Abstract
Objective
To examine the usefulness of the authors’ method involving preoperative transcatheter arterial chemoembolization followed by hepatectomy.
Summary Background Data
The presence of portal vein tumor thrombus in a patient with hepatocellular carcinoma is one of the most significant factors for a poor prognosis. No standard therapy has been established.
Methods
Forty-five of 455 patients with hepatocellular carcinoma (10%) from 1989 to 1998 were included in this study. These patients had gross portal vein tumor thrombus but no distant metastases. The 23 patients (50%) who had indications for surgery received preoperative transcatheter arterial chemoembolization: 18 underwent hepatic resection and 5 underwent ligation of the hepatic artery or portal vein on laparotomy. Among the remaining 22 patients who did not have indications for hepatectomy, 10 received regional chemotherapy and 12 underwent transcatheter arterial chemoembolization.
Results
The mean duration of survival was 3.4 ± 2.7 years in the 18 patients who received transcatheter arterial chemoembolization and hepatectomy and 0.36 ± 0.26 years in the 27 patients who did not receive hepatectomy. The survival rate of the 18 patients who received hepatic resection with preoperative transcatheter arterial chemoembolization was 82% at 1 year, 42% at 3 years, and 42% at 5 years. Portal trunk occlusion by tumor thrombus, three or more primary nodules, an indocyanine green retention rate at 15 minutes of 20% or worse, and therapeutic choice other than hepatectomy were significant predictors of a poor prognosis on univariate analysis. Hepatectomy was the only factor that was significant on multivariate analysis.
Conclusions
Patients may enjoy long-term survival if they receive hepatectomy with preoperative transcatheter arterial chemoembolization, when the number of primary nodules is no more than two, the portal trunk is not occluded by tumor thrombus, and the indocyanine green retention rate at 15 minutes is better than 20%.
Hepatocellular carcinoma (HCC) tends to invade the intrahepatic vasculature, especially the portal vein. In clinically treated series, the rate of portal invasion was 34% to 40%. 1,2 The natural history of untreated HCC is still poor, especially in patients with portal vein tumor thrombus (PVTT); the median survival of such patients was reported to be 2.7 months, whereas survival in those without PVTT was 24.4 months. 3 Chemotherapy has not yielded satisfactory results. The median survival of patients with PVTT who received systemic chemotherapy was reported to be 3.9 months, whereas that in patients who received regional chemotherapy was 9.2 months. 4,5 The safety and effectiveness of transcatheter arterial chemoembolization (TACE) for these patients have been demonstrated in selected instances, and the regression of portal thrombi has been widely accepted, although the survival benefit for these patients remains unclear and the 3-year survival rate is only 9%. 6–10 In surgically resected series, the 3-year survival rate has been reported to be 0% to 43% if patients with all degrees of PVTT are included. 2,11–13 However, if the patients are limited to those with gross PVTT (PVTT in the portal trunk, its first-order branch, or its second-order branch), the 3-year and 5-year survival rates are reportedly 15% to 28% and 0% to 10%, respectively. 14,15 In this context, none of these therapeutic modalities has provided satisfactory results, and no standard treatment policy has been established.
We assumed that if intrahepatic tumor spread could be precisely evaluated by the injection of an iodized oil (Lipiodol; Guerbet Laboratories, Aulnay Sous Bois, France), and if rapid growth of the tumor and PVTT could be interrupted by TACE, this combination therapy might enable us to select patients for hepatic resection and achieve improved survival. During the past decade, we have treated patients with gross PVTT using this combination therapy. To verify this assumption and to clarify the criteria for hepatic resection, we evaluated the long-term results and clinical and pathologic data.
METHODS
From 1989 to 1998, 455 patients with HCC were admitted to the Division of Hepato-Biliary-Pancreatic Surgery, Postgraduate School of Medicine, University of Tokyo. Of these, 45 patients (10%) were included in this study because they had gross PVTT either in the main portal vein, its first-order branches, or its second-order branches, and had no distant or extrahepatic metastases, as verified by abdominal ultrasound, computed tomography (CT), or angiography. The existence of gross PVTT was confirmed histopathologically by resected specimens in 18 instances. The study population consisted of 41 men and 4 women with a mean age of 62 years (range 45–77). The hepatitis B surface antigen was positive in 8 patients (18%), and the hepatitis C antibody was positive in 18 of 31 evaluated patients (58%). Both viruses were positive in one patient. The size and distribution of HCC were diagnosed by ultrasound, CT, and angiography. Postarterial portography and color Doppler ultrasonography were performed to determine the size and location of PVTT and impairment in portal flow. Intraoperative ultrasound was the final diagnostic procedure in patients undergoing laparotomy. Each nodule of HCC was determined to be either a primary nodule or a daughter nodule (intrahepatic metastasis) according to Kanai et al’s criteria. 16 Extrahepatic metastases were diagnosed by chest x-ray, chest CT, and bone scintigraphy. Liver functional reserve was assessed by serum biochemical data (total protein, albumin, total bilirubin (TB), prothrombin time) and the indocyanine green retention rate at 15 minutes (ICG-R15). Our criteria for hepatectomy were that ascites was not detected or was controllable by diuretics and the serum TB level was less than 2.0 mg/mL. The resection volume was decided based on TB and ICG-R15. Patients with a TB level of 1.1 to 1.9 mg/mL, or those with an ICG-R15 of 30% or more were selected for limited resection or enucleation. In patients with a TB level of 1.0 mg/mL or less, two thirds of nontumorous liver parenchyma could be removed if the ICG-R15 was 10% or less, and less than a third of it could be resected if it was 10% to 19%; patients with an ICG-R15 of 20% to 29% received Couinaud’s single segmentectomy or less. 17,18
Within these limitations, if oncologically radical surgery could be performed, the patients were selected for hepatectomy. In general, patients in whom the main portal trunk was completely occluded or HCC extended to all four sectors were excluded from hepatectomy. Among the 45 patients with gross PVTT, 23 (50%) were selected for surgery according to these selection criteria.
Before surgery, all the patients underwent TACE. The regimen for TACE has been reported elsewhere. 19 The interval between TACE and surgery was 11 to 70 days (average 30). Serum α-fetoprotein (AFP) and des-γ-carboxy prothrombin (PIVKA-2) level were analyzed 1 to 5 days before TACE and surgery in all patients.
The 23 selected patients underwent laparotomy for hepatectomy: 18 patients underwent hepatic resection and the remaining 5 did not undergo hepatectomy. The reasons for nonresection included liver involvement too extensive to permit complete resection (four patients) and the presence of lymph node metastases (one patient). The treatments for these five patients were ligation of the right hepatic artery in four patients and ligation of the right portal vein in one patient. One patient received portal embolization before hepatectomy to prevent postoperative liver failure. All of these 18 patients underwent anatomical dissection using Pringle’s maneuver. The surgical procedures undertaken were right or extended right hepatectomy (n = 8), left hepatectomy (n = 3), anterior segmentectomy (n = 5), posterior segmentectomy (n = 1) and central bisegmentectomy (n = 1). In 13 of 18 patients, PVTT was located in the resection area. In three patients, PVTT protruded into the proximal portal vein beyond the resection line approximately 1 to 2 cm and was pulled out from the stump of the portal vein. In another two patients, PVTT extended into the main portal trunk, so the right portal vein, the left portal vein, and the main portal trunk were exposed and were clumped to hamper PVTT, the portal vein was incised at the bifurcation of the right and left portal veins, and PVTT was removed. After confirmation that no PVTT remained, the stump was continuously sutured
The therapeutic modalities for the 22 patients who did not have indications for surgery were regional chemotherapy in 10 patients from 1989 to 1991 and TACE in 12 from 1992 to 1998. Regional chemotherapy was applied as follows: under epidural anesthesia, using the lateral femoral circumflex artery, a heparin-coated catheter (Anthron P-U; Toray, Tokyo, Japan) was inserted to the proper hepatic artery with the aid of fluoroscopy, and the port (Port-A-Cath; Pharmacia Deltec, St. Paul, MN) was placed at the lower abdomen. The regimen of chemotherapy included one-shot infusion of 4 mg mitomycin C and 500 mg 5-fluorouracil on the day of surgery, followed by a weekly infusion of 2 mg mitomycin C and 250 mg 5-fluorouracil. The method used for TACE in these 12 patients was the same as that for preoperative TACE.
Statistical Analysis
Overall survival analysis was performed on patients grouped as a function of hepatectomy and other therapeutic modalities using the Kaplan-Meier (product-limit) method. Survival curves were compared with the log-rank test. A multivariate Cox regression analysis was performed to identify significant contributors that were independently associated with death among the factors that were significant on univariate analysis. The features of HCC, biochemistry, and ICG-R15 in the hepatectomy group and the nonhepatectomy group were compared using the Mann-Whitney test. The level of tumor markers (AFP, PIVKA-2) before TACE versus afterward were compared by the Wilcoxon matched-pairs test. P < .05 was considered significant.
RESULTS
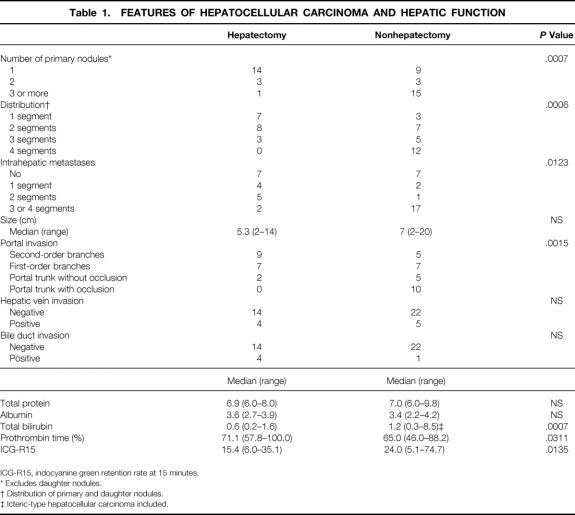
The features of HCC, biochemistry, and ICG-R15 grouped according to hepatectomy are shown in Table 1. In the 18 patients who underwent TACE and hepatectomy, the median AFP 1 to 5 days before TACE was 1,447 ng/mL (range 5–103,907) and that 1 to 5 days before operation was 471 ng/mL (range 5–22,851). The AFP level was significantly decreased by TACE (P = .0038). The level of PIVKA-2 was also decreased by TACE (P = .001): the median level was 1141 mAU/mL (range 63–80,000) before TACE and 63 mAU/mL (range 63–15,620) afterward.
Table 1. FEATURES OF HEPATOCELLULAR CARCINOMA AND HEPATIC FUNCTION
ICG-R15, indocyanine green retention rate at 15 minutes.
* Excludes daughter nodules.
† Distribution of primary and daughter nodules.
‡ Icteric-type hepatocellular carcinoma included.
Among the 27 patients who did not undergo hepatic resection, one patient was still alive as of this writing, 14.6 months after the first TACE procedure. The remaining 26 patients died of HCC. The mean survival time of these 27 patients was 0.36 ± 0.26 years; the 1-year survival rate was 7% (Fig. 1). These patients were grouped according to therapeutic modalities. The mean survival time of the 12 patients who received regional chemotherapy was 3.8 ± 3.7 months, that of the 10 who received TACE was 4.8 ± 3.7 months, and that of the 5 who underwent ligation was 5.2 ± 2.2 months. There was no survival difference in these three groups (Fig. 2).
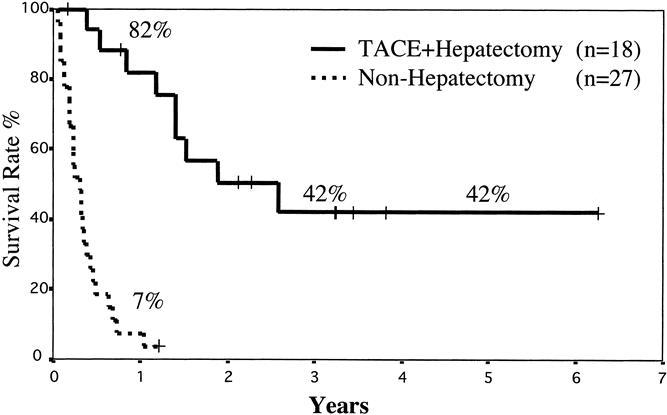
Figure 1. Overall survival of patients grouped according to whether they underwent hepatic resection. There was a statistically significant difference in survival (P < .0001).
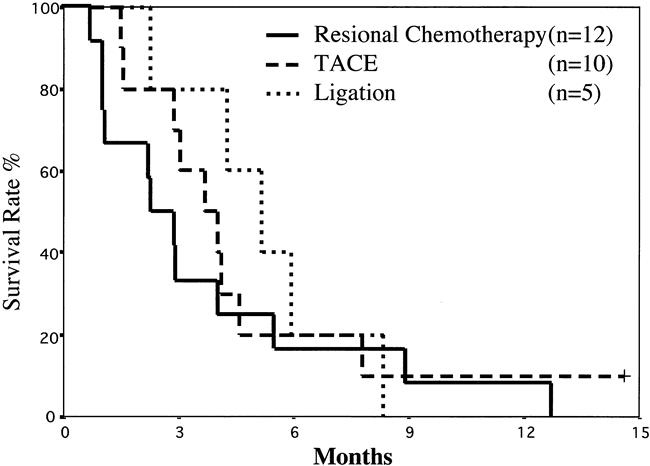
Figure 2. Survival rates of patients who did not undergo hepatectomy stratified according to the therapeutic modalities: regional chemotherapy vs. transcatheter arterial chemoembolization, regional chemotherapy vs. ligation, and transcatheter arterial chemoembolization vs. ligation. There were no significant differences.
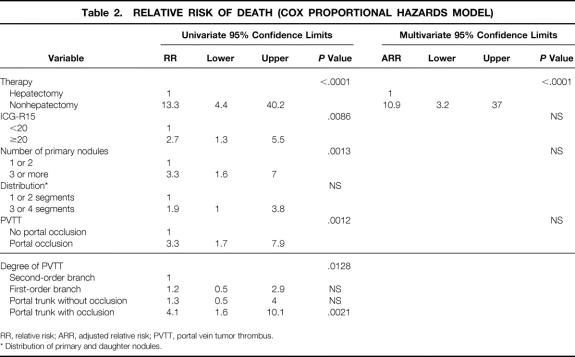
In the 18 patients who underwent hepatic resection, one patient developed liver failure and died on postoperative day 58 in 1990. The remaining 17 patients were discharged without major complications. As of September 1999, 10 of 17 patients had had recurrence in the liver (n = 7), lung (n = 2), and diaphragm (n = 1). The time between hepatic resection and recurrence in these 10 patients was 81 to 636 days (mean 233 days). Nine of the 10 patients with recurrence died of HCC, even though the recurrence in the diaphragm was resected and the patient with recurrence in the lung underwent limited resection. Seven of these 17 patients had not had a recurrence, but one died of colon cancer 2 years and 4 months after the hepatic resection, and no recurrence of HCC was observed at autopsy. The remaining six patients were alive without recurrence as of September 1999, with survivals of 1.7 to 6.3 years. The mean survival of the 18 patients who underwent TACE and hepatectomy was 3.42 ± 2.67 years, and the 3-year and 5-year survival rates were 42% (see Fig. 1). The relative risk of death with the Cox proportional hazards model is shown in Table 2. When the portal trunk was not occluded, there was no significant difference in relative risk regardless of where PVTT existed. When the portal trunk was occluded by PVTT, the relative risk increased to 4.1 (P = .0021). On multivariate analysis, hepatectomy was the only significant prognostic factor for a favorable prognosis (P < .0001).
Table 2. RELATIVE RISK OF DEATH (COX PROPORTIONAL HAZARDS MODEL)
RR, relative risk; ARR, adjusted relative risk; PVTT, portal vein tumor thrombus.
* Distribution of primary and daughter nodules.
DISCUSSION
Our results show that patients who had HCC and gross PVTT had a mean survival of 3.42 years with TACE and surgery compared with 0.36 years in the nonhepatectomy group. The features of the tumors, the grade of portal thrombus, and hepatic function differed between these two groups. Seven of the 17 patients (41%) in the hepatectomy group have not had a recurrence; 1 patient has survived for 6.3 years and 4 have survived more than 3 years; the longest survivor in the nonhepatectomy group lived for 1.22 years. Thus, the prognosis of patients with gross PVTT is not always grim. We believe one explanation for this low rate of recurrence and the long survivals is the complete removal of HCC nodules and PVTT. CT performed approximately 2 weeks after TACE and intraoperative ultrasound played an important role. CT depicted small HCC nodules and PVTT by the accumulation of an iodized oil, greatly increasing the sensitivity of the diagnosis. 20 Careful examination by ultrasound and intraoperative ultrasound also greatly improved the accuracy of diagnosis. 21,22 Another reason might be the effects of TACE. Although it has been reported that TACE prevented the growth of HCC into the portal vein and was also effective in the regression of portal thrombus, the survival benefit for these patients remains questionable. 6,8,9 In our series, the tumor makers such as AFP and PIVKA-2 were significantly decreased by TACE. Accumulation of iodized oil in PVTT, not only in HCC nodules, was seen in many patients (Fig. 3), and necrosis of PVTT was detected by pathologic examination. In this context, it seems reasonable to suppose that TACE impaired the rapid growth of PVTT until surgical intervention.
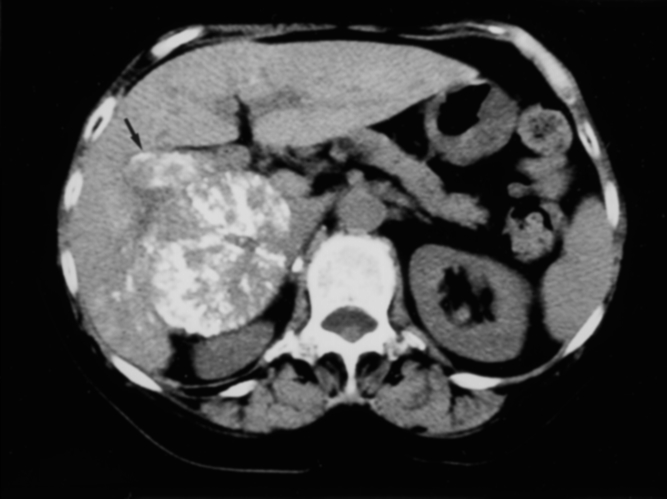
Figure 3. On the plain computed tomography scan taken 2 weeks after transcatheter arterial chemoembolization, an iodized oil accumulated in portal vein tumor thrombus located at the right portal vein (arrow), and also in a nodule of hepatocellular carcinoma.
To clarify the influence of TACE on prognosis, a comparative study, such as patients who had resection without TACE versus those with TACE, is necessary. The patients who received hepatectomy alone did not exist in our series; however, the 5-year survival rates of patients who were treated with hepatectomy alone have been reported to be 0% to 10%, 14,15 whereas the survival rate of patients undergoing TACE in our series was 42%. It may be said that TACE had a favorable effect on the prognosis. Of course, to justify the survival benefit of our treatment policy, randomized controlled studies are necessary. However, no other therapeutic modalities have provided satisfactory results. In our study, hepatectomy with preoperative TACE was the only factor that significantly predicted a favorable prognosis on multivariate analysis. It is likely that our combination therapy may be able to improve patient survival if the patients are selected carefully. PVTT is one of the most important factors in a poor prognosis, and such patients have a poor prognosis overall. 1–3 However, it is incorrect to exclude these patients from hepatectomy for this reason. Instead, it is important to select patients for whom survival can be improved by surgery with preoperative TACE. In comparing the hepatectomy group with the nonhepatectomy group, most of the patients who underwent hepatectomy had one or two primary nodules (except intrahepatic metastasis), and these were confined to one or two segments in 15 of 18 patients; none of the patients had portal occlusion.
With regard to hepatic function, 15 of 18 patients in the hepatectomy group had an ICG-R15 of better than 20%. To remove gross PVTT, hepatectomy in which the relevant portal area is resected is necessary. This is one of the reasons why patients must have an ICG-R15 of better than 20%.
Hepatic resection with preoperative TACE yielded satisfactory results for selected patients. To our knowledge, this is the first report of long-term survivors. Our results suggest that patient survival can be improved by this combination therapy when there are no more than two primary nodules on a CT scan performed approximately 2 weeks after TACE with an iodized oil, the portal vein is not occluded on arterial portography, and the ICG-R15 is better than 20%.
Acknowledgment
The authors thank Dr. Chikuma Hamada, Department of Pharmacoepidemiology, Graduate School of Medicine, University of Tokyo, for his help with the statistical analysis.
Footnotes
Correspondence: Masatoshi Makuuchi, MD, Department of Hepato-Biliary-Pancreatic Surgery, Department of Artificial Organ and Transplantation, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-8655, Japan.
E-mail: makuuchi-tky@umin.u-tokyo.ac.jp
Accepted for publication July 12, 2000.
References
- 1.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer 1996; 77: 2217–2222. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229: 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999; 29: 62–67. [DOI] [PubMed] [Google Scholar]
- 4.Okada S, Okazaki N, Nose H, et al. Prognostic factors in patients with hepatocellular carcinoma receiving systemic chemotherapy. Hepatology 1992; 16: 112–117. [DOI] [PubMed] [Google Scholar]
- 5.Ando E, Yamashita F, Tanaka M, et al. A novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal vein. Cancer 1997; 79: 1890–1896. [DOI] [PubMed] [Google Scholar]
- 6.Chung JW, Park JH, Han JK, et al. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. Am J Roentgenol 1995; 165: 315–321. [DOI] [PubMed] [Google Scholar]
- 7.Harada T, Matsuo K, Inoue T, et al. Is preoperative hepatic arterial chemoembolization safe and effective for hepatocellular carcinoma? Ann Surg 1996; 224: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetter D, Wenger JJ, Bergier JM, et al. Transcatheter oily chemoembolization in the management of advanced hepatocellular carcinoma in cirrhosis: results of a Western comparative study in 60 patients. Hepatology 1991; 13: 427–433. [PubMed] [Google Scholar]
- 9.Groupe d’Etude et de Traitement du Carcinome H. A comparison of Lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med 1995; 332: 1256–1261. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier G, Roche A, Ink O, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol 1990; 11: 181–184. [DOI] [PubMed] [Google Scholar]
- 11.Shimada M, Takenaka K, Kawahara N, et al. Surgical treatment strategy for patients with stage IV hepatocellular carcinoma. Surgery 1996; 119: 517–522. [DOI] [PubMed] [Google Scholar]
- 12.Fuster J, Garcia-Valdecasas JC, Grande L, et al. Hepatocellular carcinoma and cirrhosis. Results of surgical treatment in a European series. Ann Surg 1996; 223: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanaka N, Okamoto E, Toyosaka A, et al. Prognostic factors after hepatectomy for hepatocellular carcinomas. A univariate and multivariate analysis. Cancer 1990; 65: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 14.Ikai I, Yamaoka Y, Yamamoto Y, et al. Surgical intervention for patients with stage IV-A hepatocellular carcinoma without lymph node metastasis: proposal as a standard therapy. Ann Surg 1998; 227: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994; 106: 720–727. [DOI] [PubMed] [Google Scholar]
- 16.Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer 1987; 60: 810–819. [DOI] [PubMed] [Google Scholar]
- 17.Makuuchi M, Takayama T, Kubota K, et al. Hepatic resection for hepatocellular carcinoma—Japanese experience. Hepato-Gastroenterology 1998; 45: 1267–1274. [PubMed] [Google Scholar]
- 18.Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol 1993; 9: 298–304. [DOI] [PubMed] [Google Scholar]
- 19.Ohtomo K, Furui S, Kokubo T, et al. Transcatheter arterial embolization (TAE) in treatment for hepatoma: analysis of three-year survivors. Radiat Med 1985; 3: 176–180. [PubMed] [Google Scholar]
- 20.Takayasu K, Moriyama N, Muramatsu Y, et al. The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients. Am J Roentgenol 1990; 155: 49–54. [DOI] [PubMed] [Google Scholar]
- 21.Makuuchi M, Takayama T, Kosuge T, et al. The value of ultrasonography for hepatic surgery. Hepato-Gastroenterology 1991; 38: 64–70. [PubMed] [Google Scholar]
- 22.Takayama T, Makuuchi M, Hirohashi S, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology 1998; 28: 1241–1246. [DOI] [PubMed] [Google Scholar]