Abstract
Objective
To describe the clinical recognition, pathology, and management of Richter’s hernia and to review the relevant literature of the past 400 years.
Summary Background Data
The earliest known reported case of Richter’s hernia occurred in 1598 and was described by Fabricius Hildanus. The first scientific description of this particular hernia was given by August Gottlob Richter in 1778, who presented it as “the small rupture.” In 1887, Sir Frederick Treves gave an excellent overview on the topic and proposed the title “Richter’s hernia.” To his work—a cornerstone to modern understanding—hardly any new aspects can be added today. Since then, only occasional case reports or small series of retrospectively collected Richter’s hernias have been published.
Methods
The authors draw on their experience with 18 prospectively collected cases treated in the ICRC Lopiding Hospital for War Surgery in northern Kenya between February and December 1998 and review the relevant literature of the past 400 years.
Results
The classic features of Richter’s hernia were confirmed in all case studies of patients: only part of the circumference of the bowel is entrapped and strangulated in the hernial orifice. The involved segment may rapidly pass into gangrene, yet signs of intestinal obstruction are often absent. The death rate in the authors’ collective was 17%.
Conclusion
Richter’s hernia is a deceptive entity whose high death rate can be reduced by accurate diagnosis and early surgery. Considering the increasing incidence at laparoscope insertion sites, awareness of this special type of hernia with its misleading clinical appearance is important and of general interest.
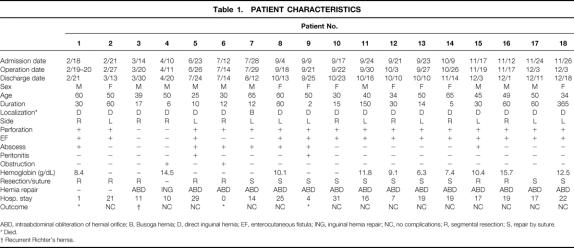
Richter’s hernia may be defined as an abdominal hernia in which only part of the circumference of the bowel is entrapped and strangulated in the hernial orifice (Fig. 1). The segment of the engaged bowel is nearly always the lower portion of the ileum, 1 but any part of the intestinal tract, from the stomach 2 to the colon, including even the appendix, 3–5 may become incarcerated.
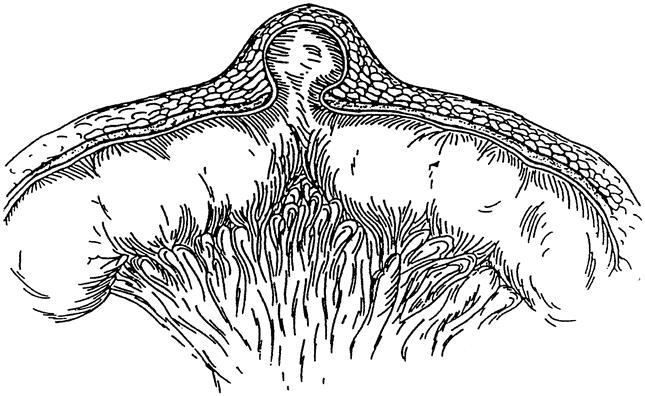
Figure 1. Partial entrapment of bowel wall with preservation of luminal continuity.
The precondition for the formation of this particular hernia, as stated by Richter, is determined by the size and consistency of the hernial orifice: it must be big enough to ensnare the bowel wall, but small enough to prevent protrusion of an entire loop of the intestine, and the margin of the hernial ring must be firm or, in Richter’s words, “possess strong spring-force.”6 According to others, the presence of a tight constricting ring is a prerequisite for strangulation and compromised blood circulation, which finally leads to ischemia and gangrene of the involved bowel. 7,8 Richter’s hernias tend to progress more rapidly to gangrene than ordinary strangulated ones. This can be observed in the series of Horbach, 9 who found 45 Richter’s hernias among 146 strangulated hernias. In these 45, he found necrosis of the bowel wall in 31 (69%); among the 101 ordinary strangulated hernias, he found bowel necrosis in only 25 (25%). This may be explained not only by the firm constricting ring that exerts direct pressure on the bowel wall, but also by the anatomical peculiarity that, as a rule, it is the free border of the intestine opposite the mesentery with the predominance of terminal arterioles that is involved. It can also be explained by the time factor. In most cases, where less than two thirds of the circumference of the bowel wall is involved,* the lumen of the gut remains free and an alarming intestinal obstruction is absent. This insidious pathologic feature of Richter’s hernia often leads to late diagnosis or even misdiagnosis, thus allowing time for bowel necrosis to develop.
This hernia is rare, but not so rare as to be merely a curiosity. Moreover, the dramatic increase in the use of laparoscopic surgery, creating a new site for development of Richter’s hernia, will undoubtedly be followed by an increase in its incidence. As our knowledge of this type of hernia increases, diagnosis will be made more easily and the condition suspected more often, thus avoiding the serious consequences of delay in treatment.
The first description of a case of Richter’s hernia was made by Fabricius Hildanus (1560–1634) in 1606 10 (Fig. 2). Because this case illustrates a typical clinical presentation of a perforated Richter’s hernia, and because we could not find a correct citation and interpretation of his interesting and detailed report in all the papers referring to it, we offer the following translation from its original Latin source:

Figure 2. Fabricius Hildanus (1560–1632).
“Gangrene resulting from an intestinal hernia with perforation and subsequent cure
Margarete of Gléresse, a noble-woman of about 63 years of age suffering from a right-sided inguinal hernia for 17 years, was bothered by severe pain since December 1597. The intestine, after finally rupturing through the abdominal wall, provoked not unimportant pain and, at the same time, inflammation and other burdensome symptoms. Called to her in January 1598, I found the right inguinal region affected with gangrene. After having scarified the tumour and done other things which are necessary to the healing of gangrene, there was callous and pussy flesh falling out of the rupture. In consequence, the symptoms—pains, inflammation, fever, swoon, nausea and vomiting—faded away. But for approximately two months the contents of the intestine, ileum or coecum, were excreted through the fistula. The sick woman, however, to the great astonishment of the persons present, returned to good health with the help of God and was perfectly cured from the rupture without any residual fistula or hernia.
Hildanus then quotes several prominent men as witnesses of this miraculous cure. He concludes from his observation that intestinal wounds are not always incurable, which was at that time still unbelievable. In the complete edition of his works of 1646, 11 he notes that the noblewoman lived until late 1613, when she died of the plague.
METHODS
Study Place and Patient Collective
Lopiding Hospital in Lokichokio in northern Kenya, is a surgical hospital for victims of the Sudanese war, run by the International Committee of the Red Cross near the southern border of Sudan. The hospital was built in 1987 with a capacity of 35 beds; because of the increasing number of war victims, it has since expanded to a 600-bed capacity. Because the hospital draws from an area the size of Spain and France and is the only hospital in this remote area, not only are war victims admitted, but patients with all kinds of trauma and abdominal and obstetric surgery emergencies as well.
We collected clinical details prospectively on all patients with strangulated hernias who were admitted to this hospital between February and December 1998. The patients were kept in the hospital until we considered them healed. In this rural Central African region, postoperative follow-up is impossible, mainly because of the lack of transportation and the enormous distances between the patients’ homes and the hospital.
Twenty-one patients with strangulated hernias were admitted to the hospital. In four patients (19%), a normal strangulated hernia was present; in 17 patients (81%), a Richter’s hernia was present. One patient was admitted twice, the second time with a recurrent Richter’s hernia. The approximate mean age of patients with Richter’s hernia was 45 years (range 25–65); exact information about age was rarely available because the patients did not know their age, and birth certificates do not exist in southern Sudan. The male-to-female ratio was 10:7 (59%:41%). The time span from onset of the symptoms to hospital admission ranged from 6 days to 2 months, with an average of approximately 3 weeks (one patient suffered from an enterocutaneous fistula for 6 months). Because we were the first doctors to see the patients, this was always due to “patient’s delay.”
All patients were either very lean or malnourished and probably hypoproteinemic. Anemia was widespread as a result of endemic malaria. Nevertheless, hemoglobin was not routinely tested before surgery, except in cases of suspected severe anemia with a probable need for substitution. Blood transfusions were administered only occasionally because of lack of blood donors and the high prevalence of syphilis, hepatitis B, and human immunodeficiency virus. They were considered only for patients in whom the hemoglobin level was less than 6 g/dL, and only blood that tested negative for hepatitis B, syphilis, and human immunodeficiency virus was transfused. Patients with a chronic enterocutaneous fistula were fed a high-protein diet (i.e., eggs and meat, as a supplement to the normal meal) for 1 to 3 weeks before surgical intervention to improve their general condition and their immunologic resistance. Because of the limited equipment of a field hospital, the diagnosis was established mainly by means of patient history, clinical examination, and the professional experience of the surgeon. Simple laboratory tests (hemoglobin, white blood cell count, malaria smear) and conventional radiographs (no ultrasound or computed tomography) were available.
In 17 patients, we found a direct inguinal hernia and in one patient a so-called Busoga hernia, which will be discussed later. Sixteen of 18 patients (89%) had a perforated Richter’s hernia: in 11 there was an enterocutaneous fistula in the groin; in one, the hernia perforated through the labia majora; and in four, there was a scrotal abscess with skin necrosis, reaching the grotesque size of a melon (Fig. 3). These four patients all showed signs of septic/toxic shock. Only two patients (11%) had intestinal obstruction.
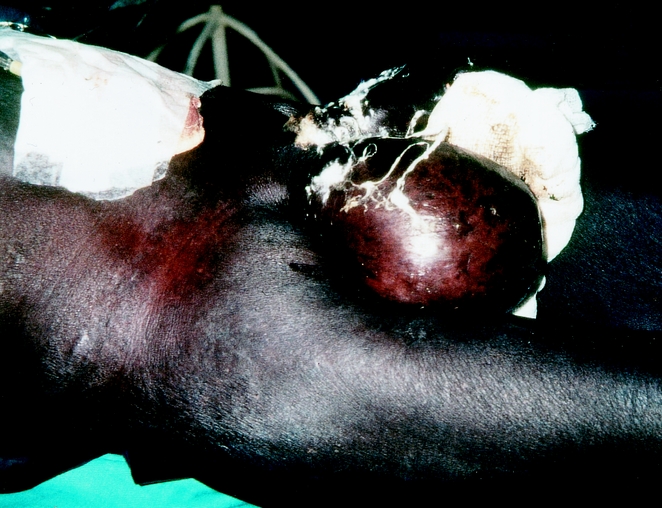
Figure 3. Grotesque scrotal abscess after perforated Richter’s hernia.
Surgical Treatment
Patients with perforated hernia (89%) required timely and sometimes aggressive preoperative resuscitation, consisting of isotonic rehydration and antibiotic therapy. In some cases, positive preoperative balances up to 9,000 mL were necessary. All patients were routinely treated with penicillin (20 million U/day) on admission. If there were signs of septicemia or peritonitis, gentamicin and metronidazole were added. These preparations for surgery normally took 12 to 36 hours.
All patients underwent surgery based on accepted principles of hernia surgery. Because of the limitations of a field hospital, no artificial biomaterial was used. In only two instances was the hernia repaired anatomically using the inguinal approach. In all other instances, the hernial orifice was obliterated from inside by interrupted Vicryl sutures because the infected inguinal area was unsuitable for reconstructive hernia repair and additional surgery could be avoided. In one patient, obliteration of the hernial orifice was accidentally omitted.
Laparotomy was necessary in all but the one patient with Busoga hernia (94%) because, in the presence of an enterocutaneous fistula, the bowel was so intensely adherent to the hernial ring that mobilization from an inguinal approach was impossible. Whenever possible, we tried to avoid resection of the intestine by directly repairing the bowel defect with extramucosal sutures, sometimes after minor resection of the involved margin of the bowel wall. This was possible in 10 of 17 instances (59%) in which not more than two thirds of the circumference of the gut was strangulated. In seven instances (41%) involving more extensive defects, segmental bowel resection was necessary. In one patient, the strangulated area of the gut was still viable and did not require any further surgical treatment.
RESULTS
Of the 15 patients who survived, the average hospital stay was 18 days (range 7–31) (Table 1). Patients were considered healed when the following conditions were fulfilled: normal oral feeding, no signs of infection, no fever or pain, and closure of the inguinal wound (or almost closed and granulating nicely, so that the patient could change the dressing on his or her own). This result was achieved in 83% of the patients (15/18).
Table 1. PATIENT CHARACTERISTICS
ABD, intraabdominal obliteration of hernial orifice; B, Busoga hernia; D, direct inguinal hernia; EF, enterocutaneous fistula; ING, inguinal hernia repair; NC, no complications; R, segmental resection; S, repair by suture.
* Died.
† Recurrent Richter’s hernia.
The death rate was 17% (3/18). These three patients died of septic shock shortly after surgery. Patient 6, who had a spontaneously perforated scrotal abscess of a grotesque size, was in such bad general condition due to septicemia that a favorable outcome was doubtful from the start. He died 6 hours after surgery.
For patient 9, who had an enterocutaneous fistula excreting more than 1,000 mL/day, surgery was delayed for 12 days in an attempt to improve the preoperative nutritional status. During this period, however, the continuous discharge of highly aggressive histiolytic bowel content caused ulceration at the perforation site in the groin and led to a leak into the peritoneal cavity. As a result of the intestinal discharge, the patient developed severe peritonitis (Fig. 4) and died 4 days after surgery.
Figure 4. Severe purulent and fibrinous peritonitis.
Patient 1 had to undergo surgery twice because in the first procedure, the local abscess in the right iliac fossa was misinterpreted as resulting from appendicitis and the bowel perforation was overlooked. This resulted in a severe generalized peritonitis with heavy fecal contamination. The patient died 24 hours after the repeat surgery, which this time was performed correctly but too late.
The patient in whom obliteration of the hernial orifice was omitted developed a recurrent Richter’s hernia 6 months later, once more with an enterocutaneous fistula (case 2 and 13). To our knowledge, this represents the first such case described.
DISCUSSION
In his famous Treatise on the Ruptures6 in 1785, August Gottlob Richter (1742–1812) (Fig. 5) gave the first comprehensive description of hernias in which only part of the circumference of the bowel is strangulated, and termed them “the small ruptures.” The nomenclature of this hernia subsequently resulted in confusion because many English authors described it without a special name or spoke of it as Littre’s hernia.* This term was also used by most German and French surgeons, but was applied to a variety of ruptures quite distinct from what we describe here. Only 100 years later, in 1887, did the famous London surgeon Sir Frederick Treves (Fig. 6) distinguish these types of hernias from herniation of a Meckel diverticulum, which was classically described by Littre. 12 Treves credited Richter with the distinction of having given the first scientific description of this particular lesion and suggested the term Richter’s hernia, “(partly) because with Richter must rest the main credit of establishing the individuality of this lesion.”13

Figure 5. August Gottlob Richter (1742–1812).

Figure 6. Sir Frederick Treves (1853–1923) in 1884.
Treves’ unparalleled scholarly contribution to the subject remains, after more than a century, the cornerstone of modern understanding. Not only did he provide a detailed clinical description based on his own surgical experience, but he also exhaustively treated the topic by citing 52 authors since 1606 in his analytic and historical review of the subject. He then modestly proposed Richter, not himself, as deserving eponymous recognition for this hernia. All of this exemplifies his honest and scientific approach to research and medicine. With the two remarkable events in his life that made him widely known—the rescue of the “Elephant Man” and the life-saving intervention in King Edward VII’s appendicitis on the eve of the coronation—we ought not to forget his role as a medical pioneer. His outstanding contributions to anatomy and surgery during his distinguished career are evidenced by his authorship of more than 250 books and medical articles. Also considering his untiring energy and immense working capacity as a staff surgeon at the London Hospital while leading a life of almost spartan simplicity, along with his literary abilities and his humanitarian commitment, he must surely be recognized as a shining example of all-around medical and personal excellence. 14–16
Recent literature 17,18 reveals little new information about the incidence and sites of Richter’s hernia and confirms the classic reports of Treves, Frankau, 19 and Gillespie et al, 20 which, except for Africa, remain the best sources of information. According to these large series, one can derive the general rule that approximately 10% of strangulated hernias are Richter’s hernias (5–15%). Normally, patients with Richter’s hernia are 60 to 80 years old, 21 but cases have been described even in infants. 22,23 The predilection of the femoral hernial site manifests itself in the sex distribution, in that in whites, women are generally overrepresented: 58% in Treves’ series and 57% in the series of Kadirov et al. 17
Richter’s hernia may occur in any usual hernial site, but it seems most likely to occur in small hernial rings with firm margins. In whites, the most common site is the femoral ring (36–88%), followed by the inguinal canal (12–36%) and abdominal wall incisional hernia (4–25%). 13,19,20,24 Rare sites, such as umbilical, 25 obturator, 26 supravesical, 8 spigelian, 27,28 triangle of Petit, 29 sacral foramen, 30 Morgagni, 31 internal, 32 or (traumatic) diaphragmatic hernias, 33 have also been described. The growing popularity of laparoscopic surgery has led to a new possible site for development of a Richter’s hernia: since the first description of a Richter’s-type herniation through a laparoscopy incision in 1977, 34 similar case reports have increasingly been published. 35–41 The conditions for development of a Richter’s hernia seem to be ideally fulfilled by the size and quality of the insertion sites of laparoscopic instruments, especially those with a diameter of 10 to 20 mm.
The situation is different in Central Africa, where Richter’s hernias occur much more frequently. In our collective in Lokichokio, which was selected only for logistical reasons (emergency cases only, difficult and tedious evacuation procedure), we found the exceptionally high rate of 81% of Richter’s-type hernias among our strangulated hernias. This cannot be explained by a selection bias for Richter’s hernias with only partial obstruction, which would ensure better chances of patient survival and of timely arrival at the hospital. First, the 11% of patients with totally obstructed Richter’s hernias in our series arrived at our hospital in time as well, and the rate of 11% for total obstruction in Richter’s hernia is consistent with the rate reported in the literature. Second, all patients with abdominal emergencies received immediate approval for their admission request by telex and, because all patients were transported by plane, arrived at the hospital in time for surgery. Rarely did we observe a “natural” selection of patients because of logistic reasons. In our opinion, the high occurrence of Richter’s hernias in this region is more likely explained by the obviously widespread anatomical peculiarity of the hernial orifice, with its predisposing diameter and firmness, combined with poor nutritional status.
Tomaszewski, 42 Hancock, 43 and Horbach, 9 all with relatively large series of unselected patients from Central Africa, found Richter’s hernias in 10.3%, 25%, and 31% of patients, respectively. The mean age of our patients was 45 years (range 25–65), with a male-to-female ratio of 59%. Tomaszewski’s patients were 20 to 45 years old, and he found a clear predominance of male patients (95%). The remaining authors gave no information regarding age and sex distribution.
There is again a different pattern concerning the herniation site. In our series, direct inguinal hernias were by far the most common sites (94%), a pattern confirmed by several other authors, who found inguinal hernias in 78% to 88% of their patients. 9,44,43 The sparse statistical data concerning the less common sites allow only estimates. In the available papers, femoral (9–13%) and indirect inguinal hernias (9%) occur with similar frequency.
In one patient, we found a peculiar form of an inguinal hernia with typical anatomical features, characterized by a defect in the conjoined tendon, which is the site of herniation, just above and lateral to the pubic tubercle. Eckhart, 44 Hancock, 43 and Horbach 9 found a high incidence of this typical hernia in the Busoga district of Central Uganda and named this hernia, unknown to Western surgeons, Busoga hernia. In a study to find the cause of this typical hernia, Eckhart suggested genetic factors in the Busoga tribe. It is, however, not congenital, because it has never been described before puberty, and Fellows 45 found no direct hernias in 200 stillbirth dissections in Kampala. He suggested environmental factors in the area around the source of the Nile at Jinja. His theory has not been validated, so the cause remains a matter of dispute.
The question of whether the quality and the condition of the intestinal wall play a role in the development of bowel entrapment has not been discussed in the literature. It seems logical, however, to assume that the more elastic the bowel wall is (consider the difference between inflating a thin-walled and a thick-walled balloon), the more easily a partial enterocele is formed. This hypothesis is supported by our observation that all our patients in Lokichokio showed a strikingly flabby bowel—possibly caused by malnutrition. Adhesions between the hernial sac and intestinal wall, as postulated by White 46 in 1912, however, were rarely observed and could well be considered a consequence of a chronic Richter’s hernia rather than a cause for it.
Making the diagnosis of Richter’s hernia may be difficult because of the apparently innocuous initial symptoms and sparse clinical findings; the diagnosis may remain presumptive until clearly confirmed at surgery. The first mild symptoms, such as vague abdominal pain and slight malaise, may not be appreciated, resulting in delayed diagnosis. 7,8,21 There may be nausea and vomiting, but they are on the whole less common and less severe than in the usual form of strangulation because obstruction is rarely complete. Clinical and radiologic signs of an ileus are present in approximately 10% of patients; in the absence of a complete mechanical obstruction, this can be due to paralysis. Local signs may be absent or discrete and, if present, are easily overlooked or misinterpreted. Throughout the literature, the most constant physical finding remains tenderness or swelling over a potential hernial orifice. Overlying erythema should heighten the index of suspicion. Because the tumor in these cases is small, it is difficult to examine, but when large enough it presents nothing more than the features of an ordinary strangulated hernia. 13,47 A small hernia in the femoral canal, the most common site of Richter’s hernia (in whites), is sometimes masked by body fat or an enlarged lymph node or is mistaken for acute lymphadenitis. If local gangrene of the intestinal wall occurs, the classic signs of inflammation appear (painful swelling, redness of the overlying skin, and local heat). In the early stage, this tumor can be discrete and can appear as a local abscess or even a subcutaneous emphysema caused by anaerobic infection. 48,49 If a hernia happens to perforate through the inguinal canal into the scrotum, corresponding to an advanced stage, as we observed several times in our African patients, grotesque scrotal abscesses with gangrene may develop; these are usually fatal because of the high toxin load. Similarly, gangrenous inflammation of the vulva can occur. 50
If surgery is performed too late or not at all, natural healing may occur in the form of drainage through an enterocutaneous fistula. Under certain conditions (a self-limiting septic process, a low-output fistula, and free intestinal passage), the fistula may spontaneously close, as observed by Fabricius Hildanus, but it may also persist for months, as reported by others. 18,51 Perforation into another compartment, such as the scrotum, vulva, thighs, or peritoneal cavity, 52 may also occur, producing a severe clinical course with considerable morbidity and a high death rate. With the appearance of peritonitis, the prognosis becomes highly unfavorable. Gangrene has been found as early as the third day of strangulation, 13 but the development of an enterocutaneous fistula may take as long as several months. 18
In addition to patient history and careful physical examination, radiology may be helpful in establishing the diagnosis. The value of ultrasound and computed tomography in particular patients is undisputed. 33,53 In our experience, however (and on this point Sir Frederick Treves would probably agree), diagnosis can usually be suspected on clinical grounds. The crucial point is to keep this condition in mind, much the same as gallstone ileus must be considered in obese patients with unexplained subacute symptoms and signs of intestinal obstruction.
According to localization and the mode of herniation and entrapment, the clinical picture and course can vary considerably. Nevertheless, the cases can be divided into four main groups based on clinical responses. To the three groups established by Gillespie et al, 20 we add a fourth to cover the situation we found in Central Africa:
The obstructive group, in which the dominant clinical presentation of intestinal obstruction leads to early diagnosis and therapy, resulting in an excellent prognosis;
The danger group, in which symptomatology is vague and nonspecific: initial examinations with or without contrast fail to reveal impending disaster, and the subsequent delay in surgery is chiefly responsible for the high rates of death and complications;
The postnecrotic group, in which local strangulation and perforation leads to formation of an enterocutaneous fistula, similar to that described by Fabricius Hildanus 400 years ago; the fistula may close spontaneously or remain chronic; and
The “unlucky perforation” group, in which the postnecrotic abscess, as a result of unlucky anatomical constellations, accidentally finds its way into another compartment, resulting either in a large abscess with severe septic/toxic load or in peritonitis; both of these would lead to a high death rate.
There is but one treatment: surgery. Richter’s hernias in the groin without apparent signs of perforation can be handled as ordinary inguinal hernias. Preliminary attempts at manual reduction should be avoided because the viability of the hernial sac can be determined by direct inspection only. In perforated hernias, laparotomy is usually necessary to deal correctly with the bowel defect. When possible, we avoid a segmental resection and close the defect after a careful debridement of the margins. This is possible in most cases in which the involved area does not exceed half (sometimes even two thirds) of the circumference of the gut. However, the rule regarding strangulation (“When in doubt, resect”) should be respected. This is especially true in patients with chronic enterocutaneous fistulas or considerable inflammation of the affected bowel segment, which could compromise free intestinal passage and lead to a leak of the anastomosis.
The main goal in these patients should be to reduce the systemic toxin load from the gangrenous herniated tissues and to prevent contaminated material from spilling into the peritoneal cavity. To accomplish these objectives, we recommend that the hernia be approached initially from its interior aspect through laparotomy to secure control of both the intestine and the vascular supply before release of the hernia. In patients with disastrous necrotizing infections of the surrounding tissue (e.g., the scrotal abscesses we observed in Lokichokio), a two-stage approach may be preferred: debridement and drainage of the abscess followed by bowel and hernia repair when the infection has subsided. With inguinal or femoral perforated hernias, either a preperitoneal or a midline approach can be used. This is a matter of personal preference, with advantages and disadvantages in either case; the surgeon, however, should always bear in mind that early surgery and careful handling of the bowel are far more important for the final outcome. As shown by one of our patients, obliteration of the hernial orifice by suture must never be omitted if recurrence of a Richter’s hernia is to be avoided.
Horbach 9 points to an interesting alternative to the bowel resection under certain conditions. If the typical “coin lesion” of a bowel wall is nonviable but not yet perforated, does not affect more than 50% of the circumference, does not extend to the mesenteric border, and shows viable and pliable margins, he recommended an invagination procedure without opening of the intestine. The gangrenous area is invaginated and the margins are sutured together. The necrotic part will de-slough inside the bowel after some days, while the united margins are fusing. This procedure, also suggested by Litler Jones in 1904 54 and Cattell, 47 proved to be quick, reliable, and well tolerated in all 23 patients.
CONCLUSION
Richter’s hernia is associated with a strikingly high death rate, as shown throughout the medical literature. The reduction of the death rate from 62.2% reported by Treves to 21.4% found by Kadirov et al 17 and 17% in our series, more than 100 years later, emphasizes the seriousness of this condition. Irrespective of the availability of sophisticated diagnostic facilities, the prognosis can be improved significantly only by including this deceptive disease in the differential diagnosis of uncharacteristic abdominal pain, specifically in patients with a history of laparoscopic surgery. In accordance with the conclusion of Kadirov et al, we repeat that imaging modalities, such as computed tomography or water-soluble contrast studies, are of doubtful usefulness in the early diagnosis of Richter’s hernia. The entrapped small segment of bowel wall would be difficult to visualize on computed tomography, and contrast studies would be unrevealing in the early stages, when there is still patency. Therefore, awareness during the clinical examination remains the key for proper diagnosis and timely surgery. Once the diagnosis is established, the acute infection phase is under control, and the patient undergoes successful surgery, the outcome does not differ from that of ordinary strangulated hernias. Based on our experience in Central Africa, where we witnessed the natural course of this disease, we fully appreciate the significance of Treves’ concluding statement: “One cannot, however, fail to be struck with the frequency with which spontaneous cure has followed in cases which have been practically left to themselves.” Such is the miracle of life.
Acknowledgments
The authors thank the International Committee of the Red Cross (ICRC) in Geneva for permission to publish the data. The authors especially thank Sven Almér, MD, Chris Giannou, MD, Martin Herrmann, MD, and Günther Wimhöfer, MD, all surgeons of the ICRC, as well as Toril Parelius, head nurse of Lopiding Hospital, for their support and help in collecting patient data during and after our war-surgery mission. Special thanks go to Hubert Steinke, MD, Institute for History of Medicine, University of Berne, for providing the medical historical materials, and to Dennis Gibbs, FRCP, Appleford, U.K., for his help concerning Sir Frederick Treves. The authors also thank Hannes Schwarz, Professor of Surgery and ICRC Delegate, for his motivating spirit and encouragement in humanitarian work.
Footnotes
Correspondence: Wolfgang Steinke, MD, Kantonsspital, Chirurgische Abteilung, CH-8200 Schaffhausen, Switzerland.
3 *There is no reason for adding an accent on the terminal “e” of this distinguished surgeon’s name (who at his time signed his name “De Litre”). This error has been continued in countless textbooks and articles during the past 150 years.
Accepted for publication February 23, 2000.
References
- 1.Tito WA, Allen WC. Richter and Littre Hernia. In: Nyhus JB, Condon RE, eds. Hernia, 3rd edition, Philadelphia: Lippincott; 1989:305–310.
- 2.Giokas G, Karakousis CP. Richter hernia of the stomach. J Surg Oncol 1998; 69 (1): 51–53. [DOI] [PubMed] [Google Scholar]
- 3.Duari M. Strangulated femoral hernia: a Richter’s type containing caecum and base of appendix. Postgrad Med J 1966; 42: 726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe PH, Hunter-Craig C. Richter’s hernia in a direct inguinal sac in a female. J R Coll Surg Edinb 1984; 29: 264. [PubMed] [Google Scholar]
- 5.Newerla GJ, Connally EF. Gangrenous appendicitis in femoral hernia of Richter’s type. Am J Surg 1943; 61: 154–156. [Google Scholar]
- 6.Richter AG. Abhandlung von den Brüchen. Göttingen: Johann Christian Dieterich; 1785.
- 7.Pualwuan F. Operative aspects of Richter’s hernia. Surg Gynecol Obstet 1958; 106: 358–362. [PubMed] [Google Scholar]
- 8.Keynes WM. Richter’s hernia. Surg Gynecol Obstet 1956; 103: 496–500. [PubMed] [Google Scholar]
- 9.Horbach JM. Invagination for Richter-type strangulated hernias. Trop Doct 1986; 16 (4): 163–168. [DOI] [PubMed] [Google Scholar]
- 10.Fabricius Hildanus. Observationum et Curationum Chirurgicarum Centuriae. Basileae: Sumptibus Ludovici Regis; 1606(1st ed):159.
- 11.Fabricius Hildanus. Opera Observationum et Curationum Medico-Chirurgicarum. Francofurtt: Sumptibus Joannes Beyert; 1646(complete ed):45–46.
- 12.Littre A. Observation sur une nouvelle espèce de hernie. Paris: Mém. de l’Acad. Royal des Sciences; 1700:300.
- 13.Treves F. Richter’s hernia or partial enterocele. Med Chir Tr 1887; 17: 149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibbs DD. Sir Frederick Treves: surgeon, author and medical historian. J R Soc Med 1992; 85: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trombley S. Sir Frederick Treves: The Extra-Ordinary Edwardian. London: Routledge; 1989.
- 16.Evans J. Sir Frederick Treves: surgeon extraordinary. London Hosp Gaz 1997; 22: 9–13. [Google Scholar]
- 17.Kadirov S, Sayfan J, Friedman S, Orda RJ. Richter’s hernia: a surgical pitfall. Am Coll Surg 1996; 182 (1): 60–62. [PubMed] [Google Scholar]
- 18.Fückiger R, Huber A. Die Richter’sche Hernie. Der Chirurg 1993; 64: 822–826. [PubMed] [Google Scholar]
- 19.Frankau C. Strangulated hernias: a review of 1487 cases. Br J Surg 1931; 19: 176–191. [Google Scholar]
- 20.Gillespie RW, Glas WN, Mertz GH, Musselman M. Richter’s hernia: its etiology, recognition and management. Arch Surg 1956; 73: 590–594. [PubMed] [Google Scholar]
- 21.Kadirov S, Sayfan J, Orda R. Partial enterocele (Richter’s hernia). Eur J Surg 1995; 161: 383–385. [PubMed] [Google Scholar]
- 22.Melmed EP, Cornes SM, Henley FA. Strangulated Richter’s hernia of the inguinal canal in a six-week-old infant. Surgery 1966; 59: 1120–1123. [PubMed] [Google Scholar]
- 23.Shanbhogue LK, Miller SS. Richter’s hernia in the neonate. J Pediatr Surg 1986; 21: 881–882. [DOI] [PubMed] [Google Scholar]
- 24.Lyall D, Luomanen R. Richter’s Hernia. Am J Surg 1948; 65: 828–833. [DOI] [PubMed] [Google Scholar]
- 25.Folimer HC. Umbilical Richter’s hernia. Rocky Mt Med J 1966; 63: 61–62. [PubMed] [Google Scholar]
- 26.Cook MD, Yarrington CD. Strangulated Richter’s hernia of the obturator canal: report of two cases. Am Prac 1962; 13: 115. [PubMed] [Google Scholar]
- 27.Naylor J. Combination of Spigelian and Richter’s hernia: a case report. Am Surg 1978; 44: 750–752. [PubMed] [Google Scholar]
- 28.Hiller N, Alberton Y, Shapira Y, Hadas-Halpern I. Richter’s hernia strangulated in a spigelian hernia: ultrasonic diagnosis. J Clin Ultrasound 1994; 22: 503—505. [DOI] [PubMed] [Google Scholar]
- 29.Millard DG. Richter’s hernia through the inferior lumbar triangle of Petit: a radiographic demonstration. Br J Radiol 1959; 32: 693. [DOI] [PubMed] [Google Scholar]
- 30.Botsford TW. Richter hernia in a sacral foramen: new site for Richter hernia. Arch Surg 1977; 112: 304–305. [DOI] [PubMed] [Google Scholar]
- 31.Kimmelstiel FM, Holgersen LO, Hilfer C. Retrosternal (Morgagni) hernia with small bowel obstruction secondary to a Richter’s incarceration. J Pediatr Surg 1987; 22: 998–1000. [DOI] [PubMed] [Google Scholar]
- 32.Fiirgaard B, Agertoft A. Internal Richter’s hernia due to congenital peritoneal defect: case report. Acta Chir Scand 1988; 154: 537. [PubMed] [Google Scholar]
- 33.Baker ME, Ungerlaider R, Cooper C, Dunnick NR. Computed tomography of a traumatic, diaphragmatic Richter’s hernia: findings mimicking an abscess. J Comput Tomogr 1988; 12 (1): 42–44. [DOI] [PubMed] [Google Scholar]
- 34.Bourke JB. Small-intestinal obstruction from a Richter’s hernia at the site of insertion of a laparoscope. Br Med J 1977; 26: 1393–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart D. Richter’s hernia at site of insertion of laparoscope. Br Med J 1977; 2: 1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins DM, Paluzzi M, Scott TE. Postlaparoscopic small bowel obstruction. Surg Laparosc Endosc 1993; 3: 139–141. [PubMed] [Google Scholar]
- 37.Kiilholma P, Makinen J. Incarcerated Richter’s hernia after laparoscopy: a case report. Eur J Obstet Gynecol Reprod Biol 1988; 28: 75–77. [DOI] [PubMed] [Google Scholar]
- 38.Wegener ME, Cheung D, Crans C, Chung D. Small bowel obstruction secondary to Incarcerated Richter’s hernia from laparoscopic hernia repair. J Laparoendosc Surg 1993; 3: 173–176. [DOI] [PubMed] [Google Scholar]
- 39.Hass BE, Schrager RE. Small bowel obstruction due to Richter’s hernia after laparoscopic procedures. J Laparoendosc Surg 1993; 3: 421–423. [DOI] [PubMed] [Google Scholar]
- 40.Radcliffe AG. Richter’s herniation of the small bowel through the trocar site following laparoscopic surgery. J Laparoendosc Surg 1993; 3: 520–522. [DOI] [PubMed] [Google Scholar]
- 41.Williams MD, Flowers SS, Fenoglio ME, Brown TR. Richter hernia: a rare complication of laparoscopy. Surg Laparosc Endosc 1995; 5: 419–421. [PubMed] [Google Scholar]
- 42.Tomaszewski P. W sprawie wystepowana przepukliny typu Richtera u ludnosci nigerii [Incidence of Richter’s hernia among the population of Nigeria]. [Article in Polish]. Wiad Lek 1988; 41: 974–975. [PubMed] [Google Scholar]
- 43.Hancock BD. Basoga hernia prevalence in abdominal emergencies in Busoga, Uganda. Trop Geogr Med 1974; 26: 15–25. [PubMed] [Google Scholar]
- 44.Eckhart P. The incidence of strangulated hernia in Busoga, Uganda. East Afr Med J 1964; 41: 59–62. [PubMed] [Google Scholar]
- 45.Fellows GJ. Observations on strangulated direct inguinal hernias at Kampala and Jinja. East Afr Med J 1968; 45: 516–522. [PubMed] [Google Scholar]
- 46.White CS. Richter’s hernia. Surg Gynecol Obstet 1912; 14: 46–48. [Google Scholar]
- 47.Cattell RB. Richter’s hernia. Surg Gynecol Obstet 1933; 56: 700–704. [Google Scholar]
- 48.Munoz E, Rodriguez JM, Bardaji M, Martinez J, Veloso E, Marco C. Emphysema of the right thigh secondary to strangulated crural hernia (Richter’s hernia). Eur J Surg 1995; 161: 697–698. [PubMed] [Google Scholar]
- 49.Agster BE, Christiansen KH. Subcutaneous emphysema as a sign of a Richter’s hernia: report of a case. Dis Colon Rectum 1974; 17: 87–88. [DOI] [PubMed] [Google Scholar]
- 50.Bätz W, Dzieniszewski GP, Neher M. Nekrotisierende Entzündung der Vulva: Symptom einer Richter’schen Darmwandhernie. Geburtsh Frauenheilk 1984; 44: 518. [DOI] [PubMed] [Google Scholar]
- 51.Udofot SU. Multiple fecal and urinary fistulae as a complication of native treatment of inguinal hernia. Trop Geogr Med 1990; 43: 105. [PubMed] [Google Scholar]
- 52.Limjoco UR, Grubbs JM, Thomas MD. Richter’s hernia with bowel perforation. Am Fam Physician 1998; 58: 352–354. [PubMed] [Google Scholar]
- 53.Middlebrook MR, Efthekari F. Sonographic findings in Richter’s hernia. Gastrointest Radiol 1992; 17: 229–230. [DOI] [PubMed] [Google Scholar]
- 54.Litler Jones TC. Lancet 1904; I: 1281. [Google Scholar]