Abstract
Objective
To examine porcine acellular dermal matrix (ADM) as a xenogenic dermal substitute in a rat model.
Summary Background Data
Acellular dermal matrix has been used in the treatment of full-thickness skin injuries as an allogenic dermal substitute providing a stable wound base in human and animal studies.
Methods
Xenogenic and allogenic ADMs were produced by treating porcine or rat skin with Dispase and Triton X-100. Full-thickness skin defects (225 mm2) were created on the dorsum of rats (n = 29), porcine or rat ADMs were implanted in them, and these were overlain with ultrathin split-thickness skin grafts (STSGs). In two adjacent wounds, 0.005- or 0.017-inch-thick autografts were implanted. In other experiments, the antimicrobial agent used during ADM processing (azide or a mixture of antibiotics) and the orientation of the implanted ADM (papillary or reticular side of ADM facing the STSG) were studied. Grafts were evaluated grossly and histologically for 30 days after surgery.
Results
Significant wound contraction was seen at 14, 20, and 30 days after surgery in wounds receiving xenogenic ADM, allogenic ADM, and thin STSGs. Contraction of wounds containing xenogenic ADM was significantly greater than that of wounds containing allogenic ADM at 30 days after surgery. Graft take was poor in wounds containing xenogenic ADM and moderately good in those containing allogenic ADM. Wound healing was not significantly affected by the antimicrobial agent used during ADM preparation or by the ADM orientation.
Conclusion
Dispase–Triton-treated allogenic ADM was useful as a dermal substitute in full-thickness skin defects, but healing with xenogenic ADM was poor.
In extensive deep burns and other full-thickness skin wounds, permanent replacement of lost skin remains a major challenge. Several methods of wound closure are in use, and each has its own advantages and disadvantages. Porcine skin and preserved cadaver skin are used for temporary wound coverage, but 1 to 2 weeks after grafting, these tissues undergo immune-mediated rejection. 1,2 Permanent wound coverage is usually accomplished using meshed, split-thickness autografts harvested from undamaged regions of skin. Extensively burned patients have limited donor sites, so thin split-thickness autografts are harvested repeatedly from the same sites. This results in substantial donor-site problems resulting from pain, infection, scarring, and sometimes keloid formation. 3 Very thin (0.005-inch-thick) meshed autografts can be used, but the lack of a sufficient dermal bed often results in extensive wound contraction at the recipient site. 4 The harvest of thicker split-thickness skin grafts (STSGs) reduces this contraction problem but causes increased problems at donor sites. Alternatively, cultured epidermal autografts expanded using cell culture methods can be used for wound coverage, but this technique is expensive, and cultured epidermal autografts often fail to survive or result in poor-quality wound healing. 5–8 Many of these difficulties with STSGs and cultured epidermal autografts stem from the lack of an adequate layer of dermis. The dermis is important for the stability of skin grafts and the healing of skin wounds because it inhibits wound contraction and provides both mechanical strength and elasticity to the skin.
In recent years, attempts have been made to produce a dermal substitute capable of supporting thin STSGs or cultured epidermal autografts. 5,9–14 Dermal substitutes that use denatured xenogenic collagen gels have been studied extensively in humans. Gels composed of bovine type I collagen with or without shark chondroitin sulfate 15 and with or without human fibroblasts and keratinocytes 16–18 have been found to have efficacy in the treatment of partial- or full-thickness skin injuries. Few adverse reactions directly related to the implant materials have been reported with these grafts. Antibovine collagen and antibovine serum titers do not increase in recipients, 17,19 nor is evidence of immediate or delayed immune reactivity to xenogenic collagen observed. 18 Although in some instances the implanted gels have been shown to degrade rapidly 20 and the space previously occupied by the gels may not necessarily be replaced by neodermis, 21 successful neodermis formation has been reported using collagen-glycosaminoglycan gels. 20,22–24
Alternatively, deepidermized xenograft porcine or human skin has been found to have modest value in treating full-thickness skin wounds in conjunction with immediate STSG autografts. 25,26 Such matrices may exhibit residual antigenicity as a result of the incomplete removal of cellular debris from the dermis or processing-dependent alterations in the connective tissue matrix. To avoid such antigenicity, different types of acellular dermal matrix (ADM) have been studied. ADM is derived from split-thickness skin from which the cellular components (keratinocytes, sweat glands, sebaceous glands, fibroblasts, vascular endothelium, and smooth muscle) have been extracted or removed. 27–29 ADM consists primarily of extracellular matrix proteins and collagen 30 and is thought to be weakly immunogenic or nonimmunogenic for the purposes of allogenic implantation. 28 Allogenic ADM prepared by different methods 27,28 has been used as a substitute for dermis in animal models 27–29 and in clinical trials for burn wound management. 31,32 These studies suggest that allogenic ADM functions as a permanent dermal transplant and enhances the healing of thin skin grafts applied to deep burn wounds while reducing donor-site healing time. However, the limited availability of cadaver skin, the expense of commercially available human ADM, and the risk of disease transmission limit the use of allogenic dermal matrix as a dermal substitute.
The use of xenogenic skin as a source of ADM might alleviate some of these problems and make ADM more readily available for use in surgical procedures. Porcine skin is already used clinically to provide physiologic coverage for skin wounds; because it is structurally and immunologically similar to rat and human skin, 33,34 it was studied here. We hypothesized that porcine xenograft ADM would facilitate healing comparably to that seen with rat allograft ADM when these matrices were implanted into full-thickness skin wounds in rats. In addition, the effects of certain variations in the methods of preparation and implantation of ADM were evaluated using allogenic and xenogenic dermal matrices.
METHODS
Animals and Tissue Harvesting
Male Sprague-Dawley rats (350–400 g; Harlan Sprague-Dawley, Indianapolis, IN) anesthetized with ketamine (40–45 mg/kg administered intramuscularly) were used as skin donors for allogenic ADM. The dorsal hair was shaved and then more completely removed using surgical hair-remover cream (Sparta Instrument, Hayward, CA). The rats were killed by exsanguination and the skin was scrubbed with povidone–iodine solution (Triadine; H&P Industries Inc., Franklin, WI). Donor skin for preparation of allogenic ADM, 0.012 inches thick, was removed using a dermatome and was meshed at a 2:1 ratio using a Padgett Electro-Dermatome (Padgett Instruments, Inc., Kansas City, MO). The removed skin was washed three times in sterile phosphate-buffered saline and processed as described below. Alternatively, commercially available (Brennen Medical, St. Paul, MN) frozen, meshed porcine skin (0.012 inches thick) was processed to produce xenogenic ADM as described below. Cryopreserved porcine skin has been exposed to sodium hypochlorite and glycerin as part of its preparation by the supplier (B. Klein, Brennen Medical, personal communication). To control for such differences between cryopreserved porcine and fresh rat skin, we also prepared ADM from fresh porcine skin and implanted this material into rat skin wounds. Wound healing in rats implanted with ADMs derived from fresh porcine skin and from cryopreserved porcine skin were equivalent (unpublished data), and results for cryopreserved porcine ADM only are reported here. Male Sprague-Dawley rats weighing 300 to 350 g were used as transplant recipients in all experiments. All animal studies were performed with Institutional Animal Care and Use Committee approval and in accordance with guidelines established by the United States Department of Agriculture.
Preparation of ADM
Donor rat skin and thawed, cryopreserved porcine skin were treated with 2.5 U/mL Dispase II (Boehringer Mannheim, Indianapolis, IN) at 4°C for 24 hours with continuous shaking to remove the epidermis and other cellular components from the dermal matrix. Subsequently, the dermis was incubated in 0.5% Triton X-100 (US Biochemicals, Cleveland, OH) for 24 hours at room temperature with continuous shaking. Either sodium azide (0.02% wt/vol) or a cocktail of antibiotics (300 U/mL penicillin, 0.3 mg/mL streptomycin, 0.75 μg/mL Fungizone (Gibco Laboratories, Grand Island, NY), 50 mg/mL gentamycin) was present at all times during both of these steps to preclude microbial growth. 27 The resulting ADM was then extensively washed and stored in sterile phosphate-buffered saline at 4°C before implanting. All solutions were filter-sterilized and all procedures were performed aseptically. To assure uniformity in the thickness of the ADMs, a digital micrometer was used to measure the thickness of 10 pieces of each type of ADM in three different places. The average thickness (± standard error) for rat ADM (0.0108 ± 0.0003 inches) and cryopreserved porcine ADM (0.0106 ± 0.0003 inches) was not significantly different (paired t tests, n = 10).
Skin Grafts Placed onto Implanted ADM
The healing of wounds treated with ADM (porcine or rat) was compared with that of wounds receiving only autologous STSGs. Four full-thickness skin defects (15 × 15 mm; 225 mm2 each) were produced on the dorsum of each rat by excising down to the panniculus carnosus. Three sets of experiments were performed to evaluate the effects of the antimicrobial agent used during ADM processing (azide or antibiotic mixture), the orientation of the implanted ADM (papillary or reticular side of ADM facing the STSG), and xenogenic (porcine) ADM implantation in rat. For the first set of experiments, one pair of wounds was implanted with rat or porcine ADM prepared in the presence of azide and the second pair of wounds with ADM prepared in the presence of antibiotics but no azide (n = 6). For the second set of experiments, one pair of wounds was implanted with rat or porcine ADM oriented with the papillary side of the dermis superficial and the second pair of wounds with ADM oriented with the reticular side superficial (n = 6). For both of these sets of experiments, all ADMs were 0.012 inch thick and 225 mm2 in area and were covered with 0.005-inch-thick STSGs at the time of surgery.
For the third set of experiments, the healing of wounds implanted with ADM (porcine or rat) was compared with that of wounds receiving only STSGs (n = 29). One wound was implanted with porcine (xenogenic) ADM and another with rat (allogenic) ADM (each ADM was 0.012 inches thick, 225 mm2 in area), and each of these was covered with an ultrathin (0.005-inch-thick) STSG autograft (obtained from the flank). In all cases, the autografts were not meshed and the ADMs were meshed but not expanded. As controls, STSGs (0.005 or 0.017 inches thick) were implanted in two adjacent wounds (Fig. 1). All wounds were then covered with Xeroform (Sherwood, St. Louis, MO) and saline-soaked bolster dressings, which were tied over to fix the grafts.
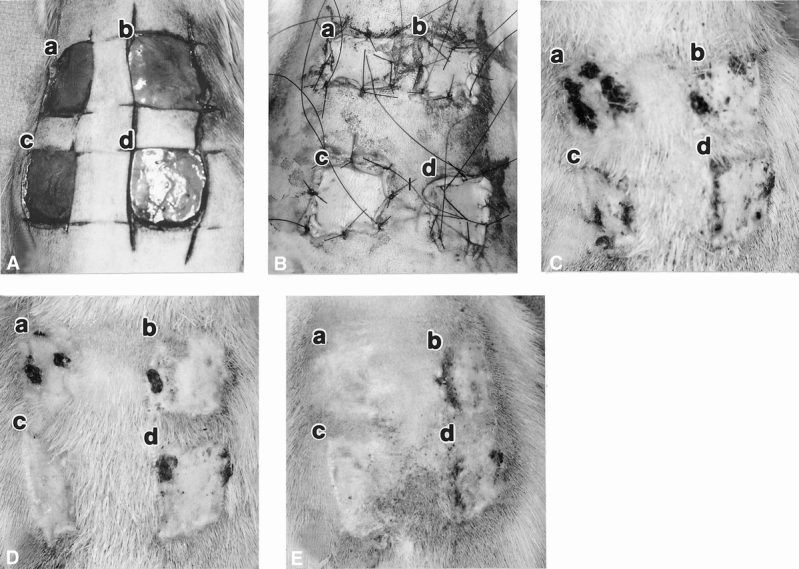
Figure 1. Macroscopic appearance of full-thickness wounds on rat dorsum implanted with acellular dermal matrix (ADM) with immediate onlay split-thickness autografts (STSGs) on day of surgery showing open full-thickness wounds (A), closed wounds containing implants (B), day 14 (C), day 20 (D), and day 30 (E). In each picture: (a) xenogenic ADM with STSG (0.005 inches thick), (b) STSG control (0.005 inches thick), (c) allogenic ADM with STSG (0.005 inches thick), (d) STSG control (0.017 inches thick). Dark areas on the wounds are regions of graft necrosis.
All grafts were examined grossly at 10, 14, 20, and 30 days after grafting for wound contraction and necrosis. So as not to disturb the healing of these wounds, a separate group of animals (n = 4), wounded and treated in like fashion, was used to obtain punch biopsies (4 mm in diameter) of the wounds at different times after surgery. The biopsies were fixed in 10% buffered neutral formaldehyde and processed for routine histology with hematoxylin and eosin stain. The percentage of graft survival was determined using the paper template technique of Sasaki and Pang. 35 The percentage of graft take was quantified by subtracting the area of graft rejection from the total area of skin graft; dividing by the total area of skin graft; and multiplying by 100. The total graft area was that observed on any given day (surgery, 10, 14, 20, or 30) and the area of necrotic tissue was that observed on the same day.
Histopathologic Evaluation of Inflammation
Biopsy specimens from each wound were evaluated by two persons under masked conditions using the following scale: 0, little or no inflammation; 1, aggregates of inflammatory cells occupying less than 25% of the sample; and 2, aggregates of inflammatory cells occupying 25% or more of the sample. Three slides per biopsy sample were observed, and the number of biopsy samples taken was 4 (days 14 and 20) or 19 (day 30).
Data Analysis and Statistics
Because the measure of graft take used was influenced by the degree of wound contraction, we combined both measurements to give a take rate normalized for wound contraction by dividing the graft area on day x by the graft area on the day of surgery, and multiplying by the take on day x. Histopathologic grading of the inflammation observed in biopsy specimens was averaged by dividing the sum of the biopsy specimen scores on day x by the total number of specimens evaluated on day x; the total number of biopsies evaluated was between 4 and 19. Paired t tests or Kruskal-Wallis nonparametric analysis of variance with Dunn multiple comparison tests were used to evaluate the statistical significance of data using InStat software (GraphPad, San Diego, CA). Before using paired t tests for nonparametric data (percentages), all were transformed using a reciprocal or log transform and tested for homogeneity of variance using the Bartlett test to ensure that the standard deviations were equal. Averaged inflammation scores were compared using the nonparametric, two-tailed Mann-Whitney statistic. P < .05 was considered significant.
RESULTS
Method of ADM Preparation Did Not Affect Wound Healing
We have found that ADM prepared in the presence of azide, although rinsed extensively, is mildly toxic to cells in tissue culture. 36 Thus, we compared wound healing in rats using ADMs prepared in the presence of azide or of antibiotics using porcine (n = 6) or rat ADM (n = 6). During a 30-day period, no differences were noted in wound size or in graft survival as reflected in the normalized take rate (data not shown) relative to these different ADM preparation methods. This was evident for both rat and porcine ADMs implanted into wounds on rats, although the normalized take rate was noticeably decreased in the wounds implanted with porcine ADM.
ADM Orientation Did Not Affect Wound Healing
The ADM is asymmetric or “sided” because it has residual basement membrane components on the papillary side and because the connective tissue architecture of the papillary and reticular dermis differs. 30,37 For optimal healing, it may be necessary to maintain the original papilloreticular orientation of the ADM on implantation into a wound. We tested the effect of ADM orientation on wound healing of skin defects in rats implanted with either porcine (n = 6) or rat (n = 6) ADMs with immediate STSG coverage. ADMs prepared from either porcine or rat skin were implanted into full-thickness wounds with either the papillary or reticular side of the dermis oriented superficially (toward the overlying STSG). During the 30-day follow-up, no differences were noted in wound size or graft survival as reflected in the normalized take rate (Fig. 2) relative to ADM orientation. Again, the normalized take rate was noticeably decreased in the wounds implanted with porcine ADM compared with those implanted with rat ADM.
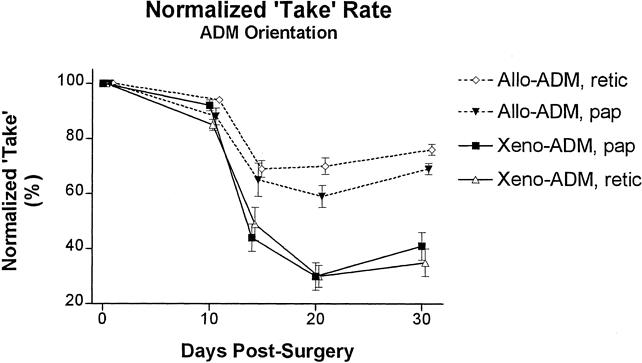
Figure 2. Effect of orientation on the normalized take rate (combined graft survival and wound area) for wounds implanted with porcine acellular dermal matrix (ADM) and split-thickness autografts (STSG; Xeno-ADM) and rat ADM and STSG (Allo-ADM). ADMs were implanted into full-thickness wounds either with the papillary side or the reticular side of the dermis oriented superficially (toward the overlying STSG). Shown are means ± standard error (n = 6).
Wounds Implanted with Xenogenic ADM Contracted Extensively
Graft areas for the xenogenic, allogenic, and thin STSG groups were significantly less (P < .05, paired t tests) than that of the thick STSG group on days 14, 20, and 30. On day 30, graft areas for the xenogenic ADM group were significantly (P < .01, paired t tests) less than those of the other three groups. Whole, untreated allogenic rat STSGs (0.017 inches thick) and frozen-thawed porcine skin (0.012 inches thick) were used as controls in some animals (n = 2). Wounds implanted with this material (Fig. 3) showed extensive contraction compared with the 0.017-inch-thick autografts (approximately 67% smaller than autograft STSGs on day 30).
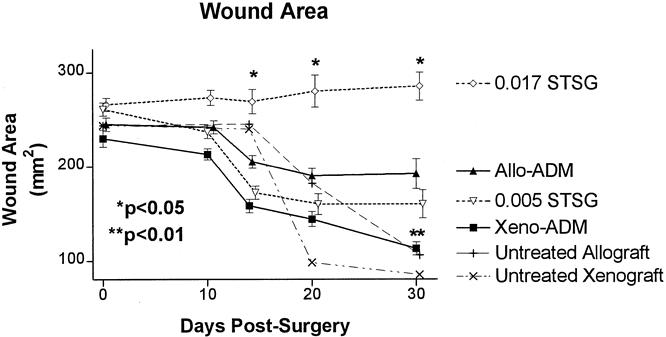
Figure 3. Areas for wounds implanted with porcine acellular dermal matrix (ADM) and split-thickness autografts (STSG; Xeno-ADM), rat ADM and STSG (Allo-ADM), thin STSG control (0.005 STSG), thick STSG control (0.017 STSG), untreated allograft (rejection control), and untreated xenograft (rejection control) groups. The wound area of the xenogenic ADM was significantly less (** P < .01, paired t test) than that of the 0.017 STSG and allogenic ADM groups on day 30. The wound area of the 0.017 STSG was significantly greater (* P < .05, paired t test) than the Allo-ADM, 0.005 STSG, and Xeno-ADM. Shown are means ± standard error (n = 29).
Graft Take Was Poor in Wounds Implanted with Xenogenic ADM
Graft survival (as a percentage of STSG area) was significantly (P < .01, paired t tests) less for the xenogenic ADM group on days 14 and 20 compared with the allogenic ADM or either STSG group on these days (Fig. 4). However, the survival of the grafts overlying the allogenic ADM was not significantly different from that of either STSG group on days 14 or 20. On day 30 after surgery, graft survival was less in the xenogenic ADM group than in the other three groups, but this difference was not significant. In controls implanted with cellular grafts, graft survival was poor (approximately 59% of the total wound area) for the allografts on day 30 and very poor for the xenografts (0%) on days 14 and 20, compared with 96% survival in the autograft STSG groups.
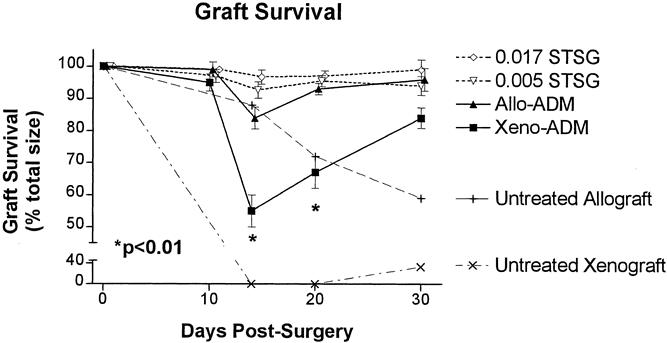
Figure 4. Graft survival (percentage of total wound area) for wounds implanted with porcine acellular dermal matrix (ADM) and split-thickness autografts (STSG; Xeno-ADM), rat ADM and STSG (Allo-ADM), thin STSG control (0.005 STSG), thick STSG control (0.017 STSG), untreated allograft (rejection control), and untreated xenograft (rejection control) groups. Graft survival of the xenogenic ADM was significantly less (P < .01, paired t test) than that of each of the other three groups on days 14 and 20. Shown are means ± standard error (n = 29).
Normalized Take Showed Poor Healing in Wounds Implanted with Xenogenic ADM
A sharp decrease in the normalized take (Fig. 5) was seen in the allogenic ADM, xenogenic ADM, and thin STSG wounds on day 14, whereas that of wounds covered with thick STSGs did not change significantly between the day of surgery and day 30. The normalized take for the xenogenic ADM group was significantly less (P < .01; paired t tests) than that for the allogenic ADM and the 0.017-inch-thick STSG group on days 14, 20, and 30. It was also less than that for the 0.005-inch-thick STSG group on days 20 and 30, but this difference was not significant.
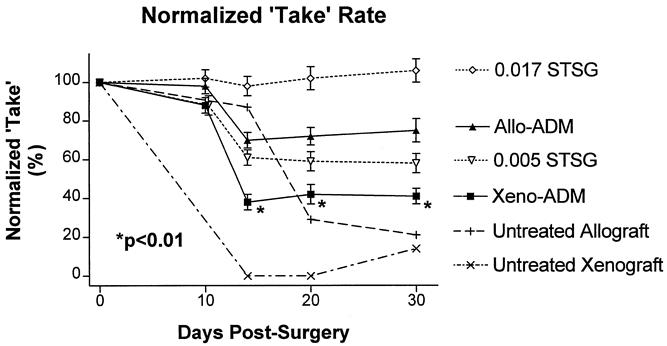
Figure 5. Normalized take rate (combined graft survival and wound area) for wounds implanted with xenogenic acellular dermal matrix (ADM) and split-thickness autografts (STSG; Xeno-ADM), allogenic ADM and STSG (Allo-ADM), thin STSG control (0.005 STSG), thick STSG control (0.017 STSG), untreated allograft (rejection control), and untreated xenograft (rejection control) groups. The normalized take for the xenogenic ADM group was significantly less (P < .01; paired t tests) than that for the allogenic ADM and the 0.017 STSG group on days 14, 20, and 30. Shown are means ± standard error (n = 29).
Extensive Inflammation Was Seen in Wounds Implanted with Xenogenic ADM
Both xenogenic and allogenic ADMs became infiltrated with fibroblasts and vascularized by 14 days after surgery (Figs. 6 and 7). Histologically, little inflammation was seen in the wounds implanted with thick STSGs and only scattered aggregates of inflammatory cells were seen in those with allogenic ADM or thin STSGs. However, extensive inflammation, frequently involving the entire biopsy specimen, was observed in wounds implanted with xenogenic ADM (Table 1). Significant increases in inflammation were evident in the wounds implanted with xenogenic ADM compared with other types of wounds on day 20 (P < .03, Mann-Whitney) and day 30 (P < .001 or P < .0001, Mann-Whitney). The inflammatory infiltrate consisted mainly of lymphocytes and plasma cells with occasional neutrophils in the specimens observed on days 14, 20, and 30. In the xenogenic ADM group, neutrophils and eosinophils were also observed on day 14, and foreign body giant cells were seen on days 20 and 30. Few eosinophils and no foreign body giant cells were observed in the other groups.
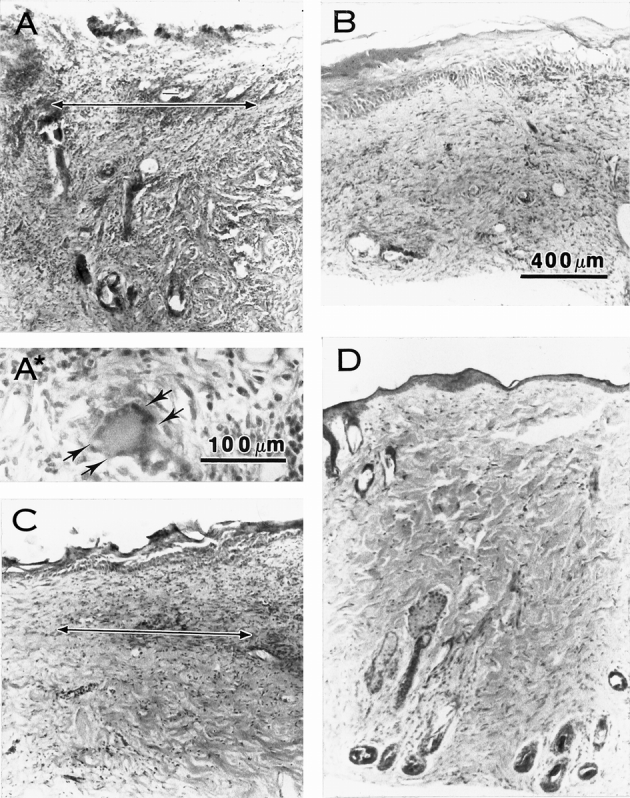
Figure 6. Microscopic appearance of acellular dermal matrix (ADM) with onlay split-thickness skin grafts (STSG) in full-thickness wounds in rat on postoperative day 20. A two-headed arrow shows the junction of the ADM and STSG. Extensive inflammatory infiltrate, tissue disruption, loss of the epithelium, and a foreign body giant cell (arrows in A*) are seen in the sample from the wound treated with xenogenic ADM and STSG (A). Minimal inflammation is seen in samples from wounds implanted with thin STSG (B), allogenic ADM and thin STSG (C), or thick STSG (D). Hematoxylin and eosin stain. Magnification bars = 100 μm (A*) or 400 μm (A, B, C, D).
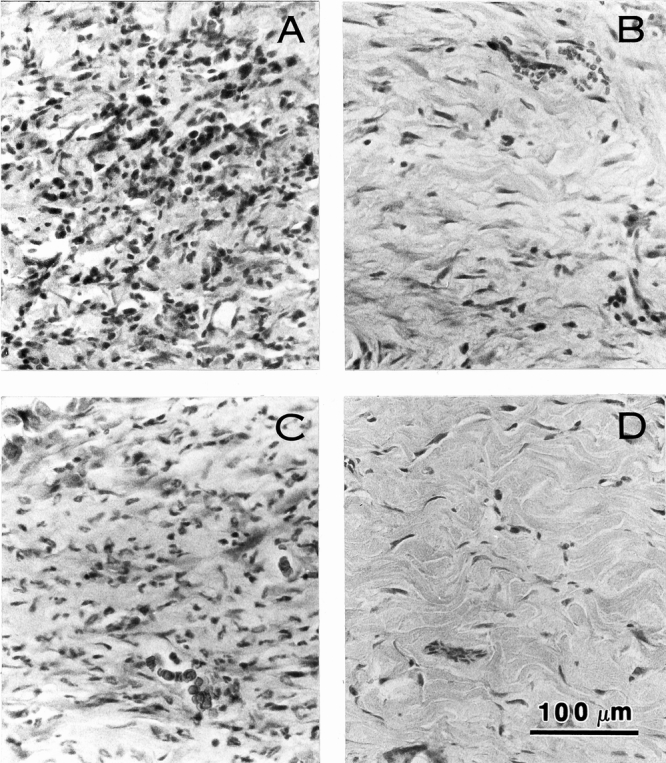
Figure 7. Microscopic appearance of acellular dermal matrix (ADM) with onlay split-thickness skin grafts (STSG) in full-thickness wounds in rat on postoperative day 20. Extensive inflammatory infiltrate is seen in the sample from the wound treated with xenogenic ADM with STSG (A) and limited inflammation is seen in samples from wounds implanted with thin STSG (B) or allogenic ADM and thin STSG (C). No inflammation or tissue disruption is seen in the thick STSG (D) group. Hematoxylin and eosin stain. Magnification bar = 100 μm.
Table 1. INFLAMMATORY REACTION IN WOUND BIOPSY SAMPLES
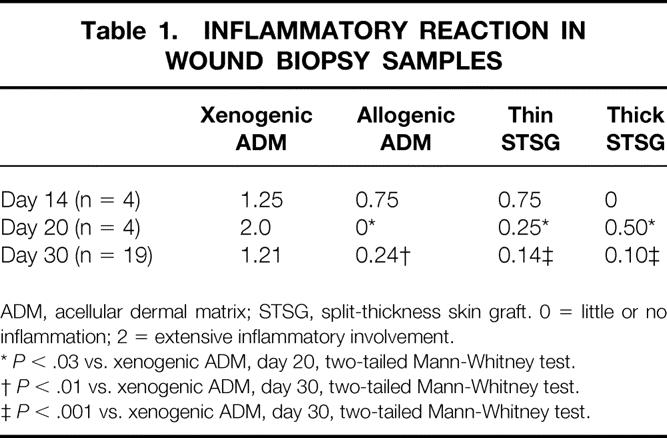
ADM, acellular dermal matrix; STSG, split-thickness skin graft. 0 = little or no inflammation; 2 = extensive inflammatory involvement.
*P < .03 vs. xenogenic ADM, day 20, two-tailed Mann-Whitney test.
†P < .01 vs. xenogenic ADM, day 30, two-tailed Mann-Whitney test.
‡P < .001 vs. xenogenic ADM, day 30, two-tailed Mann-Whitney test.
DISCUSSION
In this study, xenogenic ADM was prepared from cryopreserved, meshed porcine skin and allogenic ADM from fresh rat skin using Dispase and Triton X-100. 27,30 Two antimicrobial agents (azide or antibiotics) were used during ADM preparation and were found to give similar results with regard to wound contraction and graft survival. The effect of ADM orientation on wound healing was found to be inconsequential. In general, little wound contraction was seen on day 10 but significant contraction was seen on days 14, 20, and 30 in wounds receiving xenogenic ADM, allogenic ADM, and thin (0.005-inch-thick) STSGs. Contraction of wounds containing xenogenic ADM was significantly greater than that of wounds containing allogenic ADM at day 30 (49% and 78% of initial wound size, respectively). Graft take was poor in wounds containing xenogenic ADM on day 14 (52% of wound area) and moderately good in those containing allogenic ADM (82% of wound area). Wound healing by secondary intention was seen in both ADM groups at 20 and 30 days after surgery, but the wounds containing xenogenic ADM never healed as completely as those containing allogenic ADM (83% and 95% of wound area epithelialized, respectively, on day 30). As a result, the normalized take for xenogenic ADM was 38% to 42% from day 14 through 30; for allogenic ADM, it was 70% to 75% during this period. Wounds implanted with xenogenic ADM showed significant inflammatory involvement, occasional foreign body giant cells, and swelling and disorganization of collagen bundles in all biopsy samples, whereas those containing allogenic ADM showed mild inflammation on day 14 and little or no inflammation in late biopsy samples.
Several previous studies have suggested that the use of xenogenic material in skin grafts may be feasible. Livesey et al 28 extracted most or all cellular material from porcine dermis and implanted a small piece (5 mm in diameter) of the resulting ADM subcutaneously in rats. After 3 weeks, they found no evidence of local or systemic changes based on necropsy, histologic examination, and cardiac and liver enzyme levels, but they did observe a fibrous capsule sequestering the implanted xenogenic ADM. Boyce et al 19 grew fibroblasts and keratinocytes in and on bovine collagen-glycosaminoglycan gels and grafted this material onto skin wounds in patients. They detected no antibodies to bovine collagen or glycosaminoglycans in patient sera obtained 4 weeks after grafting, and they reported good graft survival. Similarly, others 17,18 have used allogenic human keratinocytes and fibroblasts in conjunction with a bovine collagen gel and found no increase in antibovine collagen or antibovine serum antibodies or evidence of rejection in patients at 28 days after grafting.
In contrast, the results of other studies show that difficulties may be encountered when performing xenograft skin implantation. In rats, Fang et al 38 applied immediate overlay, unmeshed isografts onto either xenodermis (cryopreserved, meshed human skin) or allodermis (fresh rat skin) that had been deepithelialized using Dispase. They found significantly greater wound contraction in the xenograft and allograft dermis groups than in wounds receiving isografts only. In our study, wounds containing xenograft ADM contracted extensively, but we observed reduced contraction in wounds implanted with allogenic ADM plus thin STSG compared with those implanted with thin STSG alone. This improvement may be attributable to the more thorough removal of cellular matter from dermal matrix prepared using Dispase followed by detergent. 27 The resulting ADM is likely to be much less antigenic than skin that has been deepidermized using Dispase only, which still contains cells associated with hair follicles, glands, and blood vessels. As a result, inflammation and related tissue damage may account for the increased wound contraction seen by Fang et al 38 for wounds implanted with allogenic dermis.
Wang et al 39 also reported the use of immediate split-thickness isografts in conjunction with trypsin-treated xenogenic (porcine) and autologous reticular dermal matrix in rats. Wounds from both dermis groups contracted to approximately 75% to 80% of their original areas, but wounds implanted with STSG alone contracted significantly more during the 6-week study period. This difference is understandable in view of the great (6:1) expansion ratio used for the STSGs, which would result in extensive contraction of the sparsely covered STSG wounds. This STSG expansion, together with the 1.5:1 expansion of the dermal matrices used in that study, may have combined to obscure the differences between allogenic and xenogenic ADMs observed in our study, where wounds were implanted with unexpanded ADMs and were covered with unmeshed STSG. Wang et al 39 did report that the autologous dermis group showed chronic inflammation, whereas the xenodermis group showed acute inflammation with significant disruption of the dermal collagen meshwork. These histologic findings are similar to those reported here for xenogenic ADM, showing that an intense local inflammatory response is induced by xenogenic dermal matrix. Further, the results of our early rat studies 40 showed that the use of xenogenic porcine ADM results in inferior STSG graft survival and significantly increased wound contraction.
The cause of the inflammatory reactivity in the xenograft ADM is of considerable interest. We showed previously that HLA type I and II antigens, cell-associated antigens (talin, vimentin, desmin) and type VII collagen are completely extracted from human skin when it is processed to make ADM by the method used here. 30 This, together with thorough histologic examination of such ADMs, 27 shows that the processing steps used to prepare ADM have removed all cells and cellular debris. Thus, it seems unlikely that cellular antigens are the source of antigenicity in xenograft ADM. However, our previous study 30 also showed that type IV collagen, laminin, some fibronectin, and acid glycosaminoglycans (such as hyaluronic acid) remain in ADM, albeit in reduced amounts compared with normal skin. We are performing immunostaining studies using antiporcine ADM antibodies to determine the source of antigenicity in porcine ADM.
Several potential problems must be considered when using xenogenic transplants. First, certain similarities 41,42 and differences exist among rat, porcine, and human skin with respect to epidermal and dermal structure, 33,34,37,43 but the differences are probably minimized once the epidermis and dermal cells are removed to produce ADM. Second, there is a potential for cross-species transmission of pathogens, especially viruses, when using xenografts. 44 Although the possibility of such transmission cannot be excluded, the methods used to prepare ADM may inactivate viruses. Finally, although hyperacute graft rejection is not a great risk in skin grafting, grafts are susceptible to cell-mediated and late antibody-mediated rejection after revascularization. 45 This susceptibility is thought to be related to the presence of xenogenic endothelial cells, other cell types, or extracellular matrix material present in the graft. With ADM, the last of these three possibilities is the only concern because all cellular material has been extracted during ADM preparation. Nonetheless, we found that the use of xenogenic ADM resulted in inferior STSG graft survival and significantly increased wound contraction during the 4-week postsurgical period studied here. This, together with our preliminary data (not shown) demonstrating that a strong humoral immune response against porcine ADM occurs in this porcine-rat model, suggests that the porcine extracellular matrix is antigenic. Whether similar results would be found if porcine ADM were to be implanted in humans is not known, but our results show that the use of xenogenic ADM as a permanent dermal substitute in humans may confer suboptimal results.
Footnotes
Correspondence: Robert J. Walter, PhD, Department of Surgery, Hektoen Institute for Medical Research, Cook County Hospital, 627 S. Wood St., Chicago, IL 60612.
E-mail: rwalter@rush.edu
Accepted for publication August 7, 2000.
References
- 1.Elliot RA, Hoehn JG. Use of commercial porcine skin for wound dressing. Plast Reconstr Surg 1973; 52: 401–405. [PubMed] [Google Scholar]
- 2.Kreis RW, Vloemans AFPM, Hoekstra MJ. The use of non-viable glycerol-preserved cadaver skin combined with widely expanded autografts in the treatment of extensive third-degree burns. J Trauma 1989; 29: 51–54. [DOI] [PubMed] [Google Scholar]
- 3.Nanchahal J, Ward CM. New grafts for old? A review of alternatives to autologous skin. Br J Plast Surg 1992; 45: 354–363. [DOI] [PubMed] [Google Scholar]
- 4.Cohen IK, Diegelmann RF, Lindblad WJ. Wound healing: biochemical and clinical aspects. Philadelphia: W.B. Saunders; 1992.
- 5.Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts as skin replacements after burn injury. Lancet 1986; 1 (8490):1123–1124. [DOI] [PubMed] [Google Scholar]
- 6.Gallico GG, O’Connor NE, Compton CC, et al. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med 1984; 311: 448–451. [DOI] [PubMed] [Google Scholar]
- 7.Munster AM. Cultured skin for massive burns. A prospective, controlled trial. Ann Surg 1996; 224: 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci USA 1979; 76: 5665–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansbrough JF, Dore C, Hansbrough SWB. Clinical trials of a living dermal tissue replacement placed beneath meshed, split-thickness grafts on excised burn wounds. J Burn Care Rehab 1992; 13: 519–529. [DOI] [PubMed] [Google Scholar]
- 10.Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns. Ann Surg 1988; 208: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroyanagi Y, Kenmochi M, Ishihara S, et al. A cultured skin substitute composed of fibroblasts and keratinocytes with a collagen matrix: Preliminary results of clinical trials. Ann Plast Surg 1993; 31: 340–351. [DOI] [PubMed] [Google Scholar]
- 12.Grillo HC, McKhann CF. The acceptance and evolution of dermal homografts freed of viable cells. Transplantation 1964; 2: 48–59. [DOI] [PubMed] [Google Scholar]
- 13.Grinnell F, Toda K, Lamke-Seymour C. Reconstruction of human epidermis in vitro is accompanied by transient activation of basal keratinocyte spreading. Exp Cell Res 1987; 172: 439–449. [DOI] [PubMed] [Google Scholar]
- 14.De Vries HJC, Middelkoop E, Mekkes JR, et al. Dermal regeneration in native non-cross-linked collagen sponges with different matrix molecules. Wound Repair Regen 1994; 2: 37–47. [DOI] [PubMed] [Google Scholar]
- 15.Burke JF, Yannas IV, Quinby WC, et al. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg 1981; 194: 413–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell E, Sher S, Jull B, et al. The reconstitution of living skin. J Invest Dermatol 1983; 81: S2–10. [DOI] [PubMed] [Google Scholar]
- 17.Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (Graftskin) for surgical wounds. A clinical experience. Dermatol Surg 1995; 21: 839–843. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins LM, Watson SR, Prosky SJ, et al. Development of a bilayered living skin construct for clinical applications. Biotech Bioengin 1994; 43: 747–756. [DOI] [PubMed] [Google Scholar]
- 19.Boyce ST, Goretsky MJ, Greenhalgh DG, et al. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann Surg 1995; 222: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke KE, Naughton G, Waldo E, et al. Bovine collagen implant: histologic chronology in pig dermis. J Dermatol Surg Oncol 1983; 9: 889–895. [DOI] [PubMed] [Google Scholar]
- 21.Compton CC, Butler CE, Yannas IV, et al. Organized skin structure is regenerated in vivo from collagen-GAG matrices seeded with autologous keratinocytes. J Invest Dermatol 1998; 110: 908–916. [DOI] [PubMed] [Google Scholar]
- 22.Orgill DP, Butler C, Regan JF, et al. Vascularized collagen-glycosaminoglycan matrix provides a dermal substrate and improves take of cultured epithelial autografts. Plast Reconstr Surg 1998; 102: 423–429. [DOI] [PubMed] [Google Scholar]
- 23.Murphy GF, Orgill DP, Yannas IV. Partial dermal regeneration is induced by biodegradable collagen-glycosaminoglycan grafts. Lab Invest 1990; 62: 305–313. [PubMed] [Google Scholar]
- 24.Yannas IV, Lee E, Orgill DP, et al. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci USA 1989; 86: 933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prunieras M. To reconstitute skin: theme and variations. Matrix 1991; 11: 302–305. [DOI] [PubMed] [Google Scholar]
- 26.Prunieras M. The reconstruction of skin: how and why. Intl J Dermatol 1990; 29: 553–556. [DOI] [PubMed] [Google Scholar]
- 27.Takami Y, Matsuda T, Yoshitake M, et al. Dispase/detergent-treated dermal matrix as a dermal substitute. Burns 1996; 22: 182–190. [DOI] [PubMed] [Google Scholar]
- 28.Livesey SA, Herndon DN, Hollyoak MA, et al. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation 1995; 60: 1–9. [PubMed] [Google Scholar]
- 29.Walter RJ, Jennings LJ, Matsuda T, et al. Dispase/Triton-treated acellular dermal matrix as a dermal substitute in rats. Curr Surg 1997; 54: 371–374. [Google Scholar]
- 30.Walter RJ, Matsuda T, Reyes HM, et al. Characterization of acellular dermal matrices (ADMs) prepared by two different methods. Burns 1998; 24: 104–113. [DOI] [PubMed] [Google Scholar]
- 31.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995; 21: 243–248. [DOI] [PubMed] [Google Scholar]
- 32.Wainwright D, Madden M, Luterman A, et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehab 1996; 17: 124–136. [DOI] [PubMed] [Google Scholar]
- 33.Wollina U, Berger U, Mahrle G. Immunohistochemistry of porcine skin. Acta Histochem 1991; 90: 87–91. [DOI] [PubMed] [Google Scholar]
- 34.Lavker RM, Dong G, Zheng P, et al. Hairless micropig skin: a novel model for studies of cutaneous biology. Am J Pathol 1991; 138: 687–697. [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki GH, Pang CY. Hemodynamics and viability of acute neurovascular island skin flaps in rats. Plast Reconstr Surg 1980; 65: 152–158. [DOI] [PubMed] [Google Scholar]
- 36.Jennings LJ, DeSagun E, Hanumadass M, et al. Growth of human keratinocytes on acellular dermal matrix in vitro. J Burn Care Rehabil 1998; 19: S171. [DOI] [PubMed] [Google Scholar]
- 37.Vardaxis NJ, Brans TA, Boon ME, et al. Confocal laser scanning microscopy of porcine skin: implications for human wound healing studies. J Anat 1997; 190: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang CH, Robb EC, Yu GS, et al. Observations on stability and contraction of composite skin grafts: xenodermis or allodermis with an isograft onlay. J Burn Care Rehab 1990; 11: 538–542. [DOI] [PubMed] [Google Scholar]
- 39.Wang H-J, Chen T-M, Cheng T-Y. Use of a porcine dermis template to enhance widely expanded mesh autologous split-thickness skin graft growth: preliminary report. J Trauma 1997; 42: 177–182. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava A, Jennings LJ, Hanumadass M, et al. Xenogenic acellular dermal matrix as a dermal substitute in rats. J Burn Care Rehab 1999; 20: 382–390. [DOI] [PubMed] [Google Scholar]
- 41.Heinrich W, Lange PM, Stirz T, et al. Isolation and characterization of the large cyanogen bromide peptides from the α1- and α2-chains of pig skin collagen. FEBS Lett 1971; 16: 63–67. [DOI] [PubMed] [Google Scholar]
- 42.Struck H. Immunological investigations of antigenicity and specificity of soluble collagen fractions. IV. Anaphylaxis and allergy experiments. Eur J Surg Res 1976; 8: 243–240. [DOI] [PubMed] [Google Scholar]
- 43.Meyer W, Schwarz R, Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Prob Dermatol 1978; 7: 39–52. [DOI] [PubMed] [Google Scholar]
- 44.Sikorski R, Peters R. Xenotransplanters turn xenovirologists. Science 1997; 276: 1893–1893. [PubMed] [Google Scholar]
- 45.Auchincloss H Jr. Xenogeneic transplantation. Transplantation 1988; 46: 1–20. [DOI] [PubMed] [Google Scholar]


