Abstract
Objective
To review the authors’ experience with a new approach for type I diabetic uremic patients: simultaneous cadaver-donor pancreas and living-donor kidney transplant (SPLK).
Summary Background Data
Simultaneous cadaver kidney and pancreas transplantation (SPK) and living-donor kidney transplantation alone followed by a solitary cadaver-donor pancreas transplant (PAK) have been the transplant options for type I diabetic uremic patients. SPK pancreas graft survival has historically exceeded that of solitary pancreas transplantation. Recent improvement in solitary pancreas transplant survival rates has narrowed the advantage seen with SPK. PAK, however, requires sequential transplant operations. In contrast to PAK and SPK, SPLK is a single operation that offers the potential benefits of living kidney donation: shorter waiting time, expansion of the organ donor pool, and improved short-term and long-term renal graft function.
Methods
Between May 1998 and September 1999, the authors performed 30 SPLK procedures, coordinating the cadaver pancreas transplant with simultaneous transplantation of a laparoscopically removed living-donor kidney. Of the 30 SPLKs, 28 (93%) were portally and enterically drained. During the same period, the authors also performed 19 primary SPK and 17 primary PAK transplants.
Results
One-year pancreas, kidney, and patient survival rates were 88%, 95%, and 95% for SPLK recipients. One-year pancreas graft survival rates in SPK and PAK recipients were 84% and 71%. Of 30 SPLK transplants, 29 (97%) had immediate renal graft function, whereas 79% of SPK kidneys had immediate function. Reoperative rates, early readmission to the hospital, and initial length of stay were similar between SPLK and SPK recipients. SPLK recipients had a shorter wait time for transplantation.
Conclusions
Early pancreas, kidney, and patient survival rates after SPLK are similar to those for SPK. Waiting time was significantly shortened. SPLK recipients had lower rates of delayed renal graft function than SPK recipients. Combining cadaver pancreas transplantation with living-donor kidney transplantation does not harm renal graft outcome. Given the advantages of living-donor kidney transplant, SPLK should be considered for all uremic type I diabetic patients with living donors.
Simultaneous cadaver kidney pancreas transplantation (SPK) and sequential pancreas after kidney transplantation (PAK) are typically the only options for uremic or posturemic Type 1 diabetic patients who wish to undergo pancreas transplantation. Together they account for more than 99% of all pancreas transplants for uremic or posturemic diabetic patients. 1 SPK transplantation is more widely used than KTA followed by PAK, because SPK is a single operation and there is an “immunologic advantage” for the pancreas because the kidney can serve as a reliable marker for rejection of the pancreas. 2 However, some advocate PAK transplantation if there is a willing living kidney donor. 3 Use of a well-matched living-donor kidney can double the expected renal allograft survival half-life. 4 Living kidney donation also shortens the waiting time for transplantation and expands the organ donor pool. 5
The 1-year pancreas graft survival rate for SPK transplantation is now 83%. 1 During the past 3 to 4 years, the 1-year pancreas graft survival rate for PAK recipients has improved from 54% survival to 71%, shrinking the “immunologic advantage” of combining a cadaver pancreas with a kidney from the same donor. 1,3,6 The use of percutaneous pancreas biopsy coupled with tacrolimus-based immunosuppression results in equivalent success of solitary pancreas and SPK transplantation. 3
Largely because of these results, and because of the distinct advantages of living kidney donation, we have developed a new approach for uremic Type 1 diabetic patients: simultaneous cadaver-donor pancreas and living-donor kidney transplantation (SPLK). More than half of our uremic type I diabetic patients who desire pancreas transplantation now opt for SPLK. Selection of SPLK is generally limited only by the availability of a living donor. As a single procedure, SPLK has obvious advantages over the standard living-donor kidney transplant followed by PAK. Moreover, because the SPLK kidney is from a living donor, there may be both short-term and long-term benefits over SPK transplantation. Potential benefits of SPLK for Type 1 diabetic uremic patients include a shorter waiting time for transplantation and better early and long-term renal graft function. Generalized use of SPLK transplantation would expand the renal organ donor pool, thus benefiting all patients waiting for a kidney transplant. The main drawback to SPLK, coordination of a living donor nephrectomy with a cadaver pancreas transplant, is easily overcome.
This paper describes the technique of SPLK and reviews the results of our first 30 consecutive cases. Comparison is made with contemporaneous consecutive series of primary SPK and PAK transplants.
METHODS
From May 1998 to September 1999, 66 primary pancreas transplants were performed for uremic or posturemic Type 1 diabetic patients. Informed consent, consistent with the ethical standards of the 1975 Helsinki Declaration, was obtained from all patients. Thirty patients (45%) received an SPLK transplant. All of the 30 living-donor kidneys were procured laparoscopically. Contemporaneous with the SPLK transplants, 19 (29%) uremic diabetic patients underwent cadaver SPK transplantation, and 17 (26%) pancreas transplants were performed after a successful kidney transplant (PAK). Of 17 patients undergoing PAK, 13 (76%) had received kidneys from living donors. In the SPLK group, recipient age averaged 38 ± 7 years, and 23 (76%) were men (Table 1). Living kidney donors for SPLK recipients were 43 ± 8 years old, and 65% were women (Table 2). Of 30 SPLK recipients, 11 (37%) were receiving dialysis at the time of transplant, compared with 15 (79%) of the SPK recipients (P < .05).
Table 1. RECIPIENT DEMOGRAPHICS
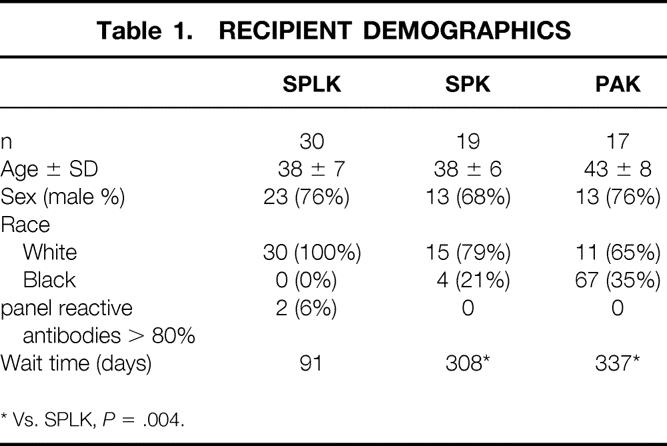
* Vs. SPLK, P = .004.
Table 2. DONOR DEMOGRAPHICS
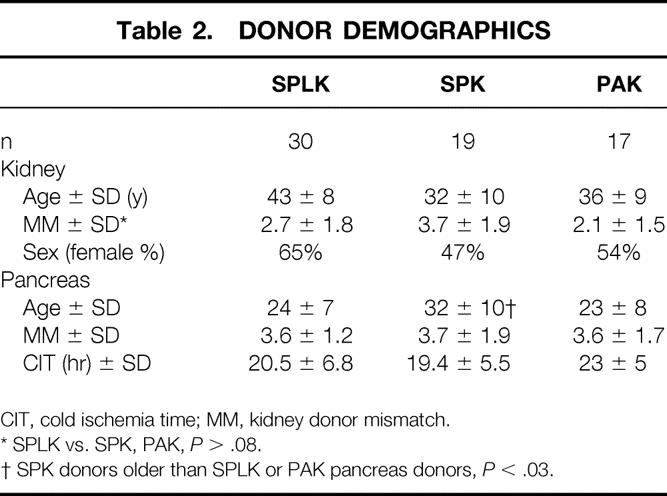
CIT, cold ischemia time; MM, kidney donor mismatch.
* SPLK vs. SPK, PAK, P > .08.
† SPK donors older than SPLK or PAK pancreas donors, P < .03.
Selection of cadaver pancreas donors for SPLK transplantation was based primarily on the quality of the cadaver donor (donor age and pancreas anatomy). Donors ages 10 to 50 years whose pancreases lacked significant fatty infiltration were preferred. Absolute contraindications for use of a donor included malignancy and positive serology for human immunodeficiency virus. ABO blood compatibility was mandatory. Human leukocyte antigen (HLA) matching was not used as a donor acceptance criterion. Cadaver pancreas donor age and degree of HLA mismatch did not differ significantly between the SPLK, PAK, and SPK groups (P > .45, see Table 2).
Graft survival was determined by Kaplan-Meier analysis. Means were compared by independent-sample t tests. Medians were compared by the Mann-Whitney test.
Technique
The selection for SPLK transplantation was based entirely on the patient’s preference and the availability of a living kidney donor. Pretransplant workup of the potential recipient included routine laboratory assessment, cardiac stress testing, and any further tests as indicated by the evaluation. Distance from the medical center was not used to exclude patients for SPLK. Twelve (40%) of the donor–recipient pairs lived more than 8 hours from the University of Maryland, and only nine (30%) lived less than 4 hours away.
The transplant surgical preparation of the pancreas is performed first. This ensures that the pancreas is of high quality and fit for implantation before the living kidney donor is anesthetized. Early examination of the pancreas is particularly important if the pancreas is from out of the region, but it serves a dual purpose: finishing the pretransplant preparation first also helps with the timing of the donor and recipient operations.
The technical aspects of the pretransplant preparation have been described. 7 Portal venous drainage was used in nearly all instances (28/30). Therefore, the arterial reconstruction was done with a longer segment of Y graft than commonly used for systemic venous drainage. If the recipient had a large abdomen, an extra segment of donor external iliac artery was anastomosed to the end of the donor common iliac artery to allow tension-free bridging of the distance between the pancreas, through the small bowel mesentery to the right common iliac artery.
On nearing completion of the bench work of the pancreas, the operating room personnel are instructed to send for both the recipient and the living kidney donor. The recipient and donor operations are started simultaneously. The aim is to complete the pancreas transplant procedure before receipt of the living donor kidney. Occasionally the living donor nephrectomy ran ahead of the pancreas implantation. In this event, the donor nephrectomy was completed and the donor kidney was flushed with a standard preservation solution and kept on ice until ready for implantation.
Laparoscopic Living Kidney Donation
Since laparoscopic donor nephrectomy was first performed in 1995, the procedure has rapidly gained acceptance in both the transplant and urology communities as an alternative to open donor organ procurement. The procedure is straightforward and essentially duplicates the steps used during open donor nephrectomy, but considerable experience is necessary to avoid damage to the kidney, ureter, and renal vessels. Because of the limited window of opportunity defined by the availability and acceptable cold ischemia time of a cadaver pancreas, laparoscopic donor nephrectomy must be performed on an urgent basis for SPLK transplantation. Three laparoscopic donor surgeons share call to provide coverage for the program. More than 50% of the procedures in this series occurred outside of regular hours.
In our elective donor nephrectomy experience of 400 cases from March 1996 through October 1999, donor hospital stay, pain medication requirements, return to normal activity and work, and risk of major and minor complications have all been superior to historical open controls. 8–10
The operation is performed using general anesthesia. Preoperative and intraoperative intravenous hydration prevents intraoperative oliguria during laparoscopic insufflation. Mannitol is also administered during the procedure. With the patient in the lateral position, three operating ports are arranged in an arc equidistant from the target organ and one extraction port is placed in the lower midline. The cephalad port is used for video camera visualization, and the caudad ports serve as working ports. Medial visceral rotation of the colon, adrenal and superior renal pole dissection, renal vascular dissection, ureteral dissection, and division of lateral attachments to the diaphragm and iliopsoas muscle are the essential steps of the procedure. Extraction of the kidney is performed using an endoscopic retrieval bag placed through a lower abdominal midline or Pfannenstiel incision. A hand-port or endoscopic sleeve device is not routinely used for kidney extraction. Donors are allowed clear liquids immediately after donation. On postoperative day 1, the bladder catheter is removed. Most patients no longer require parenteral analgesia and are tolerating a regular diet without difficulty by 24 to 48 hours. For the first 300 patients, surgical time averaged 216 minutes; warm ischemia time averaged 152 seconds and estimated blood loss was 147 mL. Mean length of stay was 66.6 hours.
Recipient Operation
Except for two patients in the SPLK group who were transplanted with systemic venous and enteric drainage, all pancreases were implanted with portal venous and enteric drainage. As previously described by Gaber et al, 11,12 either the superior mesenteric vein or a major branch of the recipient superior mesenteric vein is used for portal anastomosis (Fig. 1). The arterial Y graft is then passed through the small bowel mesentery to the recipient’s proximal right common iliac artery, where the Y graft is implanted. After the pancreas is reperfused and hemostasis is ensured, exocrine drainage is performed by duodenojejunostomy. Most centers use a diverting Roux-en-Y for the enteric anastomosis, but we have not found this to be necessary (see Fig. 1). 13 The pancreas allograft is then packed away and the left iliac vessels are exposed for a standard living-donor renal implantation. After the kidney transplant, all surgical sites are reexamined for hemostasis and the abdomen is irrigated with bacitracin and kanamycin in saline and amphotericin in water.
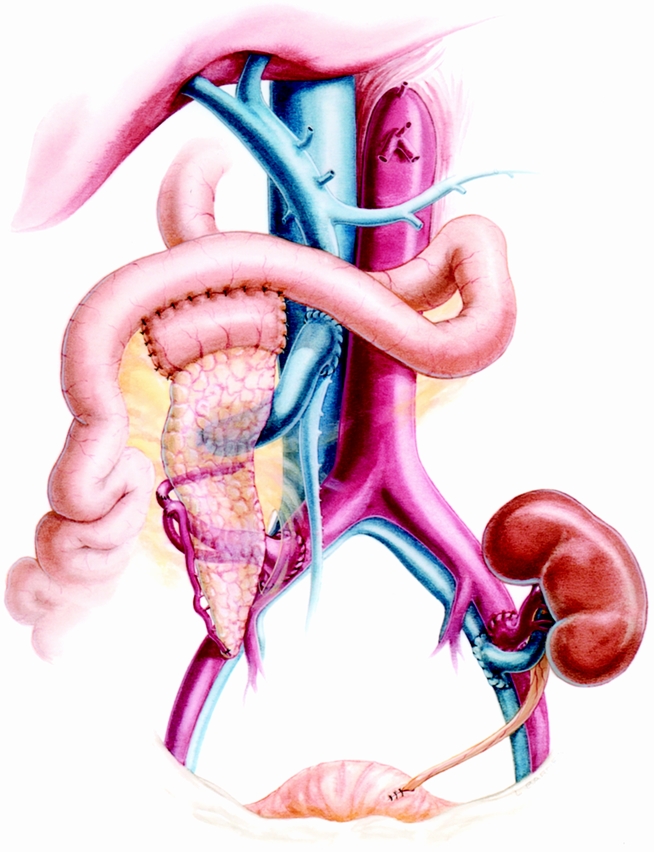
Figure 1. Simultaneous cadaver pancreas and living-donor kidney transplantation (SPLK). The pancreaticoduodenal transplant is performed while the living donor is undergoing laparoscopic donor nephrectomy. We do not use a Roux-en-Y for enteric drainage.
Anticoagulation
SPLK and SPK recipients who were uremic but did not receive dialysis receive an intravenous loading dose of heparin followed by an intravenous heparin sodium drip (300–500 units/hour), with the goal of keeping the partial thromboplastin time at 35 to 50 seconds. On approximately postoperative day 5, heparin is replaced by enteric-coated aspirin. Patients who were receiving dialysis before the transplant are anticoagulated with aspirin only. Because of a higher risk of thrombosis in solitary pancreas transplant, 1 PAK recipients are anticoagulated with 300 to 500 U/hour heparin sodium and then converted to coumadin after postoperative day 5 (International Normalized Ratio 2.0–2.5).
Immunosuppression
All pancreas recipients receive tacrolimus, mycophenolate mofetil, and prednisone as maintenance immunosuppression. Blood levels of tacrolimus are monitored weekly. For PAK and SPLK transplants, the target blood level of tacrolimus is 15 to 20 ng/mL. The tacrolimus goal for SPK recipients is 12 to 15 ng/mL. A standard prednisone taper is used. Except for some SPK recipients in a no-induction study, and other SPK and SPLK recipients in an interleukin-2 receptor antagonist study, all pancreas recipients received 10 days of OKT3.
RESULTS
Of 66 primary pancreas transplants performed for uremic or posturemic diabetes, 10 pancreas grafts (15%) were lost during a median follow-up of 6 months (range 1–16). The overall pancreas graft survival rate was 84% at 1 year (Kaplan-Meier). Three pancreas grafts (10%) were lost in the SPLK group. One SPLK pancreas transplant was lost at 4 months because of death with function (sudden cardiovascular death). Two other pancreas grafts were lost because of rejection (one hyperacute rejection in a high-panel reactive antibodies patient with a past positive cross-match, and one acute rejection associated with thrombosis 2 weeks after transplant). The 1-year actuarial graft survival rate for SPLK transplantation was 88%.
The patient with the past positive pancreas cross-match was in a special protocol to eliminate anti-HLA antibodies specific for the kidney donor. On pretransplant testing, she had a positive cross-match with her living kidney donor as a result of IgG antibodies against donor HLA-Bw4. The patient entered a special plasmapheresis protocol that made the anti-HLA-Bw4 antibodies undetectable by standard cross-match and also enzyme-linked immunosorbent assay. The cadaver pancreas donor that became available shared HLA-Bw4 with the living kidney donor, and cross-matches to both donors at the time of transplant were negative (both T and B cell). Despite a negative cross-match achieved with plasmapheresis, and despite success of the living-donor kidney, the patient had pathologically proven hyperacute pancreas rejection, and the graft was removed less than 24 hours after transplant. Histologic review of the explanted pancreas graft showed extensive coagulation and enzymatic necrosis. Most arteries showed endothelial infiltration by neutrophils, and there were areas of arterial transmural arteritis and focal fibrinoid necrosis.
Of 19 SPK pancreas transplants during the same period, 3 grafts (16%) were lost (2 early thrombosis, 1 nonanastomotic duodenal cuff leak).
The 1-year pancreas graft survival rate in the SPLK group did not differ significantly from the that of 19 contemporaneous SPK transplants (88% vs. 84%, P = .84, Fig. 2). Pancreas graft survival in the SPLK group exceeded pancreas graft survival in contemporaneous PAK recipients (71% 1-year survival), but this difference was not significant (P = .09). During follow-up, 6 (20%) SPLK recipients had biopsy-confirmed pancreatic rejection (Table 3). Hyperacute rejection occurred in one high-PRA patient, and another patient was found to have rejection after removal of a thrombosed graft 2 weeks after transplant. The other four rejections were predicted by a rise in serum lipase levels and were successfully treated. A smaller but not significantly different number of SPK recipients developed pancreas rejection: two (11%) recipients who had biopsy-confirmed rejection were treated successfully (vs. SPLK, P = .38). Two additional SPK recipients and one SPLK recipient developed renal rejection. Overall, rejection occurred in seven (23%) SPLK and four (22%) SPK patients (P = .9, see Table 3). The 1-year patient survival rate in the entire group was 96%.
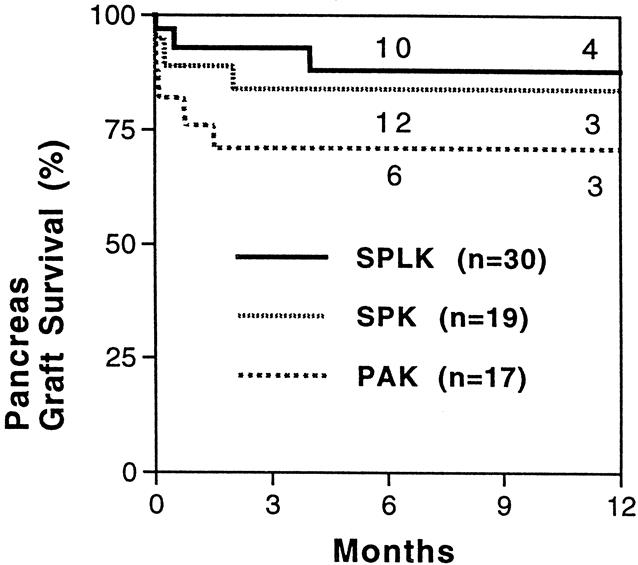
Figure 2. Pancreas graft survival rates. One-year pancreas graft survival rates were 88%, 84%, and 71%, respectively, for simultaneous cadaver-donor pancreas and living-donor kidney transplantation (SPLK), simultaneous cadaver kidney and pancreas transplantation (SPK), and living-donor kidney transplantation alone followed by a solitary cadaver-donor pancreas transplant (PAK) (P = .09). Numbers above plot lines represent the number of patients in follow-up at the time indicated.
Table 3. COMPLICATIONS
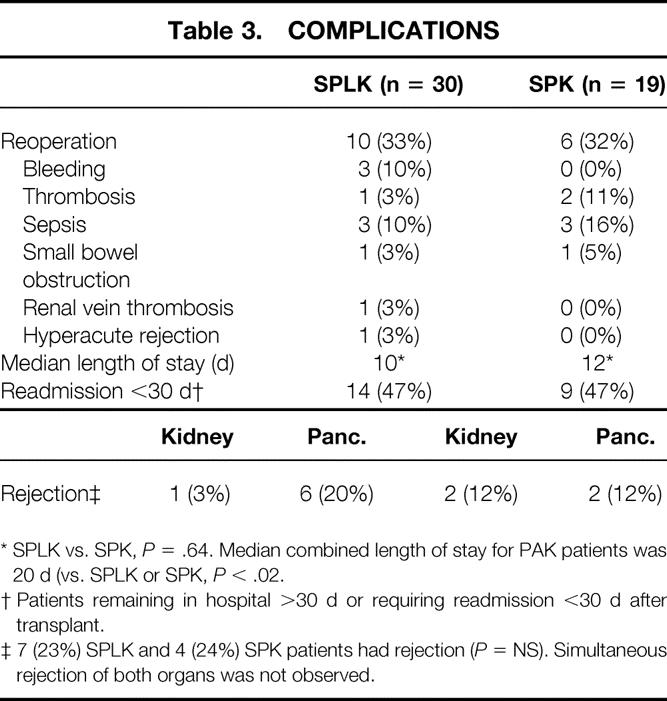
* SPLK vs. SPK, P = .64. Median combined length of stay for PAK patients was 20 d (vs. SPLK or SPK, P < .02.
† Patients remaining in hospital >30 d or requiring readmission <30 d after transplant.
‡ 7 (23%) SPLK and 4 (24%) SPK patients had rejection (P = NS). Simultaneous rejection of both organs was not observed.
Of 66 recipients, 2 patients died. Despite excellent graft function, one patient in the SPLK group with known severe autonomic neuropathy died 4 months after transplant from cardiac sudden death. One SPK recipient died 2 months after transplant from a pulmonary embolism (SPLK vs. SPK, P = .99). The 1-year actuarial patient survival rates were 95%, 94%, and 100% in the SPLK, SPK, and PAK groups, respectively (Fig. 3).
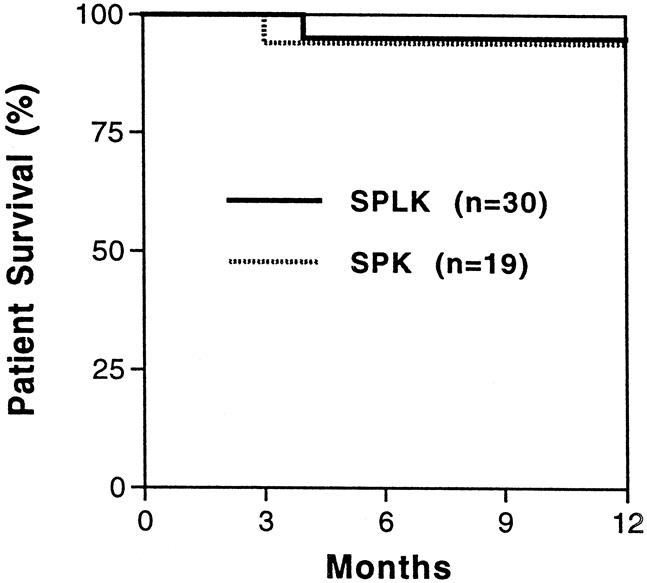
Figure 3. Patient survival rates. One-year patient survival rates were 95% and 94% for simultaneous cadaver-donor pancreas and living-donor kidney transplant (SPLK) and simultaneous cadaver kidney and pancreas transplant (SPK) recipients. The patient survival rate was 100% in living-donor kidney transplantation alone followed by a solitary cadaver-donor pancreas transplant (PAK) recipients (not shown).
Death with function accounted for the loss of two renal allografts, one each in the SPLK and SPK groups. Another SPK kidney was lost to thrombosis, resulting in a 1-year renal graft survival rate of 95% for SPLK transplantation and 89% for SPK transplantation (P = .37, Fig. 4).
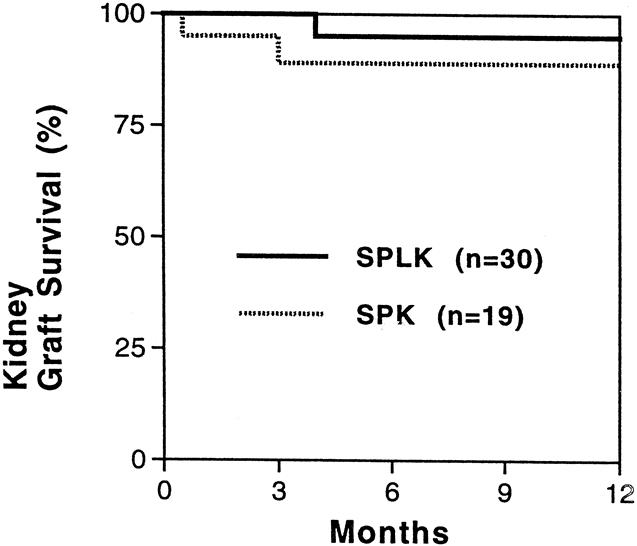
Figure 4. Kidney graft survival rates. One-year kidney graft survival rates were 95% and 89% for simultaneous cadaver-donor pancreas and living-donor kidney transplant (SPLK) and simultaneous cadaver kidney and pancreas transplant (SPK) recipients (P = .37). The only SPLK loss was death with function. No living-donor kidney transplantation alone followed by a solitary cadaver-donor pancreas transplant (PAK) kidney grafts were lost (not shown).
Of 30 SPLK recipients, 29 (97%) had immediate renal allograft function (see Table 3). One SPLK recipient of a three-artery living-donor kidney required short-term dialysis after partial renal vein thrombosis and thrombectomy. Delayed graft function (need for dialysis) occurred in four (21%) recipients of cadaver kidneys in the SPK group and one (3%) living-donor kidney in the SPLK group (P = .046). Early renal function, measured by creatinine level, appeared to be better in recipients of living-donor kidneys. At day 3 after the transplant, the mean creatinine level was 1.8 (mg/dL) in SPLK recipients and 3.3 (mg/dL) in SPK recipients (P = .03). At 30 and 90 days after transplant, the differences in SPLK and SPK renal function disappeared (creatinine 1.5 vs. 2.0 mg/dL, 1.4 vs. 1.5 mg/dL, P > .2, Table 4). The difference in early creatinine levels at day 3 after transplant may be partly explained by the greater number of SPK recipients who were receiving dialysis before transplant (79% of SPK vs. 37% of SPLK, P < .05).
Table 4. RENAL ALLOGRAFT FUNCTION
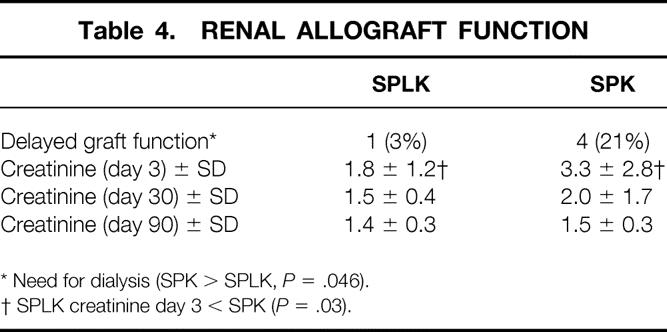
* Need for dialysis (SPK > SPLK, P = .046).
† SPLK creatinine day 3 < SPK (P = .03).
Waiting time differed significantly between SPLK and SPK recipients. On average, SPLK patients waited 91 days from initial evaluation for transplantation. Part of this wait time included the time needed for living kidney donor evaluation (approximately 30 days). SPK recipients waited an average of 308 days for a cadaver kidney and pancreas donor (vs. SPLK, P = .004, see Table 1). Pancreas donors were older for SPK recipients (32 years, range 8–52) than for SPLK (24 years, range 9–44) or PAK (21 years, range 16–41) recipients (P < .05).
Reoperations were required in 10 (33%) SPLK recipients and in 6 (32%) SPK recipients (P = .9, see Table 3). In the SPLK group, postoperative bleeding and infection were the most common reasons for relaparotomy: three (10%) patients underwent exploration for bleeding and three (10%) for intraabdominal sepsis. Other reasons for reoperation in SPLK recipients included one pancreatic thrombosis, one small bowel obstruction, and one thrombectomy for partial renal vein thrombosis. In the SPK group, intraabdominal sepsis was the most common reason for relaparotomy. Three (16%) SPK recipients underwent relaparotomy for infection (one duodenal cuff leak). One SPK recipient underwent exploration for pancreatic thrombosis and another for small bowel obstruction.
Readmission to the hospital was assessed as a measure of complications. Of 30 SPLK recipients, 11 (37%) required readmission within 30 days of transplant (3 additional patients had an initial hospital stay after transplant of >30 days; see Table 3). Gastrointestinal complaints (nausea, vomiting, and diarrhea) were the most common causes of readmission. Five (26%) of the 19 SPK recipients were readmitted within 30 days after transplant, but 4 patients’ initial hospital stay exceeded 30 days (vs. SPLK P = .45). Initial median hospital length of stay did not differ between the SPLK and SPK groups (10 vs. 12 days, P = .64). However, the combined hospital lengths of stay for both the KTA and the PAK procedures (20 days) exceeded that for the single initial admission needed for the simultaneous transplants (P < .021).
DISCUSSION
SPLK transplantation represents a new approach for Type 1 diabetic patients with renal failure. Except for the simultaneous living-donor pancreas and kidney transplant reported by the University of Minnesota, virtually all uremic diabetic patients who opt for living kidney donation and also desire pancreas transplantation have historically undergone sequential cadaver-donor pancreas transplant (PAK). 14,15 We began a program of SPLK because of increasing familiarity with laparoscopic donor nephrectomy, 8,16 the ability to perform live-donor nephrectomy 24 hours a day, and the dramatically improved results of solitary pancreas transplantation. 1,3 Because the early results of solitary pancreas transplantation now rival those of SPK (several centers report >75% 1-year PTA and PAK graft survival rates), 3,17–19 we reasoned that combining the cadaver pancreas transplant with a living-donor kidney would be an advantageous alternative for Type 1 diabetic uremic patients. At the start of the series, it was anticipated that the main difficulty might be logistical. However, although the laparoscopic donor nephrectomy technique was used, it has not proven logistically difficult to coordinate the recipient and donor operations. In addition, prolonged preservation time has not been an issue, even though many of the recipient–donor pairs live more than 8 hours from the medical center.
The main reason to consider SPLK over SPK is the option of living kidney donation. The results of living kidney transplantation significantly exceed those of cadaver kidney transplantation. According to the United Network for Organ Sharing (UNOS)’s Scientific Renal Transplant Registry, the average life span for a cadaver donor kidney transplanted in 1995–96 was 10.4 years, compared with 16.7 years for a living-donor kidney. 4 For kidney transplants performed between 1991 and 1997, even mismatched living unrelated donor kidneys significantly outlive cadaver donor kidneys (approximately 15 years vs. 9 years). 4 In this report, SPLK and SPK recipients had similar actuarial kidney graft survival rates at 1 year. However, SPLK recipients had less delayed graft function than contemporaneous SPK recipients. Whether this translates into differences in long-term function can be determined only by long-term follow-up. Our 1-year graft survival rate for SPLK kidneys (95%) is similar to that reported for living-donor kidney transplants alone (living-donor KTA). 4 The addition of a cadaver-donor pancreas transplant at the time of living-donor kidney transplant does not adversely affect the renal graft outcome.
Although we believe that the potential for increased kidney graft longevity is the major reason to consider SPLK, waiting time is also a fundamental issue. According to UNOS data, patients added to the waiting list in 1996 had a median wait time of 331 days for a primary SPK and more than 1,000 days for a primary cadaver-donor kidney transplant. 5 These waiting times are likely to increase as more patients are added to the waiting list than undergo transplantation each year. The average waiting time for our SPK recipients, 308 days, was similar to the SPK UNOS data. However, SPLK recipients had a much shorter average waiting time, only 91 days (P = .005). The obvious difference is that SPK patients must wait for a kidney, whereas SPLK patients wait only for a solitary pancreas.
The rate of major complications, as assessed by relaparotomy after transplant, was 33% in the SPLK recipients and 32% in the contemporaneous SPK recipients. Reoperation for bleeding was necessary in three SPLK patients. The risk for postoperative bleeding was increased in these patients by the use of heparin. Two SPLK recipients underwent exploration for clinical signs of sepsis, and peritonitis was confirmed in one. Abdominal sepsis was the most common reason for relaparotomy in SPK recipients, accounting for three of the five patients who needed reoperation. Because of the small numbers in each group, one cannot conclude that there are different patterns of complications after SPLK and SPK transplants; however, the overall complication rate appears similar. Length of initial hospital stay and the likelihood of readmission to the hospital within 30 days were similar for the SPLK and SPK recipients. Patients undergoing simultaneous transplants (both SPLK and SPK) had a shorter length of stay than those undergoing combined transplants (PAK recipients).
Despite different donors for kidney and pancreas, the rate of pancreas graft survival in the SPLK recipients was statistically indistinguishable from that of pancreas graft survival in the SPK group (88% vs. 84% at 1 year). The follow-up of this series is short, and the long-term immunologic risk for the “solitary” pancreas transplant in SPLK recipients may exceed that for SPK transplantation. Results reported to the International Pancreas Transplant Registry have consistently shown greater immunologic risk for solitary pancreas transplants, with PTA transplantation demonstrating the greatest risk. 1 It is doubtful, but possible, that simultaneous transplants, even from different donors, may show improved long-term function simply because the transplants are performed simultaneously. For example, uremia might have an impact on graft outcome through effects on the immune system. 20,21 SPLK and SPK pancreas graft survival rates exceeded the pancreas graft survival rate in the PAK recipients, but the difference did not reach statistical significance and was not due to immunologic failure. Historically, the major cause of graft loss in solitary pancreas transplantation has been immunologic. 6 We have previously found, however, that use of percutaneous biopsy and tacrolimus-based immunosuppression raises solitary pancreas transplant results to those of SPK. 3 Tacrolimus and mycophenolate mofetil significantly improve the graft survival of solitary pancreas transplants. 22,23
Two points are important to emphasize. First, addition of the pancreas as an SPLK puts the living-donor kidney at no greater risk than does a PAK. Second, the success of an SPLK pancreas, logically, should not be different than for a PAK. In fact, the data suggest that SPLK pancreas results exceed those for PAK.
In summary, early pancreas, kidney, and patient survival rates after SPLK are similar to those after SPK transplantation. SPLK recipients have extremely low rates of delayed renal graft function and appear to have better early renal function than SPK recipients. Combination cadaver pancreas transplantation and living-donor kidney transplantation does not adversely affect the living-donor renal outcome. Our previously published report showing the equivalent success of SPK and solitary pancreas transplantation, 3 the International Pancreas Transplant Registry data showing progressive improvement in PAK outcome, 25 and the SPLK results in this study support the contention that solitary pancreas graft results are approaching those of SPK transplantation. Given the significant potential advantages of living-donor kidney transplant, SPLK is an ideal approach in uremic Type 1 diabetic patients with living donors. Because SPLK is a single procedure, it may be preferable to the living-donor KTA and PAK procedures.
Acknowledgment
The authors thank Kandy Cannon for her work in preparing this manuscript.
Footnotes
Correspondence: Alan C. Farney, MD, PhD, Joseph and Corrine Schwartz Division of Transplantation, 29 S. Greene St., Suite 200, Baltimore, MD 21201.
E-mail: afarney@smail.umaryland.edu
Accepted for publication March 2, 2000.
References
- 1.Gruessner A, Sutherland D. Analysis of United States (US) and non-US pancreas transplants as reported to the International Pancreas Transplant Registry (IPTR) and to the United Network for Organ Sharing (UNOS). In: Terasaki P, Cecka J, eds. Clinical Transplants 1998. Los Angeles: UCLA Tissue Typing Laboratory; 1999: 53–71. [PubMed]
- 2.Gruessner R, Nakleh R, Tzardis P, et al. Rejection patterns after simultaneous pancreaticoduodenal-kidney transplants in pigs. Transplantation 1994; 57: 756–760. [PubMed] [Google Scholar]
- 3.Bartlett S, Schweitzer E, Johnson L, et al. Equivalent success of simultaneous pancreas kidney and solitary pancreas transplantation. Ann Surg 1996; 224: 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecka J. The UNOS Scientific Renal Transplant Registry. In: Cecka J, Terasaki P, eds. Clinical Transplants 1998. Los Angeles: UCLA Tissue Typing Laboratory; 1999: 1–16.
- 5.Harper A, Rosendale J, McBride M, et al. The UNOS OPTN Waiting List and Donor Registry. In: Cecka J, Terasaki P, eds. Clinical Transplants 1998. Los Angeles: UCLA Tissue Typing Laboratory; 1999: 73–90. [PubMed]
- 6.Gruessner A, Sutherland D. Pancreas transplant results in the UNOS United States of America Registry compared with non-USA data in the International Registry. In: Terasaki P, Cecka J, eds. Clinical Transplants 1993. Los Angeles: UCLA Tissue Typing Laboratory; 1994: 47–68. [PubMed]
- 7.Farney A, Brayman K, Sutherland D. Surgical approaches for treatment of diabetes mellitus. In: Braasch J, Tompkins R, eds. Surgical Diseases of the Biliary Tract and Pancreas. St. Louis: Mosby-Year Book; 1994: 658–699.
- 8.Flowers J, Jacobs S, Cho E, et al. Comparison of open and laparoscopic live donor nephrectomy. Ann Surg 1997; 226: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho E, Jacobs S, Flowers J, et al. Laparoscopic live donor nephrectomy: review of 125 cases. Presented at Society of American Gastrointestinal Endoscopic Surgeons, Seattle, WA, 1998:47.
- 10.Jacobs S, Cho E, Dunkin B. Caparoscopic donor nephrectomy: current role in renal allograft procurement. Urology 2000; 55 (b):807–11. [DOI] [PubMed] [Google Scholar]
- 11.Gaber A, Shokouh-Amiri M, Hathaway D, et al. Results of pancreas transplantation with portal venous and enteric drainage. Ann Surg 1995; 221: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaber A, Shokouh-Amiri M, Grewal H, Britt L. A technique for portal pancreatic transplantation with enteric drainage. Surg Gynecol Obstet 1993; 177: 417. [PubMed] [Google Scholar]
- 13.Di Carlo V, Castoldi R, Gristallo M, et al. Techniques of pancreas transplantation through the world: an IPITA center survey. Transplant Proc 1998; 30: 231–241. [DOI] [PubMed] [Google Scholar]
- 14.Humar A, Gruessner R, Sutherland D. Living related donor pancreas and pancreas–kidney transplantation. Br Med Bull 1997; 53: 879–891. [DOI] [PubMed] [Google Scholar]
- 15.Gruessner RW, Kendall DM, Drangstveit MB, et al. Simultaneous pancreas–kidney transplantation from live donors. Ann Surg 1997; 226: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs S, Flowers J, Dunkin B, et al. Living donor nephrectomy. Curr Opin Urol 1999; 9: 115–120. [DOI] [PubMed] [Google Scholar]
- 17.Gruessner R, Sutherland D, Najarian J, et al. Solitary pancreas transplantation for nonuremic patients with labile insulin-dependent diabetes mellitus. Transplantation 1997; 64: 1572–1577. [DOI] [PubMed] [Google Scholar]
- 18.Eubanks J, Shokouh-Amiri M, Elmer D, et al. Solitary pancreas transplantation using the portal-enteric technique. Transplant Proc 1998; 30: 446–447. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland D, Gruessner R, Najarian J, Gruessner A. Solitary pancreas transplants: a new era. Transplant Proc 1998; 30: 280–281. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz P, Gomez F, Schreiber A. Impaired function of macrophage Fc receptors in end-stage renal disease. N Engl J Med 1990; 322: 717–722. [DOI] [PubMed] [Google Scholar]
- 21.Goldblum S, Reed W. Host defenses and immunologic alterations associated with chronic hemodialysis. Ann Intern Med 1980; 93: 597–613. [DOI] [PubMed] [Google Scholar]
- 22.Gruessner R, Burke G, Stratta R, et al. A multicenter analysis of the first experience with FK506 for induction and rescue therapy after pancreas transplantation. Transplantation 1996; 61: 261–273. [DOI] [PubMed] [Google Scholar]
- 23.Gruessner RW, Sutherland DE, Drangstveit MB, et al. Mycophenolate mofetil and tacrolimus for induction and maintenance therapy after pancreas transplantation. Transplant Proc 1998; 30: 518–520. [DOI] [PubMed] [Google Scholar]
- 24.Newell K, Bruce D, Cronin D, et al. Comparison of pancreas transplantation with portal venous and enteric exocrine drainage to the standard technique utilizing bladder drainage of exocrine secretions. Transplantation 1996; 62: 1353–1356. [DOI] [PubMed] [Google Scholar]
- 25.Gruessner A, Sutherland D. Pancreas transplants for United States (US) and non-US cases as reported to the International Pancreas Transplant Registry (IPTR) and to the United Network for Organ Sharing. In: Terasaki P, Cecka J, eds. Clinical Transplants 1996. Los Angeles: UCLA Tissue Typing Laboratory; 1997: 45–59. [PubMed]


