Abstract
Objective
To assess the influence of preoperative portal vein embolization (PVE) on the long-term outcome of liver resection for hepatocellular carcinoma (HCC) in injured liver.
Summary Background Data
On an healthy liver, PVE of the liver to be resected induces hypertrophy of the remnant liver and increases the safety of hepatectomy. On injured liver, this effect is still debated.
Methods
During the study period, 10 patients underwent preoperative PVE and 19 patients did not before resection of three or more liver segments for HCC in injured liver (cirrhosis or fibrosis). PVE was performed when the estimated rate of remnant functional liver parenchyma (ERRFLP) assessed by computed tomographic scan volumetry was less than 40%.
Results
In all patients, PVE was feasible. There were no deaths or complications. The ERRFLP after PVE was significantly increased compared with the pre-PVE value. Liver resection was performed after PVE in 9 of 10 patients, with surgical death and complication rates of 0% and 45%, respectively. PVE increased the number of resections of three or more segments by 47% (9/19). Overall actuarial survival rates with or without previous PVE (89%, 67%, and 44% vs. 80%, 53%, and 53% at 1, 3 and 5 years, respectively) and disease-free actuarial survival rates (86%, 64%, and 21% vs. 55%, 17%, and 17% at 1, 3, and 5 years respectively) after hepatectomy were comparable.
Conclusion
With the use of PVE, more patients with previously unresectable HCC in injured liver can benefit from resection. Long-term survival rates are comparable to those after resection without PVE.
Hepatocellular carcinoma (HCC) is one of the most common neoplasms worldwide, with an estimated incidence of approximately 1 million new cases annually. 1 In Western countries, the age-specific incidence has recently shifted toward younger patients, 2 and its incidence has substantially increased. 2–5 Curative resection remains the only chance of long-term survival for patients with this cancer, and our strategy is to increase the number of patients amenable to surgery. However, because of the very frequent underlying liver disease, namely fibrosis and above all cirrhosis, resection has the two contradictory aims: to be curative, with a safe tumor-free margin, and to preserve as much functioning liver parenchyma as possible. The reported resectability rate is approximately 10% in Western countries. 6 The tumor size is one of the most important determinants of resectability, and we have shown that resection can be achieved in some patients after downstaging by effective transarterial chemoembolization (TACE). 7 The surgical death rate from liver failure after hepatectomy for patients with HCC in injured liver has decreased with experience. 8 However, for major resection, it still ranges from 0%9,10 to 15% to 32%11–13 in recent series reported by experienced surgeons. 6 Liver failure is the main cause of these deaths, directly related to the rate of remaining functional liver. 14–16 Thus, when an HCC is technically resectable by means of a major hepatectomy, resection may still be contraindicated in some patients even after downstaging by TACE because the future remnant liver would be too small, with a prohibitive risk of severe postoperative liver failure. For this group of patients, portal vein embolization (PVE) of the future resected liver has been proposed to induce homolateral atrophy and contralateral compensatory hypertrophy of the remnant liver, thus preventing postoperative liver failure. 17 Although the concept of PVE appears to be well accepted when performed on an healthy liver, 18,19 its use in injured liver is still debated and has been reported only in Asian series 18,20–23 (Table 1).
Table 1. SERIES OF PORTAL VEIN EMBOLIZATION BEFORE MAJOR LIVER RESECTION FOR HEPATOCELLULAR CARCINOMA IN INJURED LIVER
* Series with five or more patients. NM, not mentioned.
The aim of the present study was to assess the feasibility, safety, and efficiency of preoperative PVE in terms of increased resectability by large resections of HCC in injured liver damaged by cirrhosis or fibrosis. The effect of preoperative PVE on patient outcomes was assessed.
PATIENTS AND METHODS
Patient Population
From May 1993 to August 1999, 29 patients with HCC in injured liver that was technically resectable by a major resection (i.e., resection of three or more Couinaud segments 24) were evaluated in our unit. Ten of these patients were not candidates for surgery because the remnant liver was deemed to be too small. PVE was proposed to these 10 consecutive patients, who represented the study population. Seven of them had cirrhosis and three had mild or moderate fibrosis. The 19 patients with sufficient remnant liver volume underwent major liver resection without previous PVE. The resections extended to three, four, and five segments in 3, 12, and 4 patients, respectively. Three of these 19 patients had postoperative liver insufficiency; in 1 it was fatal. The two groups, with and without PVE before major resection, were comparable in terms of sex, age, underlying liver injury, liver function test results before surgery, tumor node metastasis classification for HCC of the International Union Against Cancer (UICC), 25 and number of courses of neoadjuvant TACE (see below for protocol). The main characteristics of the entire series of patients are shown in Table 2. During the study period, we also performed 74 minor liver resections for HCC in injured liver.
Table 2. PATIENT CHARACTERISTICS
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ICGR 15, indocyanine green retention rate at 15 minutes; PVE, portal vein embolization; TACE, transarterial chemoembolization.
Definition of Liver Injury
Hepatic fibrosis was quantified on a nontumor liver biopsy taken at a distance from the tumor using the METAVIR scoring system. 26 All histologic specimens were reviewed by the same pathologist (J.F.E.). Patients with a fibrosis score of F3 or F4 were classified as having liver cirrhosis, whereas those with a lower score (F1 or F2) were classified as having mild or moderate fibrosis.
Technique of Transarterial Chemoembolization
Transarterial chemoembolization was performed according to our protocol. 27 In brief, conventional mesenteric arteriography was performed first to check for the presence of a right hepatic artery and to outline the portal circulation in the venous phase films. The celiac artery was then catheterized, and after assessment of the hepatic vascular anatomy a mixture of 10 mL Lipiodol Ultrafluide (Guerbet Laboratories, Aulnay-sous-Bois, France) and 50 mg doxorubicin (Adriamycine, Farmacia Laboratories, Saint-Quentin-en-Yvelines, France) or 50 to 70 mg cisplatin (Cisplatyl, Lilly Laboratories, St. Cloud, France) was given. Embolization was performed with gelatin pellets (Gelfoam, Upjohn Laboratories, Kalamazoo, MI).
Planned Hepatectomy
According to the morphologic evaluation before PVE, all 10 patients would need a resection of at least three liver segments. 24,28 The planned hepatectomy was right-sided in nine patients (right hepatectomy, n = 8; right lobectomy, n = 1) and left-sided in one patient (left hepatectomy). The classification of planned hepatectomies according to the number of segments is shown in Figure 1.
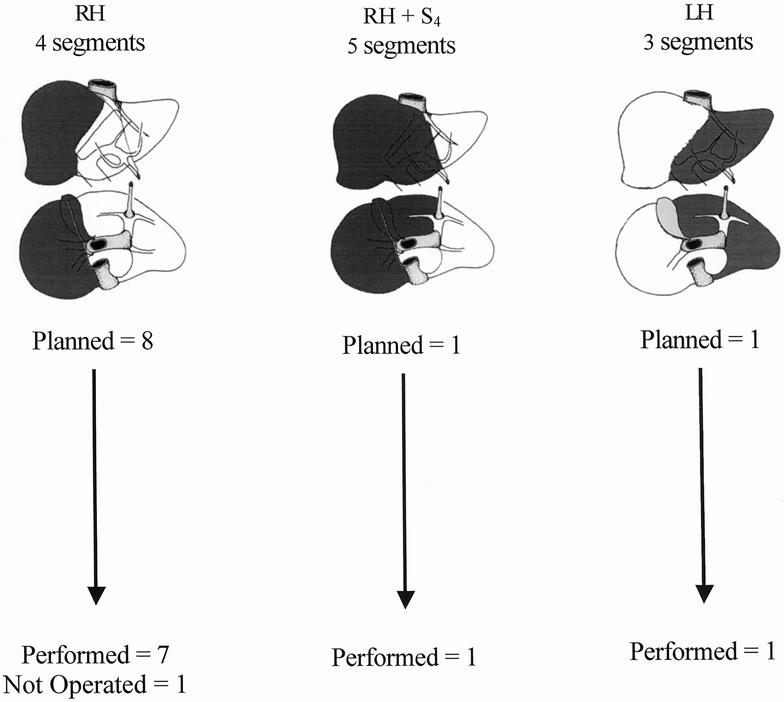
Figure 1. Relation between planned hepatectomy (darkened on anterior and inferior view of the liver) before portal vein embolization (above) and actual management (below) in 10 patients with hepatocellular carcinoma in cirrhosis (n = 7) or fibrosis (n = 3). RH, right hepatectomy; LH, left hepatectomy; Sx, segment number.
Criteria for Portal Vein Embolization
Our criteria for major hepatectomy in patients with injured liver are as follows: age younger than 70 years; indocyanine green retention rate at 15 minutes after injection of the dye (0.5 mg/kg) 10% or less; normal liver function test results, including serum albumin concentration at least 30 g/L and serum total bilirubin concentration at below 20 μmole/L, and prothrombin time at least 80% of normal; and an estimated rate of remnant functional liver parenchyma (ERRFLP) of more than 40%. According to these criteria, all patients who underwent PVE had at least one risk factor for primary surgery (2.5 ± 0.9; median 2; range 1–4 risk factors). The main indication for PVE was the need for resection of a large part of the functioning liver parenchyma, with the surgery technically feasible but contraindicated because the remnant liver would be too small, with a prohibitive risk of postoperative liver failure, the prime cause of death after hepatectomy. 14,29,30 The final decision to proceed with PVE was made only after careful evaluation of computed tomographic scan volumetry. PVE was performed systematically when the ERRFLP was 40% or less. Finally, PVE was performed at least 2 weeks after the last course of TACE.
Portal Vein Embolization
The technique of percutaneous PVE was reported in detail elsewhere. 31 In summary, after selectively catheterizing a small portal vein branch contralateral to the tumor or tumors under ultrasound guidance, control venous portography was performed under fluoroscopy and a guidewire was placed into the main portal branch homolateral to the tumor. Embolization was then performed with a mixture of enbucrilate (Histoacryl, Braun Lab, Melsungen, Germany) and lipiodol. The catheter was removed while injecting 2 mL fibrin glue (Tissucol, Immuno AG, Vienna, Austria) into the needle tract under ultrasound control.
Follow-Up of Portal Vein Embolization
Liver function tests including total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and prothrombin time, were performed before PVE, daily for 5 days thereafter, and before surgery. Computed tomographic scan volumetric measurements were performed before PVE and before surgery. The indocyanine green retention rate was measured before PVE and before surgery.
Computed Tomographic Scan Volumetric Measurements
Computed tomographic estimations of liver volumes were performed as previously described. 32–35 Briefly, computed tomography scans of the liver were obtained on a Siemens Somaton model HiQ (Siemens, Paris, France). Serial transverse scans at 1-cm intervals from the dome of the liver to the most inferior part of the organ were obtained, with enhancement by intravenous bolus injection of contrast and with the patient suspending respiration in expiration. Each slice of the liver was traced with a cursor, and the corresponding area was calculated by computer. The middle hepatic vein and gallbladder were used as landmarks to define the borders between the right and left livers. Segment 4 volume was measured using the middle hepatic vein and the umbilical portion of the left portal vein as landmarks. The total volumes measured (whole liver volume, tumor volume, and remnant liver volume) were calculated by multiplying the area of each part by the interval thickness and by adding all the interval volumes of each part.
The ERRFLP was systematically assessed before PVE and before surgery for nine patients who underwent surgery and at approximately 3 months for the patient who did not undergo surgery. It was calculated as follows: (remnant liver volume × 100)/(volume of entire liver − tumor volume). The increase in percentage of remnant liver volume was calculated as follows: (remnant liver volume before surgery − remnant liver volume before PVE) × 100/(remnant liver volume before PVE).
Decision to Perform Surgery with the Intention of Curative Hepatectomy
All computed tomographic scan investigations were reviewed by the same two radiologists, highly specialized in hepatic imaging. Hepatic resection was reconsidered when hypertrophy of the future remnant liver was considered to have reached a plateau on repeated computed tomography and provided that, first, the ERRFLP had increased to more than 40% and second, the liver function test results had returned to values comparable to those before PVE.
Surgical Technique
The technique of liver resection for HCC in injured liver in our unit has been standardized since 1984.36 At surgery, exploration of the abdominal cavity is performed to detect extrahepatic spread. Enlarged hepatic and celiac lymph nodes are excised for frozen section histology. Systematic liver ultrasound is carried out and biopsy samples are taken from any suspicious nodules. Provided a tumor-free margin of 1 cm can be obtained, parenchymal dissection is done using the ultrasonic dissector (CUSA, Cavitron Ultrasonic Aspirator, Valley Lab Inc., Boulder, CO), and resections are usually performed under intermittent clamping of the portal triad.
Definition of Postoperative Complications
Patients were defined as having postoperative liver insufficiency when at least two of the following parameters were observed concurrently: total bilirubin > 60 μmole/L, asterixis, alteration of consciousness, and prothrombin time < 30% of normal level. Other complications sought included intraabdominal hemorrhage requiring reoperation, biliary fistula, gastrointestinal hemorrhage, massive ascites (abdominal drain output >500 mL/day), renal insufficiency (with serum creatinine >150 mmol/L), infection (intraabdominal, extraabdominal, or systemic).
Data Analysis
Results are given as mean ± standard deviation unless stated otherwise. Statistical analysis was performed as indicated with a statistical analysis program package (StatView 4.5 software, Abacus Concepts, Inc., Berkeley, CA). Paired Student t tests were used. Survival rates were calculated using the Kaplan-Meier method, and groups were compared with the log-rank test. P < .05 was considered to be significant.
RESULTS
Portal vein embolization was feasible in all 10 patients. Right PVE was performed in nine patients. The branches to segment 4 (caudate lobe) were not embolized in the patient in whom this segment was to be resected. Left PVE was performed in one patient. None of the patients died within 2 months of PVE, and there were no complications from PVE.
Figure 2 shows that maximum values of total bilirubin, AST, and ALT in the 5 days after PVE were significantly increased compared with pre-PVE values, whereas prothrombin time diminished significantly. Before surgery, all liver function test results had returned to values comparable to those before PVE. The indocyanine green retention rate was not modified by PVE (13.9 ± 9.4% vs. 14.3 ± 8%, P = .9, before PVE and before surgery, respectively).
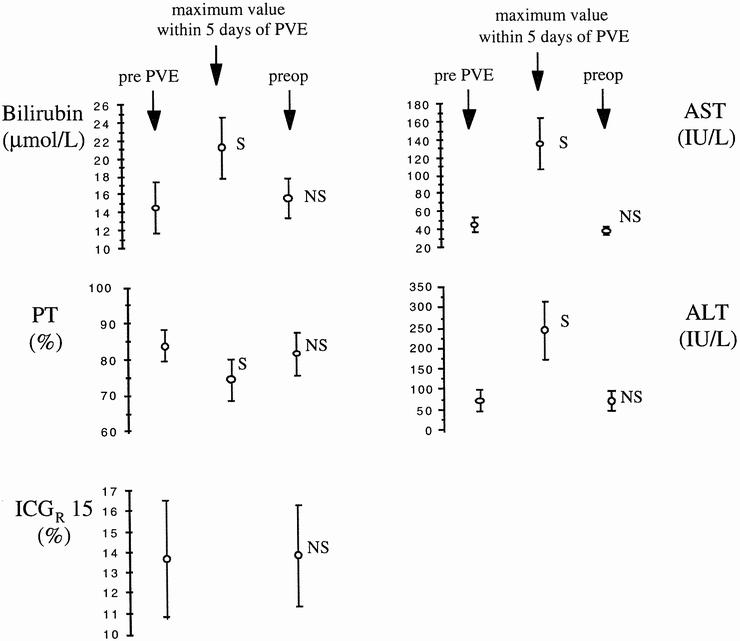
Figure 2. Chronologic course of liver biochemical parameters. Data expressed as mean ± standard error of the mean. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ICG R 15, indocyanine green retention rate at 15 minutes; PT, prothrombin time (% of normal level); S, NS, significantly (P < .05, paired t test) and not significantly different from value before portal vein embolization).
Before PVE, the ERRFLP was less than 40% in 9 of the 10 patients and 60% in the remaining one. In the latter patient, PVE was performed despite a priori sufficient remnant liver because of the risk of hepatectomy in this patient (77 years old, indocyanine green retention rate 16%, prothrombin time 69%). The main result was the significant increase in ERRFLP from 36 ± 9% to 52 ± 12% (P < 10−4; gain = 46 ± 24%). This gain was less in patients with cirrhosis than in those with mild or moderate fibrosis, but this difference did not reach statistical significance (40 ± 23% vs. 60 ± 28%, respectively, P = .3).
Based on the volumetric assessment, 9 of the 10 patients were considered to have sufficient induced liver reserve (ERRFLP > 40%) for the hepatectomy initially planned. One patient with hemochromatosis and cirrhosis (score of fibrosis = F4) had an insignificant change in ERRFLP after PVE (34% before PVE, 36% at 3 months).
Liver Resection
Figure 1 shows the relation between the planned hepatectomy before PVE and the actual management of the 10 patients. Nine patients underwent surgery a mean of 84 ± 54 days after PVE (median 65, range 32–209). Seven patients underwent right hepatectomy (four segments), one right lobectomy (five segments), and one left hepatectomy (three segments). One patient did not undergo surgery: in this patient, as already mentioned, PVE had no significant effect. He died of diffuse metastatic disease and terminal liver failure 11 months after PVE.
Impact of Portal Vein Embolization on the Feasibility of Liver Resection
Overall, preoperative PVE allowed resection of three or more segments in 9 of the 10 patients. During the same period, 19 patients with HCC in injured liver (13 with cirrhosis and 6 with mild or moderate fibrosis) underwent hepatectomy of three or more segments without preoperative PVE. Thus, during the study period, PVE increased the number of this type of hepatectomy by 47% (9/19).
Postoperative Course
There were no deaths within 60 days of surgery. No intraoperative difficulties were encountered in any patient with regard to the previous PVE. The duration of surgery, the total duration of portal triad clamping, and the intraoperative volume of blood transfusion were comparable to those without PVE. Postoperative liver insufficiency as already defined did not occur in any patient. No patient had encephalopathy. Five patients had eight complications (45%). This was comparable to the group of patients without PVE (19 complications in 11 patients). The maximum value of total bilirubin and the minimum prothrombin time during the hospital stay were respectively 48 ± 33 μmole/L and 52 ± 14% of normal. The mean hospital stay, 23 ± 16 days, was comparable to that of patients without PVE. At discharge, one of the nine patients had jaundice (total bilirubin at discharge, 33 ± 33 μmole/L) and the mean prothrombin time was 70 ± 19%. Table 3 summarizes the main intraoperative events and postoperative complications in patients with and without PVE.
Table 3. PERIOPERATIVE CHARACTERISTICS AND COMPLICATIONS
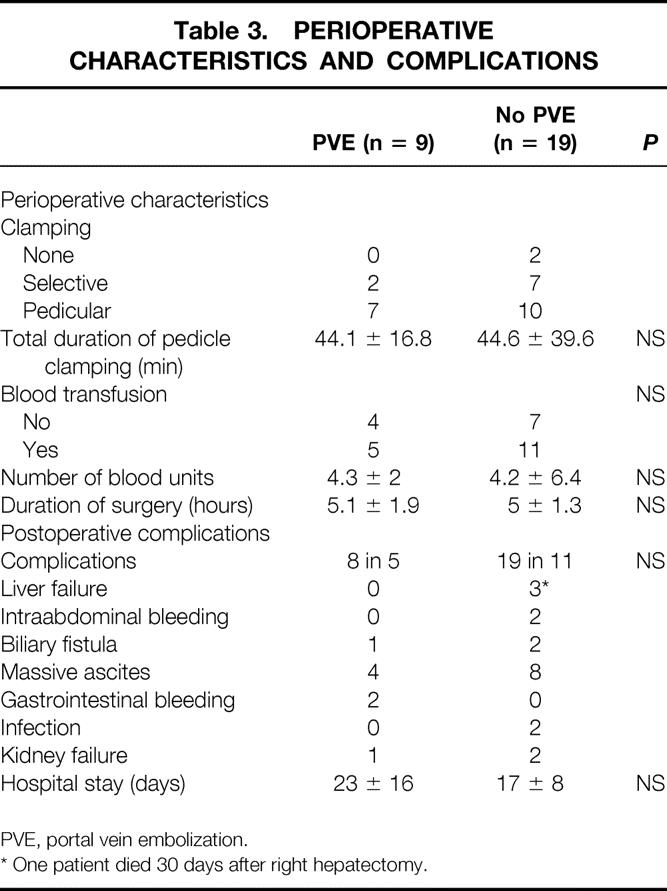
PVE, portal vein embolization.
* One patient died 30 days after right hepatectomy.
Patient Outcome
The overall actuarial survival rate of the nine patients who underwent resection after PVE was 89%, 67%, and 44% at 1, 3, and 5 years, respectively. These rates were comparable to those of the 19 patients who underwent resection without PVE (80%, 53%, and 53% at 1, 3, and 5 years respectively, P = .99, log-rank), as shown in Figure 3. Disease-free survival rates after resection with or without previous PVE were also comparable (86%, 64%, and 21% vs. 55%, 17%, and 17% at 1, 3, and 5 years, respectively, P = .2, log-rank).
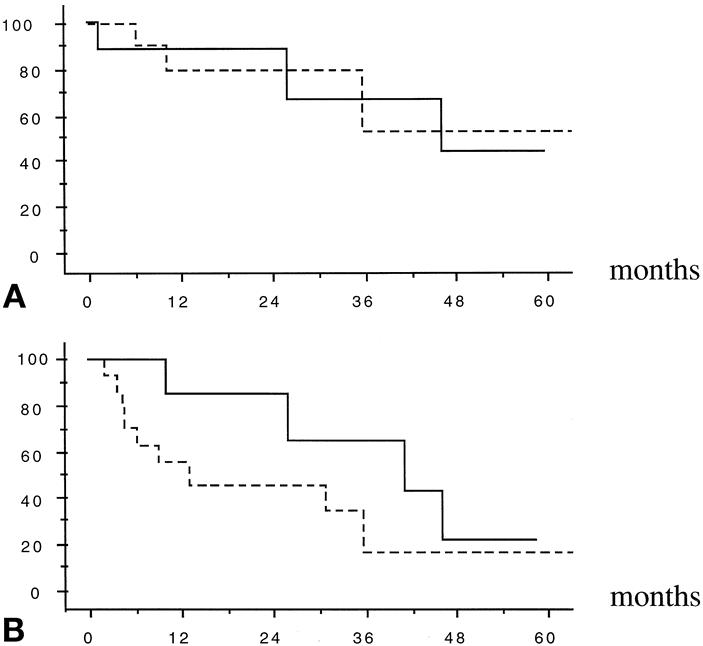
Figure 3. Overall (A) and recurrence-free (B) actuarial survival (%) after major liver resection for hepatocellular carcinoma in injured liver in 19 patients without (dashed lines) and in 9 patients with preoperative portal vein embolization (solid lines). (P = .99 and .2, respectively, log-rank)
DISCUSSION
Portal vein embolization as a preparation for large hepatic resection in patients with injured liver was feasible in all patients in this Western series. There were no deaths or complications. The significant but transient deterioration of liver function test results (bilirubin, transaminases, and prothrombin time) after PVE may be explained by the extensive peribiliary inflammation and hepatocyte necrosis, as demonstrated by De Baere et al 37 when using cyanoacrylate. By inducing hypertrophy of the remaining liver, PVE allowed resection with curative intent in nine patients (six of seven with cirrhosis and all three with mild or moderate fibrosis). There was a greater increase in the percentage of remnant liver volume for patients with mild or moderate fibrosis than in those with cirrhosis. The absence of statistical significance of this difference might be due to sample size. In one patient with cirrhosis, resection could not be performed after PVE because of the lack of induced hypertrophy. Because resection gives the only chance of long-term survival and because of the success of PVE from previous experience in Asia that was recently confirmed 18,20–23 (see Table 1), we considered it unethical to design a randomized study in which some patients would not receive PVE. This series is the first to report a long-term survival rate comparable to that after equivalent primary resections. These actuarial survival rates are comparable to those reported for major resection of large HCC in injured liver (i.e., 20% to 30% at 5 years) from our center 7,38 and from others. 6,11,12,39–41
Because without preoperative PVE our patients would have been treated conservatively or by noncurative resection to save as much liver parenchyma as possible, it can be assumed that PVE increased the feasibility of liver resection of three or more segments by 47% during the study period. This indicates that this technique can be used in a significant subset of patients, in whom it introduces the possibility of safer curative resection. Preoperative PVE in this type of patient was used before major resection in 7 of 19 instances (37%) and 15 of 39 instances (38%) by Shimamura et al 23 and Shuto et al, 12 respectively.
The fact that PVE is well tolerated might broaden its indications in the future. PVE could be used not only for patients with a small remnant liver but also to increase the safety of large liver resections, even when already feasible in terms of remnant liver volume. Other arguments to broaden the indications for preoperative PVE for HCC are first, to increase the anticancer effect of TACE 42–44 and second, to help prevent metastatic spread by the portal vein, as has already been suggested. 12,21
We used cyanoacrylate as the embolizing material because it ensures definitive obstruction of the portal vein. The concomitant use of lipiodol in our method allows visualization of the embolized portal cast by plain radiography. 31 Other materials have been used with success, 44–47 including most recently absolute ethanol. 23 The rationale for the latter in patients with HCC is that this tumor may easily involve the portal vein, and absolute ethanol has an antitumor effect together with peripheral permeation and thrombogenicity in the hepatic area. 23 Controversial points remain, such as the approach (homolateral or contralateral) to the portal vein to be embolized, 48,49 the route (percutaneous, laparoscopic, 50 or by laparotomy 18), and the optimal delay between PVE and resection. However, the latter seems to be longer for injured than for healthy livers. In our experience with PVE before major resection on healthy liver, 19 the mean delay was 49 days; in this series, in injured liver, it was 65 days. This is not surprising because regeneration in an injured liver takes longer. 51–53 Yamakado et al 21 reported a significant increase in hypertrophy of the unembolized injured liver when PVE was combined with TACE. The design of our study could not address this point.
The present series shows that a significant subset of patients who would have been a priori excluded from curative major resection may undergo surgery using a multimodal approach. This approach includes neoadjuvant TACE to diminish or at least limit the tumor burden 7 and preoperative PVE to increase the volume of the remaining liver.
In conclusion, our results demonstrate the safety and efficacy of PVE in injured liver as a preparation for major hepatectomy. This two-step approach separates the two insults: the “functional hepatectomy” accomplished by PVE, and the actual anatomical hepatectomy. The progressive atrophy of the embolized territory, triggering compensatory hypertrophy of the future remaining parenchyma, prevents postoperative liver failure and allows safe liver resection. By alleviating the contraindication to liver resection of an insufficient remnant liver, PVE increases the resectability of HCC in injured liver with a benefit in survival comparable to that obtained with primary liver resection.
Footnotes
Correspondence: Daniel Azoulay, MD, PhD, Centre Hépato-Biliaire, Hôpital Paul Brousse, 94804 Villejuif, France.
E-mail: daniel.azoulay@pbr.ap-hop-paris.fr
Accepted for publication February 11, 2000.
References
- 1.Marsh JW, Dvorchik I, Subotin M, et al. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: a pilot study. Hepatology 1997; 26: 444–450. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999; 340: 745–750. [DOI] [PubMed] [Google Scholar]
- 3.Bellanttani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos study. Hepatology 1994; 20: 1442–1449. [DOI] [PubMed] [Google Scholar]
- 4.Taylor-Robinson SD, Foster GR, Arora S, et al. Increase in primary liver cancer in the UK. Lancet 1997; 350: 1142–1143. [DOI] [PubMed] [Google Scholar]
- 5.Deuffic S, Poynard T, Buffet L, Valleron AJ. Trends in primary liver cancer. Lancet 1998; 351: 214–215. [DOI] [PubMed] [Google Scholar]
- 6.Mor E, Tur Kaspa R, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med 1998; 129: 643–653. [DOI] [PubMed] [Google Scholar]
- 7.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 1997; 226: 688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glasgow RE, Showstack JA, Katz PP, et al. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg 1999; 134: 30–35. [DOI] [PubMed] [Google Scholar]
- 9.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital mortality. Ann Surg 1999; 229: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients. Arch Surg 1999; 134: 984–992. [DOI] [PubMed] [Google Scholar]
- 11.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229: 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuto T, Hirohashi K, Kubo S, et al. Efficacy of major hepatic resection for large hepatocellular carcinoma. Hepato-Gastroenterology 1999; 46: 413–416. [PubMed] [Google Scholar]
- 13.Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease. A reappraisal. Ann Surg 1999; 229: 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto E, Kyo A, Yamanaka N, et al. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery 1984; 95: 586–592. [PubMed] [Google Scholar]
- 15.Yamanaka N, Okamoto E, Kuwata K, Tanaka N. A multiple regression for prediction of posthepatectomy liver failure. Ann Surg 1984; 200: 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999; 188: 304–307. [DOI] [PubMed] [Google Scholar]
- 17.Makuuchi M, Le Thai B, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990; 107: 521–527. [PubMed] [Google Scholar]
- 18.Imamura H, Shimada R, Kubota M, et al. Preoperative portal vein embolization: an audit of 84 patients. Hepatology 1999; 29: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 19.Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 2000; 231: 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KC, Kinoshita H, Hirohashi K, et al. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg 1993; 17: 109–115. [DOI] [PubMed] [Google Scholar]
- 21.Yamakado K, Takeda K, Matsumara K, et al. Regeneration of the unembolized liver parenchyma following portal vein embolization. J Hepatol 1997; 27: 871–880. [DOI] [PubMed] [Google Scholar]
- 22.Wakabayashi H, Okada S, Maeba T, Maeta H. Effect of preoperative portal vein embolization on major hepatectomy for advanced-stage hepatocellular carcinomas in injured livers: a preliminary report. Surg Today Jpn J Surg 1997; 27: 403–410. [DOI] [PubMed] [Google Scholar]
- 23.Shimamura T, Nakajima Y, Una Y, et al. Efficacy and safety of preoperative percutaneous transhepatic portal embolization with absolute ethanol: a clinical study. Surgery 1997; 81: 135–141. [DOI] [PubMed] [Google Scholar]
- 24.Couinaud C. Le Foie: Etudes Anatomiques et Chirugicales. Paris: Masson et Cie; 1957.
- 25.Carr BI, Flinckinger JC, Lotze MT. Cancer of the liver. In DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 5th ed. Philadelphia: Lippincott-Raven; 1997:1087–1114.
- 26.The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994; 20: 15–20. [PubMed] [Google Scholar]
- 27.Bismuth H, Morino M, Sherlock D, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg 1992; 163: 387–394. [DOI] [PubMed] [Google Scholar]
- 28.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg 1982; 6: 3–9. [DOI] [PubMed] [Google Scholar]
- 29.Detroz B, Sugarbaker PH, Knol JA, et al. Causes of death in patients undergoing liver surgery. Cancer Treat Res 1994; 69: 241–257. [DOI] [PubMed] [Google Scholar]
- 30.Zimmermann H, Reichen J. Hepatectomy: preoperative analysis of hepatic function and postoperative liver function. Review Dig Surg 1998; 15: 1–11. [DOI] [PubMed] [Google Scholar]
- 31.Azoulay D, Raccuia JS, Castaing D, Bismuth H. Right portal vein embolization in preparation for major hepatic resection. J Am Coll Surg 1995; 181: 267–269. [PubMed] [Google Scholar]
- 32.Heymsfield SB, Fulenwider, Nordlinger B, et al. Accurate measurement of liver, kidney, and spleen volume mass by computerized axial tomography. Ann Intern Med 1979; 90: 185–187. [DOI] [PubMed] [Google Scholar]
- 33.Henderson JM, Heymsfield SB, Horowitz J, Kutner MH. Measurement of liver and spleen volume by computed tomography. Radiology 1981; 141: 525–527. [DOI] [PubMed] [Google Scholar]
- 34.Van Thiel DH, Hagler NG, Schade RR, et al. In vivo hepatic volume determination using sonography and computed tomography. Gastroenterology 1985; 88: 1812–1817. [DOI] [PubMed] [Google Scholar]
- 35.Nagino N, Nimura Y, Kamiya J, et al. Right or left trisegment portal vein embolization for hilar duct carcinoma. Surgery 1995; 117: 677–681. [DOI] [PubMed] [Google Scholar]
- 36.Reference not provided.
- 37.De Baere TD, Roche A, Vavasseur D, et al. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology 1993; 188: 73–77. [DOI] [PubMed] [Google Scholar]
- 38.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993; 218: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg 1991; 15: 270–285. [DOI] [PubMed] [Google Scholar]
- 40.Iwatsuki S, Starzl TE, Shehan DG, et al. Hepatic resection vs. transplantation for hepatocellular carcinoma. Ann Surg 1991; 214: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akriviadis EA, Llovet JM, Efremidis SC, et al. Hepatocellular carcinoma. Br J Surg 1998; 85: 1319–1331. [DOI] [PubMed] [Google Scholar]
- 42.Nakao N, Miura K, Takahashi H, et al. Hepatocellular carcinoma: combined hepatic, arterial, and portal venous embolization. Radiology 1986; 161: 303–307. [DOI] [PubMed] [Google Scholar]
- 43.Oi H, Kim T, Kishimoto H, et al. Effective cases of transcatheter arterioportal chemoembolization with high-dose iodized oil for hepatocellular carcinoma. Caner Chemother Pharmacol 1994; 33 (suppl): S69–S73. [DOI] [PubMed] [Google Scholar]
- 44.Yamakado K, Hirano T, Kato N, et al. Hepatocellular carcinoma: treatment with a combination of transcatheter arterial chemoembolization and transportal ethanol injection. Radiology 1994; 193: 75–80. [DOI] [PubMed] [Google Scholar]
- 45.Makuuchi M, Kosuge T, Lygidakis NJ. New possibilities for major liver surgery in patients with Klatskin tumors and primary hepatocellular carcinoma, an old problem revisited. Hepato-Gastroenterology 1991; 38: 329–336. [PubMed] [Google Scholar]
- 46.Kawasaki S, Makuuchi M, Miyagawa S, Kakazu T. Radical operation after portal embolization for tumor of the hilar bile duct. J Am Coll Surg 1994; 178: 480–486. [PubMed] [Google Scholar]
- 47.Nagino N, Nimura Y, Kamiya J, et al. Changes in hepatic lobe volume in biliary tract cancer patients after right portal vein embolization. Hepatology 1995; 21: 434–439. [PubMed] [Google Scholar]
- 48.Nagino M, Nimura Y, Hayakawa N. Percutaneous transhepatic portal embolization using newly devised catheters: preliminary report. World J Surg 1993; 17: 520–524. [DOI] [PubMed] [Google Scholar]
- 49.Nagino M, Nimura Y, Kamiya J, et al. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology 1996; 200: 559–563. [DOI] [PubMed] [Google Scholar]
- 50.Tsuge H, Mimura H, Kawata N, Orita K. Right portal vein embolization before extended right hepatectomy using laparoscopic catheterization of the ileocolic vein: a prospective study. Surg Laparoscop Endoscop 1994; 4: 258–263. [PubMed] [Google Scholar]
- 51.Nagasue N, Yukaya H, Ogawa Y, et al. Human liver regeneration after major hepatic resection: a study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg 1987; 206: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen MF, Hwang TL, Hung CF. Human regeneration after major hepatectomy: a study of liver volume by computed tomography. Ann Surg 1991; 213: 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamanaka N, Okamoto E, Kawamura E, et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology 1993; 18: 79–85. [PubMed] [Google Scholar]