Abstract
Objective
To determine whether female sex steroids have any salutary effects on the depressed cardiovascular and hepatocellular functions following trauma and hemorrhage in male animals.
Summary Background Data
Studies indicate that gender difference exists in the immune and cardiovascular responses to trauma-hemorrhage, and that male sex steroids appear to be responsible for producing immune and organ dysfunction, but it remains unknown if female sex steroids produce any salutary effects on the depressed cellular and organ functions in males following trauma and hemorrhage.
Method
Adult male Sprague-Dawley rats underwent a midline laparotomy (i.e., trauma induction), and were bled to and maintained at a mean arterial pressure of 40 mmHg until 40% of the maximum bleed-out volume was returned in the form of Ringer’s lactate (RL). Animals were then resuscitated with RL at 4 times the shed blood over 60 minutes. 17β-Estradiol (50 μg/kg) or an equal volume of vehicle was injected subcutaneously 15 minutes before the end of resuscitation. The maximal rate of ventricular pressure increase or decrease (±dP/dtmax), cardiac output, and hepatocellular function (i.e., maximal velocity and overall efficiency of in vivo indocyanine green clearance) were assessed at 24 hours after hemorrhage and resuscitation. Plasma levels of interleukin (IL)-6 were also measured.
Results
Left ventricular performance, cardiac output, and hepatocellular function decreased significantly at 24 hours after trauma-hemorrhage and resuscitation. Plasma levels of IL-6 were elevated. Administration of 17β-estradiol significantly improved cardiac performance, cardiac output, and hepatocellular function, and attenuated the increase in plasma IL-6 levels.
Conclusion
Administration of estrogen appears to be a useful adjunct for restoring cardiovascular and hepatocellular functions after trauma-hemorrhage in male rats.
Severe hemorrhage, which often occurs with trauma, is known to produce many life-threatening sequelae. Patients who survive the initial traumatic insult remain susceptible to sepsis, septic shock, multiple organ failure, and death. 1 Cellular dysfunction occurs in many organ systems, including the cardiovascular, liver, gut, and adrenal, after hemorrhagic shock, and remain depressed for a prolonged period of time. 2–4 Moreover, a marked depression in both specific and nonspecific cell-mediated immunity, which could explain the enhanced susceptibility to sepsis after such events, has been reported. 5
Sex hormones are known to modulate immune function in animals and in humans under normal and various stress conditions. 6 Studies have shown that female mice maintain their immune responses after trauma-hemorrhage, but male mice have markedly depressed responses. 7 Although the precise mechanism for this sexual dimorphism remains unknown, studies have demonstrated that male sex steroids appear to be responsible for producing the depression in cell and organ functions after trauma-hemorrhage and resuscitation. 8,9 Additional support for this notion comes from studies that showed that castration of male animals 14 days before hemorrhagic shock prevented the depression in myocardial functions and immune responses observed under those conditions in noncastrated animals. 8,10 Furthermore, administration of flutamide, a testosterone receptor antagonist, improved the depressed immune responses and cardiac and hepatic functions in male animals after trauma and severe hemorrhage. 11 These studies suggest that male and female sex steroids such as testosterone and estradiol play an opposite role in the development of cell and organ dysfunction after injury.
Estradiol is the predominant circulating sex hormone in females, and has been shown to have protective effects after adverse circulatory conditions such as organ ischemia and reperfusion. 12,13 Moreover, the estrogen receptor is expressed in several organs in male animals such as the cardiovascular system and the liver. 14
The aim of this study therefore was to determine whether or not administration of estradiol to male animals after trauma-hemorrhage has any salutary effect on the depressed cardiovascular and hepatocellular functions under those conditions.
MATERIALS AND METHODS
Experimental Procedures
Our previously described nonheparinized model of trauma-hemorrhage in the rat was used. 2 Briefly, male Sprague-Dawley rats (275–325 g, Charles River Labs, Wilmington, MA) were fasted overnight before the experiment but were allowed water ad libitum. The rats were anesthetized by methoxyflurane (Mallinckrodt Veterinary Inc., Mundelein, IL) inhalation prior to the induction of soft tissue trauma via 5-cm midline laparotomy. The abdomen was closed in layers, and catheters were placed in both femoral arteries and the right femoral vein (polyethylene [PE-50] tubing; Becton Dickinson & Co., Sparks, MD). The wounds were bathed with 1% lidocaine (Elkins-Sinn Inc., Cherry Hill, NJ) throughout the surgical procedure to reduce postoperative pain. Rats were then allowed to awaken, and bled to and maintained at a mean arterial pressure (MAP) of 40 mmHg. This level of hypotension was continued until the animals could not maintain MAP of 40 mmHg unless extra fluid, in the form of Ringer’s lactate, was given. This time was defined as maximum bleed-out, and the amount of withdrawn blood was noted. Following this, the rats were maintained at MAP of 40 mmHg until 40% of the maximum bleed-out volume was returned in the form of Ringer’s lactate. The animals were then resuscitated with four times the volume of the withdrawn blood over 60 minutes (about 45 mL/rat) with Ringer’s lactate. The shed blood was not used for resuscitation. Fifteen minutes before the end of the resuscitation period, the rats received 50 μg/kg body weight 17β-estradiol (β-estradiol 3-benzoate; Sigma, St. Louis, MO) subcutaneously or an equal volume of the vehicle (0.5 mL; corn oil, Sigma). The catheters were then removed, the vessels ligated, and the skin incisions closed with sutures. Sham-operated animals underwent the same groin dissection, which included the ligation of the femoral artery and vein, but neither hemorrhage nor resuscitation was carried out.
After returning the rats to their cages, they were allowed food and water ad libitum. At 24 hours after the completion of fluid resuscitation or sham-operation, the animals were anesthetized with methoxyflurane and catheterized via the right jugular vein. Under continued general anesthesia with pentobarbital sodium (25–30 mg/kg BW), cardiac output and hepatocellular function were measured in each animal. All animal experiments were performed according to the guidelines of the Animal Welfare Act and The Guide for Care and Use of Laboratory Animals from the National Institutes of Health. This project was approved by the Institutional Animal Care and Use Committee of Rhode Island Hospital (Providence, RI).
Measurement of Cardiac Output
A 2.4-French fiberoptic catheter was placed into the right carotid artery and connected to an in vivo hemoreflectometer (Hospex Fiberoptics, Chestnut Hill, MA), as described previously. 2 Indocyanine green (ICG; Cardio Green, Becton Dickinson) solution was injected via the catheter in the jugular vein (1 mg/mL aqueous solution as a 50-μL bolus). Twenty ICG concentrations per second were recorded for approximately 30 seconds with the aid of a data acquisition program (Asystant+; Asyst Software, Rochester, NY). The area under the ICG dilution curve was determined to calculate cardiac output (CO), which was then divided by the body weight to determine cardiac index.
Stroke volume (SV) was calculated as:
Total peripheral resistance (TPR) was calculated as:
Measurement of Hepatocellular Function
Hepatocellular function was measured by the in vivo ICG clearance technique 15; ICG was administered by bolus injection (50 μL) of 1, 2, and 5 mg/mL ICG in aqueous solvent. The arterial concentration of ICG was recorded each second for 5 minutes. The initial velocity of ICG clearance for each dose was then calculated after performing a nonlinear regression of the ICG clearance curves according to an e-raised second order polynomial function.2 The initial velocities of ICG clearance were then plotted against the ICG doses according to the methods of Lineweaver-Burk. 16 This resulted in a straight line, allowing the determination of a maximum of ICG clearance (Vmax) and the Michaelis-Menten constant (Km). In this active hepatocellular membrane transport system, Vmax represents the functional hepatocyte ICG receptors and Km represents the efficiency of the active transport process. 2
Measurement of In Vivo Heart Performance
A polyethylene (PE-50) catheter was placed in the right carotid artery and carefully advanced into the left ventricle. The position of the catheter tip was confirmed by recording the characteristic left ventricular pressure curves. Data were analyzed using an in vivo heart performance analyzer (Micro-Med, Louisville, KY), as described in a previous publication. 17 Various left ventricular performance parameters, such as maximal rate of the pressure increase (+dP/dtmax) and decrease (−dP/dtmax), were determined.
Measurement of Plasma Interleukin-6
Blood samples were drawn from the carotid catheter into a heparinized syringe at the end of each experiment. Plasma was separated by centrifugation at 12,000 g for 15 minutes at 4°C and stored at −70°C until assayed. Plasma interleukin (IL)-6 was measured using an enzyme-linked immunosorbent assay (ELISA) kit specific for rat IL-6 (Biosource, Camarillo, CA).
Statistical Analysis
Results are presented as mean±standard error of the mean (SEM). There were eight animals in both sham groups, and seven or eight animals in the vehicle- or estradiol-treated hemorrhaged group, respectively. One-way analysis of variance (ANOVA), Tukey test, and Fisher exact test were used, and the differences were considered significant at P ≤ .05.
RESULTS
Effects of Estradiol on Hemodynamic Parameters
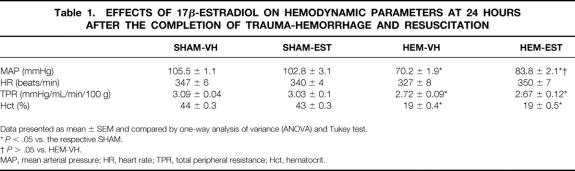
The results in Figure 1A indicate that cardiac index was 34.1±0.4 and 33.9±0.8 mL/min/100g in sham-operated animals receiving vehicle or estradiol, respectively. Cardiac index decreased by 27.3% (P < .05) in hemorrhaged and vehicle-treated animals at 24 hours after the completion of fluid resuscitation. Administration of estradiol after hemorrhage, however, restored the depressed cardiac index to sham levels. Similarly, SV decreased in hemorrhaged and vehicle-treated animals, whereas estradiol administration significantly improved SV as compared to vehicle-treated animals, and the values were similar to shams (Fig. 1B). Mean arterial pressure (MAP) decreased significantly at 24 hours after the completion of hemorrhage and resuscitation in both groups of hemorrhaged animals, in comparison to sham-operated animals (Table 1). However, animals treated with estradiol during resuscitation had a significantly higher MAP compared to vehicle-treated hemorrhaged rats. In contrast, heart rate did not differ significantly between the various groups. Total peripheral resistance was decreased in hemorrhaged animals in comparison to shams, irrespective of estradiol administration. Hematocrit decreased by more than half after trauma-hemorrhage and resuscitation in both hemorrhaged groups. Estradiol treatment in sham-operated animals had no effect on various hemodynamic parameters (Fig. 1, Table 1).
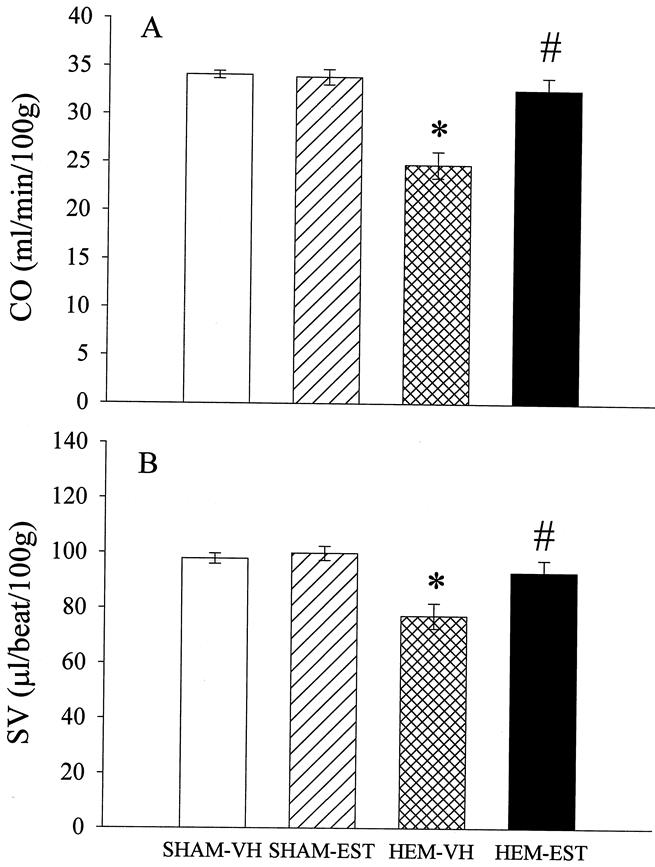
Figure 1. Effects of estradiol administration on (A) cardiac output (CO) and (B) stroke volume (SV) at 24 hours after sham-operation or trauma-hemorrhage and resuscitation, showing the comparison of sham-operated rats treated with vehicle (SHAM-VH) or estradiol (SHAM-EST), as well as hemorrhaged animals treated with vehicle (HEM-VH) or estradiol (HEM-EST) (7 or 8 animals/group). Data presented as mean±SEM and compared by one-way ANOVA and Tukey test. *P < .05 as compared to the respective shams; #P < .05 as compared to hemorrhaged and vehicle-treated animals.
Table 1. EFFECTS OF 17β-ESTRADIOL ON HEMODYNAMIC PARAMETERS AT 24 HOURS AFTER THE COMPLETION OF TRAUMA-HEMORRHAGE AND RESUSCITATION
Data presented as mean ± SEM and compared by one-way analysis of variance (ANOVA) and Tukey test.
*P < .05 vs. the respective SHAM.
†P > .05 vs. HEM-VH.
MAP, mean arterial pressure; HR, heart rate; TPR, total peripheral resistance; Hct, hematocrit.
Effects of Estradiol on Heart Performance
The maximal rate of left ventricle pressure increase (+dP/dtmax) was significantly decreased after trauma-hemorrhage (Fig. 2A), but estradiol treatment increased +dP/dtmax after trauma-hemorrhage and resuscitation, showing no statistical difference from the sham-operated animals. The maximum rate of left ventricle pressure decrease (−dP/dtmax) in the hemorrhaged group was also significantly decreased compared to the sham group, and −dP/dtmax in the estradiol-treated group increased significantly and was not different from sham values (Fig. 2B). Estradiol treatment in sham-operated animals affected neither +dP/dtmax nor −dP/dtmax.
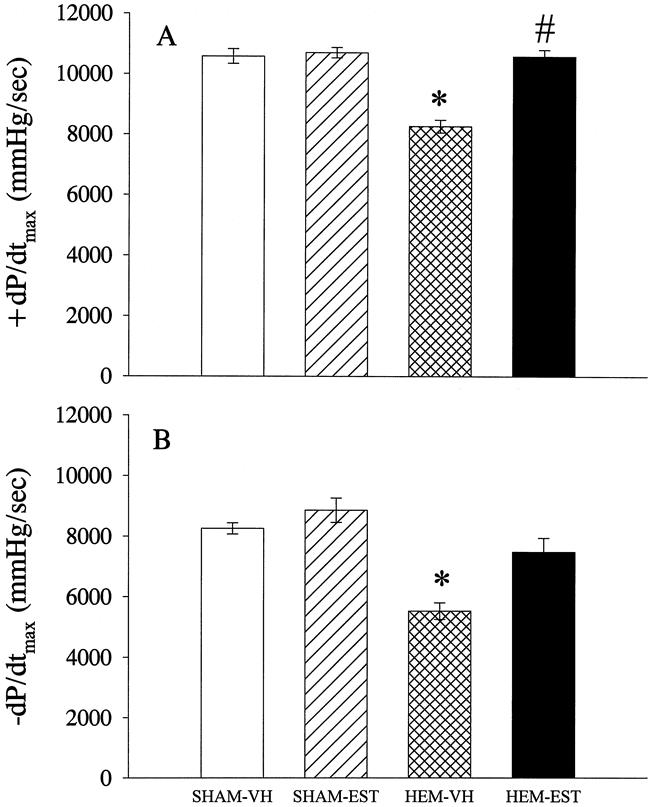
Figure 2. Effects of estradiol administration on the maximal rate of pressure (A) increase (+dP/dtmax) and (B) decrease (−dP/dtmax) in the left ventricle at 24 hours after completion of fluid resuscitation. Data presented as mean±SEM and compared by one-way ANOVA and Tukey test. *P < .05 as compared to the respective shams; #P < .05 as compared to hemorrhaged and vehicle-treated animals.
Effects of Estradiol on Hepatocellular Function
The values of the maximal velocity of ICG clearance (Vmax) were 1.29±0.1 and 1.28±0.07 mg/kg/min in sham-operated animals receiving vehicle or estradiol, respectively (Fig. 3A). In hemorrhaged and vehicle-treated rats, Vmax decreased by 73% (P < .05) at 24 hours after trauma-hemorrhage. In contrast, hemorrhaged and estradiol-treated animals had Vmax values similar to sham animals. As indicated in Figure 3B, Km was 3.0±0.2 and 3.1±0.3 mg/kg in sham-operated animals receiving vehicle or estradiol, respectively, and it decreased by 62% (P < .05) after trauma-hemorrhage and resuscitation in vehicle-treated rats. Estradiol administration significantly improved Km at 24 hours after the completion of resuscitation as compared to vehicle-treated animals, and the values were similar to shams (Fig. 3B). Estradiol administration in sham-operated animals had no effect on hepatocellular function.
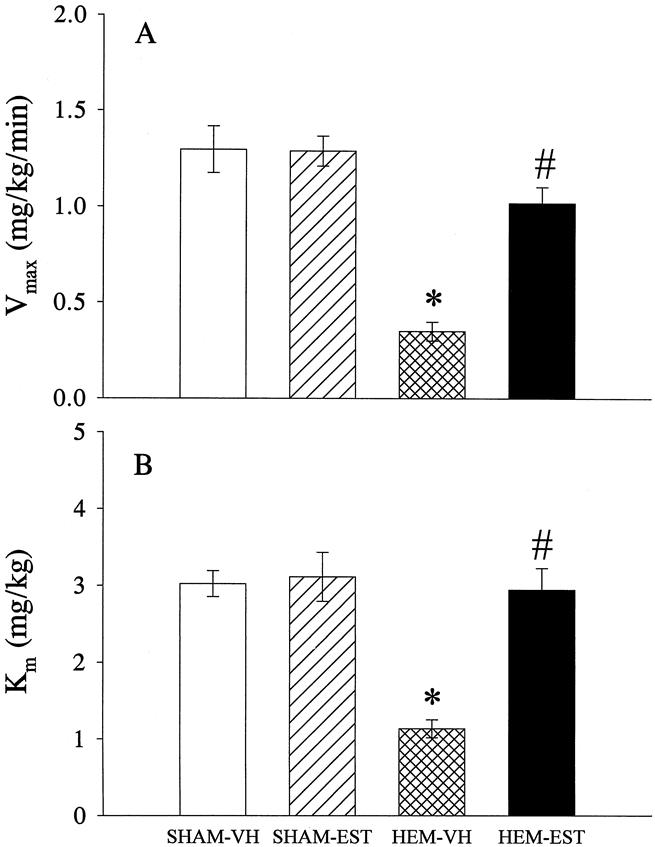
Figure 3. Effects of estradiol administration on the active hepatocellular function at 24 hours after sham operation or trauma-hemorrhage and resuscitation as measured by indocyanine green (ICG) clearance technique. (A) Vmax represents the maximal velocity of ICG clearance and (B) Km represents the overall efficiency of the ICG transport. Data presented as mean±SEM and compared by one-way ANOVA and Tukey test. *P < .05 as compared to the respective shams; #P < .05 as compared to hemorrhaged and vehicle-treated animals.
Effects of Estradiol on Plasma Levels of IL-6
Plasma levels of IL-6 increased by 691% (P < .05) at 24 hours after resuscitation in hemorrhaged and vehicle-treated animals in comparison to the respective sham group (Fig. 4). In estradiol-treated animals, however, plasma levels of IL-6 did not differ significantly from the levels found in sham-operated rats at 24 after the completion of hemorrhage and resuscitation.
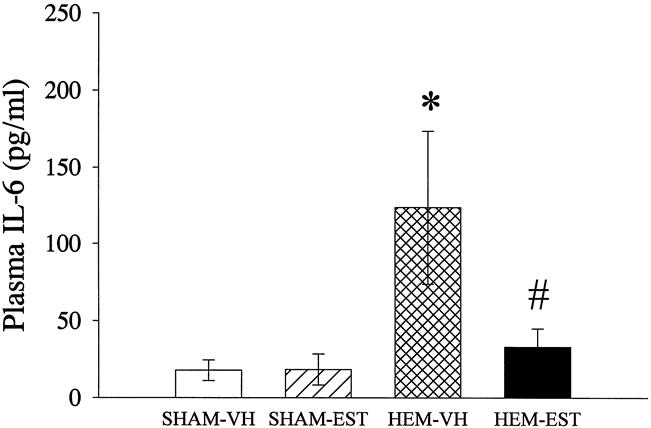
Figure 4. Alterations in plasma levels of interleukin (IL)-6 at 24 hours after sham operation or trauma-hemorrhage, measured by a specific ELISA. Data presented as mean±SEM and compared by one-way ANOVA and Tukey test. *P < .05 as compared to the respective shams; #P < .05 as compared to hemorrhaged and vehicle-treated animals.
Effects of Trauma-Hemorrhage on Mortality
The mortality rate in the vehicle-treated hemorrhaged group was 30% (3 of 10 animals) and 11% (1 of 9) in the estradiol-treated group. However, due to the relatively small number of animals, this difference was not statistically significant (P = .582).
DISCUSSION
Recent studies have shown that organ functions such as cardiac output, heart performance, adrenal responsiveness to exogenous corticotrophin, and hepatocellular clearance of ICG are significantly depressed in male animals after soft tissue trauma and severe hemorrhage. 2,3,17 Female rats during the proestrus state, however, a state in which plasma levels of estradiol were found to be the highest, showed no depression in cardiac output and hepatocellular function at 24 hours after severe hemorrhagic shock. 18 Alternatively, ovariectomized females displayed depression in organ functions after hemorrhage similar to males. Thus, it appears that the female sex steroid estradiol may have salutary effects on depressed organ functions observed after trauma-hemorrhage and resuscitation.
The ovaries are the predominant source of estradiol production in females; the testes and peripheral aromatization of testosterone and androstenedione account for the low levels of estradiol in males. Significantly reduced cardiovascular morbidity and mortality has been reported in postmenopausal women receiving hormone replacement therapy. 19 Moreover, studies have indicated that 17β-estradiol is involved in various physiologic processes such as vascular response modulation. We therefore hypothesized that administration of estradiol after trauma-hemorrhage and resuscitation in males would have salutary effects on the depressed cardiovascular and hepatocellular functions under those conditions.
The results of this study indicate that left ventricular performance, as measured by ±dP/dtmax, was significantly depressed after trauma-hemorrhage and resuscitation. 17β-estradiol–treated hemorrhaged animals displayed a restored +dP/dtmax, and an improved −dP/dtmax at 24 hours after the completion of fluid resuscitation. Moreover, the improved cardiac contractility was reflected by the restored cardiac index in treated rats. Because administration of estrogen after trauma-hemorrhage and resuscitation did not significantly alter heart rate, the improvement of the cardiac index under such conditions must be the result of an improvement in SV. Furthermore, hepatocellular function was also significantly improved, as evidenced by restoration of the Vmax and Km of ICG clearance after trauma-hemorrhage in estradiol-treated animals.
These improvements in organ functions also resulted in a better survival rate in estradiol-treated animals. The 24-hour mortality in this model of trauma and severe hemorrhagic shock was 30% in vehicle-treated animals, whereas it was 11% in the group receiving 17β-estradiol. Due to the small number of animals, however, this was not statistically significant. Moreover, it should be pointed out that we did not observe any adverse or beneficial effects of estradiol treatment in sham-operated animals.
The precise mechanism underlying the beneficial effects of estrogen on cardiovascular and hepatocellular function after trauma-hemorrhage and resuscitation remains unknown. Expression of the estrogen receptor has been reported in a number of cells and tissue in males. 20 Several studies have shown that the beneficial effects of estrogens on the cardiovascular system include both rapid nongenomic and long-term genomic mechanisms. 21–23 The rapid effects of 17β-estradiol include endothelial nitric oxide (NO) production, presumably by increasing the expression or activity of the constitutive isoform of nitric oxide synthase (cNOS). 22 The reduced release of endothelium-derived NO under various adverse circulation conditions is most likely due to the decreased activity of endothelial cNOS. 24 In this regard, our previous studies have indicated that vascular endothelial cell function (i.e., the release of vascular endothelium-derived NO) is depressed early after the onset of hemorrhagic shock. Furthermore, it has been demonstrated that administration of l-arginine (the substrate for cNOS) restores the depressed cardiac output and organ blood flow after trauma-hemorrhage. 25 Thus, it is possible that the beneficial effects of estrogen on cardiovascular and hepatocellular functions after trauma-hemorrhage are due to the up-regulation of cNOS.
In the present study, we used a single subcutaneous injection of 17β-estradiol-benzoate (50 μg/kg body weight), and observed improved organ functions after trauma-hemorrhage. It remains unknown, however, whether lower or higher doses of estradiol than the dose used in this study also would have any salutary effects on the depressed cardiovascular and hepatocellular functions after trauma-hemorrhage and resuscitation. Furthermore, organ functions in the present study were measured at 24 hours after trauma-hemorrhage and resuscitation. Whether or not salutary effects of a single dose of estradiol administration are also observed at an earlier time point, and whether they persist for the time points beyond that used in this study, remains to be determined. Regarding later time points, however, it could be argued that it is unlikely that the restored organ functions would deteriorate again. The salutary effects of estradiol treatment are also apparent by low plasma IL-6 levels. Recent studies from our laboratory have shown that the depression in liver function is already present during maximal bleed-out, concomitantly with increased levels of proinflammatory cytokines. Nonetheless, it remains to be determined how early the salutary effects on cytokine release and organ functions are observed after subcutaneous injection of estradiol.
Plasma levels of IL-6 were significantly elevated at 24 hours in vehicle-treated and hemorrhaged animals, whereas estradiol treatment during resuscitation down-regulated IL-6 to values that did not differ significantly from those in sham-operated animals. Recent studies have shown that there is a significant correlation between IL-6 and Vmax of ICG clearance. 26 Therefore, down-regulation of this inflammatory cytokine may be responsible for restoring the depressed hepatocellular function under such conditions. The studies by Deshpande et al 27 have shown that estradiol attenuates cytokine production by inhibiting activation of the transcription factor NF-κB in murine macrophages. Several lines of evidence suggested that Kupffer cells (KC) are the major source of inflammatory cytokine release after adverse circulatory conditions, 28 because reduction of KC by administration of gadolinium chloride reduced IL-6 release after trauma-hemorrhage. 28 Moreover, our recent studies have indicated that estradiol inhibits KC IL-6 release, whereas dihydrotestosterone enhances its production. 29 Thus, it could be postulated that estradiol inhibited KC IL-6 release, and due to the close proximity of this cell population to hepatocytes, thereby improved hepatocellular function. Nevertheless, the exact mechanisms of the salutary effect of estradiol administration after trauma-hemorrhage remain to be determined.
In summary, our results indicate that the administration of estrogen attenuated cardiovascular and hepatocellular dysfunction after trauma-hemorrhage and fluid resuscitation. Thus, administration of estrogen appears to be a useful and novel adjunct to fluid resuscitation for restoring organ functions after trauma-hemorrhage in male rats.
Acknowledgment
The authors thank Mr. Zheng F. Ba for his superb technical assistance during the experiments.
Footnotes
Correspondence: Irshad H. Chaudry, PhD, Department of Surgery, University of Alabama at Birmingham, 1670 University Blvd., Volker Hall, Room G094, Birmingham, AL 35294-0019.
Supported by National Institutes of Health Award R37 GM 39519.
Dr. Wang is the recipient of NIH Independent Scientist Award KO2 AI 01461.
E-mail: irshad.chaudry@ccc.uab.edu
Accepted for publication January 19, 2000.
References
- 1.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock 1998; 10: 79–89. [DOI] [PubMed] [Google Scholar]
- 2.Wang P, Hauptman JG, Chaudry IH. Hepatocellular dysfunction occurs early after hemorrhage and persists despite fluid resuscitation. J Surg Res 1990; 48: 464–470. [DOI] [PubMed] [Google Scholar]
- 3.Wang P, Ba ZF, Jarrar D, et al. Mechanism of adrenal insufficiency following trauma and severe hemorrhage: role of hepatic 11beta-hydroxysteroid dehydrogenase. Arch Surg 1999; 134: 394–401. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Wang P, Chaudry IH. Intestinal alkaline phosphatase: role in the depressed gut lipid transport after trauma-hemorrhagic shock. Shock 1997; 8: 40–44. [PubMed] [Google Scholar]
- 5.Chaudry IH, Ayala A, Ertel W, Stephan RN. Hemorrhage and resuscitation: immunological aspects. Am J Physiol 1990; 259: R663–R678. [DOI] [PubMed] [Google Scholar]
- 6.Homo-Delarche F, Fitzpatrick F, Christeff N, et al. Sex steroids, glucocorticoids, stress and autoimmunity. J Steroid Biochem Mol Biol 1991; 40: 619–637. [DOI] [PubMed] [Google Scholar]
- 7.Wichmann MW, Zellweger R, DeMaso CM, et al. Enhanced immune responses in females, as opposed to decreased responses in males following haemorrhagic shock and resuscitation. Cytokine 1996; 8: 853–863. [DOI] [PubMed] [Google Scholar]
- 8.Wichmann MW, Zellweger R, DeMaso CM, et al. Mechanism of immunosuppression in males following trauma-hemorrhage: critical role of testosterone. Arch Surg 1996; 131: 1186–1191. [DOI] [PubMed] [Google Scholar]
- 9.Slimmer LM, Blair ML. Female reproductive cycle influences plasma volume and protein restitution after hemorrhage in the conscious rat. Am J Physiol 1996; 271: R626–R633. [DOI] [PubMed] [Google Scholar]
- 10.Remmers DE, Cioffi WG, Bland KI, et al. Testosterone: the crucial hormone responsible for depressing myocardial function in males after trauma-hemorrhage. Ann Surg 1998; 227: 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remmers DE, Wang P, Cioffi WG, et al. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiol 1997; 273: H2919–H2925. [DOI] [PubMed] [Google Scholar]
- 12.Dubal DB, Shughrue PJ, Wilson ME, et al. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci 1999; 19: 6385–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser H, Davidge ST, Clanachan AS. Enhancement of post-ischemic myocardial function by chronic 17β-estradiol treatment: role of alterations in glucose metabolism. J Mol Cell Cardiol 1999; 31: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 14.Echeverria OM, Gonzalez MA, Traish AM, et al. Immuno-electron microscopic localization of estradiol receptor in cells of male and female reproductive and non-reproductive organs. Biol Cell 1994; 81: 257–265. [DOI] [PubMed] [Google Scholar]
- 15.Hauptman JG, Wang P, DeJong GK, Chaudry IH. Improved methodology for the evaluation of the velocity of clearance of indocyanine green in the rat. Circ Shock 1991; 33: 26–32. [PubMed] [Google Scholar]
- 16.Hauptman JG, DeJong GK, Blasko KA, Chaudry IH. Measurement of hepatocellular function, cardiac output, effective blood volume, and oxygen saturation in rats. Am J Physiol 1989; 257: R439–R444. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DA, Wang P, Chaudry IH. Pentoxifylline restores the depressed cardiac performance after trauma-hemorrhage and resuscitation. J Surg Res 1996; 66: 51–56. [DOI] [PubMed] [Google Scholar]
- 18.Jarrar D, Wang P, Cioffi WG, et al. The female reproductive cycle is an important variable in the response to trauma-hemorrhage and resuscitation [abstract]. Shock 1999; 11 (suppl1): 70. [DOI] [PubMed] [Google Scholar]
- 19.Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease: ten-year follow-up from the nurses’ health study. N Engl J Med 1991; 325: 756–762. [DOI] [PubMed] [Google Scholar]
- 20.Diano S, Horvath TL, Mor G, et al. Aromatase and estrogen receptor immunoreactivity in the coronary arteries of monkeys and human subjects. Menopause 1999; 6: 21–28. [PubMed] [Google Scholar]
- 21.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999; 340: 1801–1811. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Yuhanna IS, Galcheva-Gargova Z, et al. Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 1999; 103: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karas RH, Mendelsohn ME. Rapid vasomotor effects of estrogen: men are part of the club. Chest 1998; 114: 1508–1509. [DOI] [PubMed] [Google Scholar]
- 24.Lefer AM. Endotoxin, cytokines, and nitric oxide in shock. Shock 1994; 1: 79–80. [DOI] [PubMed] [Google Scholar]
- 25.Angele MK, Smail N, Wang P, et al. l -arginine restores the depressed cardiac output and regional perfusion after trauma-hemorrhage. Surgery 1998; 124: 394–401. [PubMed] [Google Scholar]
- 26.Remmers DE, Wang P, Cioffi WG, et al. Chronic resuscitation after trauma-hemorrhage and acute fluid replacement improves hepatocellular function and cardiac output. Ann Surg 1998; 227: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshpande R, Khalili H, Pergolizzi RG, et al. Estradiol down-regulates LPS-induced cytokine production and NF-κB activation in murine macrophages. Am J Reprod Immunol 1997; 38: 46–54. [DOI] [PubMed] [Google Scholar]
- 28.O’Neill PJ, Ayala A, Wang P, et al. Role of Kupffer cells in interleukin-6 release following trauma-hemorrhage and resuscitation. Shock 1994; 1: 43–47. [DOI] [PubMed] [Google Scholar]
- 29.Angele MK, Knoferl MW, Schwacha MG, et al. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol 1999; 277: C35–C42. [DOI] [PubMed] [Google Scholar]