Abstract
Objective
To assess the extent of disease in patients with pT1 esophageal adenocarcinoma and to evaluate the feasibility and outcomes of a limited surgical approach.
Summary Background Data
Radical esophagectomy with systematic lymphadenectomy is widely advocated as the treatment of choice in patients with early adenocarcinoma of the distal esophagus. This approach, however, is associated with substantial complications and long-term side effects. The extent of resection necessary to achieve cure in such patients is not clear.
Methods
Seventy-one patients with pT1 adenocarcinoma of the distal esophagus underwent transmediastinal or transthoracic esophagectomy with two-field lymphadenectomy. Twenty-four patients with uT1N0 tumors underwent a limited resection of the distal esophagus and esophagogastric junction, regional lymphadenectomy, and reconstruction by interposition of an isoperistaltic pedicled jejunal segment. The two groups were compared for extent and multicentricity of the primary tumor and associated high-grade dysplasia, pattern of lymph node metastases, complications, deaths, and outcome of surgical treatment.
Results
Multicentric tumor growth or associated high-grade dysplasia was observed in 60.6% of the resection specimens. Complete resection of the tumor and the entire segment with intestinal metaplasia was achieved in all patients, irrespective of the surgical approach. Patients undergoing limited resection had fewer complications. Lymph node metastases or micrometastases were present in none of the 38 patients with tumors limited to the mucosa (pT1a) versus 10 of the 56 (17.9%) patients with tumors invading the submucosa (pT1b). Distant lymph node metastases occurred only in patients with more than three positive regional lymph nodes. Lymph node metastases were prognostic, but the pT1a/pT1b category and the surgical approach were not. The mean Gastrointestinal Quality of Life Index after limited resection did not differ from that of healthy controls: 20 of the 24 patients were completely asymptomatic.
Conclusions
In patients with early adenocarcinoma in the distal esophagus, resection of the distal esophagus and esophagogastric junction, with regional lymphadenectomy and jejunal interposition, is an attractive limited surgical alternative to radical esophagectomy.
As a result of endoscopic surveillance programs in patients with known Barrett’s esophagus, early esophageal adenocarcinoma is being diagnosed with increasing frequency in the Western world. 1 Based on the observation that the presence of lymphatic spread is the single most important prognostic factor after complete tumor resection in patients with esophageal adenocarcinoma, radical esophagectomy and esophagogastrectomy with systematic lymphadenectomy are widely advocated as treatments of choice in such patients. 2–6 Although long-term survival rates after extended resection and lymphadenectomy are impressive, this approach is associated with substantial complications, deaths, and long-term compromise of quality of life. 2–6,7 The need for extensive resection in patients with early adenocarcinoma of the distal esophagus and lymphadenectomy has therefore been questioned. Recently, photodynamic therapy, 8 laser ablation, 9 and mucosal destruction by argon beam plasma coagulation or electrocautery 10 have been suggested as limited, organ-preserving treatment options for patients with high-grade dysplasia or early adenocarcinoma in Barrett’s esophagus. These techniques, however, are plagued by high recurrence rates, probably as a result of incomplete removal of the tumor and the underlying precancerous lesion (i.e., Barrett’s esophagus). In addition, these techniques do not permit histopathologic assessment of the true extent of the tumor and the presence of lymphatic spread.
To define the extent of resection necessary to achieve cure in patients with early esophageal adenocarcinoma, we evaluated the extent of the primary tumor and the prevalence and pattern of lymphatic spread in a large consecutive series of patients with early esophageal adenocarcinoma treated at a single institution. We assessed the feasibility and outcome of a limited surgical approach in these patients.
PATIENTS AND METHODS
Patient Population
Between July 1982 and December 1999, 368 patients with adenocarcinoma of the distal esophagus (mean age 61.7 years, male:female ratio 8.7:1) underwent surgical resection at the Department of Surgery, Technische Universität Munich. On histopathologic assessment of the resected specimens, there were 94 patients (mean age 60.7 years, male:female ratio 8.9:1) with a pT1 category according to the UICC/AJCC 1997 criteria. 11,12 The tumor was limited to the mucosa (pT1a) in 38 of these patients and to the submucosa (pT1b) in 56 patients.
Before July 1997, the treatment of choice in patients with early adenocarcinoma of the distal esophagus was subtotal esophagectomy with resection of the proximal stomach and en bloc lymphadenectomy of the lower posterior mediastinum and upper abdominal compartment (i.e., a two-field lymphadenectomy). 13 From July 1997 through 1999, a limited resection of the distal esophagus, esophagogastric junction (EGJ), and proximal stomach was performed in 24 patients with histologically proven adenocarcinoma of the distal esophagus, in whom the length of columnar cell metaplasia in the distal esophagus was less than 3 cm. They were staged as uT1N0 mol/L0 by preoperative endoscopy, endoscopic ultrasonography (7.5- and 12-MHz circular scanner in all patients), and computed tomography (CT) of the chest and abdomen. These patients were selected and followed up according to a prospective study protocol. During the same period, 15 patients underwent subtotal esophagectomy with lymphadenectomy for early adenocarcinoma in the distal esophagus staged as uT1 on preoperative endoscopy, endoscopic ultrasonography, and CT scan of the chest and abdomen. Reasons for not performing a limited resection of the distal esophagus, EGJ, and proximal stomach in these patients were long segments (range 3–10 cm) of Barrett’s esophagus that were considered not resectable using only a limited transabdominal approach (n = 13) and previous subtotal gastrectomy (n = 2).
The study population comprised the 71 patients who underwent a subtotal esophagectomy for stage pT1 adenocarcinoma of the distal esophagus between 1982 and 1999 and the 24 patients in the prospective protocol with stage uT1N0 mol/L0 adenocarcinoma who underwent a limited resection of the distal esophagus, EGJ, and proximal stomach. Patients who had received preoperative chemotherapy or radiation and patients with a concomitant cancer were excluded. In 82 patients in the study population, the presence of Barrett’s esophagus was confirmed by histologic demonstration of intestinal metaplasia in the distal esophagus. Four patients had known Barrett’s esophagus, treated with photodynamic therapy (n = 2) or argon plasma ablation (n = 2), and associated with high-grade dysplasia or early carcinoma (n = 3). Three patients with resected pT1 adenocarcinoma of the esophagus had undergone a previous fundoplication (1, 3.5, and 7 years before the diagnosis of adenocarcinoma). In 38 of the 95 patients, the tumor was detected during surveillance endoscopy performed for known Barrett’s esophagus.
Surgical Approach
In 60 patients, radical transmediastinal esophagectomy with resection of the proximal stomach was performed by laparotomy, with wide exposure of the lower posterior mediastinum by anterior splitting of the diaphragmatic hiatus and a left cervical incision. Lymphadenectomy comprised en bloc removal of all lymphatic tissue in the lower posterior mediastinum, along the cardia, the proximal two thirds of the lesser curvature, the fundus, and along the common hepatic and splenic artery toward the celiac axis. 14 In addition, enlarged cervical lymph nodes were removed. In 11 patients, an abdominothoracic en bloc esophagectomy with resection of the proximal stomach was performed. This procedure included an extended en bloc mediastinal lymphadenectomy 15 and abdominal lymphadenectomy, as described above. Reconstruction after subtotal esophagectomy was performed with a narrow gastric tube or colon interposition and a cervical (n = 64) or high intrathoracic (n = 7) anastomosis.
The limited resection (n = 24) was performed through a transabdominal approach with wide anterior splitting of the diaphragmatic hiatus and included a resection of the distal esophagus, EGJ, and proximal stomach (Fig. 1). Lymphadenectomy comprised an en bloc removal of all lymphatic tissue in the lower posterior mediastinum, along the cardia, the proximal two thirds of the lesser curvature, the fundus, and along the common hepatic and splenic artery toward the celiac axis. Preservation of the vagal nerves was attempted whenever possible. Reconstruction was performed by retrocolic and retrogastric interposition of a pedicled isoperistaltic jejunal segment, 10 to 15 cm long (see Fig. 1). The esophagojejunostomy was performed in end-to-side (functional end-to-end) fashion using a circular stapler. The jejunogastrostomy was performed with an end-to-end anastomosis, using an interrupted single-layer suture technique after a mechanical pyloric dilatation.
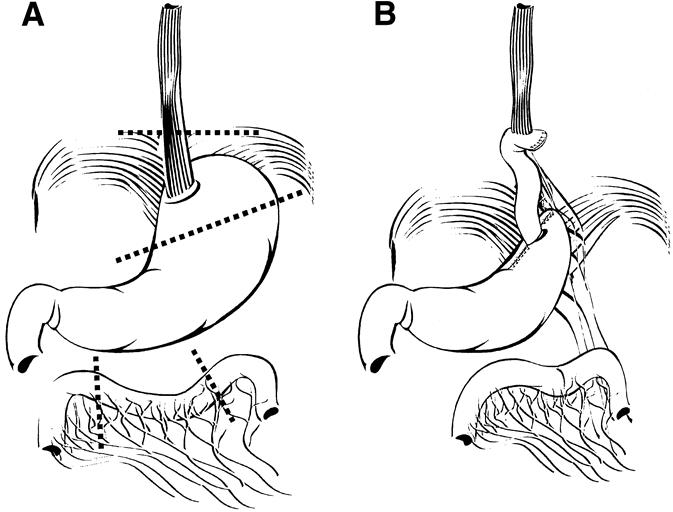
Figure 1. (A) Limited resection of the distal esophagus, esophagogastric junction, and proximal stomach. (B) Reconstruction by interposition of a pedicled isoperistaltic jejunal segment.
Histopathologic Assessment
All resected specimens were assessed by an experienced pathologist according to UICC/AJCC guidelines. 11,12 All pT1 tumors were subclassified as pT1a (limited to the mucosa) or pT1b (submucosa). Care was taken to identify multicentricity of the primary tumor and associated high-grade dysplasia. All removed lymph nodes were counted, assessed separately, and identified according to their location: at the celiac axis, along the left gastric artery, paracardial, paraesophageal distal in the posterior lower mediastinum, paraesophageal proximal in the upper mediastinum, and cervical.
In the 44 esophagectomy specimens classified as pN0 on standard histopathologic assessment that were still available for reanalysis, and in all 24 specimens from the limited resections, all removed lymph nodes were reassessed for the presence of lymph node micrometastases using immunohistochemical techniques. 16 Briefly, all specimens were fixed in formalin and embedded in paraffin. One additional section from each of the removed lymph nodes was stained by immunohistochemistry using AE1/AE3 (20:1 mixture; Boehringer, Mannheim, Germany), a monoclonal antibody cocktail that is reactive with a broad spectrum of human keratins. Because AE1/AE3 positivity has been reported in mesothelial as well as epithelial cells, an additional antibody, Ber-EP4 (immunoglobulin G1; Dako Diagnostika, Hamburg, Germany), which is reactive with epithelial cells but not mesothelial cells, was used in all cases with cytokeratin-positive cells to confirm their epithelial nature. The immunohistochemical reactions were developed with an alkaline-phosphate–antialkaline-phosphatase technique. The immunohistochemical examination was limited to one slide per lymph node for each antibody, to simulate routine histologic procedures. The negative control consisted of sections that were treated with the same protocol, but with the primary antibody omitted. Normal esophageal mucosa and the primary tumors of the specimens were used as positive controls and were consistently positive.
Follow-Up and Functional Evaluation
The survival status of all patients in the study population was ascertained between October and December 1999. Survival data were available for 66 of the 71 (92.9%) patients who underwent esophagectomy (median follow-up 67 months, range 1–190) and all 24 patients who underwent a limited resection (median follow-up 15 months, range 2–30).
In addition to endoscopy at 6-month intervals, the follow-up examination of patients after a limited resection included a detailed and standardized questionnaire inquiring about the prevalence and severity of postoperative gastrointestinal symptoms (dysphagia, heartburn, regurgitation, chest pain, epigastric pain, postprandial discomfort, diarrhea) and the course of postoperative weight. At the 12-month follow-up, a structured assessment of the postoperative quality of life was made using the Gastrointestinal Quality of Life Index, 17 a previously validated and standardized psychometric instrument with 36 questions, each with five response categories, in five subdimensions:
Core symptoms (pain, bloating, epigastric fullness, flatus, belching, bowel frequency, abdominal noise, restricted eating, enjoyment of eating, fatigue)
Physical items (strength, feeling unwell, feeling unfit, endurance, wake up at night, appearance)
Psychological items (sadness, nervousness, frustration, happiness, bothered by treatment, cope with stress)
Social items (daily activities, leisure activities)
Disease-specific items (regurgitation, dysphagia, eating speed, nausea, diarrhea, bowel urgency, constipation, blood in stool).
The global values in the patients who underwent a limited resection were compared with the global values of patients with gastroesophageal reflux disease before and after Nissen fundoplication, 18 patients with symptomatic gallstone disease before and after cholecystectomy, 17 and normal populations published in the recent literature. 17,18 All symptomatic patients were seen in person and underwent a thorough objective evaluation of the underlying cause of their symptoms. In addition, all asymptomatic patients were offered postoperative esophageal pH monitoring.
Statistical Analysis
A two-tailed Fisher exact test was used to compare proportions. Mean and median values were compared by standard statistical tests as appropriate. Survival probabilities were calculated using the Kaplan-Meier method. 19 Comparison of survival rates was performed by log-rank analysis. P < .05 was considered significant.
RESULTS
Between 1982 and 1999, patients with early (pT1) tumors accounted for 25.5% of all resections for adenocarcinoma of the distal esophagus. There were 38 patients with tumors limited to the mucosa (pT1a) and 56 patients with tumors limited to the submucosa (pT1b). The prevalence of patients with pT1 category increased markedly from 13.2% in 1982–85 to 34.9% in 1995—99. In addition, there was a marked shift toward a higher prevalence of patients with a pT1a category (i.e., tumors limited to the mucosa;Fig. 2).
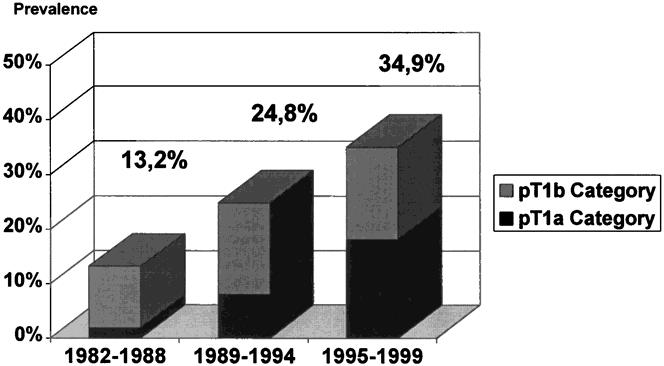
Figure 2. Prevalence of early tumors (pT1A and pT1b category) among all resected adenocarcinomas of the distal esophagus at the authors’ institution, 1982–1988 (n = 106), 1989–1994 (n = 113), and 1995–1999 (n = 149).
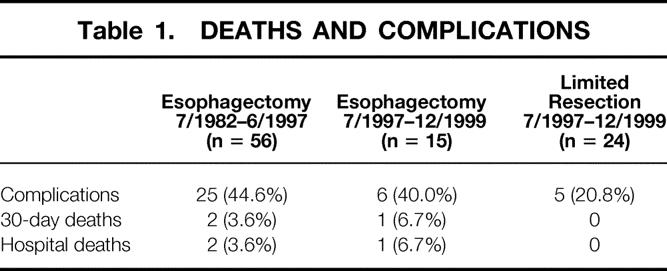
The overall postoperative 30- and 90-day death rate after subtotal esophagectomy was 4.2%. Postoperative complications after subtotal esophagectomy occurred in 31 of the 71 patients (43.7%) and included pulmonary complications (23.9%), cardiac complications (11.3%), wound complications (7.0%), sepsis (4.2%), anastomotic leaks (26.7%), and recurrent nerve paralysis (8.4%). There was no significant difference in the postoperative rates of death and complications between patients who underwent a subtotal esophagectomy before and after 1997 (Table 1). There were no deaths 30 or 90 days after limited resection. Complications occurred in 5 of 24 patients (20.8%) who underwent a limited resection; they included incidental splenectomy (n = 1), pulmonary embolus (n = 1), minor anastomotic leaks (n = 2) that could be managed conservatively, and one wound infection. The complication rate of limited resection was significantly lower than that of subtotal esophagectomy (P < .05).
Table 1. DEATHS AND COMPLICATIONS
The presence of a pT1 category was correctly predicted by a combination of endoscopy, endoscopic ultrasonography, and CT in 23 of the 24 patients who underwent a limited resection (Table 2). In one patient staged as uT1 category on preoperative assessment, histopathologic evaluation of the resected specimen showed a pT2N0 category. Preoperative staging could not reliably differentiate between pT1a and pT1b tumors. There was no significant difference in the distribution of pT1a/pT1b categories between patients who underwent a limited resection or esophagectomy after 1997 (see Table 2).
Table 2. RESULTS
* One patient staged as uT1 on preoperative endoscopy, endosonography, and computed tomography of the chest and abdomen had a pT2N0 category on histopathologic assessment of the resected specimen.
A complete tumor resection (R0 resection) and complete resection of the entire segment with intestinal metaplasia was achieved in all patients in the pT1 category, irrespective of the surgical approach (see Table 2). Histopathologic assessment showed multicentric tumor growth or areas with high-grade dysplasia adjacent to or at some distance from the invasive cancer in 60.6% of the resection specimens.
There was no significant difference in the median number of removed lymph nodes between patients who underwent a subtotal esophagectomy before and after July 1997, compared with those who underwent a limited resection (see Table 2). Standard histopathologic assessment showed no lymph node metastases in 61 of the 71 patients with pT1 tumors who underwent a transmediastinal or transthoracic esophagectomy. Reanalysis of the removed lymph nodes with immunohistochemical techniques in 44 of the patients staged as pN0 identified no additional micrometastases. Standard histopathologic assessment and analysis with immunohistochemical techniques showed no evidence of lymph node metastases or micrometastases in any of the 24 patients who underwent a limited resection.
Overall, lymph node metastases were found in none of the 38 patients with a pT1a category and 10 of the 56 patients (17.8%) with a pT1b category. The number and location of positive nodes in the 10 patients with lymph node metastases are shown in Table 3. In all patients with three or fewer positive nodes, the metastases were exclusively located in regional nodes (i.e., paraesophageal distal, paracardial, and along the left gastric artery). Lymph node metastases in distant nodes (i.e., celiac axis, paraesophageal proximal or cervical) were observed in two patients. Both also had numerous positive regional nodes—that is, there was no evidence of skip metastases to more distant lymph nodes.
Table 3. LOCATION OF POSITIVE NODES IN THE 10 PATIENTS WITH LYMPH NODE METASTASES
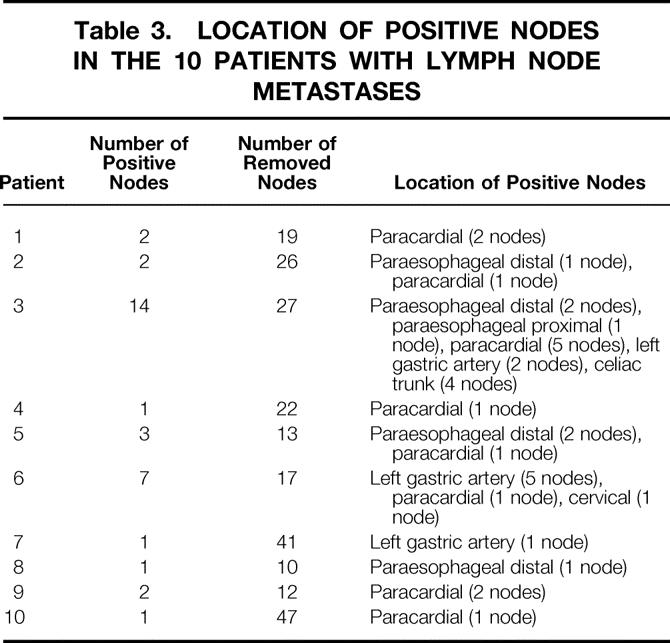
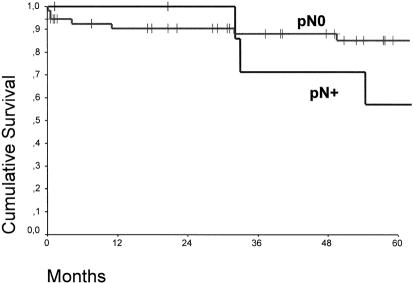
Figure 3 shows the overall survival curves of patients with pT1 adenocarcinoma in the distal esophagus who underwent a subtotal esophagectomy compared with those who underwent a limited resection. At a median follow-up of 15 months, there were no tumor recurrences or deaths in patients who underwent a limited resection. There was no significant difference in the overall survival between patients with a pT1aN0 and pT1bN0 category (Fig. 4). In contrast, the presence or absence of lymph node metastases had a significant effect on survival (P < .05) (Fig. 5).
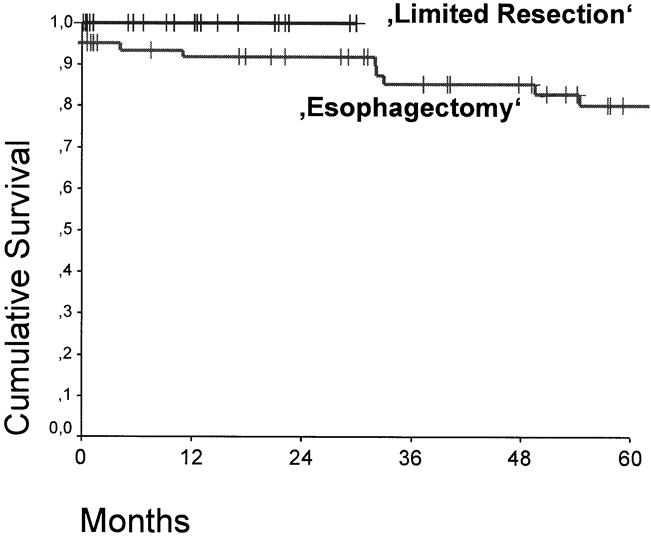
Figure 3. Cumulative survival of patients with pT1 adenocarcinoma in the distal esophagus who underwent a transmediastinal or transthoracic esophagectomy (n = 71) or limited resection (n = 24) for tumors staged as uT1. Postoperative deaths are included. There was no significant difference.
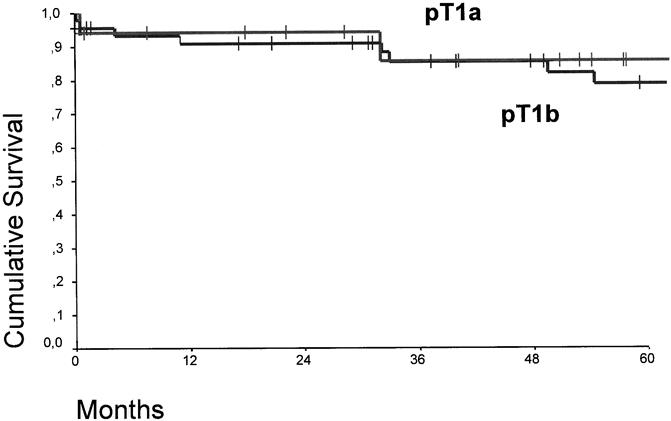
Figure 4. Cumulative survival of patients with pT1a (n = 38) and pT1b (n = 56) adenocarcinoma in the distal esophagus. There was no significant difference.
Figure 5. Cumulative survival of patients with pT1 adenocarcinoma in the distal esophagus with (pN+, n = 10) and without (pN0, n = 84) lymph node metastases (P < .05).
On symptomatic assessment, 20 of the 24 patients (83.3%) who underwent a limited resection had a good swallowing function with no evidence of dysphagia, heartburn, regurgitation, diarrhea, or postprandial epigastric complaints. Postoperative endoscopy showed no evidence of esophagitis in 23 of the 24 patients. There was no evidence of pathologic gastroesophageal reflux in six patients who volunteered for postoperative pH monitoring. Twelve of the 13 patients with follow-up of more than 12 months had regained their preoperative weight. One patient with persistent dysphagia and weight loss required endoscopic dilatation for anastomotic stricture. Of three patients with regurgitation or postprandial epigastric fullness, gastric emptying scintigraphy showed markedly delayed gastric emptying in two patients and dilatation of the interposed jejunal segment with poor peristaltic activity in the remaining one. These three patients require prokinetic medication for symptom control. One of them also had esophagitis on endoscopy and increased esophageal acid exposure on ambulatory 24-hour esophageal pH monitoring.
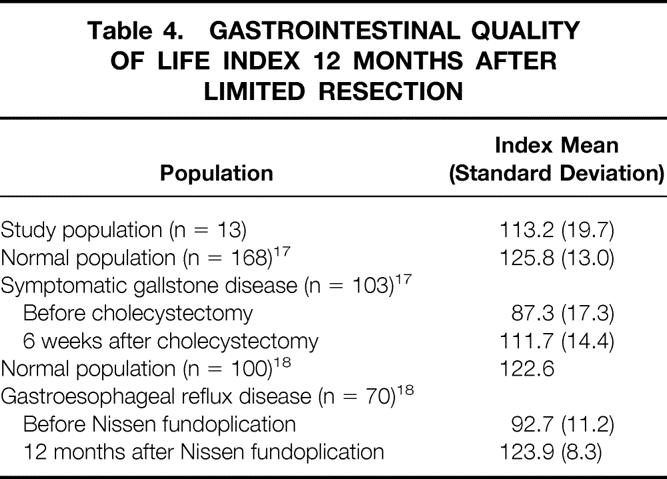
In 13 patients in the limited resection group who had more than 1 year of follow-up, mean global scores in the Gastrointestinal Quality of Life Index were not significantly different from those in the literature for normal populations, patients with symptomatic gallstone disease after cholecystectomy, or patients with gastroesophageal reflux disease after Nissen fundoplication (Table 4).
Table 4. GASTROINTESTINAL QUALITY OF LIFE INDEX 12 MONTHS AFTER LIMITED RESECTION
DISCUSSION
Early detection and complete (R0) resection are the key factors for achieving cure in patients with gastrointestinal cancer. In patients with known Barrett’s esophagus, the present results and the experience of other authors 1,20–22 show that the goal of early detection of esophageal adenocarcinoma is increasingly achieved with programs of surveillance by endoscopic biopsy. Patients with early tumor stages—tumors limited to the mucosa (pT1a category) or submucosa (pT1b category)—now account for more than one third of surgical resections for esophageal adenocarcinoma at our institution. Follow-up shows that cure can be achieved in such patients by subtotal esophagectomy and lymphadenectomy, with 5-year survival rates approaching 90%. 1,20–22 This, however, is achieved at the price of substantial complications, deaths, and a long-term compromise in the quality of life. 4–7 Consequently, the focus of recent studies has been the search for more limited procedures, with lower rates of death and complications and better long-term functional results. 8–10,22 A reduction of the extent of the procedure in a patient with a potentially curable carcinoma can be justified only if the chances for cure are not compromised by incomplete tumor removal. This requires an exact knowledge of the extent of the disease and the radicality required to achieve an R0 resection. As in a previous report, 21 our results show that multicentric tumor growth and multiple foci with high-grade dysplasia in the area of intestinal metaplasia are common in patients with early esophageal adenocarcinoma. Removal of the entire area of intestinal metaplasia in the distal esophagus must therefore be considered mandatory to avoid recurrences. Incomplete ablation, as occurs frequently with photodynamic therapy and other endoscopic techniques, or incomplete surgical removal therefore results in a high prevalence of local recurrences or new tumors. 4–7,23
Although in this study lymphatic spread was not observed in patients with tumors limited to mucosa, lymph node metastases were present in 17.8% of patients with tumors extending to the submucosa. Unfortunately, current staging modalities, including high-frequency endoscopic ultrasonography, cannot reliably discriminate mucosal from submucosal tumors. Because patients with a limited number of positive regional lymph nodes may still be cured by radical resection and lymphadenectomy, 2–5 omission of lymphadenectomy in these patients, as is the case with endoscopic ablation techniques, may compromise the chances for long-term survival.
Based on these data, the preconditions for achieving cure in patients with early adenocarcinoma of the distal esophagus are a complete macroscopic and microscopic tumor resection, including removal of the entire segment with intestinal metaplasia in the distal esophagus, and a regional lymphadenectomy. Our results show that in patients with early adenocarcinoma of the distal esophagus arising in short segments with intestinal metaplasia, these goals can be achieved by a limited transabdominal resection of the distal esophagus, EGJ, and proximal stomach. As indicated by the mean number of removed lymph nodes, the limited resection by an abdominal access only does not compromise the extent of lymphadenectomy in the lower posterior mediastinum and the upper abdominal compartment. In these patients, a benefit from more extended lymphadenectomy in the upper mediastinum and cervical region is unlikely. In our experience, lymph node metastases in the upper mediastinum or cervical region were present only in patients with numerous (more than three) positive regional nodes (i.e., patients who may not obtain a survival benefit from extended lymphadenectomy).
The potential for limited surgical resection in patients with early tumors of the distal esophagus or EGJ has also been recognized recently by several other authors. 22,24 A limited resection is, however, advantageous for the patient only if it is combined with a reconstruction procedure that provides optimal alimentary function and prevents gastroesophageal reflux. The options for reconstruction after limited resection include esophagogastrostomy, colon interposition, and interposition of a pedicled jejunal segment. Esophagogastrostomy has been plagued by poor functional results, with severe reflux after resection of the lower esophageal sphincter. Colon interposition is associated with high rates of death and complications. 25,26 Interposition of an isoperistaltic jejunal segment overcomes most of the disadvantages of esophagogastrostomy or colon interposition.
The concept of an interposed jejunal segment as a substitute for the lower esophageal sphincter was tested as an antireflux procedure and clinically introduced by Merendino and Dillard in 1955. 27 They showed that after resection of the EGJ, interposition of a 15-cm segment of isoperistaltic jejunum behaves like a physiologic sphincter and protects against gastroesophageal reflux. The use of mechanical staplers for the esophagojejunal anastomosis has made this procedure simple and safe. Low rates of death and complications and excellent long-term functional results were reported recently by several investigators in the treatment of undilatable or recurrent distal esophageal strictures and other benign lesions that require resection of the distal esophagus and cardia. 25,28–32 Histologic studies of endoscopic biopsies of the interposed jejunal loops confirmed the retention of a normal villous architecture, with Paneth cell hyperplasia but no evidence of metaplasia on long-term follow-up. 32 Our experience convincingly shows that compared with the more radical transmediastinal or transthoracic esophagectomy with gastric pull-up, this procedure offers markedly lower complication rates and excellent functional results, with a mean Gastrointestinal Quality of Life Index that does not differ from that of asymptomatic control subjects. Careful vagal nerve preservation may further reduce postoperative gastric emptying disturbances, observed in three of our patients.
Transabdominal limited resection of the distal esophagus and EGJ is not applicable in patients with early tumors arising in longer segments of intestinal metaplasia in the esophagus. Esophageal stripping with vagal nerve preservation, as suggested by Akiyama et al 33 and DeMeester et al, 2 or limited resection of the esophageal segment covered by metaplastic epithelium through a combined transabdominal and transthoracic approach with colon interposition may be more limited alternatives to subtotal esophagectomy in these patients.
CONCLUSION
Our results show that lymphatic spread does not occur in patients with adenocarcinoma of the distal esophagus limited to the mucosa but is present in up to 20% of patients with tumors invading the submucosal layer. Distant lymph node metastases were present only in patients with more than three positive regional lymph nodes (i.e., patients who would most likely not benefit from extended lymphadenectomy). A limited resection with locoregional lymphadenectomy appears to be justified in patients with T1a and T1b adenocarcinoma of the distal esophagus. Limited resection with jejunal interposition is safe, prevents gastroesophageal reflux, and is associated with good quality of life. The procedure thus represents an attractive limited surgical alternative to radical esophagectomy or endoscopic resection and ablation techniques. Long-term follow-up and further studies are required before the indications for this procedure are extended to more advanced adenocarcinoma of the distal esophagus or to adenocarcinoma arising at or below the EGJ.
Discussion
Prof. D. Skinner (New York, NY): As usual, we have heard an outstanding paper from Dr. Stein and his colleagues in Munich. I would change the title of the paper—I would say “T1 Barrett’s carcinoma: a surgical approach that is either too radical or not radical enough.” You properly divided your patients into mucosa-only tumors and those in the submucosa. With the sophisticated ultrasonography we all have these days, it is quite possible to do this separation with good accuracy. For a patient with a mucosa-only tumor, it is now widely accepted that such cases do not metastasize to lymph nodes. A limited resection, much more limited than yours (e.g., a mucosectomy in the area), would be appropriate treatment. For those in the submucosa, most series have a much higher incidence of positive lymph nodes. I suspect the fact that you only found a few lymph nodes was because you resected only a little bit of tissue, so that you did not do an adequate resection for people that have positive lymph nodes. In eight of your patients with left gastric nodes positive, only one had higher and lower nodes because you did not resect higher and lower. So I think the number of lymph nodes you counted are too few.
A couple of other comments and questions. Last week at the American Surgical Association meeting, your colleague, Prof. Siewert, presented a paper saying that this kind of metaplastic epithelium at the cardia, in other words group 2 of your cardia classification, was not really Barrett’s esophagus. And yet included in your series are only people with less than 3 cm of Barrett’s. All that middle group around the cardia are the 2-cm group. So you are operating on a group of people where your colleague says that it is not really Barrett’s. So why did you do that? Also, I think that mucosal resection is adequate for cancer limited to the mucosa. About 15 years ago, I had a chance to see a patient with a cervical adenocarcinoma in gastric epithelium, a rare case, not Barrett’s obviously. We did a 5-cm tubular resection of the mucosa only, incised the muscle, took out the cancer, did a primary anastomosis, no other reconstruction, no lymph nodes. When I last saw that patient 10 years ago, he was completely cured by just a mucosectomy. I admit this is a case report, but it makes the point that you can do less surgery than you are doing if you know that it is a pT1a case. I enjoyed this paper. I think it is an intermediate position to take. I do not think it will last. I do not think others will follow it, but I appreciate the data.
Dr. H. Stein (Munich, Germany): Thank you, Prof. Skinner, for your comments—as usual, very critical. Let me first respond concerning the classification system presented by Prof. Siewert at the American Surgical Association meeting. This is a purely topographical classification of tumors arising at, or close to, the esophagogastric junction. The presence or absence of intestinal metaplasia is not considered in this classification system. However, our experience with more 1,000 such patients shows that intestinal metaplasia in the distal esophagus is very commonly associated with esophageal adenocarcinoma (the type I tumors in our classification system) and is only rarely found in patients with true carcinoma of the gastric cardia (the type II tumors in our classification system). The patient population I presented today consists exclusively of patients with type I tumors (adenocarcinoma of the distal esophagus). The presence of specialized intestinal metaplasia in the distal esophagus (i.e., Barrett’s esophagus) was proven in more than 80% of these patients. Although the concept of limited resection may also be valid in patients with early type II (carcinoma of the gastric cardia) or type III tumors (subcardial gastric cancer), we have restricted the current analysis to patients with esophageal adenocarcinoma only in order to have a clean group of patients.
Let me come back to the first question. Was our lymphadenectomy not aggressive enough? Would we have seen more lymph node metastases in patients with pT1b tumors if we had performed a more aggressive lymphadenectomy? In each patient of our population, the number of removed lymph nodes exceeded the required minimal number of nodes for adequate staging (i.e., six nodes) in the latest version of the AJCC and UICC manuals. The median number of removed nodes with limited resection was in fact 19, and thus also exceeded the recommended number of removed nodes suggested by a recent International Society for Diseases of the Esophagus expert panel on adequate lymphadenectomy for esophageal cancer. The prevalence of patients with lymph node metastases in the pT1b group was 17.9% in our study, and thus well within the range of data reported from other centers. In fact, there is only one study that shows a markedly higher prevalence of lymph node metastases in patients with pT1b tumors. But this study is based on an analysis of only 12 patients with pT1b tumors, with a substantial number of those 12 patients having not esophageal adenocarcinoma, but adenocarcinoma of the gastric cardia, or subcardial gastric cancer infiltrating the esophagogastric junction. This one study can therefore hardly be considered representative.
Did we do a too-extensive resection in patients with pT1a tumors? Probably yes, since our study, and the available data in the literature, indicate that such patients almost never have lymphatic spread. However, in contrast to your opinion, in our hands and in the experience of many others, a T1a and T1b tumor today cannot be discriminated with a sufficient degree of certainty, even with the most sophisticated endoscopic ultrasound probes. Furthermore, multicentricity within the area of intestinal metaplasia is very common, as shown in our study. Limited surgical resection is therefore clearly a better alternative than mucosectomy alone in such patients. It removes the entire area with intestinal metaplasia, treats the underlying predisposing condition (i.e., reflux disease), and allows, in our opinion, an adequate lymphadenectomy in those patients who turn out to have a pT1b tumor on histopathological assessment of the specimen.
Will this procedure be an intermediate step towards a more differentiated tailored approach? This may be so when preoperative staging will become good enough to clearly differentiate between T1a and T1b categories. For the time being, the limited resection with jejunal interposition clearly constitutes a very good alternative to subtotal esophagectomy or to mucosal resection. In contrast to mucosectomy, limited resection certainly is an oncologically more adequate procedure. In contrast to subtotal esophagectomy, limited resection is safe and provides good quality of life.
Prof. A. Peracchia (Milan, Italy): This is an interesting study evaluating the effectiveness of the Merendino operation in patients with early adenocarcinoma arising from short-segment Barrett’s esophagus. The authors compare this procedure with the traditional esophagogastric resection, performed either through a transmediastinal or a transthoracic approach. Overall, this is a retrospective study showing that a more limited surgical approach is feasible in patients with T1 Barrett’s carcinoma. There are a few points that should be clarified. One, four patients had previously undergone endoscopic ablation. Was a limited resection or a conventional esophagectomy performed in these patients? How long a time after ablation did they have surgery? And two, it is stated that “preservation of the vagal nerves was attempted whenever possible.” It is also stated that mechanical pyloric dilatation was routinely performed. Three patients complained of disturbed postoperative gastric emptying. How can you be sure that the postoperative complaints were due to either the vagal injury or the pyloric dilatation?
Dr. Stein: Thank you, Prof. Peracchia, for these comments. We do not believe that photodynamic therapy or other types of mucosal ablation can effectively prevent cancer in patients with Barrett’s esophagus, and the four patients in the present series would certainly support this opinion. In all of these patients, ablation had been performed at other institutions, from 5 to 24 months before they presented with cancer at our department. Limited resection was performed in one patients, and three had a transmediastinal esophagectomy because of long segments with intestinal metaplasia in the distal esophagus. Gastric emptying disturbances certainly are the major problem after limited resection and jejunal interposition. I would think that vagal preservation is the key to avoiding gastric emptying disorders in these patients. We attempted to preserve the vagal innervation of the remnant stomach whenever possible, but obviously did not succeed in at least two of the patients, who turned out to have severe gastric emptying problems postoperatively, despite a mechanical pyloric dilatation. I agree that a more formal pyloroplasty may have avoided these problems and should be considered as an adjunct to limited resection in future studies.
Prof. T. Demeester (Los Angeles, California): Dr. Stein, I would certainly agree with the concept of lesser surgery for lesser disease, provided we are sure we have lesser disease. My problem with this approach is how to identify patients who have lesser disease. Our own approach is that patients who are candidates for a lesser procedure should have a biopsy of high-grade dysplasia or intramucosal carcinoma and no visible endoscopic lesion. In such patients, we have shown that the lesion is always contained by the esophageal wall, and that lymph node metastases are extremely rare (i.e., <10% of patients) and, if present, involve never more than one node. If an endoscopic lesion is present, it has been our experience that, in a significant number of such patients, the tumor extends through the wall. About 50% of patients will have lymph node metastasis, although the number of lymph nodes involved tends to be small (i.e., one or two per patient;Ann Surg 1999;230:433–40). In the absence of a visible lesion, we perform a vagus-sparing esophagectomy, without a node dissection. When a lesion is seen, we perform an en bloc resection.
I have three questions for you. First, what is the importance of a visible lesion in your approach? Second, do you really believe you can perform a proper lymphadenectomy along the lesser curve, and still preserve the vagal nerves? I question that you can. Rather, I think you are sort of berry-picking in that situation. Third, if you can’t preserve the vagus in such a situation, why not do a formal resection?
Dr. Stein (Closing Discussion): I am very well aware of your concept about visible and nonvisible lesions. The problem we have with this discrimination is the question of what constitutes a visible lesion. This very much depends on how good the endoscopist is, how good your endoscope is, and what kind of technological advances you are using to make a lesion visible. Today there are magnifying endoscopes, chip endoscopes, staining techniques, immunofluorescence endoscopy, and many more fancy things that can make lesions visible or disappear. Thus it very much depends on the quality of your endoscope and the technical approach you use to make a lesion visible. A “visible lesion” for you may therefore be something completely different for someone else. What about the extent of lymphadenectomy along the lesser curvature? In our experience, preservation of at least some of the vagal branches to the gastric antrum is possible with a limited resection of the esophagogastric junction, without compromising the quality of lymphadenectomy along the left gastric artery. Formal studies to assess whether the vagal innervation is indeed intact after this procedure and whether vagal preservation must really be preserved, if you do a pyloroplasty anyway, have not been done so far. This is the subject of further studies.
Footnotes
Correspondence: Hubert J. Stein, MD, Chirurgische Klinik und Poliklinik, Klinikum rechts der Isar der TU München, Ismaningerstr. 22, D-81675 Munich, Germany.
Presented at the Seventh Annual Meeting of the European Surgical Association, Amstel Intercontinental Hotel, Amsterdam, The Netherlands, April 14–15, 2000.
E-mail: stein@nt1.chir.med.tu-muenchen.de
Accepted for publication July 2000.
References
- 1.van Sandick JW, van Lanschot JJ, Kuiken BW, et al. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut 1998; 43: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg 1999; 117: 16–23. [DOI] [PubMed] [Google Scholar]
- 3.Holscher AH, Bollschweiler E, Bumm R, et al. Prognostic factors of resected adenocarcinoma of the esophagus. Surgery 1995; 118: 845–855. [DOI] [PubMed] [Google Scholar]
- 4.Lerut T, Coosemans W, Van Raemdonck D, et al. Surgical treatment of Barrett’s carcinoma. Correlations between morphologic findings and prognosis. J Thorac Cardiovasc Surg 1994; 107: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 5.Hagen JA, Peters JH, DeMeester TR. Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 1993; 106: 850–859. [PubMed] [Google Scholar]
- 6.Rusch VW, Levine DS, Haggitt R, Reid BJ. The management of high-grade dysplasia and early cancer in Barrett’s esophagus. A multidisciplinary problem. Cancer 1994; 74: 1225–1229. [DOI] [PubMed] [Google Scholar]
- 7.Kirby, JD. Quality of life after oesophagectomy: the patients’ perspective. Dis Esophagus 1999; 12: 168–171. [DOI] [PubMed] [Google Scholar]
- 8.Gossner L, Stolte M, Sroka R, et al. Photodynamic ablation of high-grade dysplasia and early cancer in Barrett’s esophagus by means of 5-aminolevulinic acid. Gastroenterology 1998; 114: 448–455. [DOI] [PubMed] [Google Scholar]
- 9.Gossner L, May A, Stolte M, et al. KTP laser destruction of dysplasia and early cancer in columnar-lined Barrett’s esophagus. Gastrointest Endosc 1999; 49: 8–12. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Jaffe PE, Bhattacharyya A, Sampliner RE. Laser and multipolar electrocoagulation ablation of early Barrett’s adenocarcinoma: long-term follow-up. Gastrointest Endosc 1999; 49: 442–446. [DOI] [PubMed] [Google Scholar]
- 11.Sobin LH, Wittekind C. International Union Against Cancer (UICC), eds. TNM Classification of Malignant Tumors. 5th ed. New York: John Wiley; 1997.
- 12.Fleming ID. American Joint Committee on Cancer Classification (AJCC), eds. AJCC Cancer Staging Manual. Philadelphia: Lippincott Williams & Wilkins; 1997.
- 13.Siewert JR, Stein HJ. Barrett’s cancer: indications, extent and results of surgical resection. Sem Surg Oncol 1997; 13: 245–252. [DOI] [PubMed] [Google Scholar]
- 14.Bumm R, Feussner H, Bartels H, et al. Radical transhiatal esophagectomy with two-field lymphadenectomy and endodissection for distal esophageal adenocarcinoma. World J Surg 1997; 21: 822–831. [DOI] [PubMed] [Google Scholar]
- 15.Siewert JR, Stein HJ. Lymphadenectomy for esophageal cancer. Langenbecks Arch Surg 1999; 384: 141–148. [DOI] [PubMed] [Google Scholar]
- 16.Natsugoe S, Mueller J, Stein HJ, et al. Micrometastasis and tumor cell microinvolvement of lymph nodes esophageal squamous cell cancer: frequency, associated tumor characteristics and impact on prognosis. Cancer 1998; 83: 858–866. [PubMed] [Google Scholar]
- 17.Eypasch EP, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg 1995; 82: 216–222. [DOI] [PubMed] [Google Scholar]
- 18.Kamolz T, Wykypiel H, Bammer T, Pointner R. Quality of life following laparoscopic antireflux surgery: Nissen fundoplication. Chirurgie 1998; 69: 947–950. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–462. [Google Scholar]
- 20.Peters JH, Clark GW, Ireland AP. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Sur 1994; 108: 813–822. [PubMed] [Google Scholar]
- 21.Holscher AH, Bollschweiler E, Schneider PM, Siewert JR. Early adenocarcinoma in Barrett’s oesophagus. Br J Surg 1997; 84: 1470–1473. [PubMed] [Google Scholar]
- 22.Nigro JJ, Hagen JA, DeMeester TR, et al. Occult esophageal adenocarcinoma: extent of disease and implications for effective therapy. Ann Surg 1999; 230: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riben M, Ilves R, McKenna BJ. Second primary Barrett’s adenocarcinoma after 19 years. Ann Thorac Surg 1999; 67: 1796–1798. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita K, Saito N, Saeki I, et al. Proximal gastrectomy and jejunal pouch interposition for the treatment of early cancer in the upper third of the stomach: surgical techniques and evaluation of postoperative function. Surgery 1997; 121: 278–286. [DOI] [PubMed] [Google Scholar]
- 25.Mansour KA, Bryan FC, Carlson GW. Bowel interposition for esophageal replacement: twenty-five-year experience. Ann Thorac Surg 1997; 64: 752–756. [DOI] [PubMed] [Google Scholar]
- 26.Thomas P, Fuentes P, Giudicelli R, Reboud E. Colon interposition for esophageal replacement: current indications and long-term function. Ann Thorac Surg 1997; 64: 757–764. [DOI] [PubMed] [Google Scholar]
- 27.Merendino KA, Dillard DH. The concept of sphincter substitution by an interposed jejunal segment for anatomic and physiological abnormalities at the esophagogastric junction. Ann Surg 1955; 142: 486–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerzic ZB. Modification of the Merendino procedure. Dis Esophagus 1997; 10: 270–275. [DOI] [PubMed] [Google Scholar]
- 29.Gaissert HA, Mathisen DJ, Grillo HC, et al. Short-segment intestinal interposition of the distal esophagus. J Thorac Cardiovasc Surg 1993; 106: 860–866. [PubMed] [Google Scholar]
- 30.Saeki M, Tsuchida Y, Ogata T, et al. Long-term results of jejunal replacement of the esophagus. J Pediatr Surg 1988; 23: 483–489. [DOI] [PubMed] [Google Scholar]
- 31.Picchio M, Lombardi A, Zolovkins A, et al. Jejunal interposition for peptic stenosis of the esophagus following esophagomyotomy for achalasia. Int Surg 1997; 82: 198–200. [PubMed] [Google Scholar]
- 32.Wright C, Cuschieri A. Jejunal interposition for benign esophageal disease. Technical considerations and long-term results. Ann Surg 1987; 205: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akiyama H, Tsurumaru M, Ono Y, et al. Esophagectomy without thoracotomy with vagal preservation. J Am Coll Surg 1994; 178: 83–85. [PubMed] [Google Scholar]