Abstract
Objective
To analyze the cost-effectiveness of resection for liver metastases compared with standard nonsurgical cytotoxic treatment.
Summary Background Data
The efficacy of hepatic resection for metastases from colorectal cancer has been debated, despite reported 5-year survival rates of 20% to 40%. Resection is confined to specialized centers and is not widely available, perhaps because of lack of appropriate expertise, resources, or awareness of its efficacy. The cost-effectiveness of resection is important from the perspective of managed care in the United States and for the commissioning of health services in the United Kingdom.
Methods
A simple decision-based model was developed to evaluate the marginal costs and health benefits of hepatic resection. Estimates of resectability for liver metastases were taken from UK-reported case series data. The results of 100 hepatic resections conducted in Sheffield from 1997 to 1999 were used for the cost calculation of liver resection. Survival data from published series of resections were compiled to estimate the incremental cost per life-year gained (LYG) because of the short period of follow-up in the Sheffield series.
Results
Hepatic resection for colorectal liver metastases provides an estimated marginal benefit of 1.6 life-years (undiscounted) at a marginal cost of £6,742. If 17% of patients have only palliative resections, the overall cost per LYG is approximately £5,236 (£5,985 with discounted benefits). If potential benefits are extended to include 20-year survival rates, these figures fall to approximately £1,821 (£2,793 with discounted benefits). Further univariate sensitivity analysis of key model parameters showed the cost per LYG to be consistently less than £15,000.
Conclusion
In this model, hepatic resection appears highly cost-effective compared with nonsurgical treatments for colorectal-related liver metastases.
Colorectal cancer is the second most common cancer in the United Kingdom, with an annual incidence steadily reported at approximately 57 per 100,000 population. 1–4 Prevalence increases with age (almost 50% of cases occur in patients older than 60 4) and is greater in men (approximately 60% of cases). Approximately 25% of patients with colorectal cancer have detectable liver metastases at presentation (synchronous metastases). A further 25% develop metastases during the course of their disease (metachronous metastases), usually within 2 years after initial surgical treatment of their primary tumor. 5 Metastatic disease of the liver remains a major cause of cancer-related death. 4 Overall survival is closely related to tumor burden: patients with single or multiple metastases restricted to one lobe of the liver (unilobar disease) have an expected median survival of less than 24 months, 6 and patients with bilobar disease have a median survival of less than 18 months. 7 If untreated, most patients would not be expected to survive much beyond 9 to 12 months. 8
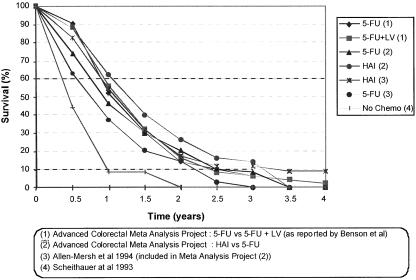
Conventional treatments for colorectal liver metastases are based on systemic chemotherapy, generally 5-fluorouracil regimens or local control with hepatic arterial chemotherapy. 4,9–12 We reviewed series and randomized trials of chemotherapy-based treatments for advanced colorectal cancer, including newer hepatic arterial therapy approaches, bolus versus continuous therapy, 5-fluorouracil plus modulator combinations, and the results of best supportive care only. Even in the most recent series, we found that there was no significant survival beyond 3 years, and that the survival benefits from chemotherapy remain small (Fig. 1). In contrast, today, surgical resection of colorectal metastases offers the prospect of long-term disease-free survival and has the potential for cure. 5,13
Figure 1. Survival benefits of various chemotherapy regimens in advanced colorectal cancer.
Because of the high death and complication rates previously associated with liver surgery, surgical removal of liver metastases has been restricted to less than 2% of patients in the United Kingdom, 4 generally those with a single, clearly defined metastasis in one lobe. However, recent advances in surgical techniques now allow safe resections in patients with multiple metastases and bilobar disease. Specialist surgical centers have reported that approximately 10% to 25% of patients with colorectal metastases undergo potentially curative resections, with overall 5-year survival rates of 20% to 45%. Gradual improvements in both surgical ability and outcomes have been noted in the past 10 to 15 years. 14
Most reports of liver resection case series come from the United States and mainland Europe. Although some patients, especially those with single metastases, do have access to specialist liver resection, there is a lack of published survival and complications data in the United Kingdom. It is possible that more patients with potentially resectable metastases remain undiagnosed or receive only standard palliative care. 15 This situation could indicate a lack of awareness of recent technical advances that increase the scope for curative resection, or the existence of resource constraints in the commissioning of specialist surgical services. Investment in these services requires consideration of both clinical efficacy and cost-effectiveness. Radical liver resections can leave patients requiring significant postsurgical care, often involving high-cost intensive care. The true costs of liver resection and the longer-term benefits in terms of extended life and avoided healthcare use remain uncertain, however.
This study reports the use of a decision analysis model to estimate the marginal direct costs and clinical benefits of liver resection. 16–18 It is part of a U.K. Development and Evaluation Committee health technology appraisal process. The model uses the outcomes of published case series data, standard unit costs, and our clinical experience of resource use in patients with liver metastases from colorectal primary cancers. This study is a cost-effectiveness analysis considering only direct costs, reported from the perspective of a healthcare provider.
METHODS
Literature Search
Randomized controlled trials, meta-analyses, literature reviews, and case series related to the surgical treatment of liver metastases from colorectal primaries were identified by systematic search in electronic databases, including MEDLINE, EmBASE, CANCERLIT, Cochrane Library, HMIC (Department of Health/King’s Fund/HELMIS), CRD (DARE/NEED), and other health technology assessment databases. This was supplemented by information obtained from Internet websites, personal contacts, and cancer-related literature databases. Search terms included combinations of the following: colon cancer, rectum cancer, liver metastasis, liver cancer, liver resection, colonic neoplasms, rectal neoplasms, liver neoplasms, hepatectomy, and resection. Although initially restricting our search to the period January 1990 to July 1998, we also included referenced case series dating as far back as 1984.
Effectiveness Data
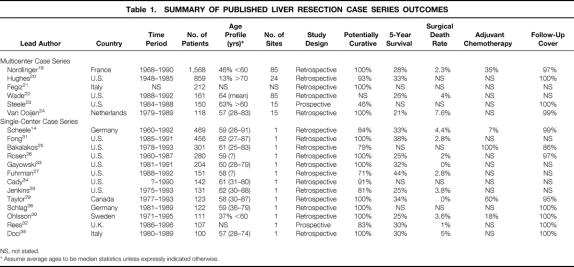
The initial search strategy identified 59 case series and 5 reviews, but no randomized controlled trials or meta-analyses. We selected case series reporting survival data for more than 100 patients, chosen as a hypothetical critical volume of clinical experience for highly specialized techniques in liver surgery. When the case series included sequential updates on patient cohorts from individual centers, only the latest reports were selected. This filtering yielded 19 independent case series representing distinct patient cohorts, with sufficient outcome data to derive overall survival, surgical complication rates, and preoperative prognostic factors (Table 1). 14,19–36 Overall 5-year survival rates ranged from 21% to 44% and were generally higher in case series from single-center studies.
Table 1. SUMMARY OF PUBLISHED LIVER RESECTION CASE SERIES OUTCOMES
NS, not stated.
* Assume average ages to be median statistics unless expressly indicated otherwise.
Model Structure
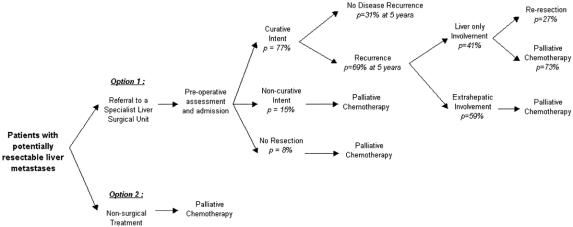
We constructed a decision model (Fig. 2) representing treatment pathways for patients judged as having liver metastases potentially resectable with curative intent. 37 Such patients are typically colorectal cancer patients after resection of the primary tumor, followed up with ultrasound or computed tomography scans and carcinoembryonic antigen levels, and presenting with new abdominal symptoms. Two basic clinical treatment decision options were considered:
Figure 2. Decision model.
Option 1: Referral to a specialist liver surgeon for further assessment of suitability for curative resection, where indicated, at laparotomy after admission
Option 2: Nonsurgical treatment with standard systemic (or locoregional) 5-fluorouracil therapy or other palliative regimens depending on previous history of adjuvant 5-fluorouracil treatment.
The model also took into account patients who were referred for surgical assessment but did not undergo curative resection. Perioperative findings at laparotomy such as the presence of previously undetected extrahepatic disease or nodal spread mean that such patients have either noncurative resections or that resection is not attempted. These patients were assumed to incur the costs of surgery and chemotherapy-based palliative treatments without gaining the survival benefits of radical surgery. Any likely quality of life gains from purely palliative resections were not taken into account.
Referral patterns for patients undergoing laparotomy were estimated from a case series considered to reflect most closely the referral and resection criteria of a typical U.K. specialist center. 32 Of the patients undergoing laparotomy, 77% underwent curative resections, as confirmed by clear resection margins at postsurgical assessment, 15% had noncurative (palliative) resection, and 8% had no resection. In contrast, most of the identified case series (see Table 1) had outcome data only for patients who underwent resection with curative intent. This implies 100% identification rates, which are unlikely to be achievable in reality.
Although repeat resections were not analyzed separately, the model included the survival data of patients who developed recurrence restricted to the liver only 14 and underwent reresection. We considered the potential costs of these reresections in the sensitivity analysis, because it is likely that such procedures would be available in a surgical resection service.
For patients having disease recurrence (estimated at 69% at 5 years post resection), 41% (or 28% of all patients who underwent initial resection) were expected to have involvement of the liver only. 32 However, the same series reported that reresection of the liver was possible in only 11% of all patients with recurrence—that is, 8% of all patients who underwent initial resection. Therefore, most patients with recurrence would be rechallenged with a second-line palliative chemotherapy regimen, depending on previous exposure to adjuvant 5-fluorouracil-based treatment.
In the sensitivity analysis, we also explored the impact of additional service costs related to increased numbers of patients undergoing follow-up after resection of the primary colorectal cancer. The value of this follow-up is under debate in the United Kingdom, 3,4 but the number of patients with regular follow-up might increase if there were more services offering potentially curative surgical resection of metastases. These costs were added to the estimated cost of the resection procedure.
The model structure was populated with data on expected resource use, associated unit costs, and expected health benefits, measured in the form of extended survival. Patients who did not undergo resection or whose resections were noncurative were assumed to have no additional survival beyond that of patients with metastatic disease treated with standard 5-fluorouracil regimens. Patients whose initial resection was potentially curative were assumed to have extended survival, corresponding to that in published case series.
Selection of Survival Data
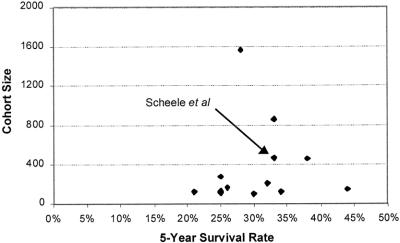
The 2-year follow-up period in our series was too short to obtain meaningful survival data. In the absence of evidence from randomized controlled trials or formal meta-analyses of existing case series, we used patient outcome data taken directly from separate published case series of patients undergoing resection of hepatic colorectal metastases. We did not pool the data because the case series were too heterogeneous. 38–40 The single-center case series reported by Scheele et al 14 was considered to be the best reflection of typical specialist practice in the United Kingdom, taking into account patient numbers (n = 463), the proportion of patients treated curatively (84%), and the complexity of the surgical procedures adopted. Full survival curve data were available, with additional descriptive patient statistics including patients older than 60 (46%), patients with synchronous metastases (41%), patients with multiple metastases (43%), patients with more than four metastases (9%), patients with bilobar disease (16%), patients with metastases larger than 5 cm in diameter (33%), and patients with extrahepatic disease (13%). Some 83% of resections were noted as “anatomic” in nature. The results of this case series have been mirrored by more recent findings from a smaller U.K. cohort study by Rees at al. 32 Further, in a simple funnel plot of the 5-year survival data taken from the identified key case series versus numbers of patients (Fig. 3), the results from Scheele et al 14 lie in the midrange of a nonskewed, apparently symmetric distribution, suggesting no clear publication bias.
Figure 3. Funnel plot of survival data (5-year survival vs. cohort size).
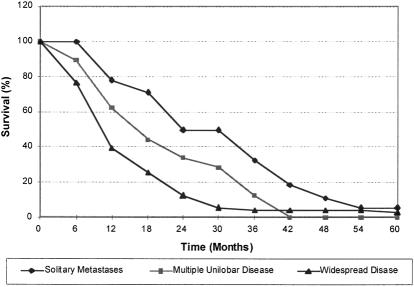
To create an historical control group for patients having liver resections, survival of patients who did not undergo resection was derived from a study of 252 such patients that had excluded patients with extrahepatic spread and significant comorbidities. 6 Median survival for patients with solitary and multiple unilobar lesions was 21 and 15 months, respectively, and fewer than 2% survived for more than 5 years (Fig. 4). Although this series is now dated, it was thought to provide the cleanest data on patients who did not undergo resection but could have potentially done so. Survival data for similar patients have remained reasonably constant in other studies during the past 20 years. 7,8
Figure 4. Overall survival of patients who did not undergo resection. (From Wagner JS, Adson MA, van Heerden JA, et al. The natural history of hepatic metastases from colorectal cancer: a comparison with resective treatment. Ann Surg 1984; 199:502–508.)
Survival data were considered for two separate groups of patients undergoing resection. The first group was defined as patients having three or fewer metastases with no evidence of extrahepatic disease, the second as those having four or more metastases, or extrahepatic disease with any number of metastases. 14 These data were compared with those of patients who did not undergo resection and were defined as having either single or multiple unilobar disease or widespread (bilobar) disease, respectively. 6
Assessment of Survival Benefits
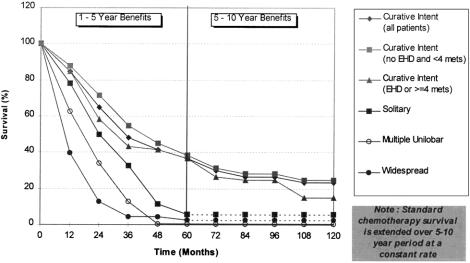
Most of the identified case series used median 5-year survival as their primary outcome measure. Although median survival estimates can be informative and allow comparisons between series, they do not reflect the overall survival experience of the whole cohort, effectively ignoring the possibility of long-term survival advantages, 41 as seen here with patients undergoing resection. We therefore used mean overall survival as our primary outcome measure, calculated directly from the published survival curves using area-under-the-curve techniques. 42 Individual survival curves were recreated in Microsoft Excel 97 using 3-month data points (Fig. 5). The area under the curve was then approximated by summation of the areas of the individual trapeziums formed. Validation of this method was performed by fitting exponential and Weibull curves to the data points, estimating the area under the curve from numeric integration. This confirmed our initial trapezoidal estimates to be accurate to within 0.01 years.
Figure 5. Comparison of survival for patients with resected and nonresected liver metastases.
Extrapolation of Clinical Benefits
Extrapolation of outcomes beyond the study data is a recognized approach for determining the lifetime benefits of treatment. 43–45 Extrapolation of published 5-year survival data up to 20 years was considered as part of the sensitivity analysis, again using exponential and Weibull mathematical curve forms (see Fig. 5), because the survival curves for patients having liver resection suggest a much longer survival than that of patients who do not undergo resection. Actuarial 20-year survival rates of more than 17% for patients undergoing potentially curative resection have been reported in case series. 14
Economic Evaluation
Form of Analysis
A cost-effectiveness analysis was performed because no reliable published quality of life data were available, precluding a formal cost-utility approach. Also, given the low overall survival rates for patients who do not undergo resection, it was thought that the key clinical benefit was an extension of life rather than a quality of life improvement.
Costs of Resection and Palliative Chemotherapy
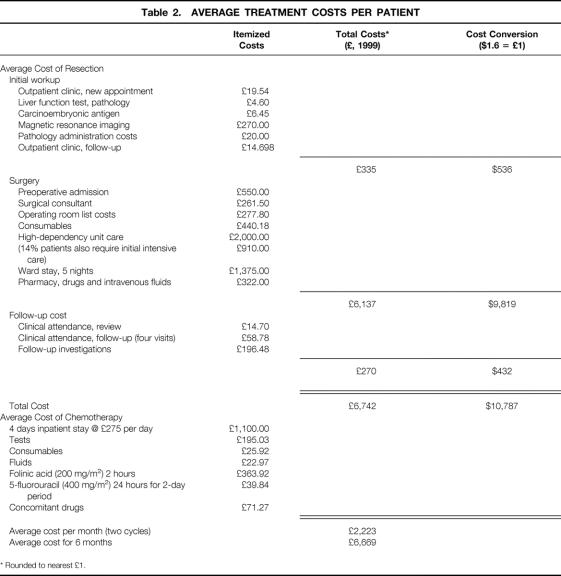
Costs were estimated from the perspective of the National Health Service as a healthcare provider and included only direct healthcare costs. To reflect the likely costs of liver resection to the National Health Service, average unit costs were applied to estimated healthcare resource use, based on 100 liver resections conducted at the hepatobiliary surgical unit at the Royal Hallamshire Hospital, Sheffield, during the period 1997 to 1999: 23 extended hepatectomies, 36 lobectomies (hemihepatectomies), and 41 segmental resections. Mean operating time was 3.5 hours. The majority (54%) of patients required no intraoperative blood transfusion; 32% required two to four units, and only 14% required more than four units of blood. The mean hospital stay was 10.3 days—5 days in the ward after a 4- to 5-day period in the high-dependency unit or, with 14% of patients, requiring additional intensive care (the high-dependency unit is significantly less expensive than the intensive care unit). Average unit cost for a resection was estimated at £6,742 ($10,787), including preoperative assessment, surgical procedures and postsurgical care allocations, and postoperative follow-up, based on regular outpatient attendance and follow-up investigations (Table 2).
Table 2. AVERAGE TREATMENT COSTS PER PATIENT
* Rounded to nearest £1.
The average unit cost of palliative chemotherapy for 3 to 6 months was estimated at £6,669 ($10,670) to £13,338 ($21,340), based on a previous U.K. study of the costs of colorectal chemotherapy 46 and substituting our local costs.
A conservative costing approach was adopted throughout, biased in favor of conventional nonsurgical treatment. We assumed that all patients who underwent resection would eventually require additional chemotherapy, although 10% to 20% of such patients will achieve long-term survival with no need for further chemotherapy, or a requirement only after several years. Although patients who had received adjuvant 5-fluorouracil chemotherapy at the time of their primary colorectal surgery would be more likely to receive other chemotherapy regimens because of tumor resistance, we assumed that same palliative treatment would be used for patients with initially irresectable metastases and with recurrence after initial hepatectomy. Accordingly, we included no difference between patients who did and did not undergo resection for the costs of later palliative chemotherapy. In the sensitivity analyses, however, we did not include chemotherapy treatment costs for patients with long-term disease-free survival (estimated at 30% of patients at 5 years). Costs of the chemotherapy avoided were estimated based on the standard De Gramont 5-fluorouracil regimen.
Discounting
Discounting is commonly used in health economics to place a present-day value on both future health gains and costs—for instance, life-years gained (LYG), treatment costs, and avoided healthcare expenditure. Because most of the costs of liver resection occur within the first year of treatment, we did not discount treatment costs in our base-case analysis. However, in the sensitivity analyses, we used the standard U.K. rate of 6% per annum to discount future costs for both reresections and differences in palliative care, because these typically take place 12 to 36 months after the initial resection. 47,48 Although it can be argued that the benefits of resection are potentially long term and should be discounted, either at the same or at a reduced rate, the relative merits and justifications for discounting future health benefits are the subject of debate. 49,50 Accordingly, we report benefits both without discount and fully discounted at 6% per annum. 47
RESULTS
Marginal Survival Benefits
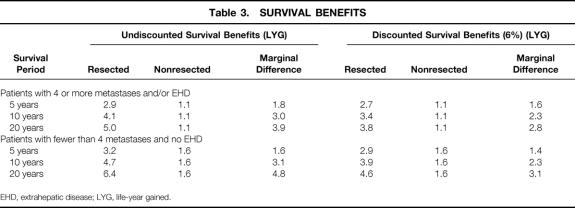
Marginal (or incremental) survival benefits are the additional benefits expected from an intervention compared with conventional or alternative treatment. Such benefits are commonly expressed in terms of LYG in survival analyses. Although for patients who do not undergo resection, survival beyond 5 years is unlikely, 10- and 20-year survival rates of approximately 1% were assigned to obtain a conservative view. When 5-year survival data were considered, patients who underwent resection had a marginal benefit of 1.6 to 1.8 LYG. There was no difference in marginal 5-year survival between the two patient groups undergoing surgery (Table 3). The marginal survival benefits in favor of resection were 3.0 to 3.1 LYG for 10-year survival and 3.9 to 4.8 LYG for 20-year survival. The extrapolation of benefits as seen in trials increases the marginal survival benefits of resection by more than 100% and improves the overall cost-effectiveness.
Table 3. SURVIVAL BENEFITS
EHD, extrahepatic disease; LYG, life-year gained.
Cost-effectiveness ratios (CERs) are derived by dividing the additional costs of a treatment by the additional health benefits and are a commonly used measure of a treatment’s health-economic worth relative to an alternative. CERs were not calculated separately for the two patient groups undergoing resection because their health benefits remained comparable, and we assumed that 80% of all patients undergoing resection in a typical U.K. practice would have three or fewer metastases with no evidence of extrahepatic disease. This resulted in an average marginal survival estimates of 1.6, 3.1, and 4.6 LYG at 5, 10, and 20 years, respectively, versus palliative chemotherapy.
Cost-Effectiveness Analysis
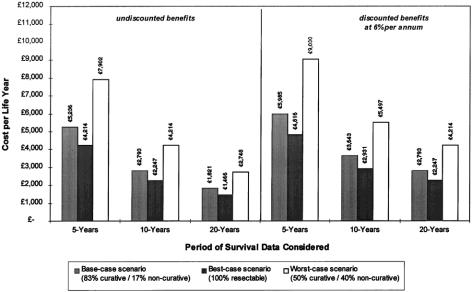
Figure 6 illustrates the incremental cost per LYG calculations. The base-case scenario is derived from the most likely mix of resection and palliation and uses conservative cost estimates, biased against resection. The base-case incremental CER for liver resection for colorectal metastases was estimated at £5,236 ($8,378) per LYG undiscounted, or £5,985 ($9,576) when discounting benefits at 6%, at 5 years after resection.
Figure 6. Incremental cost-effectiveness of liver resection.
Two alternative patient profile scenarios are also presented. The worst-case scenario assumes that only 50% of laparotomies proceed to resections with curative intent, 40% result in palliative resections, and 10% are “open-and-close” procedures. The best-case scenario assumes that presurgical assessment identifies all irresectable tumors and effectively avoids unnecessary surgery. Corresponding incremental CERs for these scenarios are £7,902 per LYG (£9,030 discounted) and £4,214 per LYG (£4,816 discounted), respectively, at 5 years.
When further extended benefits from 10- and 20-year survival expectations were included, the base-case incremental undiscounted CERs fell to £2,793 ($4,469) and £1,821 ($2,914), respectively. Even in the worst-case scenario, the discounted cost per LYG fell to less than £5,497 ($8,640) when 10-year survival data were considered.
Sensitivity Analyses
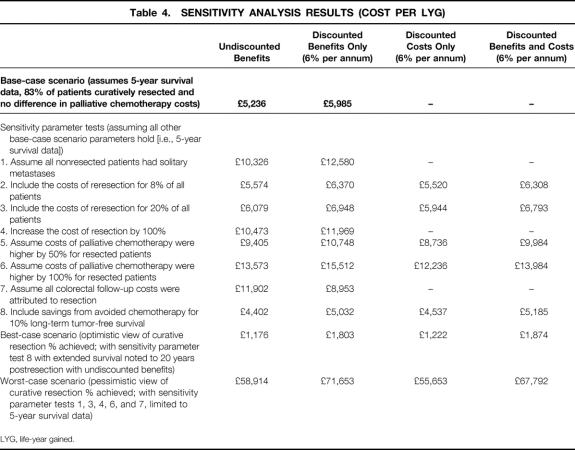
Several one-way (univariate) sensitivity analyses were conducted to examine the influence of individual parameters on cost-effectiveness results to compensate for the uncertainty of the parameters in the model. 51,52 Multivariate analyses were made on combinations of parameters to give best-case and worst-case scenarios. Table 4 lists the assumptions tested.
Table 4. SENSITIVITY ANALYSIS RESULTS (COST PER LYG)
LYG, life-year gained.
Health Benefits
The lack of directly comparable data on clinical effectiveness, differences between patient groups, and other hidden biases in case series could have affected our estimates of marginal benefits. Consequently, we modified the base-case analysis to bias it further against resection by assuming that all patients who did not undergo resection had a better prognosis because they had only solitary metastases and no evidence of extrahepatic disease. This reduced the marginal health benefits at 5, 10, and 20 years to 0.8, 2.2, and 3.7 LYG, respectively (an approximate decrease of 1 LYG for each time period). The undiscounted CER almost doubled to £10,326 ($16,521) but remained within the boundaries of what would be considered as a cost-effective treatment. 53
Costs
We explored the effect of additional costs that could be incurred as part of the treatment, including the following:
Reresections: Additional treatment costs were considered for the 8% of patients undergoing resection who could be expected to have repeat surgery for recurrence localized to the liver. The costs for repeat resection were the same as for the original procedure. The revised average treatment cost per patient with curative resection was £7,281 ($11,650) and the CER was £5,574 ($8,918) using undiscounted costs and benefits. Increasing repeat resections to 20% of cases caused the average patient cost and incremental cost-effectiveness to rise in proportion.
Regional variation in cost of liver resection: To allow for regional or national variation in costs of resection and provide a theoretical margin for future equipment and increasing sophistication in procedures, we doubled the cost of baseline values. The corresponding CER was £10,473 ($16,757).
Salvage chemotherapy: The possibility that later palliative chemotherapy costs could be higher for patients who underwent surgery than for those who did not was also considered by doubling the cost of salvage chemotherapy for patients who underwent resection. The CER increased to £13,573 ($21,717).
Additional cost of follow-up after resection of primary tumors: All patients undergoing hepatic resection for colorectal metastases were assumed to have undergone twice-yearly outpatient assessment with liver ultrasound and carcinoembryonic antigen levels, at an estimated 2-year cost of £320 ($512). We used the worst-case patient profile scenario from Table 4 to assume that 10% of patients would be potentially suitable for resection, of whom only 50% would have potentially curative resection. The entire cost of follow-up was added to the total cost of curative treatment of liver metastases, which increased the CER to £11,902 ($19,043).
Avoidance of Chemotherapy
The cost of chemotherapy was subtracted from the treatment costs of 10% of patients undergoing curative resection, instead of the 20% to 30% in the base-case scenario. Assuming a typical palliative De Gramont regimen for 6 months, the estimated reduction in average treatment cost was £1,333 ($2,132) per patient undergoing resection. 46
Extreme Scenario Analysis
Finally, multivariate sensitivity analyses were used to combine the individual model parameters to extend the scope of the worst-case and best-case scenarios to extremes with very low likelihoods. The results are shown in Table 4.
DISCUSSION
Although many retrospective case series analyses and general reviews report on the clinical effectiveness of liver resection of colorectal-related liver metastases, we are not aware of another study that has focused explicitly on the cost-effectiveness of resection. We used a simple decision-analysis model populated with published and locally derived data to conduct a cost-effectiveness analysis covering the direct health-service–borne costs and expected overall survival of patients with resectable disease.
The main cost-effectiveness analysis suggests that surgical resection of the liver for colorectal metastases is highly cost-effective compared with nonsurgical treatments, with a cost per LYG of £1,466 to £9,030 ($2,346 to $14,448). The exact figure depends on the period of survival considered (5 to 20 years) and whether the inclusion future health benefits are discounted.
Although controversy surrounds the use of league tables of cost-effectiveness, there are guidelines as to figures that can be considered reasonable. 54,55 A commonly adopted threshold for the general cost-effectiveness of new healthcare technologies in the United Kingdom is approximately £20,000 ($32,000) per LYG. 53 Above this amount, interventions are generally deemed as being high in cost; further evidence may be required before they are recommended for general commissioning.
In this study, even the worst-case scenario, with discounted 5-year data alone, provides a CER of much less than £10,000 ($16,000) per LYG, comparing favorably with many other healthcare interventions provided by the National Health Service. Taking into account the extended survival benefits beyond 5 years, the overall cost-effectiveness of resection is even greater, as shown in Figure 6.
Our univariate sensitivity analyses explored more extreme values of model parameters without forcing the CERs to prohibitive levels. The inclusion of reresection costs did not radically alter the cost-effectiveness of resection. Even the assumption that patients who did not undergo resection had the survival rates associated with solitary metastases did not take the CER beyond the limit of acceptability. By far the largest variation came from potential differences in the costs of palliative care between patients who did and did not undergo resection, at either progression or eventual recurrence. Doubling the costs of palliative care increased the CER to £13,573 ($21,717), still within the CER threshold for National Health Service treatments.
Even when the additional costs of primary surgery follow-up were included in the overall treatment costs of the patients with curative resection, the CER remained favorable. However, including the cost of follow-up after resection of the primary colorectal cancer in this way can be questioned, because this follow-up can also contribute health benefits to patients who did not undergo resection. 56
The multivariate worst-case extreme scenario was intended to indicate the level of widespread variation from baseline in model parameters and assumptions that would be required before the cost-effectiveness ratios became prohibitive to commissioning services, rather than representing a realistic view of likely treatment costs and benefits. In light of the scale of combined variance in individual modeled parameters involved, we leave the significance of this scenario open to interpretation.
As with all modeled economic evaluations, a number of limitations and caveats must be recognized. 37,57 Clinical benefits were based on distinct case series published 10 years apart because of the lack of comparative data on patients with potentially resectable disease who had nonsurgical treatment. However, we believe it is unlikely that future studies will provide any improvement on these data. The outcomes for patients who do not undergo resection that are used to estimate marginal benefits are generally better than those observed in other series, particularly for patients with single metastases, which suggests a conservative view of the benefits of resection.
Although we concentrated primarily on 5-year survival rates, there is a strong likelihood of much longer-term disease-free survival in some patients, with reports of 17% actuarial 20-year survival. 14 Extensions of health benefits to 20 years therefore seem clinically valid and can provide a fairer estimate of the lifetime benefits of liver resection. Limiting data to 10-year survival can reduce CERs to approximately 50% of those calculated under our base-case analysis.
The calculations of the resource use and unit costs for resection were based on our local clinical setting in a university teaching hospital. In principle, this introduces potential difficulties in extrapolation to other centers. However, the sensitivity analysis of increased resection costs demonstrates that resection can remain relatively cost-effective even at much higher associated costs.
This study does not take account of the set-up costs of a specialist service undertaking liver resections, and we assume that both human and surgical resources are readily available. Such costs would be entirely dependent on existing local services and circumstances. Experience in Sheffield has demonstrated that once the basic equipment is in place, additional costs are essentially those of complications from surgery; this is directly dependent on the quality of available surgical expertise. Our experience is that complication levels remain low.
We ignored any differences between the two groups undergoing resection in terms of improved quality of life. In effect, this assumes that all life-years are lived to full quality, which is unlikely after surgery, during aggressive chemotherapy, and in end-stage disease treatment. Further research on the comparative quality of life of patients with metastases and estimates of patient utility values would provide valuable information for the debate on the place of liver resection.
The criteria for defining suitable patients for liver resection remain uncertain. Many of the prognostic factors suggested in the literature are postoperative or rely on intraoperative scanning. The evidence suggests that patients most suited to resection are likely to have single metastasis or multiple metastases (restricted to a single lobe) with no evidence of extrahepatic disease—in other words, approximately 10% to 15% of those with liver metastases. Survival will be strongly influenced by the surgeon’s ability to remove the tumor with no involvement of the resection margins.
In the publicly funded National Health Service in the United Kingdom, resource use is prioritized by rational decisions based on evidence of cost and benefit. Without randomized controlled data on the effectiveness of liver resection in the treatment of colorectal metastases, commissioners of surgical services are faced with the task of considering the relative economic merits of resection on modeled case series data alone, such as those presented here. It is unlikely that randomized controlled trials of liver resection for colorectal metastases would ever be conducted because of ethical considerations, given that conventional nonsurgical treatment shows only modest survival benefits for these patients. A detailed prospective follow-up of patients undergoing resection is urgently needed to allow the real benefits and costs of resection to be judged. In the absence of such data, this modeled analysis appears to provide some evidence concerning the clinical and economic arguments for resection and supports the development of liver resection services in specialist surgical centers.
Acknowledgments
The authors thank Kath Craig, Michelle Clancey, and Sister Jane McDaid of the Surgical Directorate, Central Sheffield University Hospitals, for providing formal estimates of treatment costs.
Discussion
Prof. H. Bismuth (Villejuif, France): Mr. Majeed, your presentation was much appreciated, but it raises a considerable number of questions. The seriousness of the work and the selection of the statistical methods are not in doubt. What is of concern is the notion that when there is only one treatment that can cure the patient, we have to prove that it is less expensive than palliation. Indeed, you even add another factor in this evaluation, namely the threshold of £20,000 as the value of adding a year of life. My first question is, how did you derive this figure? Next, is it fixed for any age, or does it vary with other factors? Lastly, how do you intend the calculation to be used, and by whom? Fortunately, your conclusion was that liver resection is valuable. If it had not been, would this have meant that there would be no more liver resection in the U.K. for colorectal liver metastases?
Mr. A. Majeed (Sheffield, United Kingdom): These are very important questions, and this is really one of the reasons why the study was done. In fact, my reason for conducting this study was a personal one. When I started doing this work in Sheffield, our health authority, which determines purchasing of health services in my region, did not fund it. They said that this therapy is unproven. I had to provide my own results and also provide an economic case for the cost-effectiveness of this treatment.
Certainly, in my country, there are not very many centers that do big liver resections, and we need to do a lot more. This is the sort of economic proof that is needed to persuade health purchasers to fund this treatment, and it is very relevant in managed healthcare. Rationing in healthcare is a reality, and managers will increasingly question expensive treatments with unproven benefit. For example, I am told treating that multiple sclerosis with interferon costs £1 million per life-year gained. Convention has is that in the U.K., health economists use the value of £20,000 as a theoretical guideline for cost-effective interventions. I think this figure is arbitrary, but it has become accepted along the years. It is a figure on a scale that one uses to identify cost-benefit and varies between countries—for example, it is higher in the U.S.A., where it is $50,000. I quite agree that from a surgeon’s perspective, we know that this is the only treatment that works, but one has to prove it to those that hold the purse strings.
Prof. C. Broelsch (Essen, Germany): Mr. Majeed, I would like to congratulate you on two things—first on your excellent results, and second on introducing a new methodology to us, because, as Prof. Bismuth already pointed out, as practicing surgeons, we are not aware that you can restrict healthcare therapy to a certain group of patients, knowing that a certain treatment, for example partial hepatectomy, is better than alternative treatments.
We have to be aware that there is a political implication, by which healthcare purchasers will offer you a £20,000 line, which will include certain benefits or reimbursements for liver resections. Apparently, there has to be constant monitoring of cost-effectiveness for certain treatments. I have a couple of questions. You mentioned the 1984 study by Wagner, and the early modalities of chemotherapy as a cost baseline. Indeed, many times we have to be in consensus with the referring chemotherapists or oncologists, but in many cases that you mentioned, we have to convince them straightaway that hepatic resection is a much better treatment, although more expensive. Would this assumption change if chemotherapy becomes more complex and expensive, because they have the same cost pressure as we have for partial hepatectomy? In the second arm of your study, there is an option of having no resection. Who is to select these patients, who are potentially resectable but who undergo palliative chemotherapy instead? I think it is an important question, because for ethical reasons we will never have a randomized prospective trial on resection versus chemotherapy in resectable cases, since everybody would ask for resection as the treatment of choice.
My second question relates to whether there is an estimate of the indirect costs, like loss of salary or unemployment costs. Finally, in a more European context, I do not think your study is really comparable with other healthcare systems, such as in Germany or in the United States. There we have a mixed system for reimbursement, from both the private sector and from the social healthcare provider. While you all are working on a salary basis, your costs are considerably lower than they are in the United States, or even in Germany, where there is a larger benefit for both the patients as well as their physicians.
Mr. Majeed: Taking the last point first, we have shown that if we double the costs of treatment in our sensitivity analysis for liver resection, it is still very cost-effective. We have only examined survival in our analysis and have not considered quality of life. I am sure that “indirect” gains in terms of salary and employment from the patient’s perspective would be very important. As for Wagner’s paper on chemotherapy, we acknowledge that perhaps it is dated, but it was a very difficult task to find a cohort of patients in the literature who would have been suitable for resection but did not undergo it. Wagner’s group came closest to this. The criticism of a matched case-control group is that these were people who were going to die because of advanced disease, and they were a bad-prognosis group. Wagner specifically excluded patients with conditions that would put them at a disadvantage, or put them at a bad prognosis. So these patients would have been ideal surgical candidates who were not operated upon. That is the only reason we chose it; there are other papers in the literature, but he had the cleanest data. As for newer forms of chemotherapy, I showed a graph of various chemotherapy regimens, and the survival benefit from them is very modest. I do not see a revolutionary chemotherapy regimen on the horizon that would radically alter our conclusions.
Prof. A. Johnson (Sheffied, United Kingdom): The issue of cost-effectiveness is very important and is relevant to all health systems, even though the U.K. National Health Service may have had to focus on it first. If there is a cash limitation for certain treatments, then it is important to know which patient will benefit most as a guide to determining priorities. It is often assumed that operations are always more expensive than drug treatments, but this paper has clearly shown that this is not so.
Footnotes
Correspondence: Stephen M. Beard, MSc, School of Health and Related Research, University of Sheffield, Sheffield S1 4DA, United Kingdom.
Presented at the Seventh Annual Meeting of the European Surgical Association, Amstel Intercontinental Hotel, Amsterdam, The Netherlands, April 14–15, 2000.
Funded as part of the Trent Working Group for Acute Purchasing, a U.K. National Health Service Executive evidence-based review group, now providing input into the newly formed National Institute for Clinical Excellence.
E-mail: s.m.beard@sheffield.ac.uk
Accepted for publication July 2000.
References
- 1.Office for National Statistics. Cancer Statistics: Registrations MB1 no. 25. London: HMSO; 1992.
- 2.Colorectal Cancer in Trent, 1992–94. Trent Cancer Registry; 1998.
- 3.NHS Center for Reviews and Dissemination. Effective Health Care: The Management of Colorectal Cancer, Vol. 3, No. 6. York: University of York; 1997.
- 4.NHS Executive. Guidance on Commissioning Cancer Services: Improving Outcomes in Colorectal Cancer: The Research Evidence. London: Department of Health; 1997.
- 5.Millikan KW, Staren ED, Doolas A. Invasive therapy of metastatic colorectal cancer to the liver. Surg Clin North Am 1997; 77: 27–48. [DOI] [PubMed] [Google Scholar]
- 6.Wagner JS, Adson MA, van Heerden JA, et al. The natural history of hepatic metastases from colorectal cancer: a comparison with resective treatment. Ann Surg 1984; 199: 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengtsson G, Carlsson G, Hafstrom L, Jonsson PE. Natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg 1981; 141: 586–589. [DOI] [PubMed] [Google Scholar]
- 8.Stangl R. Factors influencing the natural history of colorectal liver metastases. Lancet 1994; 343: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 9.Benson AB. Therapy for advanced colorectal cancer. Semin Oncol 1998; 25: 2–11. [PubMed] [Google Scholar]
- 10.Scheithauer W. Randomized comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. Br Med J 1993; 306: 752–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen-Mersh TG. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet 1994; 344: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 12.Advanced Colorectal Cancer Meta-Analysis Project. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. J Clin Oncol 1992; 10: 896–903. [DOI] [PubMed] [Google Scholar]
- 13.Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984; 119: 647–651. [DOI] [PubMed] [Google Scholar]
- 14.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 15.Finch MD, Crosbie JL, Currie E, Garden OJ. An 8-year experience of hepatic resection: indications and outcome. Br J Surg 1998; 85: 315–319. [DOI] [PubMed] [Google Scholar]
- 16.Petitti DB. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. Oxford: Oxford University Press; 1994.
- 17.Tom E, Schulman KA. Mathematical models in decision analysis. Infect Control Hosp Epidemiol 1997; 18: 65–73. [DOI] [PubMed] [Google Scholar]
- 18.Buxton MJ, Drummond MF, Van Hout BA, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ 1997; 6: 217–227. [DOI] [PubMed] [Google Scholar]
- 19.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1,568 patients. Association Française de Chirurgie. Cancer 1996; 77: 1254–1262. [PubMed] [Google Scholar]
- 20.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery 1986; 100: 278–284. [PubMed] [Google Scholar]
- 21.Fegiz G, Ramacciato G, Gennari L, et al. Hepatic resections for colorectal metastases: the Italian multicenter experience. J Surg Oncol 1991; 48: 144–154. [DOI] [PubMed] [Google Scholar]
- 22.Wade TP, Virgo KS, Li MJ, et al. Outcomes after detection of metastatic carcinoma of the colon and rectum in a national hospital system. J Am Coll Surg 1996; 182: 353–361. [PubMed] [Google Scholar]
- 23.Steele G Jr, Bleday R, Mayer RJ, et al. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol 1991; 9: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 24.Van Ooijen B, Oudkerk M, Schmitz PIM, Wiggers T. Detection of liver metastases from colorectal carcinoma: is there a place for routine computed tomography arteriography? Surgery 1996; 119: 511–516. [DOI] [PubMed] [Google Scholar]
- 25.Bakalakos EA, Kim JA, Young DC, Martin EW Jr. Determinants of survival following hepatic resection for metastatic colorectal cancer. World J Surg 1998; 22: 399–404. [DOI] [PubMed] [Google Scholar]
- 26.Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 1992; 216: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuhrman GM, Curley SA, Hohn DC, Roh MS. Improved survival after resection of colorectal liver metastases. Ann Surg Oncol 1995; 2: 537–541. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins LT, Millikan KW, Bines SD, et al. Hepatic resection for metastatic colorectal cancer. Am Surg 1997; 63: 605–610. [PubMed] [Google Scholar]
- 29.Taylor M, Forster J, Langer B, et al. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg 1997; 173: 467–471. [DOI] [PubMed] [Google Scholar]
- 30.Ohlsson B, Stenram U, Tranberg K, Scheele J. Resection of colorectal liver metastases: 25-year experience. World J Surg 1998; 22: 268–277. [DOI] [PubMed] [Google Scholar]
- 31.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997; 15: 938–946. [DOI] [PubMed] [Google Scholar]
- 32.Rees M, Plant G, Bygrave S. Late results justify resection for multiple hepatic metastases from colorectal cancer. Br J Surg 1997; 84: 1136–1140. [PubMed] [Google Scholar]
- 33.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery 1994; 116: 703–710. [PMC free article] [PubMed] [Google Scholar]
- 34.Cady B, Stone MD, McDermott WV, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg 1992; 127: 561–-568. [DOI] [PubMed] [Google Scholar]
- 35.Doci R, Gennari L, Bignami P, et al. Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg 1995; 82: 377–381. [DOI] [PubMed] [Google Scholar]
- 36.Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer—competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol 1990; 16: 360–365. [PubMed] [Google Scholar]
- 37.Sheldon TA. Problems of using modelling in the economic evaluation of health care. Health Econ 1996; 5: 1–11. [DOI] [PubMed] [Google Scholar]
- 38.Jarad AR. Randomized Controlled Trials. London: BMJ Books; 1998.
- 39.Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. Br Med J 1998; 316: 469. [PMC free article] [PubMed] [Google Scholar]
- 40.Sheldon TA. Systematic reviews and meta-analyses: the value for surgery. Br J Surg 1999; 86: 977–978. [DOI] [PubMed] [Google Scholar]
- 41.Messori A, Becagli P, Trippoli S. Median versus mean lifetime survival in the analysis of survival data. Haematologica 1997; 82: 730. [PubMed] [Google Scholar]
- 42.Messori A, Trippoli S. A new method for expressing survival and life expectancy in lifetime cost-effectiveness studies that evaluate cancer patients. Oncol Rep 1999; 6: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1253–1258. [PubMed] [Google Scholar]
- 44.Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1172–1177. [PubMed] [Google Scholar]
- 45.Siegel JE, Weinstein MC, Russell LB, et al. Recommendations for reporting cost-effectiveness analyses: Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1339–1341. [DOI] [PubMed] [Google Scholar]
- 46.Ross P, Heron J, Cunningham D. Cost of treating advanced colorectal cancer: a retrospective comparison of treatment regimes. Eur J Cancer 1996; 32A: 13–17. [DOI] [PubMed] [Google Scholar]
- 47.Spackman M. Discount Rates of Return in the Public Sector: Economic Issues. London: Government Economic Service; 1991.
- 48.Department of Health. Policy Appraisal and Health. London: Department of Health; 1995.
- 49.Parsonage M, Neuburger H. Discounting and health benefits. Health Econ 1992; 1: 71–76. [DOI] [PubMed] [Google Scholar]
- 50.Drummond MF. Economic Analysis Alongside Controlled Trials. London: Department of Health; 1994.
- 51.Briggs A, Sculpher M. Sensitivity analysis in economic evaluation: a review of published studies. Health Econ 1995; 4: 355–371. [DOI] [PubMed] [Google Scholar]
- 52.Briggs A, Sculpher M, Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Econ 1994; 3: 95–104. [DOI] [PubMed] [Google Scholar]
- 53.Stevens A, Colin-Jones D, Gabbay J. Quick and clean: authoritative health technology assessment for local contracting. Health Trends 1995; 27: 37–42. [PubMed] [Google Scholar]
- 54.Mason J, Drummond M, Torrance G. Some guidelines on the use of cost-effectiveness league tables. Br Med J 1993; 306: 570–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drummond M, Torrance G, Mason J. Cost-effectiveness league tables: more harm than good? Soc Sci Med 1993; 37: 33–40. [DOI] [PubMed] [Google Scholar]
- 56.Berman JM, Cheung RJ, Weinberg DS. Surveillance after colorectal cancer resection. Lancet 2000; 355: 395–399. [DOI] [PubMed] [Google Scholar]
- 57.Sonnenberg FA, Roberts MS, Tsevat J, et al. Toward a peer review process for medical decision analysis models. Med Care 1994; 32: 52–64. [PubMed] [Google Scholar]