective tissue plate at the confluence of the left bile duct has been routinely identified (see Fig. 2). Before recognition of the left bile duct plate, a single LLS duct was believed to have been identified at the level of the round ligament in 24 of 28 cases (86%), and a single hepatojejunostomy was performed. However, five biliary radicle leaks and one stricture, all requiring reoperation, were identified in the group of RLT recipients in whom the LLS duct was misidentified (21%). Since recognition of the left bile duct connective tissue plate, we have correctly identified the principal drainage of segments 2 and 3 in 100% of patients, with individual ducts from segments 2 and 3 identified in the connective tissue transverse plate requiring separate hepatojejunostomy in 35% of recipients. From this group, only one minor biliary radicle leak was later identified at the posttransplant day 7 planned exploration.
DISCUSSION
The purpose of this clinicopathologic study was to evaluate the vascular and biliary anatomy of the LLS as applied to RLT. Despite technical improvements in surgical technique with respect to vascular complications, the incidence of biliary complications continues to be high. At the Fourth Congress of the International Liver Transplantation Society in October 1997, the Kyoto group reported a 14% incidence of biliary complications in 205 living-related transplant recipients. 22 Our group has recently reported a 15% incidence of biliary radicle leaks identified at planned exploration in 42 RLT recipients. 20 We have proposed that these complications are not the result of technical failures but involve our imperfect understanding of the segmental biliary and vascular anatomy as it applies to performance of RLT.
Ex vivo acrylic corrosion casts and cadaveric dissections were created to study the left biliary duct anatomy and its relation to the left portal vein. A constant anatomical finding was the superior location of the left bile duct to the transverse portion of the left portal vein. This orientation was maintained as the portal vein made the transition from extrahepatic to intrahepatic beyond the umbilical fissure. Four specific patterns of left biliary anatomy as applied to segments 2, 3, and 4 were identified: segment 2 and 3 bile ducts unite close to the umbilical fissure to form a single LLS duct that receives a single segment 4 duct medially (55%), segment 2 and 3 bile ducts unite medial to the umbilical fissure with two parallel segment 4 ducts joining the single LLS duct to form the left hepatic duct (30%), segment 3 duct that receives the duct from segment 4 and joins segment 2 close to the hepatic hilum (10%), and segments 2 and 3 ducts unite lateral to the umbilical fissure to form a short LLS duct that immediately receives the segment 4 duct forming the left hepatic duct (5%). The prevailing pattern, identified in 55% of specimens, is one that facilitates the reduction of grafts close to the falciform ligament. Further, the union of segments 2 and 3 bile ducts to form the LLS duct was evaluated with reference to a plane representing the umbilical fissure, which extended from the middle of the umbilical portion of the portal vein to a point on the left hepatic vein 5 mm from its outlet to the vena cava. The LLS duct originated at the umbilical fissure in 5%, to the right of the umbilical fissure in 50%, and to the left of the umbilical fissure in 45% of specimens. Therefore, performance of RLT at the umbilical fissure would be expected to yield two posterior ducts, one from segment 2 and one from segment 3, in more than half of all procedures. This finding was verified in our clinical experience through identification of a connective tissue bile duct plate at the base of the round ligament that contained separate ducts to segments 2 and 3, necessitating individual anastomoses in 35% of patients. This connective tissue bile duct plate structure at the origin of the LLS bile duct is analogous to the hilar plate at the main biliary confluence. Busuttil et al 9 recently reported two bile ducts requiring separate anastomoses in approximately 20% of split-liver procedures performed along the same surgical planes; Broelsch and Rogiers 30 reported the discovery of two distinct bile ducts from segments 2 and 3 in 30% of RLT procedures performed by dissecting to the right of the round ligament. To achieve a 90% chance of a single duct from segments 2 and 3, the plane of dissection would have to be lateral to the umbilical fissure, in segment 4, by at least 1 cm.
A second finding identified by anatomical study of liver casts and dissections was the incidence of segment 4 ducts crossing the umbilical fissure. Violation of the umbilical fissure by segment 4 ducts was noted in 30% of specimens and was most commonly associated with a left biliary duct pattern consisting of two segment 4 ducts, with one close to the umbilical fissure. When dissecting the pedicle of the graft at the level of the round ligament, segment 4 crossing ducts were consistently located anterior to the connective tissue bile duct plate and received minimal radicles from segments 2 or 3. In our clinical experience, the anterior location of these ducts has resulted in frequent oversights, with later discovery at planned exploration. However, their significance in biliary drainage is trivial, and they are readily amenable to suture-ligation. Healey and Schroy 31 reported a 20% incidence of segment 4 bile ducts crossing the umbilical fissure and described the union of segment 2 and 3 bile ducts in the umbilical fissure in 50%, lateral to the umbilical fissure in 42%, and medial to the fissure in 8% of autopsy specimens. Russel et al, 32 in a review of 838 cholangiograms and 15 liver autopsy specimens, described the union of segment 2 and 3 bile ducts immediately lateral to the plane of the falciform ligament in most specimens.
In the performance of RLT, left bile duct dissection requires a compromise between protecting the common hepatic duct in the donor (or larger graft) and optimizing the caliber and length of the duct in the segment 2–3 graft. Clarification of left bile duct anatomy is imperative because many centers are adopting RLT techniques and are likely to encounter similar complications during their development. To date, we have yet to adopt a preoperative imaging modality that accurately depicts the biliary anatomy without unwarranted risk to the donor, and we have elected not to subject living donors to endoscopic or percutaneous cholangiograms. Although the application of three-dimensional magnetic resonance imaging biliary cholangiography offers tremendous promise in the workup of the living donor, such tests are unnecessary, and they are probably impossible to obtain in the setting of an in situ split-liver procurement as described by Busuttil et al. 9 This study is an early attempt to improve our anatomical knowledge in the application of RLT.
Footnotes
Correspondence: John F. Renz, MD, PhD, UCSF Liver Transplant Program, 505 Parnassus Ave. M896, San Francisco, CA 94143-0780.
Supported by an NIH National Research Service Award (DK09283), the Association for Academic Surgery Davis & Geck Research Fellowship, and the Hefni Scholars Award.
E-mail: renz@itsa.ucsf.edu
Accepted for publication January 19, 2000.
References
- 1.Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors. Ann Surg 1991; 214: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emond JC, Heffron TG, Kortz EO, et al. Improved results of living related liver transplantation with routine application in a pediatric program. Transplantation 1993; 55: 885–840. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Uemoto S, Tokunaga Y, et al. Surgical techniques and innovations in living related liver transplantation. Ann Surg 1993; 217: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery 1984; 95: 367–386. [PubMed] [Google Scholar]
- 5.Houssin D, Couinaud C, Boillot O, et al. Controlled hepatic bipartition for transplantation for children. Br J Surg 1991; 78: 802. [DOI] [PubMed] [Google Scholar]
- 6.Broelsch CE, Emond JC, Whitington PF, et al. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg 1990; 212: 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogiers X, Malago M, Habib N, et al. In situ splitting of the liver in the heart-beating cadaveric organ donor for transplantation in two recipients. Transplantation 1995; 59: 1081–1085. [PubMed] [Google Scholar]
- 8.Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers: the ultimate expansion of the donor pool. Ann Surg 1996; 224: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss JA, Yersiz H, Shackleton CR, et al. In situ splitting of the cadaveric liver for transplantation. Transplantation 1997; 64: 871–877. [DOI] [PubMed] [Google Scholar]
- 10.Otte JB, de Ville de Goyet J, Sokal E, et al. Size reduction of the donor liver is a safe way to alleviate the shortage of size-matched organs in pediatric liver transplantation. Ann Surg 1990; 211: 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryckman FC, Flake AW, Fisher RA, et al. Segmental orthotopic hepatic transplantation as a means to improve patient survival and diminish waiting-list mortality. J Pediatr Surg 1991; 26: 422–428. [DOI] [PubMed] [Google Scholar]
- 12.Emond JC, Heffron TG, Whitington PF, Broelsch CE. Reconstruction of the hepatic vein in reduced size hepatic transplantation. Surg Gynecol Obstet 1993; 176: 11–15. [PubMed] [Google Scholar]
- 13.Kuang AA, Renz JF, Ferrell LD, et al. Failure patterns of cryopreserved vein grafts in liver transplantation. Transplantation 1996; 62: 742–747. [DOI] [PubMed] [Google Scholar]
- 14.Millis JM, Seaman DS, Piper JB, et al. Portal vein thrombosis and stenosis in pediatric liver transplantation. Transplantation 1996; 62: 748–753. [DOI] [PubMed] [Google Scholar]
- 15.Kuang AA, Rosenthal P, Roberts JP, et al. Decreased mortality from technical failures improves results in pediatric liver transplantation. Arch Surg 1996; 131: 887–891. [DOI] [PubMed] [Google Scholar]
- 16.Emond JC, Renz JF. Surgical anatomy of the liver and its application to hepatobiliary surgery and transplantation. Semin Liver Dis 1994; 14: 158–172. [DOI] [PubMed] [Google Scholar]
- 17.Ozaki CF, Katz SM, Monsour HP Jr, Wood RP. Vascular reconstruction in living-related liver transplantation. Transplant Proc 1994; 26: 167–168. [PubMed] [Google Scholar]
- 18.Emond JC, Renz JF, Ferrell LD, et al. Functional analysis of grafts from living donors: implications for the treatment of older recipients. Ann Surg 1996; 224: 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renz JF, Rosenthal P, Roberts JP, et al. Planned re-exploration of pediatric liver transplant recipients reduces post-transplant morbidity and lowers length of hospitalization. Arch Surg 1997; 132: 950–954. [DOI] [PubMed] [Google Scholar]
- 20.Reichert PR, Renz JF, Bacchetti P, et al. Biliary complications of reduced-organ liver transplantation. Liver Transpl Surg 1998; 4: 343–349. [DOI] [PubMed] [Google Scholar]
- 21.Heffron TG, Emond JC, Whitington PF, et al. Biliary complications in pediatric liver transplantation. Transplantation 1992; 53: 391–395. [DOI] [PubMed] [Google Scholar]
- 22.Matsukawa H, Egawa H, Inomata Y, et al. Biliary complications in living related liver transplantation. Abstracts from the Fourth Congress International Liver Transplantation Society, October 1997.
- 23.Uflacker R, Reichert P, D’Albuquerque LC, Silva A. Liver anatomy applied to the placement of transjugular intrahepatic portosystemic shunts. Radiology 1994; 191: 705–712. [DOI] [PubMed] [Google Scholar]
- 24.Couinaud C. Les enveloppes vasculobiliares de foie ou capsule de Glisson. Leur interet dans la chirurgie vesiculaire, les resections hepatiques et l’abord du hile du foie. Lyon Chir 1954; 49: 589–615. [Google Scholar]
- 25.Couinaud C. Le Foie, Etudes Anatomiques et Chirurgicales. Paris: Masson; 1957.
- 26.Bismuth H, Castaing D, Garden OJ. Segmental surgery of the liver. In: Nyhus L, ed. Liver Transplantation. Norwalk: Appleton & Lange; 1988: 291–310. [PubMed]
- 27.de Hemptinne B, Salizzoni M, Yandza TC, et al. Indication, technique and results of liver graft volume reduction before orthotopic transplantation in children. Transplant Proc 1987; 19: 3549–3551. [PubMed] [Google Scholar]
- 28.Broelsch CE, Emond JC, Thistlethwaite R, et al. Liver transplantation with reduced-size donor organs. Transplantation 1988; 45: 519–523. [DOI] [PubMed] [Google Scholar]
- 29.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg 1982; 6: 3–9. [DOI] [PubMed] [Google Scholar]
- 30.Broelsch CE, Rogiers X. Hepatic transplantation: special issues. In: Carter D, Russel RCG, Pitt HA, Bismuth H, eds. Rob & Smith’s Hepatobiliary and Pancreatic Surgery. London: Chapman & Hall Medical, 1996: 82–92.
- 31.Healey JE Jr, Schroy PC. Anatomy of the biliary ducts within the human liver. Arch Surg 1953; 66: 599–616. [DOI] [PubMed] [Google Scholar]
- 32.Russel E, Yrizzary JM, Montalvo BM, et al. Left hepatic duct anatomy: implications. Radiology 1990; 174: 353–356. [DOI] [PubMed] [Google Scholar]


