Abstract
Objective
To determine the effect of trauma on arginase, an arginine-metabolizing enzyme, in cells of the immune system in humans.
Summary Background Data
Arginase, classically considered an enzyme exclusive to the liver, is now known to exist in cells of the immune system. Arginase expression is induced in these cells by cytokines interleukin (IL) 4, IL-10, and transforming growth factor beta, corresponding to a T-helper 2 cytokine profile. In contrast, nitric oxide synthase expression is induced by IL-1, tumor necrosis factor, and gamma interferon, a T-helper 1 cytokine profile. Trauma is associated with a decrease in the production of nitric oxide metabolites and a state of immunosuppression characterized by an increase in the production of IL-4, IL-10, and transforming growth factor beta. This study tests the hypothesis that trauma increases arginase activity and expression in cells of the immune system.
Methods
Seventeen severely traumatized patients were prospectively followed up in the intensive care unit for 7 days. Twenty volunteers served as controls. Peripheral mononuclear cells were isolated and assayed for arginase activity and expression, and plasma was collected for evaluation of levels of arginine, citrulline, ornithine, nitrogen oxides, and IL-10.
Results
Markedly increased mononuclear cell arginase activity was observed early after trauma and persisted throughout the intensive care unit stay. Increased arginase activity corresponded with increased arginase I expression. Increased arginase activity coincided with decreased plasma arginine concentration. Plasma arginine and citrulline levels were decreased throughout the study period. Ornithine levels decreased early after injury but recovered by postinjury day 3. Increased arginase activity correlated with the severity of trauma, early alterations in lactate level, and increased levels of circulating IL-10. Increased arginase activity was associated with an increase in length of stay. Plasma nitric oxide metabolites were decreased during this same period.
Conclusions
Markedly altered arginase expression and activity in cells of the human immune system after trauma have not been reported previously. Increased mononuclear cell arginase may partially explain the benefit of arginine supplementation for trauma patients. Arginase, rather than nitric oxide synthase, appears to be the dominant route for arginine metabolism in immune cells after trauma.
Recognition that multiple metabolic pathways for arginine are expressed in cells of the immune system is a central feature of our understanding of immune competence. Arginine is the sole substrate for the production of nitric oxide, an effector molecule against bacteria, parasites, and cancer cells. 1,2 Arginine is used as substrate by arginases, which also are expressed in cells of the immune system. 3 Arginase produces ornithine, the precursor for polyamines and proline, which are conducive to an environment of growth and repair. 4,5 Studies using cultured macrophages show that arginase also can regulate the production of nitric oxide by competing with nitric oxide synthases for arginine availability. 6,7
Cytokines govern the expression of both arginase and nitric oxide synthase (iNOS). For example, gamma-interferon induces the expression of iNOS but downregulates arginase expression in immune cells. 8 In contrast, interleukin (IL)-4, IL-10, and transforming growth factor beta (TGF-β) increase arginase expression but downregulate iNOS expression. 8,9 These cytokine profiles correspond to two diametrically opposed immune states governed at least in part by the predominance of T-helper 1 (TH-1) or T-helper 2 (TH-2) lymphocytes, respectively. TH-1 immune states, which induce iNOS expression, are associated with cellular immune competence and inflammation, whereas TH-2 immune states, which induce arginase expression, are associated with downregulated cellular immunity. Therefore, the metabolism of arginine by iNOS or arginase may depend on the patient’s immune status.
Tissue injury resulting from trauma or surgery is associated with cellular immune dysfunction and a predominance of a TH-2 cytokine profile and may therefore increase arginase expression and activity in immune cells. Other evidence to support this hypothesis is as follows. 10–12 A decreased plasma arginine level in trauma patients suggests increased arginine catabolism. Polyamine excretion increases in trauma victims and plasma ornithine levels are increased or unchanged. 13,14 Ornithine and polyamines are downstream metabolites of arginase. Finally, in contrast to stress states such as sepsis, in which nitric oxide production is increased, circulating and excreted nitric oxide metabolites (nitrate and nitrite) are reduced in trauma victims. Collectively, these background data suggest that tissue injury may result in enhanced activity of arginase in the immune system.
To determine whether trauma increases immune cell arginase activity, we prospectively followed 17 trauma patients, measuring arginase expression and activity in peripheral mononuclear cells. We then compared these data with plasma arginine, ornithine, citrulline, IL-10, and nitric oxide metabolites. Outcome measures were also examined.
We report increased immune cell arginase expression and activity resulting from tissue trauma in humans. In addition, increased arginase activity coincides with depletion of arginine, citrulline, and nitric oxide metabolites from the plasma. Mononuclear cell arginase activity correlates with the severity of trauma, length of stay in the intensive care unit (ICU), and elevations in plasma IL-10. Arginase in the immune system may contribute to increased arginine catabolism and is a marker of physiologic insult after trauma.
METHODS
Population
Critically ill trauma patients (18–75 years of age) with an Injury Severity Score (ISS) of 13 or more admitted to the trauma or surgical ICU at the University of Kentucky Hospital were enrolled. The Institutional Review Board of the University of Kentucky approved the research protocol, and informed consent to participate was obtained in all patients. Of the 22 patients initially enrolled, 3 were excluded because of the use of beta-blockers and nitric oxide donors (nitroglycerin, nitroprusside) during their ICU stay, and two were excluded because malignancy was found.
The study was conducted from January 15, 1999 to April 5, 1999. All trauma admissions were screened daily by Clinical Research Center personnel to determine their eligibility for study enrollment. Patient enrollment was attempted if they were admitted to the ICU after trauma, had family available to provide informed consent to participate, and met the above inclusion and exclusion criteria. Predicted death was not a criterion for exclusion.
Enteral nutritional support was provided to all patients according to the ICU protocol at the University of Kentucky. Specialized diets, such as those containing high concentrations of arginine or other “immune-enhancing nutrients,” were not given. On average, enteral access is obtained in all trauma patients in the first 48 hours of arrival to the ICU, and caloric goal rates are met within 72 hours of arrival.
Controls consisted of volunteers among the nursing staff (15 women and 5 men, mean age 32 [range 22–43]). No one in the control group was taking beta-blockers or nitrates. Trauma and volunteer groups were comparable for age. More men were recruited in the trauma group than in the volunteers (14 vs. 5, P < .05). Using analysis of variance, we determined that the difference in gender distribution between the trauma and volunteer groups did not cause a significant bias in the results. No statistical differences were found in independent variables, arginase activity or nitrite plus nitrate, between the sexes in either group.
Injury Severity Scores were obtained by the trauma personnel involved in the trauma registry according to clinical data accrued from the chart. The nursing personnel in the emergency room and in the ICU calculate Glasgow Coma Scale and Champion Trauma Scores.
Materials
Reagents and media were obtained from Gibco, Life Technologies (Grand Island, NY) and Roche Molecular Biochemicals (Indianapolis, IN) as specified or from Sigma Chemical Co. (St. Louis, MO) if not otherwise specified.
Peripheral Blood Mononuclear Cell Isolation
Samples were collected as soon as possible after admission and verification of informed consent. On the day of injury, samples were collected an average of 8 hours after injury (range 3–16). Daily blood samples were obtained through indwelling arterial or central venous catheters from trauma patients in the ICU (up to 7 days). Plasma was obtained by centrifugation at 400 g, and the rest of the blood was suspended in Hanks balanced salt solution. Density gradient was created using Ficoll-Hypaque overlaid by blood/Hanks balanced salt solution in conical tubes centrifuged at 400 g. After two washes, the peripheral blood leukocyte pellet was frozen at −70°C for later assay.
Arginase Activity Assay
Cells were prepared in lysis buffer (Triton X-100 0.5%, HEPES 50 mmol/L [pH 7.55, Gibco], NaCl 150 mmol/L, sodium orthovanadate 1 mmol/L, trypsin-chymotrysin inhibitor 100 μg/mL, leupeptin 50 μg/mL [Roche], aprotonin 50 μg/mL [Roche], PMSF 2 mmol/L). Arginase activity was determined by conversion of arginine to ornithine by a modification of the assay described by Konarska and Tomaszewski. 15 Results are expressed as nanomoles ornithine/min/mg. Protein assay was performed according to the technique described by Bradford 16 using reagents from Bio-Rad Laboratories (Hercules, CA).
Arginase I Immunoblot Analyses
For analyses of arginase I expression, cell lysates were prepared as described for the arginase activity assay. Adult human liver extract was used as reference material for arginase I. Immunoblotting procedures were as described previously using 12.5% SDS-PAGE followed by transfer to nitrocellulose membranes. 17 Primary polyclonal chicken antibody to rat arginase I (1:50,000) and secondary peroxidase conjugated rabbit antichicken IgY (1:5,000, Jackson ImmunoResearch Laboratories, West Grove, PA) were used. Immunoblots were developed with the enhanced Chemiluminescence Plus kit (Amersham) according to the manufacturer’s recommendations.
Plasma Amino Acid Measurement
Plasma amino acid analysis for arginine, citrulline, and ornithine was performed using a Beckman Amino Acid Analyzer and postcolumn ninhydrin detection.
Nitric Oxide Metabolite Measurement
Nitric oxide is converted rapidly in biologic systems to the stable metabolites nitrite and nitrate; in hemoglobin-containing solutions, nitrate predominates. Plasma samples were injected into a reaction chamber containing hot vanadium chloride, reducing nitrite and nitrate to nitric oxide, which was then detected by the Model 280 Nitric Oxide Analyzer (Sievers Instruments, Boulder, CO). Results are expressed as micromolar concentrations of total nitrite plus nitrate.
IL-10
Plasma was obtained daily from trauma patients and from controls and preserved at −70°C until assay. IL-10 was measured using a commercially available enzyme-linked immunosorbent assay (R&D Laboratories, Minneapolis, MN) and expressed as picograms/mL (pg/mL).
Statistical Analysis
Statistical analysis was performed using Macintosh Statview and Microsoft Excel. As is often the case with human physiologic data, arginase activity values were converted to Log10 to normalize wide, unequal variances. 18 Differences between experimental and control groups were assessed by analysis of variance. Trends over time were not due to loss of patients (n) as ensured by the Kruskal-Wallace test. Age and sex differences were assessed by the Fisher plausible least significant difference test. Significance was set at P < .05. Data are presented as means ± standard error.
RESULTS
Demographics
Seventeen critically ill trauma patients admitted to the ICU at the University of Kentucky Medical Center were studied. There were 14 men and 3 women with a mean age of 35 (range 21–66). Injuries were blunt in 14 (motor vehicle collisions), and 3 patients sustained penetrating wounds (gunshot wounds to the torso). Mean ISS was 27 (range 13–45). APACHE III mean score was 41 (range 13–83). There were no deaths. Because this was a running study, the number of patients remaining decreased with time. The smallest number of patients left in the study (day 7) was 5. The maximum number of patients studied in one given day (days 1, 2) was 14.
Mononuclear Cell Arginase Activity
An early increase in mononuclear cell arginase activity occurred in the first 12 hours after trauma compared with the control group (2.12 ± 0.44 vs. 1.06 ± 0.33, P < .0001). A significant increase in mean arginase activity was maintained in the trauma population throughout the 7 days of the study compared with the control group (Fig. 1, P < .03). Eight of the 17 patients maintained elevated leukocyte arginase activity throughout their participation in the study. We conclude that an early and persistent increase in arginase activity occurs in immune cells after trauma.
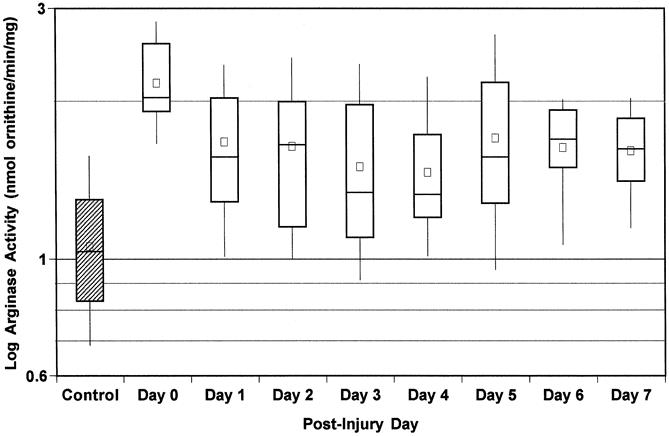
Figure 1. Peripheral blood mononuclear cell arginase activity. Peripheral blood samples from 17 trauma patients were collected daily from admission (day 0) to discharge from the intensive care unit (up to 7 days). Peripheral mononuclear cells were isolated by density gradient separation and arginase activity was measured. Each large box represents the 25th to 75th percentiles. Lines across large boxes show medians; small boxes show means. Whiskers at the top and bottom show upper and lower deciles, respectively. Arginase activity for the 20 controls is also.
Mononuclear Cell Arginase I Expression
Arginase I expression in human cells was examined by immunoblot. Mononuclear cell lysates were randomly selected from four controls and four trauma patients on postinjury day 1. Minimal arginase I expression was identified in controls and was upregulated in the trauma patients (Fig. 2).
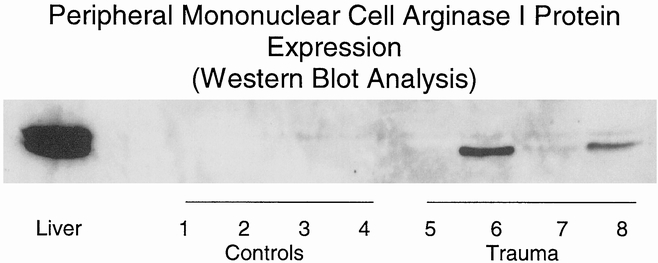
Figure 2. Human peripheral mononuclear cell arginase I protein expression. Peripheral leukocytes were isolated by density gradient from healthy volunteers (lanes 1–4) and from adult trauma victims 1 day after injury (lanes 5–8). Cell lysates were prepared and arginase I protein expression levels were determined by Western blot analysis (40 μg protein per lane). Samples from four representative controls and four trauma patients are shown. Human liver extract (1 μg) was used as a positive control.
Plasma Arginine, Citrulline, and Ornithine Levels
The plasma arginine concentration was significantly decreased on the day of injury compared with controls, and this decrease persisted through 7 days after the injury (P = .01). The plasma concentration of citrulline, the synthetic precursor of arginine, was also decreased, and this decrease persisted through 7 days after the injury (P < .0001). Ornithine is an amino acid produced from arginine by arginase. Plasma ornithine levels temporarily decreased on the day of injury and days 1 and 2 after trauma compared with controls but reached normal values by day 3 (P < .03) (Fig. 3). The correlation between leukocyte arginase activity and plasma arginine approached but did not reach statistical significance (P = .07).
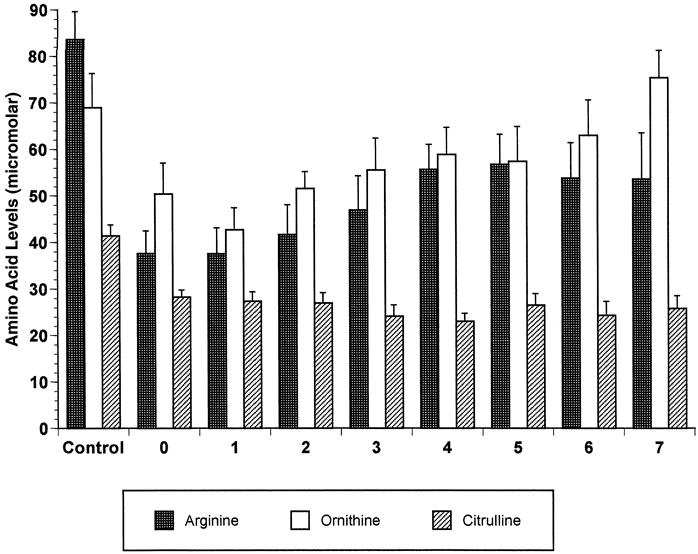
Figure 3. Postinjury changes in levels of selected amino acids. Plasma samples from trauma patients were collected daily from admission (day 0) to discharge from the intensive care unit (up to 7 days), and amino acids were measured. Values for the 20 controls are also shown.
Plasma Nitric Oxide Metabolites
Plasma nitrite plus nitrate levels were measured and compared with arginase activity. Plasma nitrite plus nitrate levels were found to be low during the first 12 hours after trauma compared with controls (23.3 ± 2.9 vs. 34.9 ± 4.7, P < .02). Plasma nitrite plus nitrate levels decreased even further by day 2 and for the next 5 days (Fig. 4)P < .03). Low plasma nitrite plus nitrate values coincided with increased leukocyte arginase activity, but there was no correlation between them.
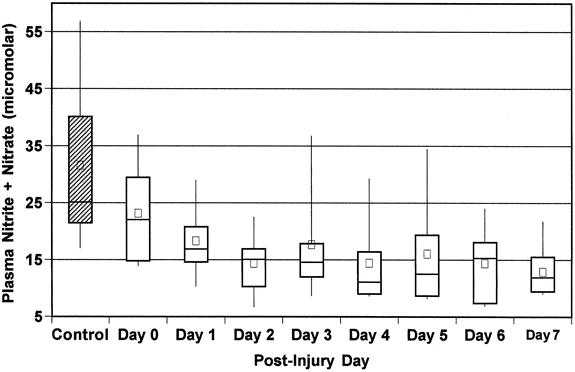
Figure 4. Peripheral blood samples from 17 trauma patients were collected daily from admission (day 0) to discharge from the intensive care unit (up to 7 days). Plasma was separated by centrifugation. Total nitrite and nitrate levels were measured by chemiluminescence. Each large box represents the 25th to 75th percentiles. Lines across large boxes show median; small dark boxes show means. Whiskers at the top and bottom show upper and lower deciles respectively. Nitrate + nitrate for the 20 controls are also shown.
Mononuclear Cell Arginase Activity Elevations Correlated with Clinical Measures of Illness
Correlation between increased arginase activity and indicators of severity of trauma was determined by linear regression analysis against known trauma scales (i.e., ISS, Champion Trauma Score, Glasgow Coma Scale). A significant inverse correlation was observed between leukocyte arginase activity on the day of injury and the Champion Trauma Score (R = 0.74, P < .01) and the Glasgow Coma Scale (R = 0.63, P < .03) but not with the ISS. In addition, a direct correlation between arginase activity on the first day after injury and the ICU length of stay was observed (R = 0.68, P < .01). These data show that mononuclear cell arginase activity increased proportionately to the severity of trauma. Arginase activity on day 1 directly correlated with the serum lactic acid level (R = 0.73, P < .04).
Mononuclear Cell Arginase Activity Elevations Correlated with Elevated Plasma IL-10
A significant increase in serum IL-10 was observed in the first 24 hours of injury (53.0 ± 15.7 pg/mL) compared with controls (6.1 ± 0.3 pg/mL) (P = .008). Circulating levels of IL-10 decreased by days 3 (18.6 ± 3.5) and 5 (14.0 ± 2.5) but remained significantly elevated at both time points compared with controls (P = .003 and P = .007, respectively). Arginase activity correlated directly with plasma IL-10 levels after trauma (R = 0.54, P = .0004). Increased arginase activity therefore occurs in this TH-2 predominant immune state characterized by increased IL-10 release.
DISCUSSION
Uncontrolled infection after tissue injury caused by trauma or surgery is an important cause of organ failure and death. 19 Much research effort has been focused on understanding why surgical patients and trauma victims are so susceptible to infection. There is now a consensus that the immune system is significantly affected in many ways by tissue trauma. A particularly important component of altered immunity after surgery or trauma is altered T-lymphocyte function. 20 Nutritional supplementation with arginine in surgical patients has been shown to improve T-lymphocyte function. Altered arginine metabolism after tissue injury therefore has significant clinical implications.
Arginine is an amino acid that has gained attention with the discovery that it serves as substrate for numerous processes in the immune system. For example, in humans, inflammation induces the expression of iNOS, an enzyme responsible for the production of large quantities of nitric oxide from arginine. 21 Nitric oxide generated by iNOS is an important effector molecule of the immune system. 2,7,22 Arginase, comprised of two isoenzymes called arginase I and II, metabolizes arginine to ornithine and urea. 3,23 Arginase I, also known as liver arginase, catalyzes an essential step of the urea cycle. Arginase I, however, can also be induced in other cells outside the liver, including macrophages and lymphocytes, as can the second isoform, arginase II. 3,8 In these extrahepatic cells, the arginases presumably play a role in the production of polyamines and proline, substances essential for cellular proliferation and tissue repair. In addition, through competition for arginine, the arginases can also modulate the production of nitric oxide. 6,7
In this study, we showed that tissue trauma increases arginase I expression and dramatically increases arginase activity in mononuclear cells to 10 or more times the values observed in controls. Coincidentally, levels of plasma arginine and its precursor citrulline are depleted. Restoration of normal cellular arginase and plasma arginine and citrulline requires up to 7 days. In contrast, plasma nitrite plus nitrate levels are decreased after trauma. Decreased circulating arginine and nitric oxide metabolites and decreased urinary excretion of nitric oxide metabolites indicate that arginine depletion is not due to accelerated synthesis of nitric oxide.
Trauma is associated with a decrease in plasma arginine and its precursor, citrulline. 24,25 This likely reflects a state of increased catabolism of arginine and a lack of compensation through endogenous production and intake. 26,27 Because nitric oxide production is dependent on the availability of arginine, an increase in arginase activity during trauma, sufficient to decrease arginine availability, could serve to control production of nitric oxide, a molecule that is essential to inflammation in trauma. 28
However, a decrease in arginine levels caused by arginase may not reflect a state of physiologic control, but rather a pathologic situation. Trauma and surgical patients have been given supplemental dietary arginine in an effort to boost their immune function. 29,30 Arginine-based diets appear to benefit patients by decreasing infection rates, antibiotic use, and length of stay. 31 However, the mechanism by which arginine repletion effects its benefits is unknown. Our data show that arginase activity in peripheral mononuclear cells is dramatically elevated, coinciding with arginine depletion. If increased arginase activity contributes to a state of acute “arginine malnutrition,” then elevated arginase expression and activity are important early alterations in the immune response that affect immune competence. These findings lend support to the concept that arginine is a “conditionally essential” amino acid that must be supplemented during stress. 32,33
A state of immune dysfunction exists after trauma as measured by decreased T-lymphocyte reactivity to mitogens and decreased production of cytokines gamma interferon and IL-2. 34 Trauma is also associated with an increase in the production of IL-4, IL-10, and TGF-β. 35,36 This has led several investigators to conclude that TH-2 lymphocytes predominate after trauma, creating an environment of immune regulation. Because these cytokines predominate after trauma, upregulated arginase activity may be the result of a predominance of TH-2 lymphocyte subpopulation activity. Correlation between elevated serum IL-10 levels and increased arginase activity supports this notion.
The clinical significance of alterations in a biochemical pathway such as arginine metabolism during disease can be evaluated by its correlation with disease severity and outcome. We have correlated the degree of increase in immune cell arginase activity with clinical measures of the severity of trauma. Arginase activity correlates with lactate levels, which are known to be independent predictors of prognosis. Finally, arginase activity correlates with the ICU length of stay, substantiating that alteration in this metabolic pathway is a marker for significant injury. These observations warrant further investigation of immune cell arginase activity and altered arginine metabolism in this and other disease processes. We have developed a satisfactory mouse model of altered arginine metabolism after trauma that will help us delineate the importance of arginase in the pathophysiology of disease. 36
In conclusion, we found a significant increase in arginase expression and activity in peripheral mononuclear cells in trauma victims coinciding with arginine and citrulline depletion, increased serum IL-10 levels, and decreased circulating nitric oxide metabolites. Increased arginase activity in cells of the immune system may be a mechanism by which arginine and nitric oxide metabolite levels decline after tissue injury. This reflects either the competition between two opposing enzymatic pathways that share a common substrate, or a dynamic in the expression of opposing enzyme systems. Increased arginase activity in peripheral mononuclear cells may have important clinical value as a marker of disease severity or predictor of outcome.
Acknowledgments
The authors thank Dr. Michael Donnelly for his statistical analyses.
Footnotes
Supported by the National Institutes of Health (KO8-00676-01 [J.B.O.] and RO1-GM 57348 [S.M.M., W.E.O.]).
Correspondence: Juan B. Ochoa, MD, Section on Trauma and Critical Care, C222 Division of General Surgery, Department of Surgery, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536-0084.
E-mail: jochoa@pop.uky.edu
Accepted for publication August 7, 2000.
References
- 1.Green SJ, Nacy CA. Antimicrobial and immunopathologic effects of cytokine-induced NO synthesis. Curr Opinion Infect Dis 1993; 6: 384–396. [Google Scholar]
- 2.Nathan CF, Hibbs JB, Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 1991; 3: 65–70. [DOI] [PubMed] [Google Scholar]
- 3.Morris SM. Arginine synthesis, metabolism, and transport: regulators of nitric oxide synthesis. In: Cellular and molecular biology of nitric oxide. Marcel Dekker, Inc.; 1999:57–85.
- 4.Mills CD, Shearer J, Evans R, et al. Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol 1992; 149: 2709–2714. [PubMed] [Google Scholar]
- 5.Seiler N, Atanassov CL. The natural polyamines and the immune system. Prog Drug Res 1994; 43: 87–141. [DOI] [PubMed] [Google Scholar]
- 6.Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol 1998; 274: H342–348. [DOI] [PubMed] [Google Scholar]
- 7.Hey C, Boucher J-L, Vadon-Le Goff S, et al. Inhibition of arginase in rat and rabbit alveolar macrophages by Nω-hydroxy-D,L-indospicine, effects on l -arginine utilization by nitric oxide synthase. Br J Pharmacol 1997; 121: 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris SM. Regulation of arginine availability and its impact on NO synthesis. In: Nitric oxide: biology and pathobiology. Academic Press; 2000:187–197.
- 9.Modolell M, Corraliza IM, Link F, et al. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol 1995; 25: 1101–1104. [DOI] [PubMed] [Google Scholar]
- 10.Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med 1999; 27: 733–740. [DOI] [PubMed] [Google Scholar]
- 11.O’Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T-helper 2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg 1995; 222: 482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherry RM, Cue JI, Goddard JK, et al. Interleukin-10 is associated with the development of sepsis in trauma patients. J Trauma 1996; 40: 613–616. [DOI] [PubMed] [Google Scholar]
- 13.Ochoa JB, Udekwu AO, Billiar TR, et al. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg 1991; 214: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeevanandam M, Young DH, Ramias L, et al. Effect of major trauma on plasma free amino acid concentrations in geriatric patients. Am J Clin Nutr 1990; 51: 1040–1045. [DOI] [PubMed] [Google Scholar]
- 15.Konarska L, Tomaszewski L. A simple quantitative micromethod or arginase assay in blood spots dried on filter paper. Clin Chim Acta 1986; 154: 7–17. [DOI] [PubMed] [Google Scholar]
- 16.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 17.Morris SM Jr, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol 1998; 275: E740–747. [DOI] [PubMed] [Google Scholar]
- 18.Winer BJ, Brown DR, Michels KM. Statistical principles in experimental design. McGraw-Hill, Inc.; 2000; 354–358. [Google Scholar]
- 19.Bone RC. The sepsis syndrome. Definition and general approach to management. Clin Chest Med 1996; 17: 175–181. [DOI] [PubMed] [Google Scholar]
- 20.Berguer R, Bravo N, Bowyer M, et al. Major surgery suppresses maximal production of helper T-cell type 1 cytokines without potentiating the release of helper T-cell type 2 cytokines. Arch Surg 1999; 134: 540–544. [DOI] [PubMed] [Google Scholar]
- 21.Morris SM Jr, Billiar TR. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol 1994; 266: E829–839. [DOI] [PubMed] [Google Scholar]
- 22.Ochoa JB, Curti B, Peitzman AB, et al. Increased circulating nitrogen oxides after human tumor immunotherapy: correlation with toxic hemodynamic changes. J Natl Cancer Inst 1992; 84: 864–867. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson CP, Grody WW, Cedarbaum SD. Comparative properties of arginases. Comp Biochem Physiol 1996; 114B: 107–132. [DOI] [PubMed] [Google Scholar]
- 24.Jeevanandam M, Ramias L, Schiller WR. Altered plasma free amino acid levels in obese traumatized man. Metabolism 1991; 40: 385–390. [DOI] [PubMed] [Google Scholar]
- 25.Moyer ED, Border JR, Cerra FB, et al. Multiple systems organ failure: IV. Imbalances in plasma amino acids associated with exogenous albumin in the trauma-septic patient. J Trauma 1981; 21: 543–547. [PubMed] [Google Scholar]
- 26.Castillo L, Sanchez M, Chapman TE, et al. The plasma flux and oxidation rate of ornithine adaptively decline with restricted arginine intake. Proc Natl Acad Sci USA 1994; 91: 6393–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu YM, Young VR, Castillo L, et al. Plasma arginine and leucine kinetics and urea production rates in burn patients. Metabolism 1995; 44: 659–666. [DOI] [PubMed] [Google Scholar]
- 28.Hierholzer C, Harbrecht B, Menezes JM, et al. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med 1998; 187: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbul A, Rettura G, Levenson SM, et al. Wound healing and thymotropic effects of arginine: a pituitary mechanism of action. Am J Clin Nutr 1983; 37: 786–794. [DOI] [PubMed] [Google Scholar]
- 30.Beale RJ, Bryg DJ, Bihari DJ. Immunonutrition in the critically ill: a systematic review of clinical outcome. Crit Care Med 1999; 27: 2799–2805. [DOI] [PubMed] [Google Scholar]
- 31.Kudsk KA, Minard G, Croce MA, et al. A randomized trial of isonitrogenous enteral diets after severe trauma. An immune-enhancing diet reduces septic complications. Ann Surg 1996; 224: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbul A, Sisto DA, Wasserkrug HL. Arginine stimulates lymphocyte immune responses in healthy humans. Surgery 1981; 90: 244–251. [PubMed] [Google Scholar]
- 33.Brittenden J, Heys SD, Ross J, et al. Nutritional pharmacology: effects of l -arginine on host defences, response to trauma and tumour growth. Clin Sci (Colch) 1994; 86: 123–132. [DOI] [PubMed] [Google Scholar]
- 34.Miller-Graziano CL, Szabo G, Griffey K, et al. Role of elevated monocyte transforming growth factor beta production in posttrauma immunosuppression. J Clin Immunol 1991; 11: 95–102. [DOI] [PubMed] [Google Scholar]
- 35.Decker D, Schondorf M, Bidlingmaier F, et al. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up- regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996; 119: 316–325. [DOI] [PubMed] [Google Scholar]
- 36.Figert P, Wracy C, Maley M, et al. Induced nitric oxide synthesis is modulated by increased arginase activity after trauma. Surg Forum 1998; 49: 58–59. [Google Scholar]


