Abstract
Objective
To assess feasibility, risks, and patient outcomes in the treatment of colorectal metastases with two-stage hepatectomy.
Summary Background Data
Some patients with multiple hepatic colorectal metastases are not candidates for a complete resection by a single hepatectomy, even when downstaged by chemotherapy, after portal embolization, or combined with a locally destructive technique. In two-stage hepatectomy, the highest possible number of tumors is resected in a first, noncurative intervention, and the remaining tumors are resected after a period of liver regeneration. In selected patients with irresectable multiple metastases not amenable to a single hepatectomy procedure, two-stage hepatectomy might offer a chance of long-term remission.
Methods
Of consecutive patients with conventionally irresectable colorectal metastases treated by chemotherapy, 16 of 398 (4%) became eligible for curative two-stage hepatectomy combined with chemotherapy and adjuvant nonsurgical interventions as indicated.
Results
Two-stage hepatectomy was feasible in 13 of 16 patients (81%). There were no surgical deaths. The postoperative death rate (2 months or less) was 0% for the first-stage procedure and 15% for the second-stage one. Postoperative complication rates were 31% and 45%, respectively, with only one complication leading to reoperation. The 3-year survival rate was 35%, with four patients (31%) disease-free at 7, 22, 36, and 54 months. Median survival was 31 months from the second hepatectomy and 44 months from the diagnosis of metastases.
Conclusions
Two-stage hepatectomy combined with chemotherapy may allow a long-term remission in selected patients with irresectable multiple metastases and increases the proportion of patients with resectable disease.
Liver resection is the only treatment known to provide long-term survival and the possibility of cure in patients with liver metastases. 1–6 However, at the time of diagnosis, most patients have irresectable tumors. Systemic chemotherapy, the current standard treatment, offers these patients a limited benefit, with median survivals not exceeding 12 to 18 months. 7–10 We combine chemotherapy with other therapeutic modalities and offer resection to patients who respond in such a way that surgery again becomes an option. In patients whose initially irresectable metastases are downstaged by neoadjuvant chemotherapy, one-stage resection provides a survival rate similar to that of patients undergoing primary resection. 11 We have also shown that cryosurgery and portal embolization can increase the resectability rate in patients with colorectal metastases. 12,13 In patients with intrahepatic multinodular diffusion of tumor, however, even with these techniques, it is not always possible to perform a resection that would be curative. An incomplete resection is not indicated because there is no significant survival benefit compared with patients who do not undergo surgery. 14
We proposed to modify this practice by using a strategy whose overall intention is curative, but in which the initial stage of the hepatic resection is intended to remove the highest possible number of metastases, but not all of them. The remnant liver hypertrophies and systemic chemotherapy limits the growth and spread of the remaining tumor deposits. The second hepatectomy is performed only if it is potentially curative, in the absence of significant tumor progression, and when adequate parenchymal hypertrophy has reduced the risk of postoperative liver failure. This approach has been mentioned in a previous publication on resection of primarily irresectable disease. 11 Here we report the specific results of two-stage hepatectomy in terms of feasibility, risks, and patient outcome.
METHODS
Study Population
From October 1992 to July 1999, 634 patients were referred to our hospital with liver metastases from colorectal cancer. Of these, 236 (37%) underwent resection. The 398 patients (63%) with irresectable disease were treated by chemotherapy, after which 105 (26%) underwent a potentially curative liver resection. The initial causes of irresectability were multinodular intrahepatic tumor spread (n = 59), unfavorable location (n = 15), size (n = 4), or concomitant extrahepatic metastases (n = 27).
Of the patients with multinodular tumors, 16 (27%) had either a partial response to chemotherapy (tumor size reduction >50% of the sum of the perpendicular diameters) or tumor stabilization (decrease <50% or increase <25% in tumor size) 15 but had contraindications to one-stage hepatectomy. These included the following: the intrahepatic diffusion of the tumor prevented removal of all tumor tissue by a single resection, even after portal embolization used when the remnant functional parenchyma was less than 40%, and ineligibility for local tumor destruction by cryosurgery or radiofrequency thermal destruction (fewer than three tumors, <3 cm, in the remnant liver). These patients gave informed consent to a planned strategy of two-stage (sequential) hepatectomy. After the initial hepatectomy, and despite chemotherapy, three patients could not undergo the second hepatectomy. The 13 patients completing both stages form the study population. Patients who underwent an unplanned repeat resection for hepatic recurrence after a first hepatectomy were not included.
Patient Characteristics
The median age of the six men and seven women at the time of the first hepatectomy was 56 years (range 45–68). The original primary tumors were colon (n = 9) and rectum (n = 4), and 10 of 13 were Dukes C. Tumor characteristics, timing of hepatectomy stages, and patient outcomes are shown in Table 1. All patients had bilobar involvement. The median number of tumors was 7 (range 3–12) and the median diameter was 37 mm (range 9–80) (Fig. 1). Before the first-stage hepatectomy, 12 patients received systemic chemotherapy, usually a combination of 5-fluorouracil, folinic acid, and either oxaliplatin or irinotecan, to control the disease. Six patients had previously received one or two previous treatments with other types of chemotherapy. Chronomodulated regimens were used in 9 of the 12 patients. The median duration of treatment was 9 months (range 4–18) and the median number of courses was 11 (range 4–18). A partial response was seen in six patients, stable disease in seven. In one patient with synchronous bilateral metastases and a partial response to chemotherapy, colectomy for the primary tumor was performed at the same time as the first hepatectomy.
Table 1. TUMOR CHARACTERISTICS, TIMING AND NATURE OF HEPATECTOMY STAGES, AND PATIENT OUTCOMES
A, alive, no recurrence; AR, alive, recurrence; D; dead; H, hepatic; mo, months; M, metachronous; P, pulmonary; S, synchronous.
* Number in parentheses indicates intervals (in months) after second stage.
† Two nodules disappeared between the stages.
‡ Two and § three additional nodules were diagnosed in the second stage.
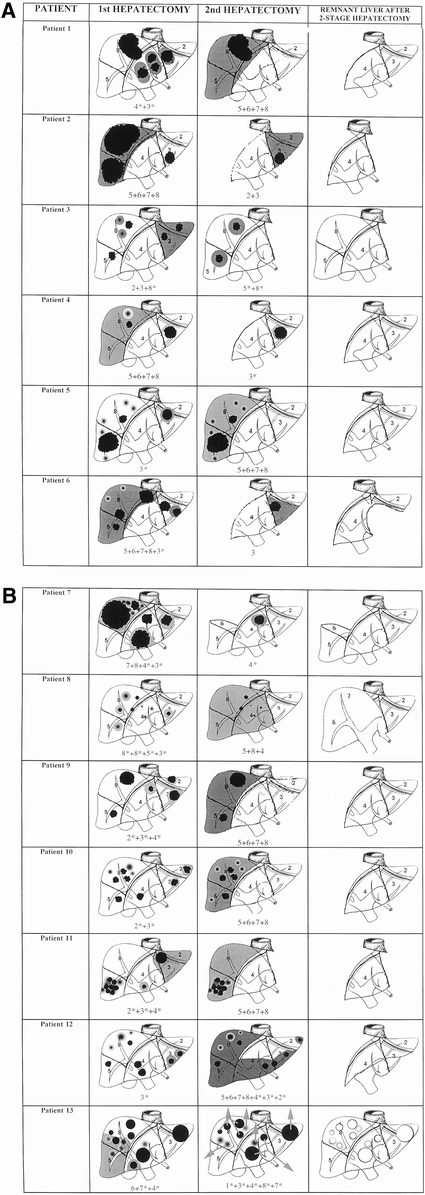
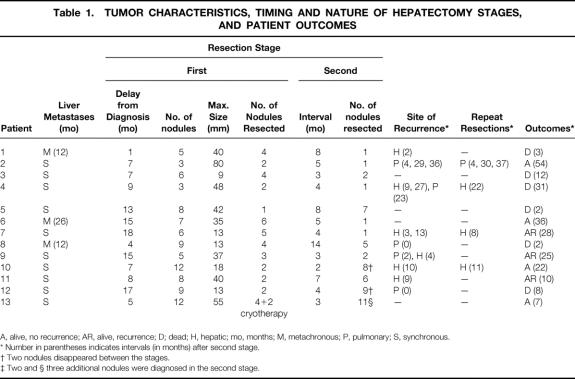
Figure 1. The 13 cases of two-stage hepatectomy, showing the liver status at the first hepatectomy, the second hepatectomy, and the remnant liver after the two-stage procedure. Gray areas represent the parts of liver resected. The segments are numbered according to the Couinaud system. (8*) means partial resection of segment 8; (8) means 8 segmentectomy.
Two-Stage Hepatectomy
The preoperative evaluation includes liver ultrasound, a spiral computed tomography scan of the chest, abdomen, and pelvis, and bone scintigraphy. Local recurrence of the primary is excluded by colonoscopy. At the laparotomy for resection, the abdomen is carefully explored for any extrahepatic tumor deposit, with fresh-frozen biopsy of any suspicious lymph node. The objective of the first hepatectomy is to make the second hepatectomy potentially curative. The liver tumors are precisely mapped, using manual palpation and intraoperative ultrasound. Mapping permits the surgeon to achieve this by resecting the highest possible number of liver tumors (the majority of patients) or by clearing tumor tissue from the less-invaded hepatic lobe, leaving the other to be further resected after regeneration. During the first-stage hepatectomy, a minimum amount of dissection is performed on the liver that will be dissected in the second stage. We avoid dividing the corresponding triangular ligament and the ipsilateral vascular structures to prevent further fibrous adhesions. Postoperative chemotherapy is given to control tumor growth, beginning 3 weeks after liver resection so that it does not interfere with initial liver regeneration. The same drug protocol is used as before surgery, except is the tumor has progressed or tumor markers have increased.Twelve patients received postoperative chemotherapy for a median of 4 courses (range 0–11).
The timing of the second hepatectomy is selected as a function of liver regeneration, control of remnant liver tumor by chemotherapy, and the probability that the second hepatectomy can be curative. The same preoperative evaluation and laparotomy protocols are used as in the first stage, with the exception of a larger dissection of the remnant liver. The median interval between the two stages was 4 months (range 2–14).
Adjuvant Procedures
As with other patients with initially irresectable disease, nonsurgical treatments were combined with chemotherapy to increase the feasibility of the two-stage hepatectomy. Cryosurgery was used in one patient for two liver metastases that were unlikely to be resectable at the second hepatectomy. Portal embolization of the liver to be resected was used in six patients in whom the extent of the expected second-stage resection exceeded 60% of the total parenchymal volume. 13 Portal embolization was also used before the first-stage hepatectomy in one patient.
Postoperative Follow-Up
Patients are followed up by serum tumor markers (carcinoembryonic antigen, CA 19–9), liver ultrasound, and spiral computed tomography scans of the chest, abdomen, and pelvis at 3-month intervals. If the overall procedure has been complete, as confirmed by histology margins and return to normal levels of tumor markers, and there is no sign of tumor recurrence, chemotherapy is discontinued after eight courses. In case of a new recurrence, chemotherapy is restarted.
End Points
The end points of the study were feasibility of the procedure, perioperative rates of death and complications, tumor recurrence, and patient survival.
RESULTS
Feasibility
Of the 16 patients, 3 were ineligible for the second-stage resection because of disease progression after the first-stage hepatectomy. One patient developed multiple bilateral pulmonary metastases, one a cerebral metastasis, and in the third patient tumor progression in the remnant liver was identified at the second laparotomy. Both patients who developed extrahepatic metastases had a high initial number of hepatic lesions (8 and 15), but otherwise they were not obviously different.
In 8 of the 13 patients who completed the full two-stage procedure, the first-stage hepatectomy was a major resection (more than three segments) (Fig. 1). No patient needed total liver vascular exclusion. Intermittent clamping of the vascular pedicle was used in six patients and intermittent hemihepatic vascular occlusion in three; no vascular clamping needed in four. The median duration of surgery was 393 minutes (range 145–615), the median units of blood transfused was 0 (range 0–6), and median hospital stay was 13.5 days (range 10–20).
In terms of the tumor behavior between the two stages, remnant tumors were stable in 12 patients, with 1 with a partial response. However, tumor marker levels increased in four patients with stable disease, leading to changes in the chemotherapy regimen.
At the second hepatectomy, there were eight major resections (62%) (Fig. 1). Total vascular exclusion was used in one patient, intermittent clamping of the hepatic pedicle in six, and intermittent hemihepatic vascular occlusion in two; vascular clamping was not needed in four patients. The median duration of surgery was 374 minutes (range 260–570), the median number of units transfused was 6 (range 0–12), and the median hospital stay was 15 days (range 9–103).
Surgical Deaths
No patient died within 2 months of the first-stage hepatectomy, but two patients died after the second-stage hepatectomy. One died at 53 days from progressive liver and renal failure after a right hepatectomy, with reoperation at day 24 for a biliary leak. This patient had undergone resection of segment 3 and of the left part of segment 2 after 18 courses of chemotherapy and right portal embolization. The left branch of the portal vein was damaged during the second surgical procedure, necessitating massive blood transfusion (10 units), and was repaired with portal triad clamping for 27 minutes. In addition, during the right hepatectomy, intermittent portal triad clamping was needed for five sessions of 15 minutes each. A second patient died of progressive liver failure at 60 days. This patient had first undergone resection of parts of segments 3, 4, 6, 7, and 8. At a subsequent right portal embolization, there was an unintentional partial left portal embolization. The second stage was a central hepatectomy (trisegmentectomy 4, 5, and 8). The nontumor liver showed portal fibrosis with fatty infiltration, possibly related to prolonged chemotherapy.
Postoperative Complications
The first hepatectomy was uneventful in nine patients. In the other four, postoperative complications included two cases of transient leakage of ascites by the abdominal drain, one perihepatic fluid collection, and one case of anaphylactic shock related to amoxicillin.
The second hepatectomy was uneventful in six patients. In the other five, postoperative complications included two cases of transient leakage of ascites by the abdominal drain, two perihepatic collections that resolved spontaneously, and one occlusion of the small intestine requiring reoperation.
Patient Outcome
After a mean follow-up of 22 months (range 3–54), an isolated hepatic recurrence was observed in four patients. Of these four, one died at 3 months, and one was receiving chemotherapy and awaiting a third hepatic resection as of this writing. Two underwent a third hepatectomy: one was disease-free at 22 months, and the other had a new hepatic recurrence but was alive at 28 months. One patient had an isolated recurrence in the lung and underwent pulmonary resections 4, 30, and 37 months after the second hepatectomy. This patient was alive and disease-free 54 months after the procedure (Fig. 2). In contrast, two patients with concomitant pulmonary metastases at the second hepatectomy died 2 and 8 months after the procedure.
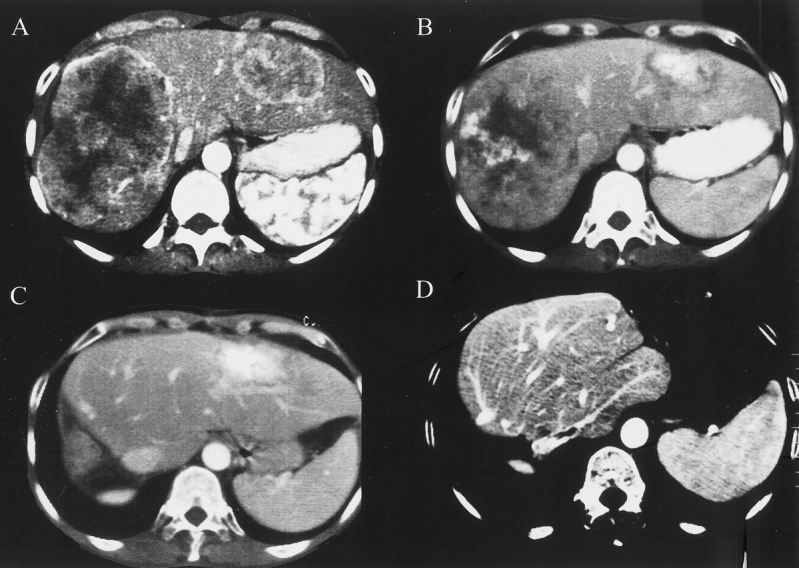
Figure 2. Comparative abdominal computed tomographs of patient 2, treated by neoadjuvant chemotherapy and two-stage hepatectomy. (A) Before chemotherapy. (B) Just before hepatectomy. (C) After the first stage (right hepatectomy). (D) After the second stage (left lobectomy). Only segments 1 and 4 remain. This patient subsequently underwent repeat pulmonary resections for lung metastases and was disease-free 54 months after the two-stage procedure.
Of two patients with combined hepatic and pulmonary recurrence, one underwent a third liver resection but died at 31 months, and the other was receiving chemotherapy 25 months after the second-stage procedure. Overall, 4 of the 13 patients were disease-free at 7 (Fig. 3), 22, 36, and 54 months after the second-stage procedure, 3 (23%) were alive with recurrence (one pulmonary, one hepatic, and one both pulmonary and hepatic), and six (46%) had died. The 3-year survival rate after the two-stage hepatectomy was 35% (Fig. 4), with median survivals of 31 months from the second hepatectomy and 44 months from the time of diagnosis of the metastases. The three patients who could not undergo the second hepatectomy died at 6, 8, and 14 months after the first liver resection.
Figure 3. Comparative abdominal computed tomographs of patient 13, treated by a two-stage hepatectomy. Liver sections (A, B) illustrate the multinodular bilobar lesions before the first hepatectomy (12 metastases). (C, D) Liver after multiple partial resection. This patient was free of recurrence 7 months after the two-stage hepatectomy.
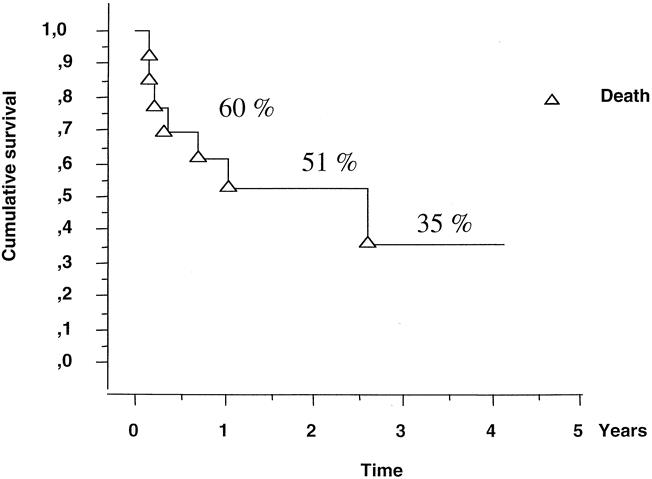
Figure 4. Kaplan-Meier survival of patients after two-stage hepatectomy.
DISCUSSION
Although liver resection is usually contraindicated if not all the tumors can be completely resected, 14 we postulated that a hepatectomy that leaves some tumor tissue in place could be justified if a second liver resection could resect the remnant tumors. This approach was briefly mentioned in our report on downstaging of colorectal metastases by neoadjuvant chemotherapy 11 but has not previously been described. The term “two-stage liver surgery” has been used recently but refers to a single hepatic resection after portal branch transection and transarterial neoadjuvant immunochemotherapy. 16
The risk/benefit assessment in two-stage hepatectomy must take into account both the potential for tumor growth after the first hepatectomy and the real survival benefit to patients with a poor prognosis as a result of widespread liver tumors. This series of 13 consecutive patients shows that multiple liver tumors can be cleared and that the usual poor prognosis of some patients with initially irresectable disease can be improved, particularly in those with multinodular metastases in the absence of extrahepatic tumor. To be eligible, their tumors had to be stabilized or downstaged by systemic chemotherapy. Accordingly, with one exception, patients considered for two-stage hepatectomy were treated initially by systemic chemotherapy. Patients with tumor progression were not eligible. Chemotherapy was continued after each hepatectomy; this was because the growth factors involved in the hypertrophy of the remnant liver induced by the first resection can also boost the growth of the tumor tissue left in place. This phenomenon has been described after liver resection 17–19 and after portal embolization 20 and might be amplified by repeat surgeries. 21 Nevertheless, because regeneration is essential to the feasibility of the second-stage resection, an interval of 3 weeks was observed after each hepatectomy to minimize the inhibition of antitumor drugs on liver regeneration. 22,23 Tumor progression after the first-stage resection was extrahepatic in two patients and in the remnant liver in a third, so that the feasibility of the procedure was 81%.
The poor natural prognosis of the patients considered for two-stage hepatectomy was confirmed by the outcomes of the three patients who did not complete the second procedure: they died 6 to 14 months after the first hepatectomy. This is in agreement with the results of previous reports on patients with irresectable disease, with a 5-year survival rate of only 0% to 1%. 3,14 In contrast, those who underwent the two-stage hepatectomy had a 3-year survival rate of 35% and a median survival of 31 months from the second hepatectomy. The median survival reached 44 months from the time of diagnosis of metastases, compared with the 15- to 18-month median survival observed with the single use of recent effective chemotherapy regimens. 8–10
This study suggests that patient selection is critical. The two patients who underwent two-stage hepatectomy but had pulmonary resectable metastases at the time of the second hepatic resection both died within 8 months, whereas sequential hepatic and pulmonary resections are currently proposed for patients with primarily resectable metastases in both locations. 23–25 One of the three patients who did not complete the procedure also developed bilateral pulmonary metastases and died within 8 months. Therefore, the presence of extrahepatic tumor, even resectable, seems to reduce the probability of survival benefit from two-stage hepatectomy.
Despite complete macroscopic resection, tumor recurred in 7 of the 13 patients, a rate similar to that observed after one-stage hepatectomies. 26 Hepatic recurrence was observed in six patients at a mean interval of 8 months after the second-stage hepatectomy. When tumor recurs after a conventional curative resection, our policy is to perform a new resection if it could be curative. 27 Of the three patients who underwent a third liver resection, one was free of disease 11 months after this repeat hepatectomy. Of the three patients who developed metachronous pulmonary metastases, one underwent repeat pulmonary resections, leading to a 54-month remission. Overall, four patients were disease-free (31%) as of this writing.
The 15% perioperative death rate of the two-stage hepatectomy strategy to clear the tumor was higher than the 1% observed in patients undergoing primary resection during the same period (data not shown). These deaths were mainly technique-related, although repeat liver resection per se may not be the reason, because the death rate of rehepatectomies is similar to that of first resections. 27–32 The higher death rate in these patients might have resulted from their diminished tolerance of perioperative complications as a consequence of their severe tumor disease or the deleterious effects of prolonged chemotherapy on the liver, 33,34 combined with the effects of adjuvant procedures to facilitate liver resection. Of the 13 patients who completed the program, 7 had undergone portal embolization and 1 had undergone cryosurgery, and all but 1 had received one to three types of neoadjuvant chemotherapy. The complications were most likely to be those of an aggressive multidisciplinary approach than those of two sequential hepatectomies.
The patients who underwent a two-stage hepatectomy represent only a small proportion of patients with irresectable liver metastases, amounting to 5% of first hepatectomies and 15% of second hepatic resections in the same period, and increased the rate of resection in referred patients to 54%. Further, this strategy has been systematically considered only in recent years, growing from 2% of first hepatic resections in the first 2 years of the study to 7% in the last 2 years. The emergence of more active chemotherapy treatments and of efficient techniques of in situ tumor destruction (cryotherapy, radiofrequency) can facilitate this two-stage strategy by better controlling the tumor process. Also, the program can be initiated at the time of colectomy when synchronous liver metastases preclude a single curative hepatectomy. An attempt to clear one hemiliver by a limited resection during the colectomy and to reserve the major hepatectomy for the second stage is a new strategy that may shorten the conventional one. This is in agreement with the current consensus for avoiding large hepatic resections at the time of colectomy. 35
Two-stage hepatectomy is a surgical modality intended for patients with primarily irresectable metastases. It is part of a multidisciplinary approach that can offer a chance of long-term remission to patients otherwise guaranteed of having a poor outcome.
Discussion
Prof. M. BÜchler (Bern, Switzerland): If we go through your numbers, you showed that you start with more than 600 patients and you end up with four patients that are so-called disease-free after 3 years, which is less than 1% of your original population. My question concerns the selection. Certainly in rare patients you can apply this aggressive kind of treatment and you will have some benefit, so what we need to know is how to make the right selection for these patients. In the end, we have four patients who are disease-free after 3 years, but at a high price: nearly everyone had morbidity, and there is also a considerable mortality. In the future, what do you think will be the criteria for selection of patients? Are there some patients who get definite advantages from this procedure? I would estimate that specific localization of the multiple metastases is the clue to the answer—namely, some on the right side and some on the left side, which, from the original intention, you might be able to resect at a second step.
Prof. R. Adam (Villejuif, France): You point out two critical issues: the patient selection and the benefit of the procedure. With respect to the first point, we address the procedure to patients with multinodular bilobar tumors, irresectable by a single hepatectomy, stabilized or downstaged by neoadjuvant chemotherapy, and amenable to a radical resection by two sequential hepatectomies. We excluded the patients in whom a single resection combined with an ablative technique could achieve radicality. This explains why the selection process was so restrictive, representing 5% of patients undergoing a first resection and 12% of patients undergoing a second resection in the same period. However, patients in whom the procedure is indicated have been increasing more recently, from 2% of primary resections in the first 2 years to 7% in the last 2 years of the study period.
With respect to the real benefit of the procedure, we should keep in mind that this series represents a subset of patients with very aggressive disease, as indicated by the fact that five of the 13 patients required two or three types of chemotherapy to achieve tumor stabilization before resection. These patients are usually destined to a very poor outcome, as confirmed by the rapid death of the three patients who could not have the second hepatectomy. To observe that four of these patients have long-term remission is promising, even if the proportion is small.
Prof. M. Malago (Essen, Germany): We have a similar problem in Essen. Did you find positive lymph nodes in these large tumors, and did they stop you? In Essen, they do not stop us. Did you try to block the tumor in the contralateral lobe instead of leaving it? We are presently using either chemoembolization or radiotherapy to block the potential growth in the contralateral lobe. Lastly, did you compare this to other one-step procedures—for example, ex vivo liver resection? We do not do ex situ liver resections anymore, but the alternative in these patients sometimes is to do an ex situ liver resection procedure and take the tumor out at once.
Prof. Adam: No patient of this series has lymph node involvement. In the other patients, our policy is to perform liver resection with lymphadenectomy when the hepatectomy is possible without a high risk. With respect to the blockage of tumor progression following the first hepatectomy, we have used only chemotherapy. We do not use chemoembolization or radiotherapy for metastatic disease. As far as comparison with ex situ liver resection is concerned, we think that the latter technique is mainly used as a single procedure for ill-located, large tumors rather than for multinodular irresectable disease. It seems to me that the two techniques concern different patients.
Prof. D. Cherqui (Créteil, France): In this series, you presented two types of lesions. Some patients had one large lesion on each lobe of the liver, allowing two sequential resections. Other patients had numerous bilateral nodules, and you mentioned one with 17 metastases. In the latter, you resected all lesions in two steps. I would like to know which technique you used to remove so many nodules while preserving intrahepatic pedicles, and also what size margins you obtained at the periphery of the lesions.
Prof. Adam: We usually try to make a partial resection with a margin of 1 cm, but for the patient with 17 nodules, the margin was much more restricted. We have assumed that to make a resection in this type of patient, even a margin of 2 to 3 mm will provide better results than not to make any resection at all.
Prof. J. Belghiti (Clichy, France): This is an interesting strategy for patients with bilobar tumors. My first question is, in what percentage of patients with bilobar tumors can you plan this two-stage strategy? Secondly, I was very impressed by the morbidity and mortality rate of your patients, especially after the second stage. This is not our experience of 17 cases. Probably it is because we adopt a different strategy with clearance of the left liver tumor together with ligation of the right portal vein during the first stage. Then we perform a right hepatectomy during the second stage, which is conducted through a different approach. Since reoperation after a right hepatectomy is difficult, do you think that performing a right resection during the first stage can explain the higher risk?
Prof. Adam (Closing Discussion): The proportion of patients with bilobar tumors previously considered as irresectable and submitted to the two-stage procedure was 27%. This proportion would be lower if all patients with bilobar tumors were included. However, when the hepatectomy did not exceed 70% of the total parenchyma, many of them were indeed resected by a single procedure.
With respect to the morbidity and mortality of our series, both result from the combined effects of prolonged chemotherapy and more technically demanding procedures. These are patients with very aggressive tumor disease who are unable to tolerate a severe complication. Your suggestion concerning the performance first of the left side to avoid the assumed higher risk of repeat resections after right hepatectomies is interesting. However, our policy has been to privilege the clearance of as much liver tumor as possible at the first stage, whichever hemiliver, to avoid dramatic tumor growth between the two hepatectomies. It is therefore difficult to say in advance which type of resection should be performed. Going further into the details of the first liver resection in our series, a pure right hepatectomy was performed in only two patients, a pure left resection in six, and combined partial left and right resection in five patients. The two patients who died in the postoperative course had had a pure left and a mixed right and left partial hepatectomy. Therefore, the risk of the procedure seems more related to the condition of the patient than to the side of the first hepatectomy.
Footnotes
Correspondence: René Adam, MD, PhD, Centre Hépato-Biliaire, Hôpital Paul Brousse, 14 Avenue PV Couturier, 94800 Villejuif, France.
Presented at the Seventh Annual Meeting of the European Surgical Association, Amstel Intercontinental Hotel, Amsterdam, The Netherlands, April 14–15, 2000.
E-mail: rene.adam@pbr.ap-hop-paris.fr
Accepted for publication July 2000.
References
- 1.Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984; 119: 647–651. [DOI] [PubMed] [Google Scholar]
- 2.Fortner JG, Silva JS, Golbey RB, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. Ann Surg 1984; 199: 306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Surgery 1988; 103: 278–288. [PMC free article] [PubMed] [Google Scholar]
- 4.Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer: biologic perspectives. Ann Surg 1989; 210: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheele J, Stangl R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19: 59–71. [DOI] [PubMed] [Google Scholar]
- 6.Jaeck D, Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Br J Surg 1997; 84: 977–980. [DOI] [PubMed] [Google Scholar]
- 7.Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med 1994; 330: 1136–1139. [DOI] [PubMed] [Google Scholar]
- 8.Levi F, Zidani R, Misset JL, for the International Organization For Cancer Chronotherapy. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 1997; 350:681–86. [DOI] [PubMed]
- 9.De Gramont A, Vignoud J, Tournigand C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48 hours continuous infusion in pretreated colorectal cancer. Eur J Cancer 1997; 33: 214–219. [DOI] [PubMed] [Google Scholar]
- 10.Rougier P, Van Cutsem E, Bajetta E, et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 1998; 352: 1407–1412. [DOI] [PubMed] [Google Scholar]
- 11.Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996; 224: 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adam R, Akpinar E, Johann M, et al. Place of cryosurgery in the treatment of malignant liver tumors. Ann Surg 1997; 225: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 2000; 231: 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990; 77: 1241–1246. [DOI] [PubMed] [Google Scholar]
- 15.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–214. [DOI] [PubMed] [Google Scholar]
- 16.Lygidakis NJ, Vlachos L, Raptis S, et al. New frontiers in liver surgery: two-stage liver surgery for the management of advanced metastatic liver disease. Hepato-Gastroenterology 1999; 46: 2216–2228. [PubMed] [Google Scholar]
- 17.Panis Y, Ribeiro J, Chretien Y, Nordlinger B. Dormant liver metastases: an experimental study. Br J Surg 1994; 19: 221–233. [DOI] [PubMed] [Google Scholar]
- 18.Slooter GD, Marquet RJ, Jeekel J, Ijzermans JNM. Tumor growth stimulation after partial hepatectomy can be reduced by treatment with tumor necrosis factor alpha. Br J Surg 1995; 82: 129–132. [DOI] [PubMed] [Google Scholar]
- 19.Picardo A, Karpoff HM, Ng B, et al. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery 1998; 124: 57–64. [PubMed] [Google Scholar]
- 20.Elias D, de Baere T, Roche A, et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg 1999; 86: 784–788. [DOI] [PubMed] [Google Scholar]
- 21.Weese JL, Ottery FD, Emoto SE. Do operations facilitate tumor growth? An experimental model in rats. Surgery 1986; 100: 273–277. [PubMed] [Google Scholar]
- 22.Ikeda Y, Matsumata T, Utsunomiya T, et al. Effects of doxorubicin on cancer cells after two-third hepatectomy in rats. J Surg Oncol 1995; 58: 101–103. [DOI] [PubMed] [Google Scholar]
- 23.Herrera MC, Palomero MF, Macias RI, et al. Comparison of the effects of bischolylglycinatechloro-platinum versus cisplatin on liver regeneration after partial hepatectomy. Anticancer Res 1998; 18: 3555–3563. [PubMed] [Google Scholar]
- 24.Regnard JF, Grunenwald D, Spaggiari L, et al. Surgical treatment of hepatic and pulmonary metastases from colorectal cancers. Ann Thorac Surg 1998; 66: 214–219. [DOI] [PubMed] [Google Scholar]
- 25.Lehnert T, Knaebel HP, Duck M, et al. Sequential hepatic and pulmonary resections for metastatic colorectal cancer. Br J Surg 1999; 86: 241–243. [DOI] [PubMed] [Google Scholar]
- 26.Ekberg H, Tramberg KG, Anderson R, et al. Patterns of recurrence in liver resection for colorectal secondaries. World J Surg 1987; 11: 541–547. [DOI] [PubMed] [Google Scholar]
- 27.Adam R, Bismuth H, Castaing D, et al. Repeat hepatectomy for colorectal cancer liver metastases. Ann Surg 1997; 225: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordlinger B, Vaillant JC, Guiguet M, et al. Survival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases. J Clin Oncol 1994; 12: 1491–1496. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Trigo V, Shamsa F, Sugarbaker P et al. Repeat liver resections from colorectal metastasis. Surgery 1995; 117: 296–304. [DOI] [PubMed] [Google Scholar]
- 30.Neeleman N, Andersson R. Repeated liver resection for recurrent liver cancer. Br J Surg 1996; 83: 893–901. [DOI] [PubMed] [Google Scholar]
- 31.Tuttle TM, Curley SA, Roh MS. Repeat hepatic resection as effective treatment of recurrent colorectal liver metastases. Ann Surg Oncol 1997; 4: 125–130. [DOI] [PubMed] [Google Scholar]
- 32.Kin T, Nakajima Y, Kanehiro H, et al. Repeat hepatectomy for recurrent colorectal metastases. World J Surg 1998; 22: 1087–1091. [DOI] [PubMed] [Google Scholar]
- 33.Sebagh M, Adam R, Lemoine A, et al. Evaluation histologique et biochimique de la toxicité hépatique d’une chimiothérapie systémique. Gastroenterol Clin Biol 1996; 20: A1.8909579 [Google Scholar]
- 34.Elias D, Lasser D, Rougier P. Frequency, technical aspects, results and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg 1995; 180: 213–218. [PubMed] [Google Scholar]
- 35.Conférence de consensus. Prévention, dépistage et prise en charge des cancers du colon. Gastroenterol Clin Biol 1998; 22: S265–S272. [PubMed] [Google Scholar]
- 36.Couinaud C. Le Foie: Etudes Anatomiques et Chirugicales. Paris: Masson et Cie; 1959.