Abstract
Objective
To evaluate in patients with Crohn’s disease, using transabdominal ultrasound, the morphologic characteristics of the diseased bowel wall before and after conservative surgery and to assess whether these characteristics and their behavior in the postoperative follow-up are useful and reliable prognostic factors of clinical and surgical recurrence.
Summary Background Data
Ultrasound is effective for evaluating the thickness of bowel wall, the most typical and constant finding of Crohn’s disease. No data are currently available concerning the behavior of the diseased intestinal wall after conservative surgery and whether the preoperative characteristics of bowel wall or its behavior after conservative surgery may predict recurrence.
Methods
In 85 consecutive patients treated with strictureplasty and miniresections for Crohn’s disease, clinical and ultrasonographic evaluations were performed before and 6 months after surgery. Assessed before surgery were the maximum bowel wall thickness, the length of bowel wall thickening, the bowel wall echo pattern (homogeneous, stratified, and mixed), and the postoperative bowel wall behavior, classified as normalized, improved, unchanged, or worsened.
Results
A significant correlation was found between a long preoperative bowel wall thickening and surgical recurrence. Bowel wall thickness after surgery was unchanged or worsened in 43.3% of patients; in these patients, there was a high frequency of previous surgery. Patients with unchanged or worsened bowel wall thickness had a higher risk of clinical and surgical recurrence compared with those with normalized or improved bowel wall thickness.
Conclusion
With the use of abdominal ultrasound, the authors found that the thickening of diseased bowel wall may unexpectedly improve after conservative surgery, and this is associated with a favorable outcome in terms of clinical and surgical recurrence. In addition to its diagnostic usefulness, ultrasound also provides reliable prognostic information concerning clinical and surgical recurrence in patients with Crohn’s disease in the postoperative follow-up.
Crohn’s disease (CD) is a chronic condition with an unpredictable course that is frequently characterized by a high rate of recurrence and in most patients a need for surgical treatment. Approximately 60% to 70% of patients require at least one surgical treatment, 1 often for bowel obstruction. In the past, wide resections have proved to be ineffective in preventing relapses, and repeated resective surgery can lead to malabsorption and short bowel syndrome in many instances. 2–5 During the past decade, strictureplasty and new bowel-sparing techniques have therefore been proposed for the conservative treatment of intestinal strictures. 4,6–9 The early postoperative results show that these techniques are effective and safe in terms of complications and death; further, long-term clinical and surgical recurrence rates are comparable with those observed after conventional resective surgery. 10–12 However, despite these encouraging results, the clinical course of CD and the behavior of the diseased intestinal walls after conservative surgery have so far been little investigated.
Ultrasonography has recently been proposed as an accurate means of evaluating bowel walls in patients with CD. 13–18 Several studies have shown that transabdominal ultrasound can detect transmural changes and the degree of thickness of intestinal bowel wall, 19–21 and this technique is as accurate as barium radiology or scintigraphy in assessing the presence, location, and extent of inflammatory bowel disease, 15,18,21 particularly in bowel segments such as the ileum that are not easily to assess by endoscopy. It has also been shown that ultrasound can detect recurrences after “radical” surgical resections 22,23 and the intestinal complications of CD. 24,25 No study has yet evaluated the behavior of the thickness of diseased intestinal wall after conservative surgery in CD, or whether the presurgical ultrasound characteristics and postsurgical behavior of bowel wall thickness (BWT) have prognostic implications on clinical and surgical recurrences.
The aims of this study were to evaluate in patients with CD using transabdominal ultrasound the morphologic characteristics of the diseased bowel wall before and after conservative surgery and to assess whether these characteristics and their postoperative behavior are useful prognostic factors for clinical and surgical recurrence after conservative surgery.
METHODS
Between February 1994 and October 1998, 119 consecutive patients with complicated or therapy-refractory CD underwent surgery in the Department of Surgery, L. Sacco University Hospital, Milan. At admission a careful clinical history was taken, including age, gender, smoking habits, site and duration of disease, number of clinical recurrences, and type and number of previous surgery. The patients were entered in a prospectively maintained database that included patient presentation, indication for surgery, location of CD, type of surgery performed in our department, histopathologic findings, and postoperative medical treatment. Depending on the patient’s status at presentation, the preoperative workup included electrocardiography, a routine blood test, and radiologic, endoscopic, and ultrasound studies.
Exclusion criteria were pure colonic location, primary indication for surgery other than intestinal stenoses (e.g., unresponsiveness to medical treatment, abscess, or fistula), and failure to obtain a preoperative ultrasound. The study was approved by the local ethics committee, and informed consent was obtained from all patients.
Ultrasound evaluations were performed before and 6 months after surgery. Postoperative recurrences were sought at 6-month intervals. Clinical recurrence was defined as symptoms related to CD, variably associated with radiologic, endoscopic, and laboratory findings, treated with a medium-high dose of steroids. Surgical recurrence was defined as the presence of medically uncontrolled disease or complications requiring a new surgical procedure (e.g., occlusive disease, intraabdominal abscesses, or high-flow fistulas). 11,12,26
Eighty percent of the patients were randomly assigned to postoperative treatment with mesalazine, azathioprine, or no medical therapy as part of another ongoing planned study.
The transabdominal ultrasonographic examinations were performed by the same physician (G.M.), who was aware of the diagnosis of CD and the previously performed surgical resection but unaware of the patient’s clinical and biochemical data, type of surgery performed, and postoperative medical treatment. The decision to perform surgery and the choice of technique were always made regardless of the ultrasound findings.
Whole abdomen scans were carried out using a real-time scanner (Ecotron-Aloka Mod. SSD-680 Tokyo, Japan). An intestinal wall thickness of 4 mm or more was considered pathologic and was evaluated with respect to the maximum BWT. The preoperative ultrasound parameters considered were the maximum BWT, the length of BWT, and the bowel wall echo pattern. The maximum preoperative BWT has been used as a surrogate index for transmural inflammation in the involved tracts, 15–19,27 and it was measured both longitudinally and transversely as previously described. 15,18 To ensure that the maximum measurement was not incidental or due to an artifact, only the measurements that could be reproduced for bowel segments of no less than 4 cm long were considered reliable. In patients with more than one lesion, the greatest BWT value was used. In patients in whom the distribution of the intestinal loops made it possible, the preoperative length of BWT was measured in centimeters. The preoperative bowel wall echo pattern, which has been correlated with the degree of endoscopic and clinical activity, 16,28 was classified into three groups: pathologic BWT with loss of stratification (homogeneous echo pattern), pathologic BWT with stratification (stratified echo pattern), and the coexistence of intestinal tracts with and without stratification (mixed echo pattern) (Fig. 1).

Figure 1. Ultrasonographic appearance of pathologic bowel wall thickening with (A) and without (B) stratification. Longitudinal (left) and transverse (right) sections. The mixed echo pattern found in some patients shows both these aspects.
In the postoperative assessment, only the BWT behavior (obtained by comparison of preoperative and postoperative BWT) was considered because of the possible changes that occur to the intestinal wall after a strictureplasty or a minimal bowel resection in terms of echo pattern and length of BWT, and because BWT is the most typical and constant finding of CD. 19,20
Normalized (BWT ≤ 4 mm) or improved BWT (reduction of ≥2 mm or 20% of preoperative value) and unchanged or worsened BWT (increase of ≥2 mm or 20% of preoperative value) after the 6-month follow-up were evaluated for their possible prognostic impact on clinical and surgical recurrences. A change of 2 mm was arbitrarily used because although good intraobserver agreement in BWT measurement has been reported in CD, 29 we considered that BWT changes of 1 mm were within the measurement variation that may occur in an individual patient.
The detailed indications and surgical techniques have been previously described. 6,8,11,12 Briefly, the conservative surgical approaches adopted were strictureplasty, minimal bowel resection, and both. In the strictureplasty group, the Heineke-Mickulicz technique was used for short ileal strictures (up to 10 cm), whereas side-to-side ileoileal plasty, ileocecal plasty, and side-to-side ileocolic plasty were used to treat long ileal segments or stenoses arising near the ileocecal valve or a previous ileocolic anastomosis. The minimal bowel resections were intestinal resections without macroscopically disease free margins, with the removal of the intestinal segment with no residual lumen or a fistula arising from a stenotic segment or an abscess close to a stenotic segment.
Variable frequency was compared using the chi-square or Fisher exact test. Cumulative probability of clinical and surgical recurrences was estimated by the Kaplan-Meier method, stratified according to the behavior of BWT. Time-to-event differences were tested using the log-rank test. To assess the relative excess risk of events and to control for confounding factors, the Cox proportional hazards model, including age, duration of disease, and previous surgical treatment, were fitted, computing hazard ratios (heart rate) and the corresponding 95% confidence intervals (95% CI). The proportional assumption was examined with log-log survival plots or by adding time-dependent interaction terms to the model.
RESULTS
Of the 119 patients consecutively treated, 7 were excluded because no ultrasound evaluation was performed before surgery, 9 were excluded for indications other than stenoses, and 18 were excluded for pure colonic involvement. The remaining 85 patients included 38 women (44.7%) and 47 men (55.3%) with a mean age of 37.7 ± 11.6 years and a mean CD duration of 81.3 ± 72.4 months. Forty-seven patients (55.3%) were current smokers, and 47 (55.3%) had undergone previous surgery for CD. The mean number of clinical recurrences was 3.2 ± 2.3. The anatomical site of CD was ileal in 63 patients (74.1%) and ileocolonic in 22 (25.9%). Forty-two patients (49.4%) had internal fistulas and 27 (31.8%) had intraabdominal abscesses secondary to the indication for surgery. Forty patients underwent minimal bowel resection, 30 underwent strictureplasty, and 15 underwent both. Patient characteristics classified by type of surgery are shown in Table 1; no significant differences were found between the groups.
Table 1. CLINICAL AND ULTRASOUND CHARACTERISTICS BY TYPE OF SURGERY
* Patients in whom it was clearly evaluable.
One patient was lost to follow-up, and it was not possible to repeat the ultrasound study 6 months after surgery in 11 patients. According to Kaplan-Meier function, the 3-year overall time-to-event estimates were 39% for clinical recurrence and 21% for surgical recurrence, with a median follow-up of 28.4 months (range 3–70). The 3-year time-to-event estimates by type of surgery were clinical recurrence 50% and surgical recurrence 26% for strictureplasty, 29% and 13% for minimal bowel resection, and 40% and 35% for the combined procedure. The log-rank test was not significant in any of the cases (P = .3).
There were no differences in the preoperative BWT and echo patterns of the patients who did and those who did not have a recurrence, but patients with surgical recurrence had a slight but significantly longer preoperative BWT (P = .04) (Table 2). BWT returned to normal or improved in 42 patients (56.7%) and remained unchanged or worsened in 32 (43.3%). In particular, it returned to normal in 15 of the patients who had undergone minimal bowel resection (62.5%), in 3 of those who had undergone the combined procedure (12.5%), and in 6 of those who had undergone strictureplasty alone (25%).
Table 2. PREOPERATIVE ULTRASONOGRAPHIC CHARACTERISTICS OF BOWEL WALLS IN PATIENTS UNDERGOING CONSERVATIVE SURGERY
* Patients in whom it was clearly evaluable.
†P = .04 vs. patients with no recurrences.
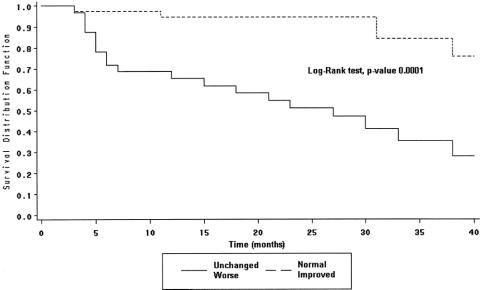
The clinical and surgical characteristics of the population by BWT behavior are summarized in Table 3. Among the demographic and clinical characteristics considered, the unchanged or worsened BWT was significantly more common in the patients who had undergone previous surgery (P = .01). The 3-year Kaplan-Meier time-to-event estimates for the patients with an unchanged or worsened BWT and those with normalized or improved BWT are shown for clinical recurrence in Figure 2 and for surgical recurrence in Figure 3 (P = .0001 and .002, respectively, log-rank test). The Cox proportional hazard model showed that patients with an unchanged or worsened BWT had a high risk of clinical (heart rate 9.98; 95% CI 3.48–28.56) and surgical (heart rate 16.15; 95% CI 2.87–90.75) recurrence (Table 4).
Table 3. BEHAVIOR OF BOWEL WALL THICKENING ACCORDING TO PREOPERATIVE VARIABLES
Figure 2. Kaplan-Meier time-to-event estimates: clinical recurrence.
Figure 3. Kaplan-Meier time-to-event estimates: surgical recurrence.
Table 4. HAZARD RATIOS OF RECURRENCE RELATED TO BOWEL WALL THICKENING
CI, confidence interval.
* Estimates from proportional hazard regression model including terms for age, duration of disease, and previous surgical treatment.
† Reference category.
DISCUSSION
Several risk factors for postoperative recurrences of CD have been proposed for their influence on the postsurgical outcome. 26,30–35 However, there is still no general agreement, probably because of the heterogeneity of patient series, the lack of randomized controlled trials, and the wide differences in preoperative and postoperative medical treatment.
Transabdominal ultrasound has so far been used as a reliable tool in the diagnosis of CD in terms of activity, location, type of complications, and postsurgical recurrence, especially for those segments such as the ileum that cannot be easily assessed by endoscopy. 15,19–22 The BWT is the most typical and constant aspect of the disease, and a good correlation between the degree of the BWT measured by ultrasound and the pathologic transmural changes of the bowel wall has been shown. 19,20 Further, if the homogeneous echo pattern has been correlated with a high degree of endoscopic and clinical activity, 16,28 nothing is known about the prognostic implications of preoperative ultrasound characteristics of the bowel wall or their postoperative changes.
We found that the preoperative BWT was significantly longer in the patients with surgical recurrence, as in other large series of patients in which the extent of disease was associated with an increased risk of recurrence. 36–38 Because the diseased bowel wall was completely or largely retained in our patients, one can postulate that this finding is related to the conservative type of surgery: in other words, the longer the diseased bowel wall left in situ, the greater the risk of relapse. Given the similarity of our findings measured using different diagnostic tools and after different surgical treatments, it can be postulated that the development of new stenoses or recurrences is not prevented by the amount of resected bowel. D’Haens et al 39 found that the radiologic appearance of patients with long preoperative bowel involvement measured at the time of recurrence was similar to that observed at baseline despite the fact that they had undergone resective surgery. Further, in a randomized controlled trial, Fazio et al 5 proved that the width of resection margins is ineffective in preventing recurrence, and it is worth remembering that the site of recurrence in most of the patients requiring reoperation after primary conservative surgery for CD is different and distant from the site of previous strictureplasties. 10
In this study, 43.3% of our conservatively treated patients had an unchanged or worsened BWT in the postoperative period. This seems to be a strong predictor of clinical and surgical outcome, because it is partially confirmed by the significantly higher frequency of previously operated patients in the unchanged or worsened group—almost as if the evolution of CD is worse in such patients.
Further, 56.7% of the patients had an improved or normalized BWT after surgery, regardless of the surgical procedure and postoperative medical therapy. The return to normal of BWT is much more surprising if we consider that thickened bowel wall can be observed in almost all the patients with active or quiescent CD, regardless of its clinical and biochemical activity. 15
Despite the different method used, our data concur with those of Poggioli et al, 40 who studied five patients undergoing a side-to-side enterocolonic anastomosis (an example of nonresective surgery such as strictureplasty). In these patients, colonoscopy and large-bowel enema performed 6 months after surgery revealed complete morphologic regression of the disease.
Alexander-Williams 41 has suggested that CD at a strictureplasty site probably improves because the release of the obstruction breaks a vicious cycle of bacterial overgrowth, increased intraluminal pressure with submucosal bacterial spread, ulcerations, and further scarring. In 89 patients with CD treated with ileal resection and then examined ileoscopically within 1 year of surgery by Rutgeerts et al, 42 the most powerful variable determining outcome was the endoscopic status of the neoterminal ileum. Our data relating to a similar follow-up period after conservative surgery shows that the ultrasound evaluation of the behavior of diseased intestinal walls may be a useful means of identifying the presence of a specifically aggressive CD pattern characterized by a higher frequency of surgical resections. 42,43
In conclusion, although further studies are necessary to clarify the implications of these results in the indication for the use of a particular surgical technique or postoperative medical treatment, ultrasound seems to be an reliable, low-cost, reproducible, noninvasive tool in the postoperative follow-up of patients with CD because it allows the identification of those at high risk of clinical and surgical recurrence.
Footnotes
Correspondence: Angelo Maria Taschieri, MD, Chairman and Head, Division of General Surgery, State University of Milan, Luigi Sacco University Hospital, Via GB Grassi, 74 -20157- Milano, Italy.
E-mail: angelo.taschieri@unimi.it
Accepted for publication August 11, 2000.
References
- 1.Sales DJ, Kirsner JB. Prognosis of inflammatory bowel disease. Arch Intern Med 1983; 143: 294–299. [PubMed] [Google Scholar]
- 2.Hellers G. Crohn’s disease in Stockholm county. 1955–1974. A study of epidemiology, results of surgical treatment and long-term prognosis. Acta Chir Scand 1979; 490 (suppl 490): 31–69. [PubMed] [Google Scholar]
- 3.Thompson JS. Strategies for preserving intestinal length in the short bowel syndrome. Dis Colon Rectum 1987; 30: 208–213. [DOI] [PubMed] [Google Scholar]
- 4.Fazio VW, Tjandra JJ, Lavery IC, et al. Long-term follow-up of stricturoplasty in Crohn’s disease. Dis Col Rectum 1993; 36: 355–361. [DOI] [PubMed] [Google Scholar]
- 5.Fazio VW, Marchetti F, Church JM, et al. Effect of resection margins on the recurrence of Crohn’s disease in the small bowel. Ann Surg 1996; 224: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taschieri AM, Cristaldi M, Elli M, et al. Description of new “bowel-sparing” techniques for long strictures of Crohn’s disease. Am J Surg 1997; 173: 509–512. [DOI] [PubMed] [Google Scholar]
- 7.Stebbling JF, Jewell DP, Kettlewell MGW, et al. Long-term results of recurrence and reoperation after strictureplasty for obstructive Crohn’s disease. Br J Surg 1995; 82: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 8.Michelassi F. Side-to-side isoperistaltic strictureplasty for multiple Crohn’s strictures. Dis Colon Rectum 1996; 39: 345–349. [DOI] [PubMed] [Google Scholar]
- 9.Hurst RD, Michelassi F. Strictureplasty for Crohn’s disease. Techniques and long-term results. World J Surg 1998; 22: 359–363. [DOI] [PubMed] [Google Scholar]
- 10.Ozuner G, Fazio VW, Lavery IC, et al. Reoperative rates for Crohn’s disease following strictureplasty. Long-term analysis. Dis Colon Rectum 1996; 39: 1199–1203. [DOI] [PubMed] [Google Scholar]
- 11.Sampietro GM, Cristaldi M, Porretta T, et al. Early perioperative results and surgical recurrence after strictureplasty and miniresection for Crohn’s disease. Dig Surg 2000; 17: 261–267. [DOI] [PubMed] [Google Scholar]
- 12.Cristaldi M, Sampietro GM, Danelli P, et al. Long-term results and multivariate analysis of prognostic factors in 138 consecutive patients operated on for Crohn’s disease using “bowel-sparing” techniques. Am J Surg 2000; 179: 266–270. [DOI] [PubMed] [Google Scholar]
- 13.Sonnenberg A, Erckenbrecht J, Peter P, et al. Detection of Crohn’s disease by ultrasound. Gastroenterology 1992; 83: 430–434. [PubMed] [Google Scholar]
- 14.Schwerk WB, Beckh K, Raith M. A prospective evaluation of high-resolution sonography in the diagnosis of inflammatory bowel disease. Eur J Gastroenterol Hepatol 1992; 4: 173–182. [Google Scholar]
- 15.Maconi G, Parente F, Bollani S, et al. Abdominal ultrasound in the assessment of extent and activity of Crohn’s disease: clinical significance and implication of bowel wall thickening. Am J Gastroenterol 1996; 91: 1604–1609. [PubMed] [Google Scholar]
- 16.Hata J, Haruma K, Suenaga K, et al. Ultrasonographic assessment of inflammatory bowel disease. Am J Gastroenterol 1992; 87: 443–447. [PubMed] [Google Scholar]
- 17.Papi C, Iscaro D, Salvatori V, et al. Sonographic evaluation of Crohn’s disease. Ital J Gastroenterol 1989; 21: 257–262. [Google Scholar]
- 18.Brignola C, Belloli C, Iannone P, et al. Comparison of scintigraphy with indium-111 leukocyte scan and ultrasonography in assessment of X-ray-demonstrated lesions of Crohn’s disease. Dig Dis Sci 1993; 38: 433–437. [DOI] [PubMed] [Google Scholar]
- 19.Kimmey MB, Wang KY, Haggitt RC, et al. Diagnosis of inflammatory bowel disease with ultrasound. An in vitro study. Invest Radiol 1990; 1085–1090. [DOI] [PubMed] [Google Scholar]
- 20.Hata J, Haruma K, Yamanaka H, et al. Ultrasonographic evaluation of the bowel wall in inflammatory bowel disease: comparison of in vivo and in vitro studies. Abdom Imaging 1994; 395–399. [DOI] [PubMed] [Google Scholar]
- 21.Maconi G, Ardizzone S, Parente F, et al. Ultrasonography in the evaluation of extension, activity and follow-up of ulcerative colitis. Scand J Gastroenterol 1999; 34: 1103–1107. [DOI] [PubMed] [Google Scholar]
- 22.Di Candio G, Mosca F, Campatelli A, et al. Sonographic detection of postsurgical recurrence of Crohn disease. AJR Am J Radiol 1986; 146: 523–526. [DOI] [PubMed] [Google Scholar]
- 23.Andreoli A, Cerro P, Falasco G, et al. Role of ultrasonography in the diagnosis of postsurgical recurrence of Crohn’s disease. Am J Gastroenterol 1998; 93: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 24.Maconi G, Bollani S, Bianchi Porro G. Ultrasonographic detection of intestinal complications in Crohn’s disease. Dig Dis Sci 1996; 41: 1643–1648. [DOI] [PubMed] [Google Scholar]
- 25.Gasche C, Moser G, Turetschek K, et al. Transabdominal bowel sonography for detection of intestinal complication in Crohn’s disease. Gut 1999; 44: 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutenbach E, Berlin JA, Lichtenstein GR. Risk factor for early postoperative recurrence of Crohn’s disease. Gastroenterology 1998; 115: 259–267. [DOI] [PubMed] [Google Scholar]
- 27.Maconi G, Parente F, Bollani S, et al. Factors affecting splanchnic haemodynamics in Crohn’s disease: a prospective study using Doppler ultrasound. Gut 1998; 43: 645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maconi G, Bollani S, Cristaldi M, et al. Clinical significance of echo pattern of the thickened bowel wall in Crohn’s disease. Digestion 1998; 59 (suppl 3): 139. [Google Scholar]
- 29.Arienti V, Arfilli L, Boriani L, et al. Inter- and intra-observer variation in ultrasonographic findings in inflammatory bowel diseases. Ital J Gastroenterol Hepatol 1997; 29 (suppl 2): A4. [Google Scholar]
- 30.Wolff BG. Factors determining recurrence following surgery for Crohn’s disease. World J Surg 1998; 22: 364–369. [DOI] [PubMed] [Google Scholar]
- 31.Greenstein AJ, Lachman P, Sachar DB, et al. Perforating and nonperforating indications for repeated operations in Crohn’s disease: evidence for two clinical forms. Gut 1988; 29: 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachar DB, Wolfson DM, Greenstein AJ, et al. Risk factors of postoperative recurrence of Crohn’s disease. Gastroenterology 1983; 85: 917–921. [PubMed] [Google Scholar]
- 33.Benoni C, Nilsson A. Smoking and inflammatory bowel disease: comparison with systemic lupus erythematosus. A case-control study. Scand J Gastroenterol 1990; 25: 751–755. [DOI] [PubMed] [Google Scholar]
- 34.Brignola C, Cottone M, Pera A, et al. The Italian Cooperative Study Group. Mesalazine in the prevention of endoscopic recurrence after ileal resection for Crohn’s disease. Gastroenterology 1995; 108: 345–349. [DOI] [PubMed] [Google Scholar]
- 35.Caprilli R, Corrao G, Taddei G, et al. Prognostic factors for postoperative recurrence of Crohn’s disease. Dis Colon Rectum 1996; 39: 3335–3341. [DOI] [PubMed] [Google Scholar]
- 36.Raab Y, Bergstrom R, Ejerblad S, et al. Factor influencing recurrence in Crohn’s disease. An analysis of consecutive series of 353 patients treated with primary surgery. Dis Colon Rectum 1996; 39: 918–925. [DOI] [PubMed] [Google Scholar]
- 37.Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in Crohn’s disease. Gastroenterology 1994; 106: 643–648. [DOI] [PubMed] [Google Scholar]
- 38.Williams JG, Wong WD, Rothenberg DA, et al. Recurrence of Crohn’s disease after resection. Br J Surg 1991; 78: 10–19. [DOI] [PubMed] [Google Scholar]
- 39.D’Haens GR, Gasparaitis AE, Hanauer SB. Duration of recurrent ileitis after ileocolonic resection correlates with presurgical extent of Crohn’s disease. Gut 1995; 36: 715–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poggioli G, Stocchi L, Laureti S, et al. Conservative surgical management of terminal ileitis: side-to-side enterocolic anastomosis. Dis Colon Rectum 1997; 40: 234–237. [DOI] [PubMed] [Google Scholar]
- 41.Alexander-Williams J. The technique of intestinal strictureplasty. Int J Colorectal Surg 1986; 1: 54–57. [DOI] [PubMed] [Google Scholar]
- 42.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990; 99: 956–963. [DOI] [PubMed] [Google Scholar]
- 43.Heimann TM, Greenstein AJ, Lewis B, et al. Prediction of early symptomatic recurrence after intestinal resection in Crohn’s disease. Ann Surg 1993; 218: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]