Abstract
Objectives
To determine the prevalence of adenomas in ileal pouches from patients with familial adenomatous polyposis (FAP) and to determine whether a correlation exists between the presence of pouch adenomas and duodenal adenomas and the site of the adenomatous polyposis coli gene mutation.
Summary Background Data
Restorative proctocolectomy can markedly reduce the risk of colorectal adenocarcinoma in FAP patients. However, adenomas with the potential to progress to adenocarcinoma can develop in the duodenum, ileum, and continent ileostomy after restorative proctocolectomy. More recently, adenomas have been described in the ileal pouch after ileoanal anastomosis.
Methods
Pouch endoscopy was offered to 167 patients with FAP who had undergone restorative proctocolectomy between January 1984 and December 1996.
Results
Adenomas were found in 35% of the 85 ileal pouches examined. No invasive carcinomas were noted. The risk of developing one or more adenomas at 5, 10, and 15 years was 7%, 35%, and 75%, respectively. Patients with adenomas were more likely to have duodenal and ampullary adenomas. No correlation was detected between adenoma development and the site of the adenomatous polyposis coli mutation.
Conclusions
Adenomas are frequently found in the ileal pouch of patients after restorative proctocolectomy for FAP. Regular endoscopic surveillance of the pouch is recommended at a frequency similar to that of upper gastrointestinal endoscopy.
Familial adenomatous polyposis (FAP) is an inherited disease characterized by the development of hundreds of adenomas in the colon and rectum. Because virtually all patients will develop adenocarcinoma if left untreated, prophylactic colectomy is indicated. Surgical options include restorative proctocolectomy with construction of an ileal reservoir (RPC) and colectomy with ileorectostomy (IR). Upper gastrointestinal and small bowel polyps may also be present. The prevalence of small bowel adenomas is underestimated compared with that of duodenal adenomas, which are more accessible for evaluation. Colonic metaplasia, adenomas, and adenocarcinomas can develop in the ileum of patients with FAP, 1–10 and the segment of ileum used to construct the ileal pouch could logically harbor such adenomas.
Familial adenomatous polyposis results from mutations in the adenomatous polyposis coli (APC) gene. The isolation of this gene on chromosome 5q has enabled a precise genetic characterization of the disease, and genetic testing can unambiguously identify affected patients. 11–13 Moreover, the site of the mutation on the APC gene correlates closely with the phenotype (e.g., profuse polyposis vs. mild polyposis) and course of disease. 12,13
Because the incidence of adenomas in the ileal pouch of patients with FAP is unknown, we began a program of pouch surveillance by endoscopy. The objectives were to evaluate the incidence of adenomatous polyps in the pelvic pouches of patients with FAP after RPC, and to determine whether the presence of these adenomas was related to the severity of the duodenal adenomas and to the site of the mutation in the APC gene.
METHODS
The medical records of all patients with FAP (n = 232) treated between January 1984 and December 1996 were reviewed. Only patients who had undergone RPC were included in the study. Patients were excluded if they died (n = 10) or if they had undergone IR (n = 22) or a proctocolectomy and ileostomy, continent or not (n = 23). Patients living outside the European community (n = 12) and patients who had previously refused follow-up endoscopy of their upper gastrointestinal tract (n = 5) were also excluded. Demographic data and data concerning the type of surgery, pathologic specimens, and upper gastrointestinal endoscopy were obtained from the medical records. The severity of duodenal polyposis was defined according to the Spigelman classification. 14 Patients were contacted and offered pouch endoscopy at our institution or at an institution nearer to their residence, according to their wishes and the authorization of their regional social security center. A flexible sigmoidoscope was used and samples of polyps were taken for biopsy. If polyps were not seen, two random mucosal samples were routinely obtained for biopsy. The number, size, histologic type, and degree of dysplasia of the polyps were recorded. Lesions found in the pouch were staged according to the classification proposed by Spigelman to assess the severity of duodenal polyposis. 14
Denaturing gradient gel electrophoresis (DGGE) 11 and the protein truncation test (PTT) were used to screen the APC gene for germline mutations in 54 independent propositi. A search for mutations in exons 1 to 14 was performed with DDGE using 18 independent polymerase chain reaction amplifications. This screened the entire coding sequence and the adjacent intronic regions of exons 1 to 14. Exon 15 was screened for mutations using DDGE with 25 independent amplifications. This screened all of exon 15 with the exception of two small regions between codons 2186 and 2213 and codons 2583 and 2628 for mutations. The PTT was also used to screen exon 15 for mutations. The PTT was performed as described by Powell et al. 15 All electrophoretic variants detected by DDGE or PTT were sequenced directly from amplified products prepared as previously described using Prism ready reaction dye primer cycle sequencing kits and an ABI 373A sequencer (both Applied Biosystems Inc., Les Ulis, France).
Continuous variables and categorical variables were compared using the Wilcoxon test or the Fisher exact test, respectively.
RESULTS
Ileal pouch endoscopy was offered to 167 patients. Eight-five of the 167 (40 men and 45 women) agreed to endoscopy. The median age at the time of RPC for these 85 patients was 27 (range 9–67; mean ± standard deviation, 28 ± 12) years. RPC was the initial procedure for 65 patients, 19 had a conversion of an IR to an RPC, and 1 had a conversion of a straight ileoanal anastomosis to an RPC. The pouch was J-shaped and hand-sewn to the dentate line after complete mucosectomy in all patients. Three patients had a colon carcinoma (one stage 2 and two stage 1 according to the Union Internationale Contre le Cancer classification 16), and two patients who were converted from IR to RPC had a stage 2 and a stage 3 rectal cancer. The mean follow-up period from the time of RPC was 85 ± 54 months. The pelvic pouch had been in place for less than 5 years in 37 patients and for 5 or more years in 48 patients.
Forty-two patients (49%) had no lesion found at pouchoscopy, but random biopsies revealed microadenomas with low-grade dysplasia in two patients.
None of the patients had clinical or endoscopic features of pouchitis. However, the histologic criteria of pouchitis were not recorded. Four patients had ulcerations of the pouch mucosa, one of which proved to be Crohn’s disease. Thirty-nine patients had polyps at pouchoscopy. Eleven of the polyps were lymphoid polyps and 28 were adenomas. Thus, a total of 30 patients (35%) had adenomas in their pouch (28 grossly visible polyps and 2 microadenomas). The features of these adenomas are detailed in Table 1. The severity of the pouch polyposis was classified as stage 1 in 1 patient, stage 2 in 23 patients, stage 3 in 5 patients, and stage 4 in 1 patient. The risk of developing pouch adenomas at 5, 10, and 15 years after RPC was 7%, 35%, and 75% (95% confidence intervals, 1–12.5%, 21.5–49.5%, 61–92.5%), respectively (Fig. 1).
Table 1. PATHOLOGIC FINDINGS IN ADENOMAS FROM ILEAL POUCH
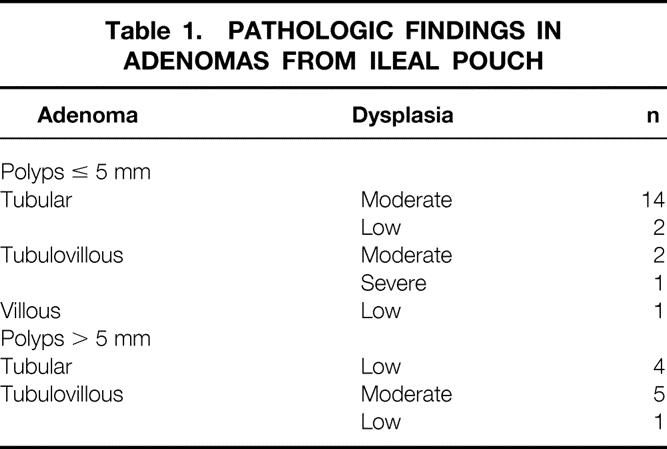
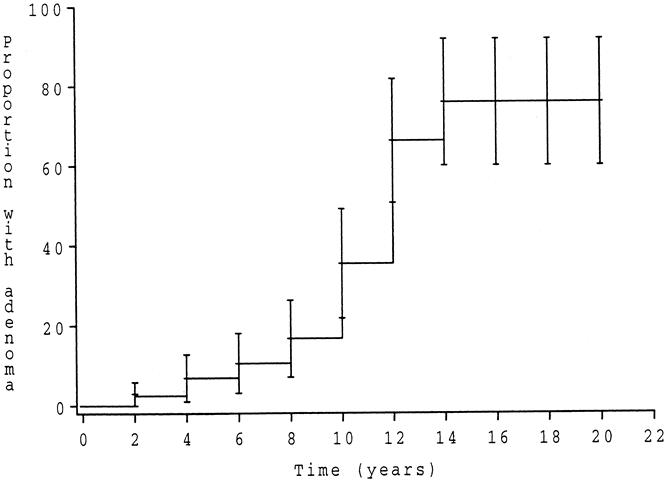
Figure 1. Risk curve to develop adenoma of the pouch after restorative proctocolectomy with construction of an ileal reservoir.
A comparison of the 30 patients with pouch adenomas (group 1) to the 55 patients without adenomas (group 2) revealed that patients in group 1 tended to have a longer follow-up since RPC (99 ± 40 vs. 77 ± 58;P = .06) and tended to be younger than those in group 2 (25 ± 9 vs. 30 ± 13 years;P = .15). Moreover, group 1 patients had adenomas of the duodenum and of the papilla more often (77% vs. 41%;P = .002) and more specifically adenomas of the papilla (50% vs. 8%;P = .001) than group 2 patients. The mean period between the last upper gastrointestinal endoscopy and the pouchoscopy was 2 ± 8 months (median 0, range 0–25). The severity of duodenal polyposis (Table 2) and incidence of colorectal carcinoma at the time of RPC did not differ between the two groups.
Table 2. SEVERITY OF DUODENAL POLYPOSIS IN PATIENTS WITH (GROUP 1) AND WITHOUT (GROUP 2) POUCH ADENOMAS

APC mutations were identified in 42 propositi. This included 49 affected patients who underwent pouch endoscopy. Patients with FAP were classified according to the site of the mutation. Twenty-two families had a mutation before codon 1250, of which 10 patients were found to have pouch adenomas. Twenty families had a mutation after this codon, of which 12 patients had pouch adenomas (P = .37).
DISCUSSION
Patients with FAP are primarily predisposed to the development of colorectal adenomas, but to a lesser extent they are also predisposed to the development of small bowel polyps. The exact incidence of ileal polyps is unknown because of the difficulty of investigating the distal small bowel by endoscopy. Lymphoid and adenomatous polyps, colonic metaplasia, and adenocarcinoma have all been described in the terminal ileum 17–20 of patients with FAP. These lesions were first described in the ileum immediately proximal to IR, 21,22 in ileostomies, and in two continent ileostomies. 9,10 Adenomas 23 and in one patient invasive carcinoma were reported after straight ileoanal anastomosis. 24 More recently, adenomas have been noted in the ileal pouch after RPC. 25–29 In this study we found that 35% of our patients had adenomas in their ileal pouch, an incidence similar to the 42% reported by Church et al. 29 This risk is high considering the life expectancy of these patients. In a group of patients who had their RPC performed in their 20s, the risk of subsequent adenoma development in the ileal pouch was 75% at 15 years of follow-up. The ideal operation for FAP would eliminate the risk of colorectal carcinoma while achieving good functional results and a low complication rate. Among the different surgical procedures available, RPC allows removal of all the colorectal diseased mucosa and achieves a functional result similar to that of colectomy with IR. Since 1984, we have favored RPC for most patients with FAP, even though a greater complication rate has been observed than after IR. 30 Restorative proctocolectomy has been recommended when patients have a rectum that is carpeted with polyps, when the follow-up is anticipated to be poor, or when upper rectal cancer is present. The strongest argument favoring RPC over colectomy with IR is that RPC should theoretically reduce the risk of rectal cancer development to a greater degree than colectomy with IR because the rectal mucosa has been removed. However, the prevalence of ileal pouch adenomas in patients with RPC, as reported here, raises the possibility of pouch cancer. The potential risk cannot be compared with the risk of rectal cancer after IR because follow-up after RPC has been less than 15 years. The risk of cancer in the remaining rectum after IR is estimated to range from 10% to 55% after 20 years of follow-up and increases after age 50 years. 31 Two pouch carcinomas have been reported after RPC. 32,33 One of these developed at the anastomotic site, but no mucosectomy had been previously performed and the likely origin of this tumor was the residual rectal mucosa. Ileal adenomas do not contraindicate RPC, but further follow-up will be necessary to assess the risk of carcinomatous transformation.
One patient in our series had severe dysplasia. She had previously developed a carcinoma in situ in residual glandular mucosa after incomplete mucosectomy. Resection of this carcinoma had been performed by completion mucosectomy and advancement of the ileal pouch. 34 Adenomas with severe dysplasia were seen at endoscopy 42 months later. Administration of sulindac resulted in a complete endoscopic response 12 months after initiation of the therapy, an observation previously reported. 28
In our study, patients who developed pouch adenomas tended to be younger than patients who did not. A more aggressive disease, expressed in younger patients and requiring earlier surgery, might be incriminated. The correlation between the presence of duodenal adenomas and the development of ileal pouch adenomas, highlighted in this series, would support this hypothesis and may suggest that a similar mechanism added to the existing APC germline mutation promotes adenoma formation in both mucosal sites. Such a mechanism would increase the risk of nonexpression of the normal allele. However, patients with pouch adenomas tended also to have a longer follow-up. It could be argued that such an event might be time-related and could be expected in all patients. Also, a selection bias might be responsible for the findings: for instance, patients with duodenal adenomas may be more likely to agree to endoscopic examination. Further investigations and longer follow-up will therefore be required to determine whether pouch adenoma is inevitable in all patients with RPC or specific to a subgroup of patients.
Molecular genetic testing has been proposed as a guide to surgical management of patients with FAP. Vasen et al 13 suggested that patients with an APC mutation before codon 1250 may have a lower risk of developing a rectal carcinoma and could have a colectomy and IR. In our series, no correlation between the site of the APC mutation and the presence of ileal pouch adenomas was found. However, the number of patients with APC mutations in this study may be too small to have the statistical power to determine whether the site of the APC mutation correlates with the tendency to develop adenomas in the ileal pouch. Patients with FAP have been routinely tested for APC mutations at our institution since 1992. Mutational screening methods such as single-strand conformational polymorphism, DGGE, RNAse protection, and protein truncation assays have been developed. Although 20% of the germline mutations that have been identified in APC patients are found clustered in two codons, the widespread distribution of the rest of the mutations in most of the first half of the coding sequence makes the direct search for mutations by DNA sequencing impractical. Monitoring of allele-specific expression of the APC gene has also been proposed. These combined approaches detect mutations in 80% to 90% of patients with FAP, 15 a proportion similar to that observed in our series (a mutation could be identified in 42 of 54 [78%] families with FAP).
In summary, our results show that endoscopy of the ileal pouch should be recommended after RPC to detect adenomas and prevent the development of carcinoma. The interval between endoscopic examinations could be the same as for duodenal surveillance, and both areas could be evaluated during the same visit.
Footnotes
Support by a grant from Bourse du Fonds D’Etudes et de Recherche du Corps Medical des Hôpitaux de Paris.
Correspondence: Professor Emmanuel Tiret, Department of Digestive Surgery, Hôpital Saint-Antoine, 184 rue du Faubourg Saint Antoine, F-75571 Paris, France.
E-mail: emmanuel.tiret@sat.ap-hop-paris.fr
Accepted for publication August 4, 2000.
References
- 1.Nakahara S, Itoh H, Iida M, et al. Ileal adenomas in familial polyposis coli. Dis Colon Rectum 1985; 28: 875–877. [DOI] [PubMed] [Google Scholar]
- 2.Roth JA, Logio T. Carcinoma arising in an ileostomy stoma: an unusual complication of adenomatous polyposis coli. Cancer 1982; 49: 2180–2184. [DOI] [PubMed] [Google Scholar]
- 3.Ross JE, Mara JE. Small bowel polyps and carcinoma in multiple intestinal polyposis. Arch Surg 1974; 108: 736–738. [DOI] [PubMed] [Google Scholar]
- 4.Primrose JN, Quirke P, Johnston D. Carcinoma of the ileostomy in a patient with familial adenomatous polyposis. Br J Surg 1988; 75: 384. [DOI] [PubMed] [Google Scholar]
- 5.Suarez V, Alexander-Williams J, O’Connor HJ, et al. Carcinoma developing in ileostomies after 25 or more years. Gastroenterology 1988; 95: 205–208. [DOI] [PubMed] [Google Scholar]
- 6.Gadacz TR, McFadden DW, Gabrielson EW, et al. Adenocarcinoma of the ileostomy: the latent risk of cancer after colectomy for ulcerative colitis and familial polyposis. Surgery 1990; 107: 698–703. [PubMed] [Google Scholar]
- 7.Gilson TP, Sollenberger LL. Adenocarcinoma of an ileostomy in a patient with familial adenomatous polyposis: report of a case. Dis Colon Rectum 1992; 35: 261–265. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JA III, Talton DS, Poole GV. Adenocarcinoma of a Brooke ileostomy for adenomatous polyposis coli. Am J Gastroenterol 1993; 88: 1122–1124. [PubMed] [Google Scholar]
- 9.Beart RW Jr, Fleming CR, Banks PM. Tubulovillous adenomas in a continent ileostomy after proctocolectomy for familial polyposis. Dig Dis Sci 1982; 27: 553–556. [DOI] [PubMed] [Google Scholar]
- 10.Stryker SJ, Carney JA, Dozois RR. Multiple adenomatous polyps arising in a continent reservoir ileostomy. Int J Colorectal Dis 1987; 2: 43–45. [DOI] [PubMed] [Google Scholar]
- 11.Olschwang S, Laurent-Puig P, Groden J, et al. Germ-line mutations in the first 14 exons of the adenomatous polyposis coli (APC) gene. Am J Hum Genet 1993; 52: 273–279. [PMC free article] [PubMed] [Google Scholar]
- 12.Olschwang S, Tiret A, Laurent-Puig P, et al. Restriction of ocular fundus lesions to a specific subgroup of APC mutations in adenomatous polyposis coli patients. Cell 1993; 75: 959–968. [DOI] [PubMed] [Google Scholar]
- 13.Vasen HFA, van der Luijt RB, Slors JFM, et al. Molecular genetic tests as a guide to surgical management of familial adenomatous polyposis. Lancet 1996; 348: 433–435. [DOI] [PubMed] [Google Scholar]
- 14.Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989; 2: 783–785. [DOI] [PubMed] [Google Scholar]
- 15.Powell SM, Petersen GM, Krush AJ, et al. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med 1993; 329: 1982–1987. [DOI] [PubMed] [Google Scholar]
- 16.International Union Against Cancer (UICC). TNM classification of malignant tumours, 5th ed. New York: Wiley-Liss; 1997: 66–69.
- 17.Heald RJ. Gardner’s syndrome in association with two tumours of the ileum. J R Soc Med 1967; 60: 914–915. [PMC free article] [PubMed] [Google Scholar]
- 18.Thomford NR, Greenberger NJ. Lymphoid polyps of the ileum associated with Gardner’s syndrome. Arch Surg 1968; 96: 289–291. [DOI] [PubMed] [Google Scholar]
- 19.Shaw EB, Hennigar GR. Intestinal lymphoid polyposis. Am J Clin Pathol 1974; 61: 417–422. [DOI] [PubMed] [Google Scholar]
- 20.Shull LN, Fitts CT. Lymphoid polyposis associated with familial polyposis and Gardner’s syndrome. Ann Surg 1974; 180: 319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton S, Bussey HJR, Mendelsohn G, et al. Ileal adenomas after colectomy in nine patients with adenomatous polyposis coli/Gardner’s syndrome. Gastroenterology 1979; 77: 1252–1257. [PubMed] [Google Scholar]
- 22.Iida M, Itoh H, Matsui T, et al. Ileal adenomas in postcolectomy patients with familial adenomatosis coli/Gardner’s syndrome. Dis Colon Rectum 1989; 32: 1034–1038. [DOI] [PubMed] [Google Scholar]
- 23.Myrhj T, Bülow S, Mogensen AM. Multiple adenomas in terminal ileum 25 years after restorative proctocolectomy for familial adenomatous polyposis. Dis Colon Rectum 1989; 32: 618–620. [DOI] [PubMed] [Google Scholar]
- 24.Hoehner JC, Metcalf AM. Development of invasive adenocarcinoma following colectomy with ileoanal anastomosis for familial polyposis coli. Dis Colon Rectum 1994; 37: 824–828. [DOI] [PubMed] [Google Scholar]
- 25.Bertoni G, Sassatelli R, Nigrisoli E, et al. First observation of microadenomas in the ileal mucosa of patients with familial adenomatous polyposis and colectomies. Gastroenterology 1995; 109: 374–380. [DOI] [PubMed] [Google Scholar]
- 26.Sheperd NA, Jass JR, Duval I, et al. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol 1987; 40: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nugent KP, Spigelman AD, Nicholls RJ, et al. Pouch adenomas in patients with familial adenomatous polyposis. Br J Surg 1993; 80: 1620. [DOI] [PubMed] [Google Scholar]
- 28.Church JM, Oakley JR, Wu JS. Pouch polyposis after ileal pouch-anal anastomosis for familial adenomatous polyposis. Dis Colon Rectum 1996; 39: 584–586. [DOI] [PubMed] [Google Scholar]
- 29.Wu JS, McGannon EA, Church JM. Incidence of neoplastic polyps in the ileal pouch of patients with familial adenomatous polyposis after restorative proctocolectomy. Dis Colon Rectum 1998; 41: 552–557. [DOI] [PubMed] [Google Scholar]
- 30.Kartheuser AH, Parc R, Penna CP, et al. Ileal pouch-anal anastomosis as the first choice operation in patients with familial adenomatous polyposis: a ten-year experience. Surgery 1996; 119: 615–623. [DOI] [PubMed] [Google Scholar]
- 31.Nugent KP, Phillips RKS. Rectal cancer risk in older patients with familial adenomatous polyposis and an ileorectal anastomosis: a cause for concern. Br J Surg 1992; 79: 1204–1206. [DOI] [PubMed] [Google Scholar]
- 32.Bassuini MMA, Billings PJ. Carcinoma in an ileoanal pouch after restorative proctocolectomy for familial adenomatous polyposis. Br J Surg 1996; 83: 506. [DOI] [PubMed] [Google Scholar]
- 33.Von Herbay A, Stern J, Herfarth C. Pouch-anal cancer after restorative proctocolectomy for familial adenomatous polyposis. Am J Surg Pathol 1996; 20: 995–999. [DOI] [PubMed] [Google Scholar]
- 34.Malassagne B, Penna C, Parc R. Adenomatous polyps in the anal transitional zone after ileal pouch-anal anastomosis for familial adenomatous polyposis: treatment by transanal mucosectomy and ileal pouch advancement. Br J Surg 1995; 82: 1634. [DOI] [PubMed] [Google Scholar]


