Abstract
Objective
To determine the optimal method of wound closure for dirty abdominal wounds.
Summary Background Data
The rate of wound infection for dirty abdominal wounds is approximately 40%, but the optimal method of wound closure remains controversial. Three randomized studies comparing delayed primary closure (DPC) with primary closure (PC) have not conclusively shown any advantage of one method over the other in terms of wound infection.
Methods
Fifty-one patients with dirty abdominal wounds related to perforated appendicitis, other perforated viscus, traumatic injuries more than 4 hours old, or intraabdominal abscesses were enrolled. Patients were stratified by cause (appendicitis vs. all other causes) and prospectively randomized to one of two wound management strategies: E/DPC (wound packed with saline-soaked gauze, evaluated 3 days after surgery for closure the next day if appropriate) or PC. In the E/DPC group, wounds that were not pristine when examined on postoperative day 3 were not closed and daily dressing changes were instituted. Wounds were considered infected if purulence discharged from the wound, or possibly infected if signs of inflammation or a serous discharge developed.
Results
Two patients were withdrawn because they died less than 72 hours after surgery. The wound infection rate was greater in the PC group than in the E/DPC group. Lengths of hospital stay and hospital charges were similar between the two groups.
Conclusion
A strategy of DPC for appropriate dirty abdominal wounds 4 days after surgery produced a decreased wound infection rate compared with PC without increasing the length of stay or cost.
Dirty surgical wounds are associated with a high rate of wound infection. 1 Postoperative wound infections have a significant impact on health resources and costs, 2,3 and the sequelae of wound infections (wound dehiscence and resulting incisional hernias) can result in significant long-term problems. 4–6 Of the many risk factors influencing postoperative wound infections, the method of skin closure has been implicated as an important factor. Delayed primary closure (DPC) and primary closure (PC) are two commonly used methods, but there is no consensus as to the optimal method. Cruse and Foord 1 found in a retrospective survey a wound infection rate of 40% among 2,093 dirty wounds, but they did not specify how skin closure was performed. Three prospective randomized studies 7–9 performed on appendectomy wounds only showed no advantage to DPC in terms of decreased wound infection compared with PC. We conducted a prospective randomized trial on patients with dirty abdominal wounds and hypothesized that a strategy of DPC of appropriate dirty abdominal wounds would result in a decreased rate of wound infection.
METHODS
Patient Population
A total of 51 patients were enrolled between January and July 1999 at the University of Miami/Jackson Memorial Hospital and the Ryder Trauma Center. The University of Miami Institutional Review Board approved the experimental protocol. All the patients were admitted to the trauma/emergency surgery or colorectal services, were 18 years of age or older, and were found to have dirty abdominal wounds at the time of surgery. Dirty abdominal wounds were defined as those that involved preexisting clinical infection, perforated viscera, or traumatic wounds with viscus injury more than 4 hours from the time of injury with retained devitalized tissue, in accordance with the Centers for Disease Control criteria. 10
Patients were first stratified into those with appendectomy wounds and those with all other abdominal wounds, and then were randomized to receive one of two strategies for wound management: PC or evaluation of delayed primary closure for appropriate wounds (E/DPC). For PC, wounds were closed with skin staples and subcutaneous tissues were not approximated. For E/DPC, wounds were packed with saline-soaked gauze and were not manipulated until postoperative day 3, at which time the dressing was changed using sterile technique and the wound was evaluated for closure. If the wound appeared pristine (showed no drainage), it was approximated the next day with adhesive strips. Otherwise, it was left open and dressing changes were instituted twice a day. If a wound infection was suspected based on the appearance or odor of the wound or systemic signs (fever, tachycardia) before postoperative day 3, the dressing was removed and the wound was inspected. Using sterile technique, the wound was repacked. Subsequently, the wound was inspected on postoperative day 3 or earlier if necessary.
Surveillance of Wound Infection
One of two study coordinators (G.G. or A.W.O.) observed all study patients daily until discharge and subsequently in the clinic at least 1 week and 1 month after discharge. A wound infection occurred when purulent drainage was observed. Both superficial incisional and deep incisional surgical site infections, as defined by Centers for Disease Control criteria, 10 were included as wound infections. A possible wound infection was noted when signs of inflammation or a serous discharge developed, as defined by the 1964 National Research Council study on wound infections. 11 All wound infections and possible wound infections in both groups were evaluated by one of three surgical attendings (S.M.C., N.N., or M.G.M.). Infected wounds were opened and packed. Possibly infected wounds were observed closely and opened if purulent drainage, increasing erythema, induration, or warmth developed.
Demographics
We tabulated underlying medical conditions (host susceptibility factors) that could contribute to infectious complications: diabetes mellitus, obesity (body mass index > 30 kg/m2), malnutrition (clinical observation of muscle wasting, prealbumin level < 20 mg/dL, albumin level < 2.5 g/dL), steroid use, cardiovascular disease (symptoms, signs, history, or diagnostic tests that revealed significant disease of the cardiovascular system), malignancy, preoperative immunocompromised state (e.g., acquired immunodeficiency syndrome), and prolonged preoperative hospital stay (≥4 days). 12
Statistical Analysis
Assuming an alpha error of 0.05 and a beta error of 0.20, a study population of 80 patients per group was required for this study, based on assumed wound infection rates of 30% for the PC group and 10% for the DPC groups. The chi-square and Fisher exact tests were used to determine whether any association between the presence of wound infection and the type of skin closure existed. Mean comparisons were performed by the two-sample Student t test. The null hypothesis was rejected when P < .05. An interim analysis after enrollment of 50 patients was planned.
RESULTS
A total of 51 patients were randomized. Two in the PC group were withdrawn because of early deaths (<72 hours after surgery), leaving 49 patients for the final analysis. All 49 patients received the allocated interventions. Both groups of patients were similar in terms of age, cause of dirty wound, and risk factors. The proportion of patients with one or more risk factors was similar (DPC 58% vs. PC 48%, P = .41 by z test). There were significantly more men in the PC group (P < .025, chi-square;Table 1).
Table 1. PATIENT DEMOGRAPHICS
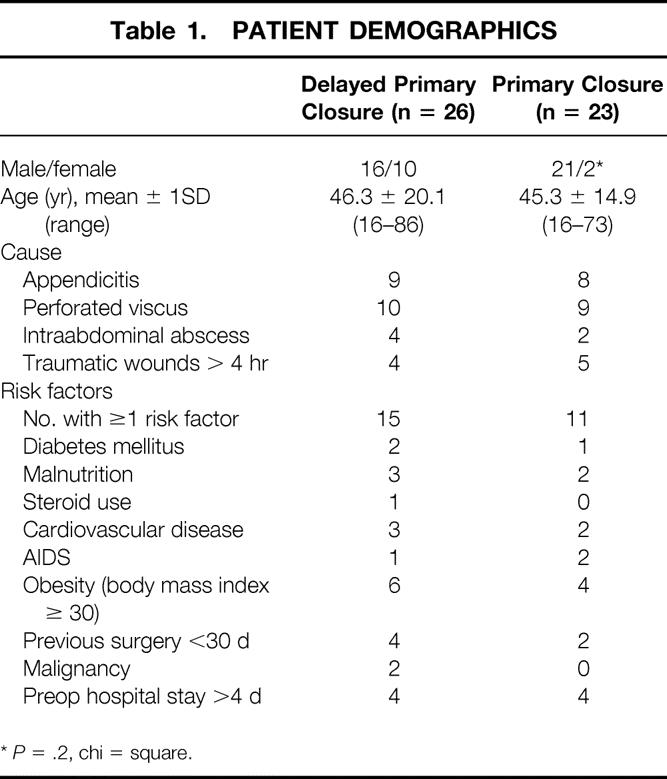
*P = .2, chi = square.
In the E/DPC group, 14 patients underwent DPC, with one wound infection and two possible wound infections. One wound infection occurred in a patient with a late pericolostomy cellulitis that tracked across to the midline wound. The wound was reopened on postoperative day 25. One of the two possible wound infections occurred after an open cholecystectomy where the gallbladder was perforated and there was free pus in the abdominal cavity. The wound was reopened on postoperative day 6 when a significant amount of serosanguineous drainage but no erythema or induration was noted. The other possible wound infection occurred after exploratory laparotomy for peritonitis resulting from a dislodged gastrostomy tube, after which the wound also developed serosanguineous drainage without erythema or induration. This wound was reopened on postoperative day 12. Twelve wounds (46%) were judged to be unsuitable for closure when inspected 72 hours after surgery as a result of excessive drainage (without evidence of infection), and dressing changes were initiated per protocol. All the other wounds were observed for at least 1 month after DPC, and none had to be reopened. Thus, the overall wound infection rate for E/DPC was 3/26 (12%) (Table 2).
Table 2. OUTCOME OF ALL DIRTY WOUNDS
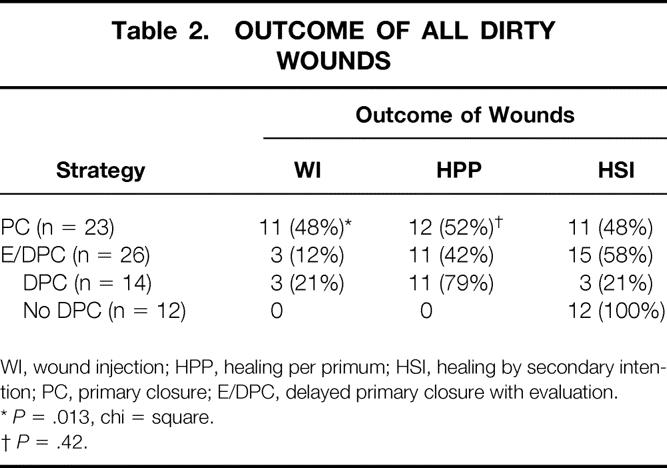
WI, wound injection; HPP, healing per primum; HSI, healing by secondary intention; PC, primary closure; E/DPC, delayed primary closure with evaluation.
*P = .013, chi = square.
†P = .42.
In the PC group similarly followed up for at least 1 month after surgery, there were 11 wound infections (48%). There was a significant association between wound infection and the type of skin closure (E/DPC 3/26 vs. PC 11/23, P = .013, chi-square).
Analyzing only appendectomy wounds (Table 3), five (55%) of the nine wounds in the E/DPC group were not closed on postoperative day 3. The overall wound infection rate was 0%. In contrast, four (50%) of the eight wounds in the PC group developed infections. A similar association was found between wound infection and type of closure (DPC 0/9 vs. PC 4/8, P = .03, Fisher exact test).
Table 3. OUTCOME OF APPENDECTOMY WOUNDS
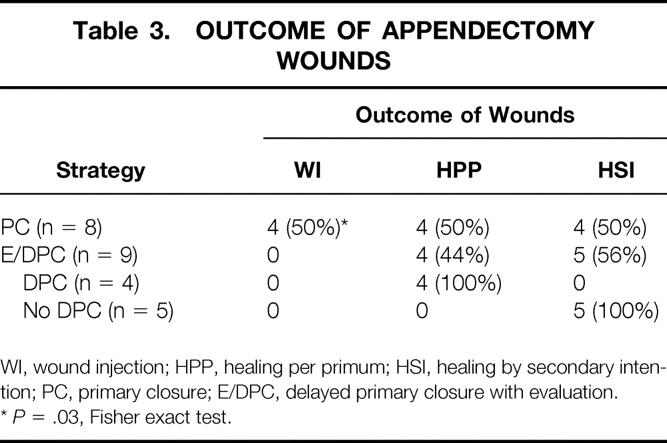
WI, wound injection; HPP, healing per primum; HSI, healing by secondary intention; PC, primary closure; E/DPC, delayed primary closure with evaluation.
*P = .03, Fisher exact test.
Analyzing patients who did not require intensive care unit care after surgery (n = 31), the length of hospital stay was not significantly different between the two groups (E/DPC 7.1 ± 3.5 days vs. PC 5.3 ± 1.4 days). Hospital charges were likewise similar (E/DPC $22,258 [range $10,001–$47,927] vs. PC $26,352 [range $5,127–$45,822]). One patient in the PC group who stayed 38 days because of social reasons was not included in the analysis.
For patients requiring postoperative intensive care unit care (n = 18), hospital charges were also not significantly different (E/DPC $227,237 [range $11,281–$1,477,043] vs. PC, $78,101 [range $31,497–$250,078]). When patients with wound infections were compared with those without wound infections or with wounds left open, there was also no significant difference in hospital charges (wound infections $55,735 [range $5,127–$250,077] vs. no wound infection or left open, $54,524 [range $10,001– $ 437,063]). There were six (12%) deaths in the study, all occurring in patients who needed intensive care unit treatment.
Results of cultures were not available for five wound infections (three in the E/DPC group, two in the PC group) because they were misplaced. In the other nine infected wounds in the PC group, there were seven gram-negative aerobic isolates (Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumanii, Klebsiella oxytoca), one anaerobic isolate (Bacteroides fragilis), eight gram-positive cocci isolates (Staphylococcus aureus, coagulase-negative Staphylococcus, Enterococcus faecalis, Enterococcus gallinarum, Streptococcus viridans, Streptococcus agalactiae), and one fungal isolate (Candida albicans).
DISCUSSION
The financial impact and complications of wound infection and its sequelae are significant. Davey and Nathwani 2 found excesses in hospital costs per wound infection of $600 for an inguinal hernia repair and $2,152 for colorectal surgery. Other authors have reported increased costs associated with the hospital stay. 13,14 Riou et al 6 reported a wound infection rate of 45% in 31 patients with fascial dehiscences among 2,761 patients undergoing major abdominal surgery, compared with 2% in a control group. The incidence of incisional hernia after repair of wound dehiscences is as high as 45%. 4 Bucknall et al 15 found a 1.7% incidence of burst abdomen and a 7.4% incidence of incisional hernia among 1,129 major laparotomies where wound infection was a significant contributing factor. Irvin et al 16 found that dehiscence and herniation occurred in 2.5% of 163 noninfected wounds and 25% of 28 infected wounds. Haddad and Macon 5 found a 31% wound infection rate among 70 wounds with dehiscence among 18,120 major abdominal surgical procedures. Necrotizing fasciitis remains a rare but potentially lethal complication of surgical wound infections. The increased incidence of significant complications associated with wound infection supports the notion that it is prudent to avoid wound infection whenever possible.
In the modern era, DPC of dirty wounds was popularized in World War I as described by Hepburn in 1919. 17 Its use in peacetime was described by Wilke 18 in 1931 and by Coller and Valk 19 in 1940. This technique became the standard of care in World War II. Wilson 20 reported a series of 305 war wounds closed 4 to 47 days after débridement and found a wound infection rate of 15% when the wounds were closed within 10 days versus approximately 49% when the wounds were closed more than 10 days after surgery. Grosfeld and Solit 21 in 1968 reviewed perforated appendiceal wounds and found a wound infection rate of 2.3% for delayed closure compared to 14.6% with PC. These studies were performed, however, before current antimicrobial regimens were available. More recently, Lemieur et al 22 found a wound infection rate in perforated appendicitis of 24% when the incision was closed primarily, and Yellin et al 23 found a wound infection rate of approximately 4% after DPC of all their advanced appendicitis wounds.
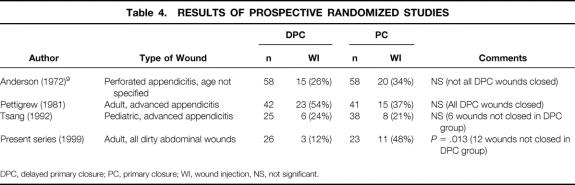
Table 4 summarizes the results of the previous three prospective randomized trials comparing DPC with PC in the literature. Tsang et al 7 studied 63 children with gangrenous or perforated appendicitis and found no difference in the rate of wound infection between the two groups. Six (24%) wounds were not closed in the DPC group because of significant exudate. Pettigrew 8 and Andersen et al 9 both randomized more than 100 patients each with gangrenous or perforated appendicitis to DPC versus PC. These authors used topical antibiotics in one or more randomized arms, and even though they found benefit in the use of these antibiotics, this may have constituted a significant confounding variable. Further, these two studies were at least 20 years old, highlighting the need to address this issue with a more recent trial.
Table 4. RESULTS OF PROSPECTIVE RANDOMIZED STUDIES
DPC, delayed primary closure; PC, primary closure; WI, wound injection, NS, not significant.
Our study is the first to randomize abdominal laparotomy wounds with appendiceal wounds constituting a minority of the subject population. Closing selected dirty wounds 4 days after surgery resulted in a significantly lower rate of infection for all wounds. Although the outcome of the two groups appeared to be similar (approximately half the patients in each group required twice-daily dressing changes at the time of discharge), we did not consider the inability to close wounds on postoperative day 4 in the DPC group to be a failure in treatment; rather, we believed that prevention of a wound infection and its sequelae was a greater benefit to the patient. Our results also showed that it was possible to determine clinically which wounds could be closed in delayed fashion with a reasonable success rate (79%).
In terms of length of stay and hospital charges, we found no difference between the two groups even when patients were stratified by the presence or absence of intensive care unit stay, showing that other variables were important in determining the hospital course. One limitation of the study was the difficulty in analyzing the use of antibiotics, because antibiotics were used not only for serious underlying intraabdominal infections, where the duration and type of antibiotics in part depended on the clinical response in each patient, but also for a variety of concomitant indications (e.g., pneumonia, line sepsis). Therefore, it was not ethical to restrict the use of antibiotics in accordance with a specified protocol.
Several other limitations of the study existed. Because of the study design, the investigators evaluating the wounds could not be masked, and this may have introduced a potential bias depending on each investigator’s preference for skin closure. The sample size may have been too small to detect any difference in length of stay or hospital charges. However, it is more likely that other factors played a more important role in determining the hospital stay than the type of skin closure. Also, long-term follow-up of wounds (>1 month) was not done routinely because this was difficult in our largely indigent patient population.
In conclusion, a strategy of DPC of dirty abdominal wounds when clinically appropriate appears to decrease the rate of wound infection when compared with PC without increasing hospital charges or the length of stay.
Footnotes
Correspondence: Stephen M. Cohn, MD, FACS, Divisions of Trauma and Surgical Critical Care, University of Miami School of Medicine, Miami, FL 33136.
E-mail: stephen.cohn@miami.edu
Accepted for publication August 4, 2000.
References
- 1.Cruse PJE, Foord R. The epidemiology of wound infection. Surg Clin North Am 1980; 60: 27–39. [DOI] [PubMed] [Google Scholar]
- 2.Davey PG, Nathwani D. What is the value of preventing postoperative infections? New Horizons 1998; 6: S64–71. [PubMed] [Google Scholar]
- 3.Renvall S, Niinikoski J, Aho AJ. Wound infections in abdominal surgery. Acta Chir Scand 1980; 146: 25–30. [PubMed] [Google Scholar]
- 4.Grace RH, Cox SJ. Incidence of incisional hernia following dehiscence of the abdominal wound. Proc R Soc Med 1973; 66: 1091–1092. [PMC free article] [PubMed] [Google Scholar]
- 5.Haddad V, Macon WL. Abdominal wound dehiscence and evisceration: contributing factors and improved mortality. Am Surg 1980; 46 (9): 508–513 [PubMed] [Google Scholar]
- 6.Riou J-PA, Cohen JR, Johnson H. Factors influencing wound dehiscence. Am J Surg 1992; 163: 324–330. [DOI] [PubMed] [Google Scholar]
- 7.Tsang TM, Tam PKH, Saing H. Delayed primary wound closure using skin tapes for advanced appendicitis in children. Arch Surg 1992; 127: 451–453. [DOI] [PubMed] [Google Scholar]
- 8.Pettigrew R. Delayed primary wound closure in gangrenous and perforated appendicitis. Br J Surg 1981; 68: 635–638. [DOI] [PubMed] [Google Scholar]
- 9.Andersen B, Bendtsen A, Holbraad L, et al. Wound infections after appendectomy. Acta Chir Scand 1972; 138: 531–536. [PubMed] [Google Scholar]
- 10.Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection. Infect Control Hosp Epidemiol 1999; 20: 247–280. [DOI] [PubMed] [Google Scholar]
- 11.Committee on Trauma, Division of Medical Sciences, National Academy of Sciences–National Research Council. Postoperative wound infections: the influence of ultraviolet irradiation of the operating room and of various factors. Ann Surg 1964; 160(suppl 1):1–192. [PubMed]
- 12.Garibaldi RA, Cushing D, Lerer T. Risk factors for postoperative infection. Am J Med 1991; 91 (suppl 3B): S158–163. [DOI] [PubMed] [Google Scholar]
- 13.Shulkin DJ, Kinosian B, Glick H, et al. The economic impact of infections. Arch Surg 1993; 128: 449–452. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen KB, Bremmelgaard A, Sorensen AI, et al. Estimated costs of postoperative wound infections. Epidemiol Infect 1994; 113: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bucknall TE, Cox PJ, Ellis H. Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. Br Med J 1982; 284: 931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irvin TT, Stoddard CJ, Greaney MG, et al. Abdominal wound healing: a prospective clinical study. Br Med J 1977; 2: 351–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hepburn HH. Delayed primary suture of wounds. Br Med J 1919; 1: 181–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilke DPD. Observations on mortality in acute appendicular disease. Br Med J 1931; 1: 253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coller FA, Valk WL. The delayed primary closure of contaminated wounds. Ann Surg 1940; 112: 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson H. Secondary suture of war wounds. Ann Surg 1945; 121: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosfeld JL, Solit RW. Prevention of wound infection in perforated appendicitis. Ann Surg 1968; 168: 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemieur TP, Rodriguez JL, Jacobs DM, et al. Wound managent in perforated appendicitis. Am Surg 1999; 65: 439–443. [PubMed] [Google Scholar]
- 23.Yellin AE, Berne TV, Heseltine PN, et al. Prospective randomized study of two different doses of clindamycin admixed with gentamicin in the management of perforated appendicitis. Am Surg 1993; 4: 248–255. [PubMed] [Google Scholar]