Abstract
Objective
To assess the feasibility and safety of laparoscopic liver resections.
Summary Background Data
The use of the laparoscopic approach for liver resections has remained limited for technical reasons. Progress in laparoscopic procedures and the development of dedicated technology have made it possible to consider laparoscopic resection in selected patients.
Methods
A prospective study of laparoscopic liver resections was undertaken in patients with preoperative diagnoses including benign lesion, hepatocellular carcinoma with compensated cirrhosis, and metastasis of noncolorectal origin. Hepatic involvement had to be limited and located in the left or peripheral right segments (segments 2–6), and the tumor had to be 5 cm or smaller. Surgical technique included CO2 pneumoperitoneum and liver transection with a harmonic scalpel, with or without portal triad clamping or hepatic vein control. Portal pedicles and large hepatic veins were stapled. Resected specimens were placed in a bag and removed through a separate incision, without fragmentation.
Results
From May 1996 to December 1999, 30 of 159 (19%) liver resections were included. There were 18 benign lesions and 12 malignant tumors, including 8 hepatocellular carcinomas in cirrhotic patients. Mean tumor size was 4.25 cm. There were two conversions to laparotomy (6.6%). The resections included 1 left hepatectomy, 8 bisegmentectomies (2 and 3), 9 segmentectomies, and 11 atypical resections. Mean blood loss was 300 mL. Mean surgical time was 214 minutes. There were no deaths. Complications occurred in six patients (20%). Only one cirrhotic patient developed postoperative ascites. No port-site metastases were observed in patients with malignant disease.
Conclusion
Laparoscopic resections are feasible and safe in selected patients with left-sided and right-peripheral lesions requiring limited resection. Young patients with benign disease clearly benefit from avoiding a major abdominal incision, and cirrhotic patients may have a reduced complication rate.
For liver resections, unlike other areas of abdominal surgery, the laparoscopic approach has not been widely developed. The reasons are presumed technical difficulties and the intraoperative hazards of bleeding and gas embolism. Another concern is the potential risk of tumor seeding in patients with malignant disease, who constitute the majority of candidates for liver resections. However, technologic refinements in laparoscopic instruments, experience in laparoscopic and hepatic surgery, and the application of the principles of oncologic surgery have led some groups, including ours, to explore the place of laparoscopic liver resections. Initial laparoscopic procedures on the liver included staging of tumors to select patients for open resection 1,2 and treatment of nonparasitic cysts by unroofing. 3 More recently, there have been reports of limited series of laparoscopic liver resections. 4–13
We initiated a prospective evaluation of laparoscopic liver resections in selected patients, and we report the results in our first 30 patients.
METHODS
Inclusion Criteria
In January 1996, a prospective study of laparoscopic liver resections was initiated. The laparoscopic approach was considered based on the size and location of the lesion or lesions and the preoperative diagnosis. Lesions that were 5 cm or less in diameter, had limited hepatic involvement, and were located in the left or peripheral right segments of the liver (Couinaud segments 2–6) were included. Preoperative diagnoses included presumed benign lesion, hepatocellular carcinoma (HCC) in cirrhotic patients, and noncolorectal metastases.
The indications for resection were not modified by the use of the laparoscopic approach. For resection of benign lesions, these were the presence of symptoms, a diagnosis of hepatic adenoma or cystadenoma, and an uncertain diagnosis on biopsy. The indications in HCC included Child class A patients with superficial tumors. The indications for metastases were the occurrence of a liver tumor during follow-up of patients with noncolorectal cancer.
Exclusion criteria included previous upper abdominal surgery, colorectal metastases, decompensated cirrhosis, and cardiac or respiratory failure.
Before the final decision to use laparoscopic resection was made, all cases were reviewed at a weekly conference attended by all staff surgeons, anesthesiologists, radiologists, and pathologists. Patients were informed of the innovative nature of the procedure and consent was obtained.
Surgical Technique
The procedures were performed by two surgeons, one an expert in hepatic surgery and the other in laparoscopic procedures. Liver resections were defined according to Couinaud’s classification, using the following terminology: left hepatectomy for resection of segments 2, 3, and 4; bisegmentectomy 2 and 3 for left lateral resection; segmentectomy for resection of one segment, according to its theoretical borders and defined by its number; and atypical resection for resection of less than one segment.
For resection of lesions in segments 2 through 5, the patient was placed in the supine position, with lower limbs apart. The surgeon was between the legs with one assistant on each side. Port sites are shown in Figure 1. For lesions in segment 6, the patient was placed in the left lateral decubitus position to expose the lateral and posterior aspect of the right lobe, as described for right adrenal resections (Fig. 2). 14 The surgeon was on the ventral side of the patient, with one assistant on the right and another on the other side of the table. Two monitors were used in most cases.
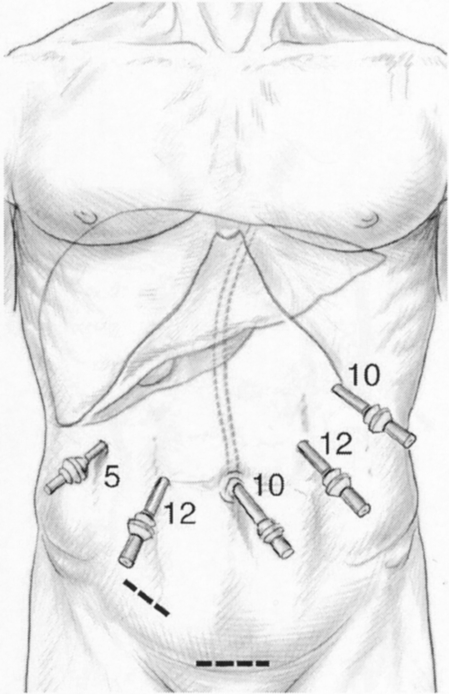
Figure 1. Typical port placement for resection of lesions located in segments 2 through 5. The patient is in the supine position with lower limbs apart, the surgeon between the legs.
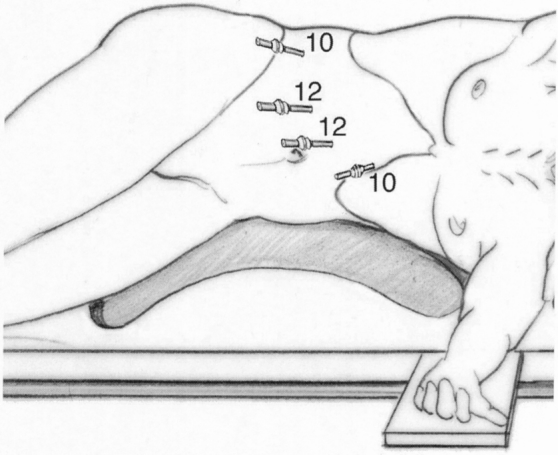
Figure 2. Typical port placement for resection of lesions in segment 6. The patient is in the left lateral decubitus position for right lobe mobilization and posterior exposure. The table can be turned to the right to tilt or flip the right lobe against the diaphragm and gain anterior access.
CO2 pneumoperitoneum was used. Abdominal pressure was monitored and maintained at less than 15 mmHg. A 30° laparoscope was used in all cases. The liver was explored visually and by laparoscopic ultrasound. Once resection had been decided on, a tape was placed around the porta hepatis and passed through a 16F rubber drain for use as a tourniquet for the Pringle maneuver. This maneuver was used systematically in cirrhotic patients, in resections of one segment or more in other patients, and whenever bleeding occurred during transection. Intermittent clamping was applied, with 15-minute clamping and 5-minute release periods. The lesser omentum was checked for the presence of a left hepatic artery. If present, it was clamped with a bulldog clamp. Hepatic transection was performed with a harmonic scalpel, which was sufficient for vascular and biliary structures up to 3 mm. Bipolar electrocoagulation was also available for minor bleeding. Larger structures were secured with clips. Portal pedicles and major hepatic veins were divided by application of a linear stapler (Fig. 3).
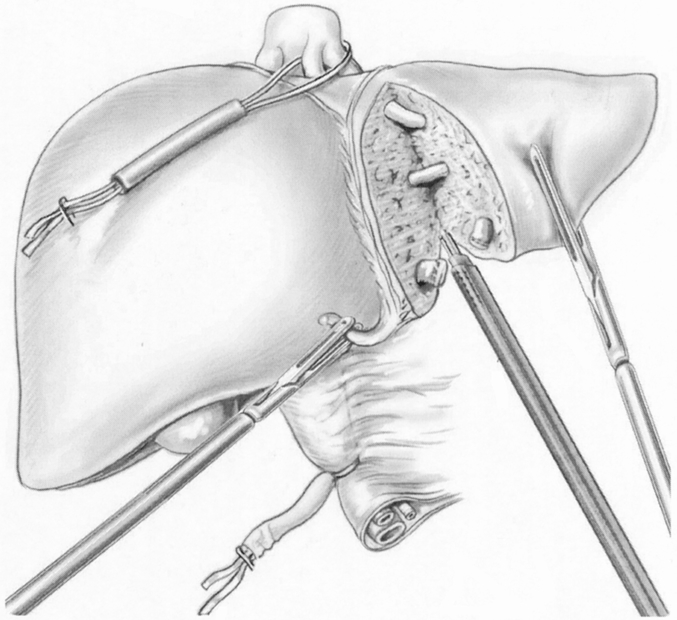
Figure 3. Laparoscopic bisegmentectomy 2 and 3. The portal triad is clamped. The middle and left hepatic veins are taped and can be readily clamped if necessary. Liver transection is performed with a harmonic scalpel. Larger structures are divided with a linear stapler.
In left-sided resections, the round, falciform, and left triangular ligaments and the lesser omentum were divided. Dissection of the falciform ligament was continued to the level of the inferior vena cava and the insertion of the hepatic veins. After the 10th procedure, extrahepatic control of the left and middle hepatic veins was used. The left lobe was lifted and the peritoneum above segment 1 was opened to expose the posterior insertion of the left hepatic vein. A soft dissecting clamp was carefully passed around the left and middle hepatic veins, which usually form a common trunk. A tape was placed around the hepatic veins and passed through a 16F rubber drain to clamp the middle and left hepatic veins, when necessary.
In right-sided resections, the right triangular ligament was divided, taking advantage of the lateral position of the patient. The dissection did not attempt to expose the inferior vena cava or the right hepatic vein because resections were limited to peripheral lesions. The operating table was then rotated to the right so that the right lobe lay on the diaphragm, and resection was performed.
The resected specimen was placed in a plastic bag and externalized through a separate incision, either along a previous appendectomy incision or a new suprapubic horizontal incision. The incision was made long enough to allow easy removal of the specimen in one piece, without fragmentation. This incision was immediately closed and the abdomen reinflated. The surgical field was irrigated and checked for bleeding or bile leak, and residual fluid was removed by suction. Abdominal drainage was usually omitted, and the pneumoperitoneum was vented.
Studied Criteria
The surgical procedure, postoperative course, and outpatient follow-up at 1, 3, and 12 months for benign disease, and beyond for malignant disease, were studied. The following data were collected prospectively: duration of surgery and clamping, blood loss, perioperative transfusions, surgical events, postoperative complications, hospital stay, recurrence, and distant events.
RESULTS
From May 1996 to December 1999, 31 eligible patients were informed of the possibility of a laparoscopic approach, and only 1 refused it. The laparoscopic approach was attempted in 30 of 159 liver resections (19%). There were 21 women and 9 men, with a mean age of 54 years (range 23–75). The lesions were located in the right liver in 13 patients and in the left liver in 17 (Fig. 4). Mean tumor size measured on the surgical specimen was 4.25 cm. The lesions were benign in 18 patients and malignant in 12 (Table 1). Examples of resected lesions are shown in Figures 5 and 6. A margin of at least 1 cm beyond tumor limits was obtained in all patients who underwent surgery for malignancy.
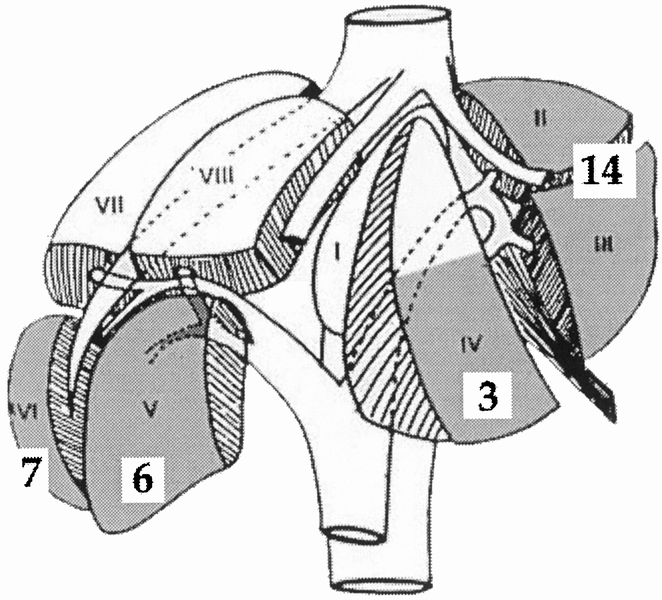
Figure 4. Locations of treated lesions according to Couinaud’s classification. Shaded areas are considered consistent with laparoscopic resection. Numbers in white squares are the number of lesions in each corresponding segment.
Table 1. HISTOLOGIC DIAGNOSIS IN 30 LAPAROSCOPIC LIVER RESECTIONS
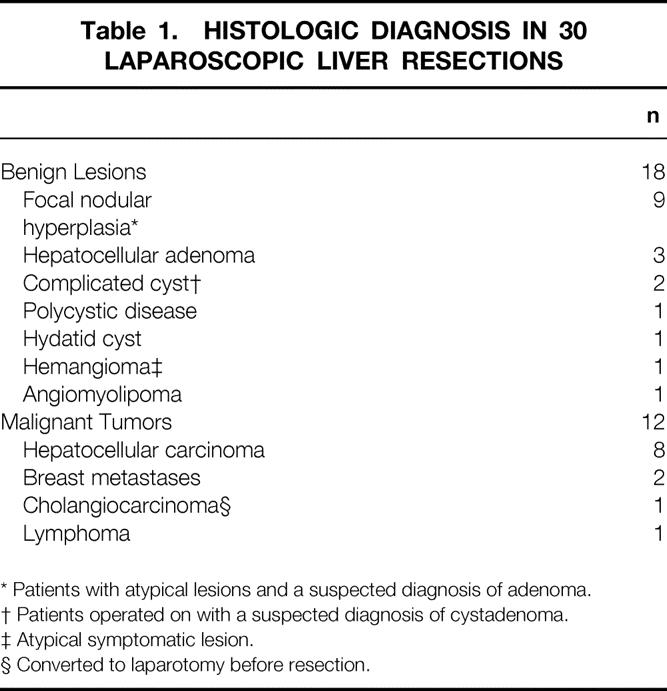
* Patients with atypical lesions and a suspected diagnosis of adenoma.
† Patients operated on with a suspected diagnosis of cystadenoma.
‡ Atypical symptomatic lesion.
§ Converted to laparotomy before resection.
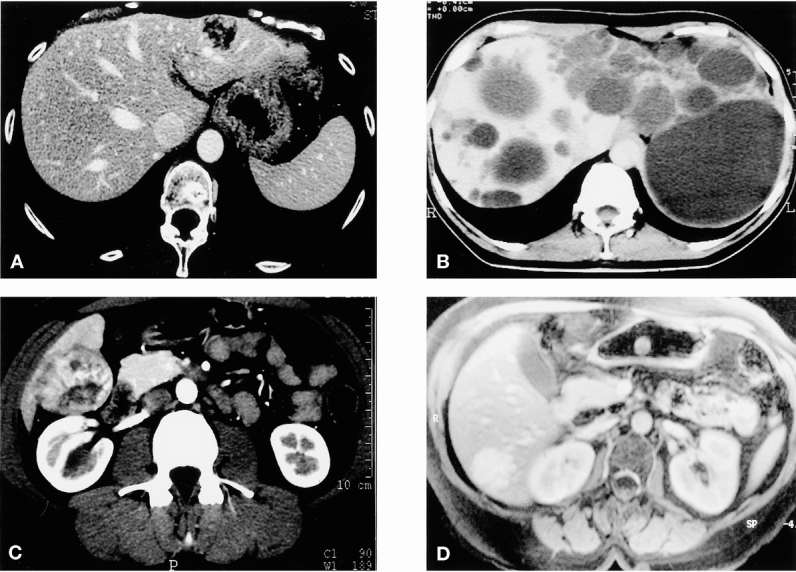
Figure 5. Examples of benign lesions. (A) Angiomyolipoma of segment 2; bisegmentectomy 2 and 3. (B) Hepatocellular adenoma of the right lobe with extrahepatic development; atypical resection. (C) Polycystic liver disease with left predominance; multiple unroofing and bisegmentectomy 2 and 3. (D) Focal nodular hyperplasia of segment 6; segmentectomy 6.
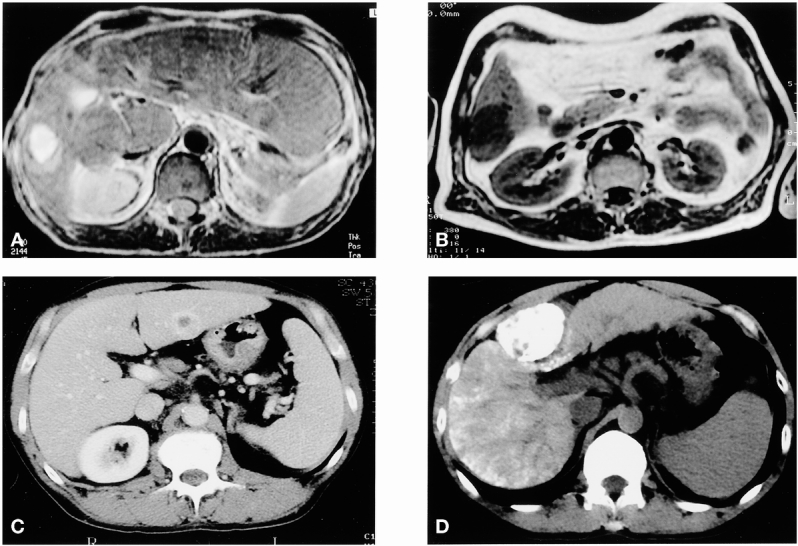
Figure 6. Examples of hepatocellular carcinomas in cirrhotic patients. (A) Lesion of segment 6 recurring after percutaneous ethanol injection in an atrophic right lobe with hypertrophy of segments 1, 2, and 3; segmentectomy 6. (B) Lesion of segment 3; segmentectomy 3. (C) Lesions of segment 6; segmentectomy 6. (D) Lesion of segment 4 with initial presentation by spontaneous rupture treated with chemoembolization and subsequent segmentectomy 4.
Indications
Of 12 patients with a presumed diagnosis of benign hepatocellular tumor, 4 had symptoms related to mass; the others underwent resection mainly with diagnostic intent. Histologic diagnosis after resection was hepatocellular adenoma in three and fibronodular hyperplasia (FNH) in nine. Patients with FNH lacked characteristic imaging and biopsy features, thus precluding preoperative diagnosis.
In two patients with a symptomatic cystic lesion, cystadenoma was suspected, leading to resection. Both cysts contained fibrinous and necrotic material, but pathologic study of the resected specimen showed epithelium without mucous secretion, more suggestive of complicated solitary cysts. One patient with polycystic liver disease had pain and food intolerance as a result of cystic disease predominant in the left lobe. Multiple bilateral cyst unroofing and bisegmentectomy 2 and 3 were performed. The patient with hydatid cyst had abdominal pain and calcified solid tumor. The patient with hemangioma had abdominal pain in the area of an atypical lesion, and the patient with angiomyolipoma had a history of melanoma and suspected metastasis. In these latter three patients, final diagnosis was obtained only after histologic study of the resected tumor.
Of the eight patients with HCC and cirrhosis, one had undergone previous treatment with percutaneous ethanol injection and one with chemoembolization. The other six patients had superficial lesions considered unsuitable for percutaneous treatment. They all were Child A, and six had grade 1 or 2 esophageal varices. In one patient with an unspecified tumor, histology showed it to be cholangiocarcinoma. Histologic study of the resected specimen confirmed a primary hepatic lymphoma in another patient. Two patients with breast metastases had liver tumor occurring in the follow-up of the primary lesion.
Intraoperative Results
The laparoscopic procedure was completed in 28 patients. Conversion to laparotomy occurred in patients 11 and 20. Patient 11 had mild hemorrhage in the area of the left hepatic vein near the end of a procedure for FNH. A short midline incision was performed, permitting safe completion of the resection. In patient 20, the conversion occurred before the resection started because exposure was considered insufficient for a safe procedure; an open resection was performed. Both patients are included in the series, but patient 20 was excluded from the analysis of surgical results because laparoscopic resection had not started.
The types of laparoscopic liver resection are summarized in Table 2. They included one major hepatectomy (three or more segments); all other resections were limited to less than three segments. Portal triad clamping was used in 20 patients and was intermittent in all of them. Its mean cumulative duration was 50 minutes (range 10–117). The left and middle hepatic veins were taped in five patients and clamped in three for 5 to 15 minutes. Mean blood loss was 300 mL (median 100, range 0–1,500).
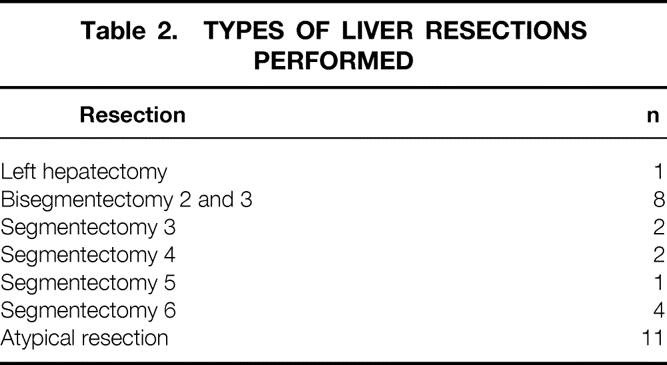
Table 2. TYPES OF LIVER RESECTIONS PERFORMED
During transection, there were four episodes of mild intraoperative bleeding, which did not differ from those observed during open resection. Three were from a branch of the left or middle hepatic vein, leading to conversion in one patient. One episode of hemorrhage occurred from insufficient stapling of a portal pedicle. During clamping release, it was not immediately diagnosed. Additional control of this pedicle resolved the problem without conversion. This patient had HCC and cirrhosis and received intraoperative transfusions of four units of packed red cells. None of the other patients received intraoperative transfusions.
There were no signs suggestive of gas embolism in any of the patients. One diaphragm tear occurred during mobilization of inflammatory adhesions of the right lobe in a patient with HCC previously injected with ethanol. It was treated by suture and pneumothorax exsufflation.
Mean surgical time was 214 ± 87 minutes (median 180, range 60–360). Mean weight of the specimen was 163 ± 98 g (median 150, range 50–420).
Postoperative Results
There were no deaths. Postoperative complications consisted of six complications (20%), five of which occurred in patients with malignant disease. There were two pulmonary complications, both in cirrhotic patients. One was due to Legionella pneumonia and required a temporary tracheostomy. The other patient, a heavy smoker, required 6 days of mechanical ventilation. These two patients received three and two units of packed red cells in the postoperative period because of mild anemia but did not develop decompensation of cirrhosis. Both recovered completely. Another cirrhotic patient developed transient ascites. The five other cirrhotic patients had a smooth postoperative course. In one patient, pyoderma gangrenosum, a rare autoimmune necrotic skin lesion requiring steroid therapy, occurred at port sites. Two incisional hernias occurred on 12-mm port orifices in obese patients, requiring minor secondary reoperation. There were no cases of postoperative bleeding or bile leak.
Mean hospital stay of the whole series was 9.6 days (median 7, range 3–40). It was 6 days (median 6, range 3–8) for uncomplicated cases and 5.2 days for benign cases (median 5, range 3–8).
All patients with benign lesions were cured of lesions and symptoms on follow-up. Two patients who underwent surgery for HCC developed recurrence after laparoscopic resection. One patient had multiple bilateral nodules at 8 months and as of this writing was receiving palliative therapy. The other developed a second solitary tumor in a remote area of the liver 14 months after resection and as of this writing was being considered for liver transplantation. The other six patients were alive without recurrence. Of the two patients with metastases of breast carcinoma, one had an asymptomatic intrahepatic recurrence diagnosed at 6 months. The patient with lymphoma had no other location at postoperative screening and was well at 9 months. After a mean follow-up of 12 months, no port-site recurrence was observed in any patient who underwent surgery for malignant disease, and no tumor recurrence could be attributed to the laparoscopic approach.
DISCUSSION
This series demonstrates the technical feasibility of limited laparoscopic liver resections in selected patients. The procedures can be considered safe because there were no deaths and no unusual complications, and only 10% of the patients required transfusions during their hospital stay.
The patients underwent surgery collaboratively by an expert in each subspecialty (hepatic surgery and laparoscopic surgery), and the complexity of resection gradually increased. Careful patient selection is essential. The safe segments for laparoscopic resection are the anterior and lateral segments (segments 2 through 6). Tumors located in the posterior and superior segments (segments 1, 7, and 8) should not be considered at present because laparoscopic exposure is difficult and there are connections with the inferior vena cava and major hepatic veins.
The lesion should be reasonably small, because large tumors are more difficult to mobilize, have more dangerous vascular connections, and have a higher risk of bleeding. Although there was one full left hepatectomy (three segments), our patient selection led to limited resections in other patients, including unisegmentectomies, bisegmentectomies, and atypical resections. This is in accordance with the literature. 4–13
Our aim was to reproduce the technique used for open liver resections. Portal triad clamping was applied in all resections of one segment or more. Intermittent clamping was used for several reasons. It is better tolerated than continuous clamping in cases of underlying liver disease or prolonged clamping. 15–17 During open surgery, we use it systematically in cirrhotic patients and when the anticipated clamping time exceeds 40 minutes. 18 During laparoscopy, intermittent clamping was used in all patients, not only in those with cirrhosis, because the duration of liver transection is usually longer, and suction for minor bleeding cannot be used permanently. Intermittent clamping allows the surgeon to take time for meticulous transection, and it can be applied for periods of more than 1 hour, 15,16 as in two of our patients.
The harmonic scalpel proved useful during laparoscopic resection because it can coagulate and divide the hepatic parenchyma during the same application. However, it should be given enough time to operate to achieve adequate hemostasis. We experienced four bleeding episodes during transection; they were not different from what can occur during open surgery. No major hemorrhage occurred. Only one patient received a transfusion during surgery; two received two to three units after surgery. As in open surgery, management of bleeding during transection requires technical experience; more importantly, adequate prophylactic measures are the best guarantee. These include inflow occlusion, but outflow control may also be required, as we previously reported. We used control of the left and middle hepatic veins in left-sided resections, as we do in open surgery, 18 and this permitted control of bleeding in some instances. Control of the right hepatic vein was not attempted because right-sided resections were only peripheral. One specific issue, however, is that when intermittent clamping is used during laparoscopy, bleeding during clamp release periods can be more easily overlooked. Continued monitoring of the surgical field remains warranted during unclamping periods.
The potential risk of gas embolism led some authors to use gasless suspension laparoscopy. 4,6,8 When CO2, which is highly soluble, is used, this accident is rare. 19,20 It has not been reported during laparoscopic liver resections or adrenalectomies, which also include dissection of the inferior vena cava or its major branches. However, precautions such as abdominal pressure monitoring and hepatic vein control are warranted.
The duration of surgery in this series of mostly minor resections was long, with a mean of 214 minutes. This has been observed for other complex laparoscopic procedures and is associated with a learning curve effect. The operations were particularly long in cirrhotic patients. To transect the fibrotic liver requires time, and segmentectomies were often performed. These procedures are often longer than straight resections, even in open surgery. Despite the long times, patient recovery was excellent: most patients, including five with cirrhosis, were discharged from the hospital in less than 1 week. There were no specific postoperative complications such as liver failure, hemorrhage, or bile leaks.
The two major complications were respiratory failures, both occurring in cirrhotic patients. These complications were due in one patient to infection with Legionella and in the other to delayed ventilator weaning. Although postoperative pulmonary function has been reported to be better preserved after laparoscopy than after laparotomy, 21,22 the length of some laparoscopic procedures may have outweighed this advantage. The other complications were more minor, but two patients had incisional hernia, emphasizing the need for adequate fascia closure of port sites of more than 10 mm, which may be difficult in obese patients.
Using the laparoscopic approach should not modify the indications for liver resections. This is particularly true for benign lesions. Cysts and hemangiomas are easily diagnosed and do not require resection, except for the rare symptomatic or doubtful cases. Our attitude in patients with benign hepatocellular tumors 23,24 includes resections of hepatic adenomas and conservative management of FNH if the diagnosis is certain. In our experience, FNH can be diagnosed on noninvasive imaging in 80% of patients and by a biopsy in 10%, with diagnostic resection required in only 10% (unpublished data). The use of laparoscopic liver resection did not change this approach and did not lead to the resection of more cases of FNH. We proposed resection to patients with symptoms, suspected adenoma, or HCC, or when a histologic diagnosis could not be made on biopsies.
Most of the indications for liver resections are malignant lesions, including metastatic disease, mostly of colorectal origin, and HCC. There is a debate about the specific risks of tumor seeding during laparoscopic surgery. Early attempts at laparoscopic resection of cancers were associated with high numbers of abdominal metastases, especially at port sites, but also in the peritoneal cavity. 25–28 The potential mechanisms for tumor seeding include direct contamination by technical errors and the role of the pneumoperitoneum, through hyperpressure, cell exfoliation, and cytokine activation. 27 More recent prospective studies ensuring proper oncologic surgery (“no-touch” technique, specimen bag, and abdominal wall protection) do not seem to confirm this trend. 29 Experimental animal studies comparing tumor cell implantation in the presence of CO2 pneumoperitoneum, gasless laparoscopy, or laparotomy have reported conflicting results and usually fail to reproduce the clinical situation. 30 However, the fact that immune response is better preserved after laparoscopy than open surgery seems established. 31 In this series, the laparoscopic approach was used to treat 12 patients with malignant disease, including HCC and noncolorectal metastases. There were neither detectable port-site or intraperitoneal metastases, nor unusually early tumor recurrences.
The aim of laparoscopic resection in cirrhotic patients was to reduce postoperative complications. Liver resections, even the most minor ones, in cirrhotic patients carry a high risk of complications. The main complication is decompensation of cirrhosis, with the development of ascites, jaundice, and encephalopathy. 32–36 Only one of eight patients, all of whom had at least one segment resected, developed mild and transient ascites, and another one had transient jaundice. These results were obtained in Child A patients with superficial tumors, but interestingly six of the eight patients had portal hypertension, as attested by the presence of esophageal varices. Portal hypertension is a major risk factor for the development of postoperative decompensation after open resection. In the study by Bruix et al 36 on Child A cirrhotic patients undergoing open resection for limited HCC, 59% of 29 had at least one sign of postoperative decompensation. The main predictor of postoperative decompensation was the presence of portal hypertension, with a 73% rate of ascites. This is also our experience with open resection, which we hesitate to perform in patients with esophageal varices. The laparoscopic approach might offer an improvement, as has been suggested by several reports. 4,6,8,12 A possible reason is the preservation of the abdominal wall, which avoids interruption of large collateral veins and the exposure of abdominal viscera, with the dual benefit of less need for fluid infusion and improved reabsorption of ascites. These results require confirmation in larger numbers of patients.
Noncolorectal metastases are not fully accepted as indications for liver resection because extrahepatic disease is usually present, and the impact of resection on survival has not been established. 37,38 However, occasional long-term survival is possible, and these patients should be managed on an individual basis. In those patients, who usually did not undergo prior laparotomy, staging as a first step of the laparoscopy allows previously undetected intrahepatic or extrahepatic disease to be identified, permitting selection of candidates for resection by laparoscopy or laparotomy. In contrast, resection of hepatic colorectal metastases clearly improves patient survival and represents a major indication for liver resection. 39 Laparoscopic treatment of colorectal cancer is controversial, and its evaluation in several multicenter randomized trials is ongoing. 30 Although we deal here with metastatic disease, we decided to exclude these patients until the role of laparoscopy in colorectal cancer is elucidated.
The advantages of laparoscopic liver resections should be those of all laparoscopic procedures, which obviously reduce the parietal damage in the abdomen. This may be associated with reductions in postoperative pain and hospital stay, reduced peritoneal adhesions, and an earlier return to previous activity. A cosmetic advantage is also clear because of the absence of long abdominal incisions, and this should be taken into account, especially in young women. The global benefit seems clear in patients with benign disease for all these reasons. These advantages also apply to cancer patients, provided they do not interfere with the primary goal of maximal tumor clearance and minimal tumor recurrence. Other potential advantages in cancer patients include less postoperative immune dysfunction and earlier access to adjuvant treatment by earlier recovery. Finally, patients who may require repeat procedures, such as repeat hepatectomy or subsequent transplantation, may benefit from an easier reoperation.
In conclusion, this study provides information about the possibilities, indications, and limits of laparoscopic liver resections. Laparoscopic resections are feasible and safe in patients with left-sided and right-peripheral lesions requiring limited resection.
Discussion
Prof. D. Jaeck (Strasbourg, France): First, Daniel, I congratulate you because I enjoyed your nice presentation. You showed that laparoscopic liver resection is feasible, and that in your hands it is safe! However, I would like to discuss the indications of this approach. It is clear that for benign lesions we are not allowed to have morbidity or any mortality. So we want to be sure to have at least the same results as in open surgery. For cancer, as you discussed particularly in the conclusion, you did not prove that it is as safe and as efficient as open surgery. So, which are the real advantages? Cost? Probably not, because we need more sophisticated instruments. Time? Certainly not, because you showed that hospital stay and duration of the procedure are the same as for open surgery. Blood loss is at least the same as with open surgery, perhaps even more. Morbidity seems to be the same, and your conclusion was that the potential benefit, if you do not consider the benign disease, could be obtained in case of hepatocellular carcinoma in cirrhotic patients. So I would like to ask if you could tell us more precisely for which type of tumor (location, stage) and in which kind (Child-Pugh stage) of cirrhotic patients you would propose a laparoscopic resection.
Prof. D. Cherqui (Créteil, France): Concerning benign lesions, I do not see that the risk has been increased in this study compared to open surgery. Indeed, there were no complications in patients with benign disease in this study. We did not perform these procedures until we were confident that we did not expose the patients to a higher risk. The operations were performed collaboratively by two surgeons, one expert in liver surgery and one expert in laparoscopic surgery. The patients were selected because they had a small and peripheral lesion. As soon as we felt that either exposure or minor bleeding was a problem, we converted to open surgery, and this occurred in two of the 30 cases.
Concerning patients with malignant lesions, who represent the majority of indications of liver resections, there is a concern about the possibility of tumor seeding. We chose to use laparoscopic resections in patients with hepatocellular carcinoma and cirrhosis with the aim of reducing morbidity, which is high in these patients. There is evidence that part of the morbidity is related to the abdominal wall incision, with division of collateral circulation. We anticipated that there would be less postoperative ascites, and indeed, although this is not controlled data, only one patient developed ascites. Ascites is usually reported in 30% to 50% of the cases, even after small resections by open surgery in cirrhotic patients. We excluded patients with colorectal metastases from this study because the use of laparoscopy is presently a controversial issue in colorectal cancer, even for the resection of the primary tumor. Patients with small colorectal metastases are operated on for cure, so at the present time we are waiting for the results of controlled studies in progress for laparoscopic resection of the primary colorectal tumors. However, patients with primary colorectal tumors may have peritoneal invasion, which has a higher risk of dissemination, while liver metastases are very rarely associated with peritoneal invasion. For patients with breast metastases, we used laparoscopy because these patients often have very small lesions that one cannot detect on preoperative imaging. Initial laparoscopy permitted us to avoid unnecessary laparotomy in such cases and to proceed with laparoscopic resection in other cases.
Prof. A. Johnson (Sheffield, United Kingdom): This is an interesting paper on technique, but the indications in about half the cases might be questioned (e.g., FNH with good MRI scans and secondary breast tumors). You state that the benefit is clear in young people, but I still think you have to prove that for the indications that would be generally agreed. The particular group in which this technique might be helpful are cirrhotic patients, but as you were only operating on grade A liver tissue, you would not expect much postoperative ascites. I wonder if you measured the metabolic response, because studies in gallbladder surgery show that the pneumoperitoneum itself produces a tremendous increase in antidiuretic and other hormones. The laparoscopic approach could actually be increasing, rather than decreasing, the problem of ascites. It is a very long operation, unnecessarily long for benign disease, and if you add up the length of all your incisions there is quite significant damage to the abdominal wall, 9 or 10 cm perhaps. One question about technique: you said you put a sling around the right hepatic and middle hepatic veins. If you did make a hole there, there is a real risk of a gas embolus with the pneumoperitoneum. Did you always do that, or was it only used on certain occasions? Two final questions: did you do any comparative costings, compared with the open procedure, and why did the patients stay in for so long after the laparoscopic resection?
Prof. D. Cherqui (Closing Discussion): Concerning the indications, I did not have time to show them to you here, but in our institution, many patients with benign lesions found incidentally at ultrasound are referred for diagnosis, and these include mainly young women. We see about 60 new cases of focal nodular hyperplasia (FNH) every year. Presently, noninvasive diagnosis is obtained in 80% of these patients just by CT and MRI scans. In the remaining 20%, diagnosis cannot be obtained by these means and a biopsy is required. Our previously reported policy is to undertake laparoscopic biopsy. The reason is that we get a large specimen for histologic diagnosis. In more than half of the cases, frozen-section study gives us the diagnosis. If it says FNH, we do not resect and the procedure is limited to laparoscopy, with the patient out of hospital the day after. So it is only a very small percentage of the patients with FNH that undergo resection, including the rare patients with symptoms and those in whom the pathologist cannot diagnose FNH. The latter are atypical FNHs that can only be diagnosed on the full specimen, therefore requiring resection for diagnosis.
For breast tumor metastases, there is no consensus so far as to the benefit of liver resection. However, in selected patients, the prognosis seems related to the liver lesions, and there are several articles in the literature advocating liver resection of isolated and limited breast metastases. Several cases are proposed every year. Only a few are operated on, but occasional long-term survival is observed. Indeed, we only operated on Child A patients because this is, for us, the only indication. In Child B patients with small hepatocellular carcinoma, we never consider resection and we would rather do transplantation. In Child A patients, there are several options. When the tumor is superficial, we believe that it is not an indication for percutaneous treatment because this would open the tumor in the peritoneum. So we propose resection of superficial tumors and do it by laparoscopy when feasible. The sling around the middle and left hepatic vein must be placed cautiously, and we did not observe any case of gas embolus. It is true that numerous port sites are required to perform this procedure, plus an extraction incision. However, this is the case for most complex laparoscopic procedures. I do not think that you can sum up all small incisions and say it is equal to one large laparotomy with fascia and muscle division. Finally, laparoscopic liver resection is certainly more expensive at the present time, but the primary goal is to be less invasive, and hopefully costs will be reduced in the future.
Footnotes
Correspondence: Daniel Cherqui, MD, Service de Chirurgie Générale et Digestive, Hôpital Henri Mondor, 94010 Créteil, France.
Presented at the Seventh Annual Meeting of the European Surgical Association, Amstel Intercontinental Hotel, Amsterdam, The Netherlands, April 14–15, 2000.
E-mail: daniel.cherqui@hmn.ap-hop-paris.fr
Accepted for publication July 2000.
References
- 1.John TG, Greig JD, Crosbie JL, Miles WF, Garden OJ. Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg 1994; 220: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahusen F, Cuesta MA, Borgstein PJ, et al. Selection of patients for resection of colorectal metastases to the liver using diagnostic laparoscopy and laparoscopic ultrasonography. Ann Surg 1999; 230: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morino M, De Giuli M, Festa V, Garrone C. Laparoscopic management of symptomatic nonparasitic cysts of the liver. Ann Surg 1994; 219: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashizume M, Takenaka K, Yanaga K, et al. Laparoscopic hepatic resection for hepatocellular carcinoma. Surg Endosc 1995; 9: 1289–1291. [DOI] [PubMed] [Google Scholar]
- 5.Gugenheim J, Mazza D, Katkhouda N, Goubaux B, Mouiel J. Laparoscopic resection of solid liver tumours. Br J Surg 1996; 83: 334–335. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery 1996; 120: 468–475. [DOI] [PubMed] [Google Scholar]
- 7.Azagra JS, Goergen M, Gilbert E, Jacobs D. Laparoscopic anatomical hepatic left lateral segmentectomy: technical aspects. Surg Endosc 1996; 7: 758–761. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe Y, Sato M, Ueda S, et al. Laparoscopic hepatic resection: A new and safe procedure by abdominal wall lifting method. Hepato-Gastroenterology 1997; 44: 143–147. [PubMed] [Google Scholar]
- 9.Rau HG, Buttler E, Meyer G, Schardey HM, Schildberg FW. Laparoscopic liver resection compared with conventional partial hepatectomy: a prospective analysis. Hepato-Gastroenterology 1998; 45: 2333–2338. [PubMed] [Google Scholar]
- 10.Huscher C, Lirici MM, Chiodini S. Laparoscopic liver resections. Sem Laparosc Surg 1998; 5: 204–210. [DOI] [PubMed] [Google Scholar]
- 11.Samama G, Chiche L, Bréfort JL, Le Roux Y. Laparoscopic anatomical hepatic resection. Surg Endosc 1998; 12: 76–78. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Atty MY, Farges O, Jagot P, Belghiti J. Laparoscopy extends the indications for liver resections in patients with cirrhosis. Br J Surg 1999; 86: 1397–1400 [DOI] [PubMed] [Google Scholar]
- 13.Katkhouda N, Hurwitz M, Gugenheim J, et al. Laparoscopic management of benign solid and cystic lesions of the liver. Ann Surg 1999; 229: 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagner M, Pomp A, Henford BT, Pharand D, Lacroix A. Laparoscopic adrenalectomy: lessons learned from 100 consecutive procedures. Ann Surg 1997; 226: 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias D, Desruennes E, Lasser P. Prolonged intermittent clamping of the portal triad during hepatectomy. Br J Surg 1991; 78: 42–44. [DOI] [PubMed] [Google Scholar]
- 16.Wu CC, Hwang CR, Liu TJ, P’eng FK. Effects and limitations of prolonged intermittent ischemia for hepatic resection on the cirrhotic liver. Br J Surg 1996; 83: 121–124. [DOI] [PubMed] [Google Scholar]
- 17.Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection. A controlled study. Ann Surg 1999; 229: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherqui D, Malassagne B, Colau PI, et al. Hepatic vascular exclusion with preservation of the caval flow for liver resections. Ann Surg 1999; 230: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskop RJ, Lubarsky DA. Carbon dioxide embolism during laparoscopic cholecystectomy. South Med J 1994; 84: 414–415. [DOI] [PubMed] [Google Scholar]
- 20.Yacoub OF, Cardona I, Coveler LA, Dodson MG. Carbon dioxide embolism during laparoscopy. Anesthesiology 1982; 57: 533–535. [DOI] [PubMed] [Google Scholar]
- 21.Delaunay L, Bonnet F, Cherqui D, et al. Laparoscopic surgery minimally impairs postoperative cardiorespiratory and muscle performance. Br J Surg 1995; 82: 373–376. [DOI] [PubMed] [Google Scholar]
- 22.Schwenk W, Bohm B, Witt C, et al. Pulmonary function following laparoscopic or conventional colorectal resection. A randomized controlled evaluation. Arch Surg 1999; 134: 6–12. [DOI] [PubMed] [Google Scholar]
- 23.Cherqui D, Rahmouni A, Charlotte F, et al. Management of focal nodular hyperplasia and hepatocellular adenoma in young women. A series of 41 patients with clinical, radiologic and pathologic correlations. Hepatology 1995; 22: 1674–1681. [PubMed] [Google Scholar]
- 24.Mathieu D, Kobeiter H, Cherqui D, Rahmouni A, Dhumeaux D. Oral contraceptive intake in women with focal nodular hyperplasia of the liver. Lancet 1998; 352: 1679–1680. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone PAS, Rohde DC, Swartz SE, Fetter JE, Wexner SD. Port-site recurrences after laparoscopic and thoracoscopic procedures in malignacy. J Clin Oncol 1996; 14: 1950–1956. [DOI] [PubMed] [Google Scholar]
- 26.Cook TA, Dehn TC. Port-site metastases in patients undergoing laparoscopy for gastrointestinal malignancy. Br J Surg 1996; 83: 1419–1420. [DOI] [PubMed] [Google Scholar]
- 27.Neuhaus SJ, Texler M, Hewett PJ, Watson DI. Port-site metastases following laparoscopic surgery. Br J Surg 1998; 85: 735–741. [DOI] [PubMed] [Google Scholar]
- 28.Jacquet P, Averbach AM, Stephens AD, Sugarbaker PH. Cancer recurrence following laparoscopic colectomy. Report of two patients treated with intraperitoneal heated chemotherapy. Dis Colon Rectum 1995; 38: 1110–1114. [DOI] [PubMed] [Google Scholar]
- 29.Poulin EC, Mamazza J, Schlachta CM, Gregoire R, Roy N. Laparoscopic resection does not adversely affect early survival curves in patients undergoing surgery for colorectal adenocarcinoma. Ann Surg 1995; 229: 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beart RW. Laparoscopy: to inflate or to lift? Cancer 1999; 86: 747–748. [PubMed] [Google Scholar]
- 31.Vittimberga FJ, Foley DP, Meyers WC, Callery MP. Laparoscopic surgery and the systemic immune response. Ann Surg 1998; 227: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bismuth H, Houssin D, Ornowski J, Meriggi F. Liver resections in cirrhotic patients: a Western experience. World J Surg 1986; 10: 311–317. [DOI] [PubMed] [Google Scholar]
- 33.Franco D, Capussotti L, Smadja C, et al. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology 1990; 98: 733–738. [PubMed] [Google Scholar]
- 34.Nagasue N, Kohno H, Chang YC, et al. Liver resection for hepatocellular carcinoma: results of 229 consecutive patients during 11 years. Ann Surg 1993; 217: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg 1993; 218: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111: 1018–1022. [DOI] [PubMed] [Google Scholar]
- 37.Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, et al. Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg 1998; 187: 4874–4893. [DOI] [PubMed] [Google Scholar]
- 38.Harrison LE, Murray FB, Newman, et al. Hepatic resection for noncolorectal, nonendocrine metastases: a fifteen-year experience with ninety-six patients. Surgery 1997; 121: 625–632. [DOI] [PubMed] [Google Scholar]
- 39.Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg 1999; 86: 158–169. [DOI] [PubMed] [Google Scholar]


