Abstract
Objective
To test the hypothesis that the duodenum is required to coordinate interdigestive insulin secretion with gastrointestinal motility and to determine whether duodenectomy alters the interdigestive cycles of plasma motilin and insulin levels and their relations to insulin secretion and motility.
Methods
Adult mongrel dogs were chronically implanted with force transducers in the stomach, duodenum, and upper jejunum to monitor contractile activity. Eight healthy mongrel dogs were divided into control and duodenectomized dogs. Insulin secretion, gastrointestinal motility, and plasma concentrations of motilin during the interdigestive period were measured in normal and duodenectomized dogs.
Results
After duodenectomy, no obvious phase III contractions were seen in the gastric antrum, but migrating phase III contractions were seen in the upper jejunum. The plasma motilin concentration did not fluctuate as it does in normal dogs, and remained low. After duodenectomy, insulin secretory cycles were not coordinated with either cycles of interdigestive motility or the plasma concentration of motilin. Exogenous motilin administration stimulated endogenous insulin release significantly compared with saline-treated controls. The contractile response of the stomach to exogenous motilin after duodenectomy was similar to that of intact dogs.
Conclusions
Duodenectomy disrupts the relation between cycles of both interdigestive gastrointestinal motility and insulin secretion. These effects of duodenectomy may be attributable to interruption of the duodenopancreatic neural connections, hormonal abnormalities, or loss of vagus-sensitive humoral factors. The duodenum, which stores motilin, seems to play an important role in the relations between gastric migrating motor complexes and the concomitant increase of insulin secretion in fasted dogs. The mechanism responsible for the effect of motilin in both duodenectomized and normal dogs may involve a cholinergic pathway.
The duodenum occupies a strategic anatomical position in the upper digestive tract and is intimately involved in the coordination of various functions of the upper gut. It influences gastric empting, 1 pancreatic and biliary secretion, 2 and the organization and initiation of gastric motor patterns 3,4 by invoking hormonal and neural mechanisms. Others have confirmed these findings. 5–7 Tanaka and Sarr 8 reported that duodenectomy disrupts not only the coordination of gastric and intestinal migrating motor complexes (MMC) but also the coordination between interdigestive motility and pancreatic secretion, and it abolishes the interdigestive cyclic variations in plasma motilin and pancreatic polypeptide (PP) levels. 9 The release of PP appears to be regulated by a complex interplay between neural input (from the vagus) and humoral factors that originate from the upper gut. 10,11
The duodenum also plays an important role by regulating the release of certain pancreatic hormones. For example, intraduodenal glucose administration causes the release of more insulin than does intravenous glucose administration. This response is in part due to the release of gastric inhibitory polypeptide (GIP), a putative regulatory hormone found in the duodenum and to some extent the upper jejunum. 12 GIP therefore has a stimulatory or “incretin” effect on insulin release. Motilin, a 22-amino-acid residue polypeptide, 13 is produced in motilin cells, which are scattered throughout the mucosal epithelium of the upper small intestine, like the GIP-producing cells. 14,15 Recently, we have shown that plasma insulin and plasma motilin concentrations fluctuated simultaneously in close association with spontaneous phase III contractions during the interdigestive state. In the dog, the cyclic change in insulin release appears to be controlled by motilin by means of vagal, cholinergic, muscarinic pathways involving 5-hydoroxytryptamine3 (5-HT3) receptors. 16
To date, most studies have focused on pancreatic exocrine secretion, 3–6,9 and there have been no reports on the relation between motilin and insulin in duodenectomized dogs. The aims of the present study, therefore, were to determine whether duodenectomy disrupts the coordination between interdigestive insulin secretion and gastrointestinal motility, and to determine whether alternations of plasma motilin levels accompany changes in insulin secretion and gastrointestinal motility after duodenectomy. In addition, we investigated the effect of exogenous motilin administration on insulin release and gastrointestinal motor activity in dogs after total duodenectomy. Measurements were made of interdigestive insulin secretion, gastrointestinal motility, and plasma concentration of motilin in normal dogs and in dogs that had undergone total duodenectomy.
METHODS
Eight healthy mongrel dogs (10–15 kg) were divided into a control group (n = 5) and an experimental groups (n = 3). All surgery was performed using aseptic techniques, anesthesia with pentobarbital sodium (30 mg/kg; Nembutal, Abbott, North Chicago, IL) and halothane, and assisted ventilation with room air.
In control dogs, force transducers 17 were implanted chronically onto the serosal surface in the direction of the circular muscle of the gastric antrum 3 cm proximal to the pyloric ring, the midduodenum at the level of the opening of the main pancreatic duct, and the upper jejunum. The lead wires of the force transducers exited through a skin incision made lateral to the right scapula through a costal subcutaneous tunnel. After closure of the abdominal cavity, a silicone tube (Silastic no. 602–205; Dow Corning, Midland, MI) was inserted chronically into the superior vena cava through the right jugular vein (jugular tube), and another tube was placed into the inferior vena cava through a branch of the femoral vein (femoral tube). These two tubes exited through skin incisions made on the neck and the back, respectively, and were then fixed with silk sutures onto the skin adjacent to each of the skin incisions. The jugular tube was used for withdrawal of blood samples, and the femoral tube was used for injection of test drug solutions.
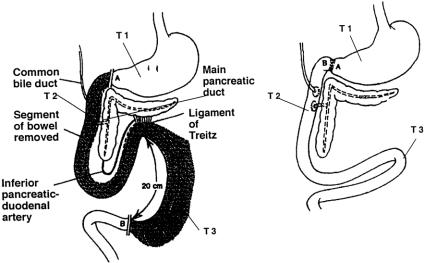
In experimental dogs, total duodenectomy was performed using a modification 8 of the technique of Sillin et al 18 (Fig. 1). The stomach was transected just proximal to the pylorus, and the jejunum was transected 20 cm distal to the ligament of Treitz. The duodenum and proximal 20 cm of jejunum were excised by ligating the blood supply to the wall of the bowel. All the connections between the duodenum and pancreas were divided by careful dissection close to the duodenal wall, thereby preserving the neurovascular supply to the pancreas. In the early phase of this dissection, the duodenum was opened laterally by a longitudinal incision to aid in identification of the ostia of the bile duct and main pancreatic duct. These ostia were preserved by a circumscribing incision, leaving small buttons (1.5 cm in diameter) of the duodenal wall and preserving the neurovascular supply to these buttons. The only remaining duodenal mucosa involved the small buttons, which encompassed the bile and pancreatic ducts. Gastrointestinal continuity was restored by an end-to-end anastomosis of the distal antrum and the proximal jejunum. The buttons containing the ostia of the bile and pancreatic ducts were reimplanted separately into the jejunum using 3-0 silk. Force transducers were implanted similarly on the gastric antrum 3 cm proximal to the pyloric ring and at two sites on the jejunum, 10 and 40 cm distal to the gastrojejunostomy line. The dogs were allowed 2 weeks to recover from surgery before further experiments.
Figure 1. Preparation of the canine model of total duodenectomy. After excision of the entire duodenum and proximal jejunum, the ostia of the bile duct and pancreatic duct were reimplanted into the jejunum. Force transducers were implanted on the gastric antrum 3 cm proximal to the pyloric ring and at two sites on the jejunum, 10 and 40 cm distal to the gastrojejunostomy.
Gastrointestinal contractile activity was recorded using a small connector under the jacket protector between the cable leads from an amplifier (WT685G; Nihon Kohden Kogyo, Tokyo, Japan) and the lead wires of the force transducers. Each of the amplifiers was adjusted so that the maximum amplitude of the phase III contractions at the transducer site was recorded fully within the span of the pen deflection. Determination of each phase of contractile activity in the stomach was made according to the following criteria. Phase I is a period of motor quiescence, representing 40% to 60% of the total cycle length. Phase II, representing 20% to 30% of the cycle length, is characterized by increasing but irregular contractions. Phase III, which starts in the gastroduodenal region and migrates down the length of the small intestine, is the most characteristic activity, showing strong, regular contractions of maximal frequency. Phase IV is a brief transitional period that occurs before another period of quiescence (i.e., phase I).
Insulin levels were measured using a microparticle capture enzyme immunoassay for high-molecular-mass analysis and fluorescence polarization immunoassay for hapten assays as reported by Flore et al. 19 Insulin was recognized specifically by a monoclonal mouse antibody against insulin, and bound/free separation was achieved using microparticles as the solid phase. When the same samples were also measured using radioimmunoassay, the correlation coefficient between the two measurements was high (r = 0.982, with a regression line y = 0.87 x −0.66). The actual assay procedures were carried out using an automated benchtop immunochemistry analyzer system (IMx; Abbott Korea, Ansan, Korea), and the minimum detectable concentration in dog plasma was 0.1 μU/mL.
Measurement of motilin
An enzyme-linked immunosorbent assay showed that our anticanine motilin (cMOT) antibody (CMIS1), which was raised in rabbits, effectively recognized the midportion of the cMOT molecule, and the antiserum did not cross-react with other hormones, including gastrin, secretin, cholecystokinin, vasoactive intestinal polypeptide, substance P, PP, peptide YY, or neuropeptide Y, as reported previously. 20 The unlabeled maximal inhibitory concentration of cMOT was 159 pg/mL, as assessed by high-performance liquid chromatography profiles of [125I-Tyr-23] cMOT. That for porcine motilin was 1.07 μU/mL, as assessed by radioimmunoassay using CMIS1, as reported previously. 20 Each tube contained 100 μL standard or unknown sample, 100 μL rabbit antimotilin serum, and either 200 μL assay buffer (phosphate-buffered saline [PBS] containing 1% bovine serum albumin and 0.05% NaN3) for the unknown sample or 100 μL assay buffer and 100 μL hormone-free serum (prepared by charcoal extraction) for the standard. The antiserum was diluted in PBS containing 2% normal rabbit serum and 0.05 mol/L ethylenediaminetetraacetic acid (EDTA); the final dilution of the antiporcine motilin serum was 1:20,000. After incubation for 24 hours at 4°C, 100 μL [125I-Tyr-23] cMOT (1,000 count per minute) was added and the sample was further incubated for 24 hours at 4°C. Two hundred microliters of a second antiserum (goat antirabbit IgG serum), which was diluted at 1:125 in PBS containing 3.5% polyethylene glycol (PEG6000; Wako Pure Chemical, Osaka, Japan) and 0.05 mol/L EDTA, was added. The sample was then incubated at room temperature for 2 hours. Bound and free [125I] motilin were separated by centrifugation at 3,000 rpm for 15 minutes. The supernatant was discarded and the precipitate was counted in a gamma counter. Intra- and interassay coefficients of variation were less than 4% and 6%, respectively. The minimum detectable concentration of motilin in dog plasma was 6.25 pg/mL.
Results are expressed as mean ± standard error. Differences between the groups were compared by the Student t test and repeated measures analysis of variance where appropriate. The level of statistical significance was set at P < .05.
RESULTS
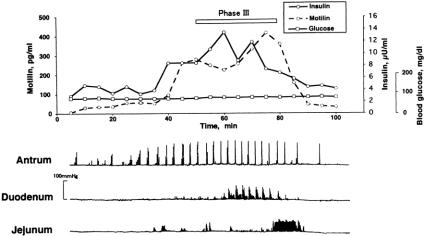
Figure 2 shows a typical example of the relation between the changes in plasma motilin, insulin, and glucose concentrations and contractile activity in the gastric antrum, duodenum, and jejunum of a conscious dog. The interdigestive migrating complex occurred at constant intervals in the gastric antrum. Insulin levels began to increase during phase II and peaked at the beginning of phase III, after which they fluctuated but gradually decreased to the phase I value. Changes in plasma motilin concentration were also found to fluctuate with each phase of contractile activity in the gastric antrum, as we have reported previously. 21
Figure 2. Spontaneous changes in plasma insulin, glucose, and motilin concentrations and contractile activity in the stomach, duodenum, and jejunum in a fasted conscious dog. Plasma motilin concentration started to increase during phase II in the duodenum and peaked at the end of phase III. Insulin concentration started to rise with the increase in motilin concentration and quickly peaked at the beginning of phase III.
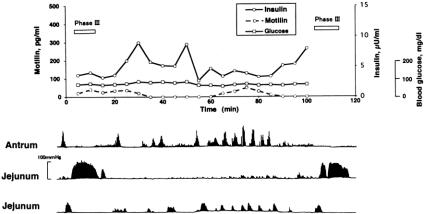
Figure 3 shows a typical example of the relation between the changes in plasma motilin, insulin, and glucose concentrations and contractile activity in the gastric antrum and jejunum of a conscious dog after duodenectomy. No obvious phase III contractions were observed in the gastric antrum, but migrating phase III contractions were seen in the upper jejunum. The plasma concentration of insulin, but not motilin or glucose, made small fluctuations in the range of 1.9 to 9.9 μU/mL; plasma insulin concentrations were not, however, correlated with the interdigestive migrating complex. The plasma motilin concentration did not fluctuate as it does in normal dogs and remained low.
Figure 3. One-hundred-minute changes in plasma insulin, glucose, and motilin concentrations and contractile activity in the gastric antrum and upper jejunum in a conscious dog 1 month after duodenectomy. No obvious phase III contractions were observed in the gastric antrum. Plasma motilin concentrations remained low, and insulin concentration exhibited small fluctuations. Plasma insulin concentrations were not correlated with interdigestive migrating complexes.
Figure 4 shows mean changes in plasma insulin, glucose, and motilin concentrations during phases I, II, and III of the interdigestive state. Plasma levels of insulin showed a consistent pattern of variation throughout the interdigestive motor cycle. The lowest insulin level occurred during phase I, and this level increased during that phase, tending to peak during early phase III (from 3.2 ± 0.4 μU/mL during phase I to 6.7 ± 0.9 μU/mL during early phase III, P < .05). The lowest plasma motilin level occurred during phase I, the highest level during late phase III. During phase I, motilin levels (58.3 ± 12.5 pg/mL) were significantly lower than during early phase III (311.5 ± 60 pg/mL). Peak levels of plasma motilin occurred later in the interdigestive cycle than peak levels of plasma insulin.
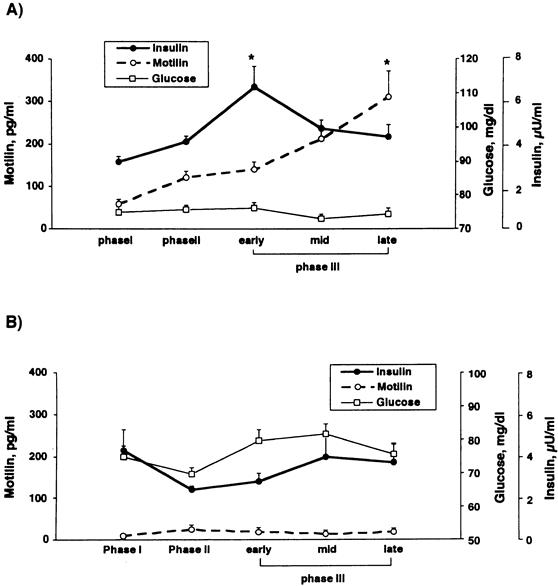
Figure 4. (A) Mean changes in plasma insulin, glucose, and motilin concentrations during phases I, II, and III of the interdigestive state in conscious dogs. Mean plasma concentrations of insulin in early phase III and mean plasma concentrations of motilin in late phase III were significantly elevated from the respective values during phase I. (B) Mean changes in plasma insulin, glucose, and motilin concentrations during phase I, II, and III of the interdigestive state in conscious duodenectomized dogs. Plasma motilin concentrations did not fluctuate as they do in normal dogs and remained low. Changes in insulin concentrations were similar to those of motilin and did not peak during the interdigestive state. Values are means ± SE; * P < .05.
In duodenectomized dogs, plasma motilin concentrations did not fluctuate as they did in the intact dogs and remained low (16.5 ± 3.7 pg/mL). Changes in the insulin concentrations were similar to those of motilin and did not reach a peak during the interdigestive state; the mean insulin concentration was 3.5 ± 0.4 μU/mL.
When a dose of 0.1 μg/kg motilin was given during phase I in normal dogs, typical phase III contractions were induced in the stomach, and 5 minutes after administration these were accompanied by a significant increase in the endogenous release of insulin (11.6 ± 3.8 μU/mL) compared with a saline-treated control (3.5 ± 0.5 μU/mL;Fig. 5). After duodenectomy, an exogenous single bolus dose of motilin also stimulated endogenous insulin release, which was followed 5 minutes after administration by a peak in plasma motilin concentration (8.2 ± 1.0 μU/mL). The motilin injection did not affect glucose concentrations significantly in either the normal control dogs or the duodenectomized dogs (data not shown).
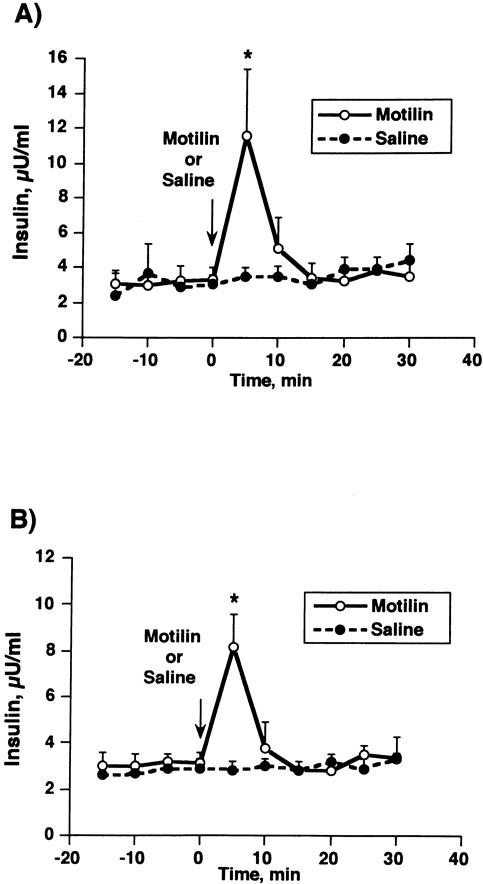
Figure 5. Effect of exogenous motilin administration on insulin release during the interdigestive state in normal conscious dogs (A) and duodenectomized dogs (B). The insulin response at 5 minutes was significantly elevated compared with that observed in the saline group in both control and duodenectomized dogs. Values are means ± SE; * P < .05.
Figure 6 shows the typical effect of a dose of 0.1-μg/kg motilin during the interdigestive state in an intact dog and a duodenectomized dog. The contractile response of the stomach to exogenous motilin after duodenectomy was similar to that of intact dogs. A 0.1-μg/kg dose of motilin also induced contractions similar to interdigestive migrating complexes in the stomach and the duodenum; these contractions then migrated through the small intestine.
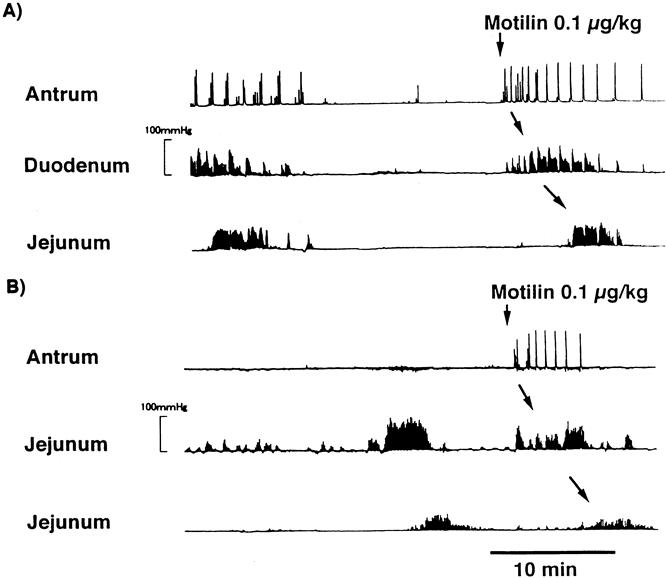
Figure 6. Typical effect of a 0.1-μg/kg dose of motilin during the interdigestive state in an intact dog (A) and a duodenectomized dog (B). The contractile response of the stomach to exogenous motilin after duodenectomy was similar to that of the intact dogs.
DISCUSSION
We showed that in the dog, selective removal of the duodenum with preservation of the entire pancreas is associated with both a loss of the cyclic variations in the plasma concentration of motilin during fasting and an absence of the characteristic gastric MMC. We also found that after duodenectomy, plasma insulin concentrations did not fluctuate.
Our results show that after duodenectomy, the gastric MMC was replaced by irregular, sparse contractions that had no cyclic pattern. The jejunal MMC returned relatively early after duodenectomy, exhibiting a cyclic pattern with a shorter period (data not shown). Motilin is a putative polypeptide that regulates gastrointestinal motility. 21 It is well known that motilin plays an important role in initiating phase III contractions in the stomach of fasted humans and dogs. Because motilin-producing cells are distributed chiefly in the duodenal wall, 14 the duodenum plays an important role in the regulation of gastrointestinal motility. Tanaka and Sarr 8 reported that the initiation and consolidation of phase III of the gastric MMC were impaired after total duodenectomy in dogs; they attributed this to the resulting reduced plasma concentration of motilin. Our observations also indicate that the duodenum is important in the control of gastrointestinal motor activity, and the cyclic release of motilin by the duodenum may serve to initiate and coordinate the timing of upper gastrointestinal motor activity.
In this study, a 0.1-μg/kg intravenous dose of motilin induced phase III-like contractions in the stomach of both normal and duodenectomized dogs. It is well known that the initiation of interdigestive phase III contractions in the stomach and duodenum is closely related to elevation of the plasma concentration of motilin in both dogs and humans. 22–24 The phase III activity of the MMC plays a role in cleaning meal residues, basal secretions, and cellular debris from the gastrointestinal tract (and is thus commonly known as housekeeping activity). 25 The absence of the housekeeping function of phase III activity of the gastric MMC could explain the large volume of gastric juice output often experienced after pylorus-preserving pancreaticoduodenectomy. Indeed, when motilin is administered to patients who have undergone pylorus-preserving pancreaticoduodenectomy, their gastric motility increases and the output of gastric juice decreases. 26
In the interdigestive state, it has been shown that cyclic changes in plasma motilin concentration in the interdigestive state are associated with changes in pancreatic and biliary output into the duodenum. 3–6,9 Motilin is thought to stimulate the exocrine pancreas through a cholinergic pathway. In fact, it has been reported that if exogenous motilin is given to dogs in phase I of the interdigestive state, pancreatic secretions increase in a dose-dependent, atropine-sensitive manner. 27 Most reports have thus far focused on pancreatic exocrine secretions, and few have described the stimulation of the endocrine pancreas by motilin. We have recently described the interdigestive phenomenon of 100-minute periodic oscillations of the endocrine pancreas (PP and insulin) and motor activity in the upper gastrointestinal tract. 16,28 We have also described the mechanism by which increases in the secretion of the endocrine pancreas are associated with increases in plasma motilin concentration. Motilin is thought to stimulate the endocrine pancreas through vagal cholinergic muscarinic receptors including 5-HT3 receptors. In this study, duodenectomy abolished cyclic changes in motilin concentration, and consequently no phase III activity was observed in the stomach of duodenectomized dogs. The plasma insulin concentration did not fluctuate. Loss of the cyclic pattern of plasma insulin concentration as a result of duodenectomy suggests that the duodenum controls the release of insulin by either a neural or a hormonal mechanism.
In this study, exogenous motilin administration stimulated the endogenous release of insulin in association with phase III contractions in the stomach. Zimmerman et al 29 reported that in dogs, extrinsic vagal innervation is essential for cyclic PP release in association with phase III and motilin release in the autotransplanted pancreas. For this reason, the direct neural connections between the duodenum and the endocrine pancreas are unlikely to control changes in insulin release in these dogs. These findings suggest strongly that the cyclic increase in insulin requires both cholinergic impulses and a critical circulating concentration of motilin.
We found no change in blood sugar levels while the insulin level was elevated. The reason for this is not clear. However, it has been shown that stimulation of the vagus nerve increases the secretion of both glucagon and insulin in a variety of species. 30 Because the results of our previous studies showed that motilin stimulates PP by means of the vagus, 28 it is likely that endogenous pancreatic glucagon is also stimulated by motilin.
Studies of the levels of immunoreactive and bioactive insulin in the blood in response to the delivery of glucose by enteric and parental routes in humans have provided evidence that signals arising in the gut serve to increase insulin secretion in response to the absorption of nutrients. 31–33 Thus far, most investigations into the effect of nutrient ingestion on enterohormonal changes and their effect on the endocrine pancreas have focused on GIP. 12,34,35 Evidence is accumulating that GIP only partly contributes to the incretin effect. Incretin is an uncharacterized hormonal factor of enteric origin that enhances the response of the beta cell to glucose. 36 Although GIP is clearly a powerful insulin secretagogue, it appears that it can only partly explain the incretin effect. In vivo and in vitro immunoneutralization studies using antisera against different epitomes of GIP abolished only 20% to 50% of the incretin effect. 37,38 Further, it has been shown that the abolition of insulin release caused by an oral glucose load leaves the incretin effect nearly untouched, 39 raising even more doubt that GIP has an exclusive role in the enteroinsular axis. We found that motilin also stimulated the endogenous release of insulin in duodenectomized dogs. Because motilin is active only during the interdigestive state, it cannot be responsible for the incretin effect. However, motilin may be involved in the enteroinsular axis because it stimulates insulin release through a neurohumoral pathway.
In conclusion, the duodenum, which stores motilin, seems to play an important role in the relation between gastric MMC and the concomitant increase of insulin secretion in fasted dogs. The mechanisms responsible for the effect of motilin in both intact and duodenectomized dogs may involve a cholinergic pathway, although further studies are needed to clarify this.
Footnotes
Correspondence: Hideki Suzuki, MD, Department of Surgery, Gunma University School of Medicine, 3-39-22 Showamachi 371, Japan.
Accepted for publication August 15, 2000.
References
- 1.Meeroff JC, Go VLW, Phillips SF. Control of gastric emptying by osmolality of duodenal contents in man. Gastroenterology 1975; 68: 1144–1151. [PubMed] [Google Scholar]
- 2.Boldyreff W. Einige neue Seiten der Tatigkeit des Pankreas. Ergeb Physiol 1911; 11: 121–185. [Google Scholar]
- 3.Dimagno EP, Hendricks JC, Go VLW, et al. Relationships among canine fasting pancreatic and biliary secretions, pancreatic duct pressure and duodenal phase III motor activity: Boldyreff revised. Dig Dis Sci 1979; 24: 689–683. [DOI] [PubMed] [Google Scholar]
- 4.Keane FB, Dimagno EP, Dozois RR, et al. Relationships among canine interdigestive exocrine pancreatic and biliary flow, duodenal motor activity, plasma pancreatic polypeptide and motilin. Gastroenterology 1980; 78: 310–316. [PubMed] [Google Scholar]
- 5.Itoh Z, Takahashi J, Nakaya M, et al. Variation in canine exocrine pancreatic secretory activity during the interdigestive state. Am J Physiol 1981; 241: G98–103. [DOI] [PubMed] [Google Scholar]
- 6.Konturek SJ, Thor PJ, Bilski J, et al. Relationship between duodenal motility and pancreatic secretion in fasted and fed dogs. Am J Physiol 1986; 250: 570–574. [DOI] [PubMed] [Google Scholar]
- 7.Magee DF, Naruse S. Characteristics of secretin-stimulated pancreatic secretion in dogs. J Physiol Lond 1984; 356: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka M, Sarr MG. Role of the duodenum in the control of canine gastrointestinal motility. Gastroenterology 1988; 94: 622–629. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Sarr MG, Spencer MP, et al. Effect of duodenectomy on interdigestive pancreatic secretion, gastrointestinal motility, and hormones in dogs. Am J Physiol 1989; 257: G415–422. [DOI] [PubMed] [Google Scholar]
- 10.Adrian TE, Besterman HS, Cooke TJC, et al. Mechanism of pancreatic polypeptide release in man. Lancet 1977; ii: 161–163. [DOI] [PubMed] [Google Scholar]
- 11.Taylor IL, Kaufman GL Jr, Walsh JH, et al. Role of small intestine and gastric antrum in pancreatic polypeptide release. Am J Physiol 1981; 240: G387–391. [DOI] [PubMed] [Google Scholar]
- 12.Creutzfeldt W. The incretin concept today. Diabetelogia 1979; 16: 75–95. [DOI] [PubMed] [Google Scholar]
- 13.Brown JC, Cook MA, Dryburgh JR. Motilin, a gastric motor activity stimulating polypeptide: the complete amino acid sequence. Can J Biochem 1973; 51: 533–537. [DOI] [PubMed] [Google Scholar]
- 14.Buchan AM, Polak JM, Capella C, et al. Electronimmunocytochemical evidence for the K-cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry 1978; 56: 37–44. [DOI] [PubMed] [Google Scholar]
- 15.Nauck M, Schmidt WE, Ebert R. Insulinotropic properties of synthetic gastric inhibitory polypeptide: interactions with glucose, phenylamine and cholecystokinin-8. J Clin Endocrinol Metab 1989; 69: 654–662. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Mochiki E, Haga N, et al. Motilin controls interdigestive cyclic release of insulin through vagal cholinergic muscarinic pathways in fasted dogs. Am J Physiol 1998; 274: G87–95. [DOI] [PubMed] [Google Scholar]
- 17.Itoh Z, Honda R, Takeuchi S, et al. An extraluminal force transducer for recording contractile activity of the gastrointestinal smooth muscle: its construction and implantation. Gastroenterol Jpn 1977; 12: 275–283. [DOI] [PubMed] [Google Scholar]
- 18.Sillin LF, Rosenbloom MS, Chung RS. Ninety-five percent duodenectomy. An experimental study. Am J Surg 1984; 148: 337–339. [DOI] [PubMed] [Google Scholar]
- 19.Flore M, Mitchell T, Doan R, et al. The Abbott IMx automated benchtop immunochemistry analyzer system. Clin Chem 1988; 34: 1726–1732. [PubMed] [Google Scholar]
- 20.Mochiki E, Satoh M, Tamura T, et al. Exogenous motilin stimulates endogenous release of motilin through cholinergic muscarinic pathways in dogs. Gastroenterology 1996; 111: 1456–1464. [DOI] [PubMed] [Google Scholar]
- 21.Itoh Z, Takeuchi S, Aizawa I, et al. Changes in plasma motilin concentration and gastrointestinal contractile activity in conscious dogs. Am J Dig Dis 1978; 23: 929–935. [DOI] [PubMed] [Google Scholar]
- 22.Peeters TL, Vantrappen G, Janssens J. Fasting plasma motilin levels are related to the interdigestive motor complex. Gastroenterology 1980; 79: 716–719. [PubMed] [Google Scholar]
- 23.Itoh Z, Honda R, Hiwatashi K. Motilin induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol. 1976; 11 (suppl 39): 93–100. [PubMed] [Google Scholar]
- 24.Vantrappen G, Janssen J, Peeters TL, et al. Motilin and interdigestive migrating motor complex in man. Dig Dis Sci 1979; 24: 497–500. [DOI] [PubMed] [Google Scholar]
- 25.Szurszewski JH. A migrating electoric complex of the canine small intestine. Am J Physiol 1969; 217: 1757–1763. [DOI] [PubMed] [Google Scholar]
- 26.Matunaga H, Tanaka M, Naritomim G, et al. Effect of leucine 13 motilin (KW5139) on early gastric stasis after pylorus-preserving pancreatoduodenectomy. Ann Surg 1998; 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magee DF, Naruse S. The role of motilin in periodic interdigestive pancreatic secretion in dogs. J Physiol 1984; 355: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mochiki E, Inui A, Satoh M, et al. Motilin is a biosignal controlling cyclic release of pancreatic polypeptide via the vagus in fasted dogs. Am J Physiol 1997; 272: G224–232. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman DW, Sarr WM, Smith CD, et al. Cyclic interdigestive pancreatic exocrine secretion: is it mediated by neural or hormonal mechanism? Gastroenterology 1992; 102: 1378–1384. [PubMed] [Google Scholar]
- 30.Ahren B, Taborsky GJ Jr. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology 1986; 119: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 31.McIntyre N, Holdsworth DC, Turner DS. New interpretation of oral glucose tolerance. Lancet 1964; 2: 20–21. [DOI] [PubMed] [Google Scholar]
- 32.Erick H, Stimmer L, Hlad CJ, et al. Plasma insulin response to oral and intravenous glucose stimulation. J Clin Endocrinol 1964; 24: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 33.Dupre J, Beck JC. Stimulation of release of insulin by extract of intestinal mucosa. Diabetes. 1966; 25: 555. [DOI] [PubMed] [Google Scholar]
- 34.Browen JC, Dryburgh JR, Ross SA, et al. Identification and actions of gastric inhibitory polypeptide. Recent Prog Horm Res 1975; 31: 487–532. [DOI] [PubMed] [Google Scholar]
- 35.Brown JC, Otte SC. Gastrointestinal hormones at the control of insulin secretion. Diabetes 1978; 27: 782–787. [DOI] [PubMed] [Google Scholar]
- 36.Creutzfeldt W. The incretin concept today. Diabetologia 1979; 16: 75–85. [DOI] [PubMed] [Google Scholar]
- 37.Ebert R, Illmer K, Creutzfeldt W. Release of gastric inhibitory polypeptide (GIP) by intraduodenal acidification in rats and humans and abolishment of the incretin effect of acid by GIP-antiserum in rats. Gastroenterology 1979; 76: 515–523. [PubMed] [Google Scholar]
- 38.Ebert R. Gut signals for islet hormone release. Eur J Clin Invest 1990; 20 (suppl 1): 20–26. [DOI] [PubMed] [Google Scholar]
- 39.Nauck M, Härter S, Ebert R, et al. Effects of four orally administered analogues of prostaglandin E1 and E2 on glucose tolerance and on the secretion of pancreatic and gastrointestinal hormones in man. Eur J Clin Invest 1989; 19: 298–305. [DOI] [PubMed] [Google Scholar]