Abstract
Objective
To assess the value of positron emission tomography with 18fluorodeoxyglucose (FDG-PET) for preoperative lymph node staging of patients with primary cancer of the esophagus and gastroesophageal junction.
Summary Background Data
FDG-PET appears to be a promising tool in the preoperative staging of cancer of the esophagus and gastroesophageal junction. Recent reports indicate a higher sensitivity and specificity for detection of stage IV disease and a higher specificity for diagnosis of lymph node involvement compared with the standard use of computed tomography and endoscopic ultrasound.
Methods
Forty-two patients entered the prospective study. All underwent attenuation-corrected FDG-PET imaging of the neck, thorax, and upper abdomen, a spiral computed tomography scan, and an endoscopic ultrasound. The gold standard consisted exclusively of the histology of sampled nodes obtained by extensive two-field or three-field lymphadenectomies (n = 39) or from guided biopsies of suspicious distant nodes indicated by imaging (n = 3).
Results
The FDG-PET scan had lower accuracy for the diagnosis of locoregional nodes (N1–2) than combined computed tomography and endoscopic ultrasound (48% vs. 69%) because of a significant lack of sensitivity (22% vs. 83%). The accuracy for distant nodal metastasis (M+Ly), however, was significantly higher for FDG-PET than the combined use of computed tomography and endoscopic ultrasound (86% vs. 62%). Sensitivity was not significantly different, but specificity was greater (90% vs. 69%). The FDG-PET scan correctly upstaged five patients (12%) from N1–2 stage to M+Ly stage. One patient was falsely downstaged by FDG-PET scanning.
Conclusions
FDG-PET scanning improves the clinical staging of lymph node involvement based on the increased detection of distant nodal metastases and on the superior specificity compared with conventional imaging modalities.
Carcinomas of the esophagus and gastroesophageal junction (GEJ) are still considered to have a poor prognosis because most patients have advanced disease. 1 Up to 80% of patients have positive lymph nodes at surgery. 2 Even in early T1b/T2 tumors, nodal involvement is common (30–50% of patients). This probably relates to the rich submucosal lymphatic network, leading to extensive and chaotic spread of tumor cells. Locoregional recurrence after resection is attributed to nodal involvement in approximately 40% of patients. 3 Nodal involvement is the most important prognostic factor in esophageal carcinoma, with a dramatic fall in the cure rate in patients with positive nodes.
In the past two decades, esophagectomy has become the mainstay of treatment for resectable tumors, offering a chance for cure or prolonged disease-free survival in many patients. 2,4,5 The generally poor outcome in the unfortunate majority of patients with advanced disease at surgery has led to a recent change in approach. Induction chemoradiotherapy followed by resection appears to offer better survival in those who respond, but the results remain difficult to interpret because of the lack of precise clinical staging. 6 Current staging modalities include spiral computed tomography (CT), endoscopy, and endoscopic ultrasonography (EUS). Although these methods provide a combined accuracy of 70% to 90% in the preoperative identification of metastatic disease, 7–18 in many patients advanced disease is detected only during surgery 19 or after minimally invasive surgical staging by both laparoscopy and thoracoscopy. 20,21
Positron emission tomography (PET) using the radiolabeled glucose analogue 18F-fluorodeoxyglucose (FDG) as a tracer is a well-established imaging technique that offers new perspectives in the staging of malignant disease. FDG-PET scanning enables the creation of metabolic images of tissues by studying the altered glucose metabolism in neoplastic cells. These images are complementary to the traditional morphologic images and may be more sensitive because the functional changes can precede the anatomical ones. 22–24
Several studies have confirmed the importance of PET in detecting occult metastases in patients with esophageal cancer 25–27 and head and neck cancer. 28 Our study group reported recently on the utility of FDG-PET scanning for staging carcinomas of the esophagus and GEJ. 29 We found that FDG-PET scanning demonstrated better accuracy compared with combined CT and EUS in the diagnosis of stage IV disease (i.e., distant lymph nodes, organ metastasis, or both) (82% vs. 64%, respectively, P = .004). This was the result of both a better sensitivity (74% vs. 47%) and specificity (90% vs. 78%). Peritumoral nodal assessment by PET showed inferior sensitivity compared with CT and EUS (33% vs. 62%) but improved specificity (89% vs. 67%). For detection of regional and distant nodal involvement as a combined entity, PET scanning achieved similar sensitivity but improved specificity (98% vs. 90%, P = .025) compared with CT and EUS. These results clearly indicated the potential value of FDG-PET scanning in clinical staging. However, the study focused on peritumoral nodes. Studies on combined regional and distant nodal involvement are needed to assess the impact of FDG-PET scanning on clinical staging according to the commonly used TNM system.
The aim of this study was to assess the value of FDG-PET scanning for assessing the extent of nodal involvement in preoperative TNM staging of resectable cancer of the esophagus and GEJ. The specific working hypotheses was that FDG-PET could more accurately diagnose distant nodal metastases than could conventional noninvasive imaging modalities, and thus could improve therapeutic management, particularly in view of neoadjuvant chemoradiation regimens.
PATIENTS AND METHODS
The patients used for this study were part of a larger prospective study assessing the value of FDG-PET scanning for preoperative staging of carcinoma of the esophagus and GEJ. 29 All these patients underwent the standard preoperative staging procedures, including history, physical examination, laboratory tests, ultrasound of the neck, a barium esophagogram, bronchoscopy, spiral CT of chest and abdomen, and a transesophageal EUS. In the same week, FDG-PET scanning was performed. The FDG-PET scan, CT and EUS findings, and all other preoperative staging data were reviewed and correlated at a multidisciplinary tumor conference involving thoracic surgeons, medical oncologists, a pathologist, radiation oncologists, a diagnostic radiologist, and a nuclear medicine physician.
Between October 1997 and December 1998, 74 consecutive patients with de novo carcinomas of the esophagus and GEJ were seen. Of these, we excluded patients with prior treatment for carcinoma of the esophagus and GEJ, diabetes mellitus, inflammatory lung disease, and inoperability for medical reasons. We included only patients who had undergone FDG-PET scanning (this technique was still investigational and thus not reimbursed for carcinomas of the digestive system at the time of the study) and also had the extent of nodal involvement verified by histologic analysis. Of the 42 patients eligible for inclusion, two-field (n = 13) and three-field (n = 26) lymphadenectomy was performed in conjunction with primary curative surgery. The three other patients had biopsy-proven distant nodal metastases for which they received neoadjuvant chemoradiation.
Spiral CT of Chest and Abdomen
In one imaging session, each patient underwent a routine examination of the chest and abdomen for tumor staging and a second examination for image fusion with the FDG-PET scan. The routine examination was always performed first, using the following acquisition parameters: slice thickness 5 mm, table speed 9 mm/rotation, pitch 1.8, and reconstruction interval 5 mm. The following intravenous contrast injection parameters were used: concentration 350 mg iodine/mL, velocity of injection 2 mL/sec, injection time 40 seconds, scan delay 35 seconds, and total volume of contrast 80 mL or, when combining chest and abdomen in a single session, 120 mL. The patient was positioned supine with the arms above the head. To dilate the esophagus for a better examination of the tumor, an oral bolus of Gastrografin was given at the time of scanning. The scanning was performed within one breath-hold. Immediately thereafter, an additional CT examination was performed to acquire the images for fusion with PET. The same modalities were used except for a different pitch (1.2) and scan range (from jaw to celiac level). The patient’s arms were placed at the sides and the patient was instructed to breathe normally.
Spherical lymph nodes with a maximum cross-sectional diameter of at least 10 mm were considered metastatic. Oval lymph nodes were considered metastatic when the longest axis measured more than 15 mm. All examination results were prospectively interpreted by a chest radiologist who was unaware of the results of the other imaging studies.
Esophageal Endoscopic Ultrasonography
In most patients, EUS was performed with a radial scanner; in some, a linear sector scan was used. Patients were premedicated with 5 to 10 mg diazepam. In patients with an obstructing tumor (n = 5), dilatation was not normally performed. The examination was limited to the part above the stenosis. In all other patients, the examination started with a search for perigastric and periceliac lymph nodes, followed by examination of the tumoral mass itself, the peritumoral region, and the periesophageal structures above the tumor. Endosonographic criteria for lymph node metastasis were based on size, shape, margins, and echo pattern. Based on these characteristics, nodes were classified as probably malignant or probably benign by one of three examiners with 4 to 12 years of experience.
FDG-PET Scan
All patients were scanned in the morning, after an overnight fast. The imaging was performed with a CTI-Siemens 931/08/12 scanner (Knoxville, TN) with an axial field of view of 10.1 cm and a spatial resolution of 8 mm. A transmission scan was obtained in five bed positions, ranging from the maxilla down to a midabdominal level. Thereafter, 6.5 MBq/kg FDG (maximum 555 MBq) was injected into an antecubital vein, and after a 60-minute uptake period, PET imaging was initiated. The emission scan was obtained in five bed positions (7 minutes per position), with a similar sequence and range as the transmission scan. All images were corrected for decay and photon attenuation and reconstructed in a 128 × 128 matrix with use of an iterative reconstruction algorithm and 32 iterations. 30,31 Transaxial, coronal, and sagittal views were evaluated by visual inspection on a high-resolution display monitor (SUN workstation, Sun Microsystems, Inc., Mountain View, CA). The visual analysis was performed prospectively by an analyst unaware of all patient data.
For precise spatial localization of the PET lesions, an automated registration of the transaxial PET and CT slices was performed. The reconstructed PET transmission images (and hence the PET emission images) and the CT images were registered using an algorithm based on information theory, maximizing the mutual information between the intensities of both images. 32
Surgery
The surgical approach was determined by the location of the proximal pole of the tumor. For tumors below the carina and tumors of the GEJ, the access consisted of an extended left thoracophrenotomy through the sixth intercostal space. The peritoneum at the dorsal side of the spleen was incised to allow complete mobilization of the tail and part of the pancreatic body. The spleen and pancreas could then be lifted and reflected to the right. All lymphatic tissue localized in the left upper abdominal quadrant, from the hiatus down the celiac axis and mesenteric artery, including the area covering the left adrenal gland down to the renal artery, could be removed while maintaining direct vision of the vascular plane of the descending aorta. The lymphadenectomy further included nodes along the left gastric artery, the splenic artery, and the hilum of the spleen. This was a spleen- and pancreas-preserving compartment II dissection.
In the chest, a so-called posterior mediastinectomy was performed, with clearance of all lymphatic tissue including the thoracic duct, subcarinal lymph nodes, aortopulmonary window, left lower paratracheal, and mainstem bronchi lymph nodes.
Because lymphatic spread in tumors of the upper esophageal half is more likely toward the upper posterior mediastinum, tumors above the level of the carina were approached by a right thoracotomy, allowing optimal tumor dissection and lymphadenectomy of the upper posterior mediastinum.
Whatever the location of the tumor, the lymphadenectomy of the upper abdominal compartment was completed by resecting the lesser curvature down to a level just proximal to the pylorus. This is frequently the site of lymph node metastasis, as shown by Akiyama et al. 2 A cervical location of the anastomosis allowed, in the meantime, a cervical lymphadenectomy to be performed, the so-called third-field lymphadenectomy. This was done through a U-shaped incision in the neck. The third-field lymph node dissection included, bilaterally, lymph nodes lateral to the carotid vessels (deep external nodes), the internal jugular and supraclavicular nodes, and the lymph nodes along the recurrent nerves (deep internal nodes; i.e., the paratracheal nodes, the brachiocephalic artery nodes, and the recurrent nerve nodes down to the point where the intrathoracic lymph node dissection ended). Reasons for not performing the cervical (third-field) lymphadenectomy were diverse and included advanced biologic age, comorbidity, peroperative instability, and high-grade dysplasia/carcinoma in situ.
Data Analysis
The results of the imaging modalities were compared to a gold standard provided by the histologic examination of routine hematoxylin–eosin-stained sections of the materials obtained during extensive two- or three-field lymphadenectomies performed in conjunction with esophagectomy in 39 patients, and in 3 patients by guided biopsies of lesions detected in distant nodal areas. Ultrasound findings, including ultrasound-guided fine-needle aspiration cytology, as indicated by any of the examinations, were not further included in the study design because at no time did they provide further relevant information.
To analyze the accuracy of the combined use of CT and EUS, the positive results of both techniques were summed. Thus, a positive result with one technique overruled a negative result with the other. In the same way, to analyze the accuracy of the overall clinical staging, results of CT, EUS, and FDG-PET were combined. Finally, all resected nodes were counted to compare the number of positive nodes counted clinically with the number of positive nodes found on pathologic examination. Clinical and pathologic lymph node staging results were compared using the latest edition of the UICC TNM classification. 33
The sensitivity, specificity, and accuracy of CT, EUS, and FDG-PET were calculated using the standard definitions. 34 Results were compared by a MacNemar test for correlated proportions. P < .05 was considered significant.
RESULTS
Patient Characteristics and Extent of Lymph Node Sampling
The 42 patients in the study population were 7 women and 35 men with a mean age of 58.5 years (range 44–76). Three patients had biopsy-proven distant nodal metastases (M+Ly) (one paratracheal mediastinal and two in the neck) and received induction chemoradiotherapy. The guided biopsies were based on the suspicion of distant nodal involvement, as indicated by imaging. Thirty-nine patients (93%) underwent extensive lymphadenectomies. The mean age of the 13 patients undergoing two-field lymphadenectomy was 63.6 years; the mean age of the 26 patients undergoing three-field lymphadenectomy was 58.3 years.
The primary tumor histology was squamous cell carcinoma in 10 patients (24%) and adenocarcinoma in 32 patients (76%). Fifteen patients (36%) had adenocarcinoma of the GEJ, 21 patients (52%) had a tumor of the distal esophagus, and 6 patients (14%) had tumor located in the middle third. The total number of examined nodes was 1,976. In one patient many nodes were removed but an exact count was not available. The mean number of nodes examined per patient who underwent lymphadenectomy was 51.5. The R0 (i.e., complete macroscopic and microscopic resection) rate was 90% (35/39 patients). Twenty-eight patients (72%) had a total number of 182 positive nodes, for a mean of 6.5 positive nodes per patient. Of the 26 patients who underwent three-field lymphadenectomy, 6 (23%) had positive cervical lymph nodes.
Table 1 lists the included patients, with the pathologic staging (gold standard) and the results of EUS and CT. The gold standard indicated N0M0 stage in 11 patients, N1–2 stage in 18 patients, and M+Ly disease in 13 patients. Table 2 shows the results of the analysis of the diagnostic accuracy, sensitivity, and specificity of FDG-PET and the combined use of CT and EUS in reference to the gold standard for the diagnosis of N1–2 or M+Ly disease.
Table 1. STAGING OF LYMPH NODES VERSUS PATHOLOGIC STAGING
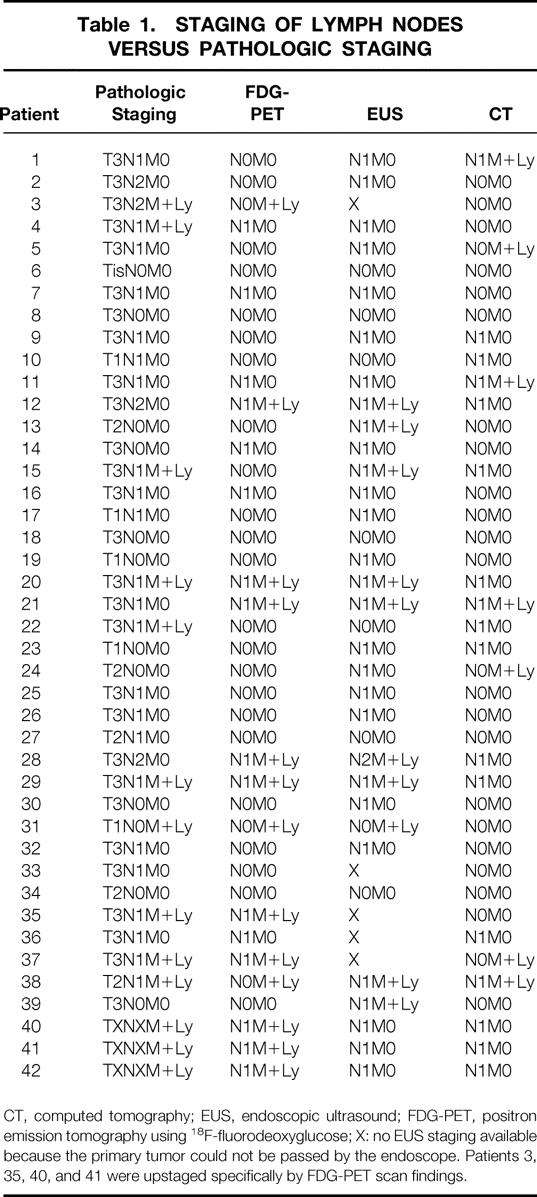
CT, computed tomography; EUS, endoscopic ultrasound; FDG-PET, positron emission tomography using 18F-fluorodeoxyglucose; X: no EUS staging available because the primary tumor could not be passed by the endoscope. Patients 3, 35, 40, and 41 were upstaged specifically by FDG-PET scan findings.
Table 2. PREOPERATIVE ASSESSMENT OF LYMPH NODE INVOLVEMENT
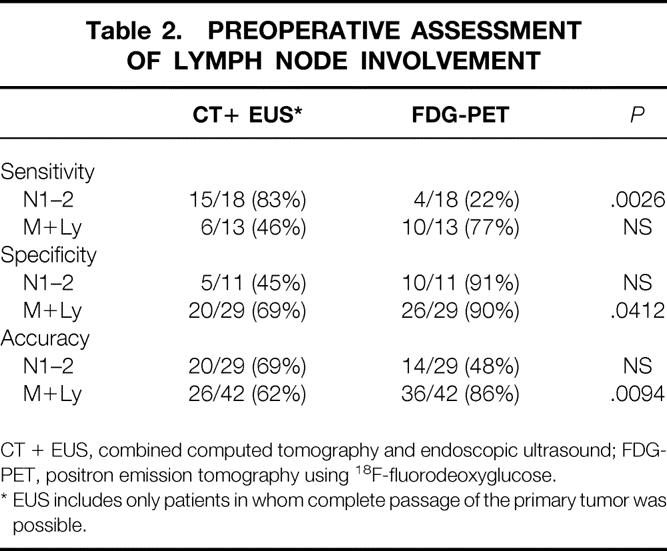
CT + EUS, combined computed tomography and endoscopic ultrasound; FDG-PET, positron emission tomography using 18F-fluorodeoxyglucose.
* EUS includes only patients in whom complete passage of the primary tumor was possible.
Sensitivity
The sensitivity of combined CT and EUS was significantly higher for locoregional nodal involvement (N1–2) than FDG-PET (83% vs. 22%, P = .0026). For distant nodal locations (M), the sensitivity was statistically similar for both diagnostic approaches (77% vs. 46% FDG-PET vs. combined CT and EUS). False-negative M+Ly lesions on FDG-PET were found in three patients. These lesions were located in the lower neck nodes in two patients and in the posterior mediastinum in one patient. The missed nodes were macroscopically not enlarged. In two of these lesions, the histology report mentioned limited microscopic invasion of the lymph nodes.
Specificity
The specificity of FDG-PET for the diagnosis of N1–2 and M+Ly involvement was 91% and 90%, respectively. For M+Ly, the specificity of FDG-PET was significantly higher compared with combined CT and EUS (69%, P = .0412). False-positive lesions were found in four patients on PET scanning. In two of these patients, histology indicated the presence of inflammation in enlarged mediastinal nodes; one patient had sarcoidosis. In another patient, the FDG-PET scan indicated the presence of a focal lesion in the hilus of the spleen; it was not evident at surgery and did not manifest itself at follow-up. The fourth patient had heterogeneous tracer uptake in the primary tumor that was incorrectly considered as reflecting local nodal involvement.
Accuracy for Diagnosis of Nodal Involvement
Overall, clinical staging was correct in 24 patients, overestimated in 13, and underestimated in 5. FDG-PET and CT tended to understage (33% and 45%), whereas EUS did both overstage (27%) and understage (32.5%). FDG-PET overstaged 4 patients (9.5%), CT 6 patients (14%), and EUS 10 patients (27%) (Table 3).
Table 3. OVERALL ACCURACY IN LYMPH NODE STAGING
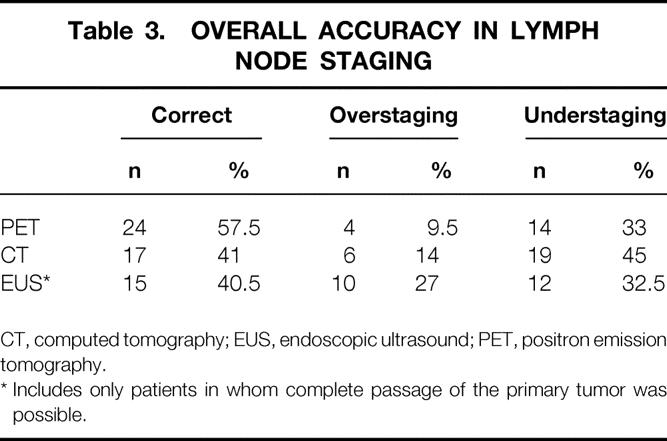
CT, computed tomography; EUS, endoscopic ultrasound; PET, positron emission tomography.
* Includes only patients in whom complete passage of the primary tumor was possible.
Patients 10 and 29 had correct clinical staging, but the location of the involved lymph node field was incorrect. One patient with pN1 disease had a positive mediastinal node on CT, whereas this was negative on the pathology examination. This patient, however, had a positive local peritumoral node not seen on PET, EUS, or CT. The second patient with pM+Ly had positive nodes around the celiac axis on EUS and FDG-PET; they were negative on the pathology examination. However, positive nodes were found in the cervical field that were missed on the FDG-PET, CT, and EUS. The accuracy for diagnosing distant nodal metastasis was significantly higher for FDG-PET than for combined CT and EUS (86% vs. 62%, P = .0094).
The result of the FDG-PET scan disagreed with the result of combined EUS and CT in 12 of the 42 patients (29%). In these patients, the FDG-PET result was correct in 11 of 12 patients; 5 patients (12%) were correctly upstaged from N0–1-2 stage to M+Ly. In two of these patients, although EUS could not pass the tumor, EUS missed positive nodes proximal to the tumor—supradiaphragmatic in one patient with a GEJ tumor (patient 3) and in the neck in another patient with a distal-third tumor (patient 35). In three other patients, the FDG-PET scan indicated positive M+Ly lymph nodes that were missed by both CT and EUS. One patient (patient 40) had a GEJ tumor with positive paratracheal mediastinal nodes. Two patients (patients 41 and 42) with a middle- and distal-third tumor, respectively, had positive nodes in the neck.
Ultrasound of the neck (including ultrasound-guided fine-needle aspiration cytology performed after PET in two patients) was negative in all patients with histology-proven positive nodes in the neck. Overall, eight patients had positive lymph nodes in the neck (19% of all patients, 23% of the patients receiving three-field lymphadenectomy). In three patients, the diagnosis was based specifically on the FDG-PET scan. Two patients had also correct cervical lymph node staging by either CT or EUS. Finally, in three others, the lymph node involvement was missed by the clinical investigation, including the ultrasound.
In six patients, FDG-PET scans were correctly downstaged, from M+Ly stage to N0–1-2 stage, compared with the combination of EUS and CT. The cause of overstaging was CT in four patients and EUS in two. One patient was falsely downstaged by FDG-PET: this patient had a GEJ tumor with positive lymph nodes in the posterior mediastinum missed on PET and diagnosed as suspicious on EUS.
Accuracy for the Number of Involved Nodes
Lymph node count (Table 4) revealed underestimation in 24 patients, overestimation in 2 patients, and a correct count in 13 patients for PET. For EUS the corresponding figures were 10, 14, and 10.
Table 4. ASSESSMENT OF NODAL INVOLVEMENT BASED ON COUNT
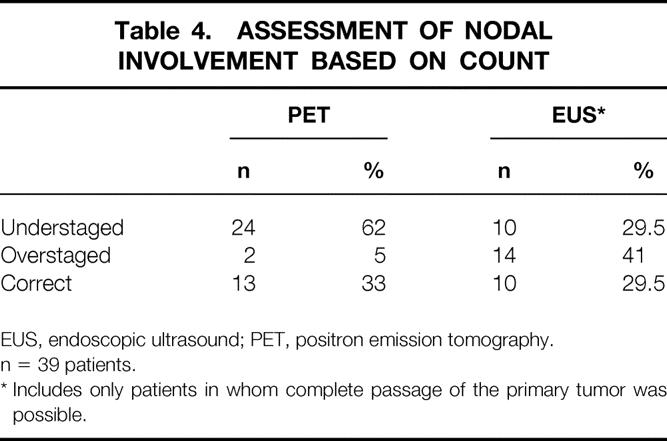
EUS, endoscopic ultrasound; PET, positron emission tomography.
n = 39 patients.
* Includes only patients in whom complete passage of the primary tumor was possible.
DISCUSSION
Accurate staging is of paramount importance in planning optimal therapeutic strategy for patients with carcinoma of the esophagus or GEJ. The mainstay of clinical staging is CT combined with EUS. However, based on the literature, the accuracy of CT and EUS is far from optimal because it can both overstage and understage lymph nodes. 13–18 Overstaging is due to the difficulties in distinguishing reactive inflammatory from neoplastic nodes, understaging to the inability to identify nonenlarged nodes as neoplastic or minimally involved. Perhaps the most important reason is the well-known excessive and chaotic lymphatic spread characteristic of cancer of the esophagus and GEJ. This was most impressively illustrated by the results from three-field lymphadenectomy, as has been reported mainly by Japanese investigators. Akiyama et al 2 extensively studied the lymph node metastasis pattern in a series of 200 patients. The frequency of nodal metastasis was studied according to the location of the tumor and each of the three dissected fields. According to the location of the tumor (upper, middle, or distal third), cervical lymph node involvement was seen in 46.3%, 29.2%, and 27% of patients; the corresponding incidence of abdominal lymph nodes was 12.2%, 39.9%, and 74%. Involvement of distant lymph nodes was unpredictable regardless of tumor location.
In our experience in the T3N+ setting, adenocarcinoma of the distal esophagus had up to 30% unforeseen cervical lymph nodes; tumors of the GEJ had up to 20%. 19 Further, many other lymph node regions (e.g., common hepatic and splenic artery areas) are difficult to label correctly on CT or EUS. 18 This understaging and overstaging of lymph nodes in a substantial fraction of tumors is a limiting factor for adequate selection of patients for neoadjuvant treatment modalities. Moreover, when using chemoradiotherapy as an induction treatment modality, the question arises whether to include the supraclavicular and cervical area or the superior abdominal compartment in the irradiation field. Enlarging the irradiation field to such a magnitude may significantly increase the rates of complications and even death before and after surgery.
A more recent trend is the development of nonsurgical endoluminal destruction techniques for early superficial tumors, especially in Barrett carcinoma, using mucosectomy, photodynamic therapy, or laser destruction. A major obstacle, however, is the lack of discrimination between T1a and T1b. T1b tumors carry a risk of lymph node involvement in up to 50% of cases, but such nodes are frequently missed in the clinical workup because of limited involvement. 35 This subset of patients, however, is known to benefit from radical surgery because of the relatively limited tumor load, with reported 5-year survival rates of more than 60%. 36 These examples illustrate that accurate staging is of crucial importance.
Refinements in the cytology technique of fine-needle aspiration under ultrasound or EUS guidance seem to open promising perspectives and may become important tools, improving clinical staging. 37,38 So far, however, these technologies seem to have limited availability and require a steep learning curve. 38 In this study all patients systematically underwent ultrasound of the neck in the clinical workup. If indicated, fine-needle aspiration cytology was performed under ultrasound guidance. However, ultrasound with or without fine-needle aspiration cytology failed to contribute to the clinical staging. EUS with fine-needle aspiration cytology was not performed in this series.
Some centers propose the routine use of minimally invasive surgery as a staging procedure, using laparoscopy and video-assisted thoracic surgery. 20,21 However, this approach is time-consuming and expensive and delays final treatment. Routine application is therefore limited. In this series, video-assisted thoracic surgery and laparoscopy were rarely used, and only on specific indication.
The use of FDG-PET using radioactive tracers to detect changes in metabolism between normal and malignant tissue also seems to open new perspectives in improved accuracy of staging. In several types of carcinoma, including esophageal carcinoma, FDG-PET applications have been reported to increase the detection of occult metastasis by 10% to 20% compared with conventional staging. 25–28,39
This study specifically addresses the potential value of FDG-PET in assessing lymph node involvement by comparing the results with those obtained with CT and EUS, and the value of the combined CT, EUS, and PET compared with the final pathologic staging in a series of 42 patients. Thirty-nine patients underwent primary resection with extensive two- and three-field lymphadenectomy. This is a unique feature compared with other published series. Indeed, all other reports included several patients who were either not considered for further primary resectional surgery or in whom resection consisted in a transhiatal esophagectomy. It is generally accepted that this approach is less adequate for assessing lymph nodes and thus underestimates potential lymph node involvement. 40
Comparing clinical with pathologic staging, the overall accuracy for lymph node staging was 57%, with a tendency for understaging in FDG-PET (33%) and confirming the tendency for both understaging (32.5%) and overstaging (27%) for EUS.
Luketich et al 26 reported an accuracy of 48% for locoregional nodal metastasis. Kole et al 41 reported an overall 90% accuracy and Flanagan et al 42 a 76% accuracy for lymph node detection. The lower accuracy in this study is most likely explained by patient selection: only patients considered for resection with curative intent, including extensive lymphadenectomy, were included.
The low 22% sensitivity of FDG-PET for locoregional lymph node (N1-N2) staging can be explained by the difficulty in discriminating the primary tumor from peritumoral lymph nodes. This is due to intense tracer accumulation and ill-defined anatomical boundaries, even when using the most accurate CT/PET image registration techniques, 29 or the limited microscopic invasion of the lymph nodes, as seen in two patients.
In staging distant LN metastasis (M+Ly), the sensitivity was higher (77.6%). The false-negative results were related to a minimal tumor load in the lymph nodes in two patients. The missed lymph nodes were macroscopically not enlarged, resulting in difficulties in resolving increased FDG uptake in lesions less than 1 cm in diameter. Similar difficulties are well known in EUS because sensitivity decreases for lymph node metastases less than 1 cm. 21 Future improvements in spatial resolution or development of new radiotracers are needed to improve the negative predictive value of lymph node involvement in FDG-PET. 42
The most important positive finding in this study was the significantly higher specificity of PET versus CT and EUS. The specificity of FDG-PET was 91% for locoregional nodes and 90% for distant lymph nodes versus 45% and 69% for CT and EUS. In assessing the accuracy for diagnosis of M+Ly, PET FDG scanning correctly upstaged five patients (12%) from N+ to M+Ly (i.e., stage IV disease). This upstaging effect is very much related to the design of the study and the extensive surgery, including three-field lymphadenectomy, the cervical field revealing positive nodes in 6 of the 26 patients (23%).
Overall, of the 42 patients, 8 had positive nodes in the neck. In three patients, FDG-PET specifically diagnosed cervical lymph node metastasis, missed in all three by CT and EUS and cervical ultrasound, including ultrasound-guided fine-needle aspiration cytology in two patients. Adding the cervical field to the lymphadenectomy again confirms the high incidence of “unforeseen” positive nodes in the neck that have been missed by conventional CT and EUS imaging. 19 Obviously, the high specificity and accuracy of FDG-PET compared with combined CT and EUS, especially for detecting distant nodal metastasis (M+Ly), provided key information and may be essential in making therapeutic plans for patients with carcinoma of the esophagus and GEJ. False-positive lesions were found in four patients, suggesting the need for caution in interpreting positive nodes on an FDG-PET scan. In two of these patients (one with sarcoidosis), histology indicated the presence of inflammation in enlarged mediastinal nodes. In another patient, the FDG-PET scan indicated the presence of a focal lesion in the hilus of the spleen. This was not confirmed at surgery and subsequent follow-up. From these observations, it is clear that positive findings on an FDG-PET scan must be confirmed by pathologic examination, whenever possible, before denying any patient a chance for a therapeutic regimen with curative intent.
A remaining obstacle is the difficulty of both EUS and FDG-PET in determining the number of involved lymph nodes, with frequent overscoring and underscoring, and incorrect labeling of the region of “positive” nodes. Although the TNM stage may be accurate, the tumor load in lymph nodes can be underscored or overscored. The number of involved nodes was underscored in 24 patients (62%) by FDG-PET and in 10 (29.5%) by EUS. The number of nodes involved was overscored in 2 patients (5%) by PET scanning and 14 (41%) patients by EUS.
The number of involved nodes is a well-documented prognostic indicator. Several studies suggest that a positivity rate more than 10% leads to a significantly worse prognosis. 43 These findings resulted in a change in the most recent UICC TNM classification, using the number of involved regional nodes as a discriminator between N1 and N2 disease for GEJ tumors.
In summary, PET scanning is not yet solving the problem of accurate clinical staging in cancer of the esophagus and GEJ. However, its high specificity for lymph node staging and its well-documented increased potential for detecting occult organ metastasis make it superior to any other available staging investigation. A good illustration is the high incidence (90%) of complete R0 resections, despite locally advanced disease in 72% of the patients. The overall R0 resection rate was 81% in our previous experience, before PET. 44 These arguments justify the routine use of FDG-PET in the clinical staging of these often complex carcinomas. However, when positive FDG-PET findings would deny a patient a chance for treatment with a curative option, confirmation by histopathology is mandatory. Extensive minimally invasive staging and extensive lymphadenectomy at the time of resection remain the reference techniques.
Discussion
Prof. T. Demeester (Los Angeles, California): I have three questions. One is that whenever you look at these tests, the way you set up the test population affects the outcome. Really, you should have a chance for the test to be equally positive or negative on your test population. So it would be nice, although it is difficult I am sure, to look at your data, and have 50% of the people with regional or distant node metastases and 50% who did not, and then analyze the data, because as you give them more negative cases, you load it to make it look better in its specificity. So I am not sure you really have a specificity that is that good. Second, is it all worth it for 10% improvement? I mean, the cost of that PET scan in our country is probably $1,000—more than the surgeon’s fee, almost. And you get all this other stuff, too. It is getting too theoretical. Third, I think a lot of this is generated by the oncologist. Their real effort is to take the patient and give preoperative chemotherapy. By the way, I think we have done enough of this. It does no good to 60% of the people, don’t you agree? You started your presentation by saying how often lymph nodes are involved. Why don’t you go straight to surgery? If patients have more than four lymph nodes or whatever degree of involvement you want to take, then treat them with postoperative chemotherapy. Now, the internist does not want to do that, because he wants to use you as a biological assay for your patients and how they are doing with their chemotherapy. That is what he wants.
Prof. T. Lerut (Levven, Belgium): I will try to come back on each of the questions. The first is a very good remark, but of course this study was set up as an evaluation of a new tool that became available. In our series, there were 28% of patients with negative nodes out of a group of 39, so that is a very small group. What you say is indeed correct, but to accumulate enough material to have significant statistical power will require quite a bit of time. To validate the full spectrum of all the possibilities is just one of the options that should come up in the future.
Is it worth 10%? I think you do not see the value of PET scan in assessing lymph node involvement on its own, disconnected from its overall value in staging, because one of the most powerful elements of the PET scan is its ability to detect occult systemic organ metastases. So it is the combination of the two. In this series, that was 18%. Then I think that it becomes worthwhile.
Yes, also in Belgium, the cost is maybe more than the fee of the surgeon. But there is more than just the surgeon. If you operate on the patient, then you have to calculate the costs of the operation, the hospital stay, and so on.
For the third question, you know that I am a true follower of the gospel according to Tom DeMeester concerning the value of surgery in esophageal cancer. I fully agree that we are poisoning our patients, once in a while, with too much chemotherapy. Nevertheless, I think it depends on how you look at those things. Of course one should not deny the patient any chance of cure and indeed go for surgery. But if you have, let us say, six lymph nodes involved, spread through three different compartments, you and I know that the chances for cure are virtually zero. So then I think you can change the treatment philosophy—unless you say to go for surgery in such a patient because you can improve disease-free survival, with good quality of life. That, of course, is a matter of how you look at the problem of treating cancer patients at an advanced stage of disease when they are getting into a palliative situation.
Prof. H. Obertop (Amsterdam, The Netherlands): I found it a little difficult to figure out what the real advantage of the PET scan is. Well, let us say it is 10%. You were not using ultrasound of the neck and fine-needle aspiration cytology; therefore, the whole conventional workup could have been better. Also, you could have used endo-ultrasound with fine-needle aspiration cytology. My first question is, do you think if you used these techniques, would there be any advantage at all of the PET scan? My second question is about the N2 lymph nodes, as mentioned in your manuscript. As far as I know, in staging esophageal cancer, there is only N1 or N0, and one of the arguments in favor of the PET scan is that it helps you to differentiate between these N1 and N2 nodes. Could you clarify this issue?
Prof. Lerut: To the second question: the N2 in the TNM system is for GE junction tumors only.
Prof. Obertop: So the GE junction tumors are staged as gastric cancers?
Prof. Lerut: That is right, but they are treated as esophageal cancers.
Prof. Obertop: OK. I know this is also a very difficult topic and we should not go into that.
Prof. Lerut: Different centers can have different attitudes. In our center, we consider that GE junction tumors behave like tumors of the esophagus, and we treat them accordingly. Concerning other diagnostic tools, in fact we did use ultrasound of the neck and, where it was feasible, fine-needle aspirates were made, but in five patients none of them was positive. Fine-needle cytology with echoendoscopy is a relatively new methodology. Our echoendoscopists are using it very recently, only in the past 6 months. It has not been incorporated in the routine evaluation in our center, and at this point I doubt that there are many centers that have sufficient experience with fine-needle aspiration using EUS. It requires a very highly trained echoendoscopist, so I think for the time being that should not be considered as a routine diagnostic tool.
Prof. M. BÜchler (Bern, Switzerland): As you know, we have done PET studies in pancreatic cancer in the early 1990s together with Hans Beger. My question goes to the following problem. You have shown us three patients where it is quite clear that there is a spot, and it is quite convincing that this is tumor, but you have not shown us most of the others. The problem I had when we studied pancreatic cancer was that it is not a “yes” or “no.” It is not that we have a big spot, and then we have no spot; it is very frequently something gray. Then the nuclear medicine people say this is positive, then in other patients they say it is not positive, it is background. So my question goes to how you determined what is positive and what is negative, because the biggest problem is in interpreting those scans that are gray, a bit more gray, and a little bit more gray, and they say this is positive and this is negative.
Prof. Lerut: That is indeed a problem related to the technology, and there is certainly a learning curve in how to use PET, which we have only started to study. One of our nuclear medicine people was familiar with the technology when we started the study, so that this learning curve was excluded. Of course I did not show you every patient; one can’t do that in a 10-minute talk on all 39 patients. But what I did show you was that the sensitivity in the regional and distal metastases group was rather low because the patients had minimal tumor loads. That, of course, has to do with the biology of the tumor. If there is a minimal tumor load, there is minimal uptake, and they do not show up on PET scan. That is definitely one of the reasons why the sensitivity is so low. It simply does not show up until it reaches a critical mass, which has an important implication. Nobody should conclude from a negative PET scan that there is no lymph node and so lymphadenectomy should not be done.
Prof. B. Kremer (Kiel, Germany): Could you show any effect of neoadjuvant or palliative treatment to the lymph nodes by PET scan?
Prof. Lerut (Closing Discussion): That is the question of evaluating downstaging after chemotherapy. This study is ongoing, maybe to be presented here next year!
Footnotes
Correspondence: Prof. Toni Lerut, MD, PhD, University Hospital Gasthuisberg, Catholic University of Leuven, Herestraat 49, 3000 Leuven, Belgium.
Presented at the Seventh Annual Meeting of the European Surgical Association, Amstel Intercontinental Hotel, Amsterdam, The Netherlands, April 14–15, 2000.
E-mail: toni.lerut@uz.kuleuven.ac.be
Accepted for publication July 2000.
References
- 1.Muller JM, Ersami H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990; 77: 845–857. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H, Masahiko T, Udagawa H, Kihyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994; 220: 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isono K, Onada S, Ishikawa T, et al. Studies on the causes of death from esophageal carcinoma. Cancer 1982; 49: 2173–2179. [DOI] [PubMed] [Google Scholar]
- 4.Lerut T, De Leyn P, Coosemans W, et al. Surgical strategies in esophageal carcinoma with emphasis on radical lymphadenectomy. Ann Surg 1992; 216: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner DB. En bloc resection for neoplasms of the esophagus and cardia. J Thorac Cardiovasc Surg 1983; 85: 59–71. [PubMed] [Google Scholar]
- 6.Bhansali MS, Vaidya JS, Bhatt RG, et al. Chemotherapy for carcinoma of the esophagus: a comparison of evidence from meta-analysis or randomized trials and of historical control studies. Ann Oncol 1996; 7: 355–359. [DOI] [PubMed] [Google Scholar]
- 7.Rice TW, Zuccaro G, Aldenstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of lymph node status. Ann Thorac Surg 1988; 65: 787–792. [DOI] [PubMed] [Google Scholar]
- 8.Sondenna K, Skaane P, Nygaard K, et al. Value of computed tomography in preoperative evaluation of resectability and staging in esophageal carcinoma. Eur J Surg 1992; 168: 537–540. [PubMed] [Google Scholar]
- 9.Consigliere D, Chua Cl, Hui F, et al. Computed tomography for esophageal carcinoma: its value to the surgeon. J R Coll Surg Edinb 1992; 37:113–117. [PubMed]
- 10.Ziegler K, Sanft C, Zeitz M, et al. Evaluation of endosonography in TN staging of oesophageal cancer. Gut 1991; 32: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boter JF, Lightdale CJ, Zauber AG, et al. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology 1991; 181: 419–425. [DOI] [PubMed] [Google Scholar]
- 12.Tio TL, Coene PPLO, Schouwink MH, et al. Esophagogastric carcinoma: preoperative TNM classification with endosonography. Radiology 1989; 173: 411–417. [DOI] [PubMed] [Google Scholar]
- 13.Grimm H, Soehendra N, Hamper K, et al. Endosonography versus computed tomography for determination of loco-regional spread in esophageal cancer: a prospective controlled study. In: Ferguson MK, Little AG, Skinner DB, eds. Diseases of the Esophagus. Vol. 1: Malignant Diseases. Mount Kisco, NY: Futura; 1990:125–134.
- 14.Vilgrain V, Mompoint D, Palazzo L, et al. Staging of esophageal carcinoma: comparison of results with endoscopic sonography and CT. Am J Roentgenol 1990; 155: 277–281. [DOI] [PubMed] [Google Scholar]
- 15.Massari M, Cioffi U, De Simone M, et al. Endoscopic ultrasonography for preoperative staging of esophageal carcinoma. Surg Laparosc Endosc 1997; 7: 162–165. [PubMed] [Google Scholar]
- 16.Pham T, Roach E, Falk GL, et al. Staging of oesophageal carcinoma by endoscopic ultrasound: preliminary experience. Aust NZ J Surg 1998; 68: 209–212. [DOI] [PubMed] [Google Scholar]
- 17.Vickers J. Role of endoscopic ultrasound in the preoperative assessment of patients with oesophageal cancer. Ann R Coll Surg Engl 1998; 80: 233–239. [PMC free article] [PubMed] [Google Scholar]
- 18.Rösch T. Endosonographic staging of esophageal cancer: a review of literature results. Gastrointest Endosc Clin North Am 1995; 5: 537–547. [PubMed] [Google Scholar]
- 19.van de Ven C, de Leyn P, Coosemans W, et al. Three-field lymphadenectomy and pattern of lymph node spread in T3 adenocarcinoma of the distal esophagus and the gastro-esophageal junction. Eur J Cardiothorac Surg 1999; 15: 769–773. [DOI] [PubMed] [Google Scholar]
- 20.Krasna MJ, Flowers JL, Attar S, et al. Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 1996; 111: 800–807. [DOI] [PubMed] [Google Scholar]
- 21.Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 1997; 114: 817–823. [DOI] [PubMed] [Google Scholar]
- 22.Warburg O. On the origin of cancer cells. Science 1956; 123: 309–314. [DOI] [PubMed] [Google Scholar]
- 23.Pauwels E, McCready VR, Stoot JH, et al. The mechanism of accumulation of tumour-localising radiopharmaceuticals. Eur J Nucl Med 1998; 25: 277–305. [DOI] [PubMed] [Google Scholar]
- 24.Dhalbom M, Hoffman EJ, Hoh CK. Whole-body positron emission tomography: methods and performance characteristics. J Nucl Med 1992; 33: 1191–1199. [PubMed] [Google Scholar]
- 25.Block M, Patterson GA, Sundaresen RS, et al. Improvement in staging esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg 1997; 64: 770–777. [DOI] [PubMed] [Google Scholar]
- 26.Luketich JD, Schauer PR, Meltzer CC, et al. Role of positron emission tomography in staging esophageal cancer. Ann Thorac Surg 1997; 64: 765–769. [DOI] [PubMed] [Google Scholar]
- 27.Luketich JD, Friedman DM, Tracey L, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg 1999; 68: 1133–1137. [DOI] [PubMed] [Google Scholar]
- 28.Stokkel MPM, ten Broek FW, Hordijk GJ, et al. Preoperative evaluation of patients with primary head and neck cancer using dual-head 18Fluorodeoxyglucose positron emission tomography. Ann Surg 2000; 231: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flamen P, Lerut A, Van Cutsem E, et al. The utility of positron emission tomography (PET) for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000; 18: 3202–3210 [DOI] [PubMed] [Google Scholar]
- 30.Shepp LA, Vardi Y. Maximum likelihood reconstruction for emission tomography. IEEE Trans Med Imaging 1982; 1: 113–122. [DOI] [PubMed] [Google Scholar]
- 31.Lange K, Carson R. EM reconstruction algorithm for emission and transmission tomography. J Comput Assist Tomogr 1984; 8: 306–316. [PubMed] [Google Scholar]
- 32.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 1997; 16: 186–197. [DOI] [PubMed] [Google Scholar]
- 33.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours, 5th ed. New York: Wiley-Liss; 1997.
- 34.Beck J. Likelihood ratios: another enhancement of sensitivity and specificity. Arch Pathol Lab Med 1986; 110: 685–686. [PubMed] [Google Scholar]
- 35.Natsugoe S, Baba M, Yoskinaka H, et al. Mucosal squamous cell carcinoma of the esophagus: a clinicopathologic study of 30 cases. Oncology 1998; 55: 235–241. [DOI] [PubMed] [Google Scholar]
- 36.Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall implications for therapy. J Thorac Cardiovasc Surg 1999; 117: 16–23. [DOI] [PubMed] [Google Scholar]
- 37.Doldi SB, Lattuada E, Zappa MA, et al. Ultrasonographic imaging of neoplasms of the cervical esophagus. Hepato-Gastroenterology 1997; 44: 724–726. [PubMed] [Google Scholar]
- 38.Hunerbein M, Dohmoto M, Haensch W, Schlag PM. Endosonography-guided biopsy of mediastinal and pancreatic tumors. Endoscopy 1998; 30: 32–36. [DOI] [PubMed] [Google Scholar]
- 39.Flamen P, Stroobants S, Van Cutsem E, et al. Additional value of whole-body positron emission tomography with fluorine-18–2-fluoro-2-deoxy-D-glucose in recurrent colorectal cancer. J Clin Oncol 1999; 17: 894–901. [DOI] [PubMed] [Google Scholar]
- 40.Hagen JA, Peters JH, DeMeester TR. Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 1993; 106: 850–859. [PubMed] [Google Scholar]
- 41.Kole AC, Plukker JT, Nieweg OE, et al. Positron emission tomography for staging of oesophageal and gastroesophageal malignancy. Br J Cancer 1998; 9: 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flanagan FL, Dehdashti F, Siegel BA, et al. Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. Am J Roentgenol 1997; 168: 417–424. [DOI] [PubMed] [Google Scholar]
- 43.Roder JD, Busch R, Stein HJ, Fink U, Siewert JR. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the esophagus. Br J Surg 1994; 81: 410–413. [DOI] [PubMed] [Google Scholar]
- 44.Lerut T. Esophageal surgery at the end of the millennium. J Thorac Cardiovasc Surg 1998; 116: 1–20. [DOI] [PubMed] [Google Scholar]


