Abstract
Objective
To evaluate the potential of isolated limb perfusion (ILP) for efficient and tumor-specific adenovirus-mediated gene transfer in sarcoma-bearing rats.
Summary Background Data
A major concern in adenovirus-mediated gene therapy in cancer is the transfer of genes to organs other than the tumor, especially organs with a rapid cell turnover. Adjustment of the vector delivery route might be an option creating tumor specificity in therapeutic gene expression.
Methods
Rat hind limb sarcomas (5–10 mm) were transfected with recombinant adenoviruses. Intratumoral luciferase expression after ILP was compared with systemic administration, regional infusion, or intratumoral injection using a similar dose of adenoviruses carrying the luciferase marker gene. Localization studies using lacZ as a marker gene were performed to evaluate the intratumoral distribution of transfected cells after both ILP and intratumoral injection.
Results
Intratumoral luciferase activity after ILP or intratumoral administration was significantly higher compared with regional infusion or systemic administration. After ILP, luciferase gene expression was minimal in extratumoral organs, whether outside or inside the isolated circuit. Localization studies demonstrated that transfection was confined to tumor cells lying along the needle track after intratumoral injection, whereas after ILP, lacZ expression was found in viable tumor cells and in the tumor-associated vasculature.
Conclusions
Using ILP, efficient and tumor-specific gene transfection can be achieved. The ILP technique might be useful for the delivery of recombinant adenoviruses carrying therapeutic gene constructs to enhance tumor control.
Recent advances in molecular engineering have enabled gene therapy to become a promising therapeutic entity for an ever-increasing number of clinical applications. Of the potential viral and nonviral vector systems for the transfer of therapeutic genes into target cells, recombinant retroviruses and adenoviruses have been most widely used in both preclinical studies and clinical trials. 1 Virus-mediated gene transfer can be accomplished by either ex vivo or in vivo approaches. The ex vivo strategy involves harvesting the target cells, genetically modifying them in vitro, and reimplanting them into the patient. The in vivo approach involves the direct transfection of target cells by recombinant viruses with transgene, using systemic, regional, or tissue-specific administration. 2
Although gene therapy was originally developed for correction of genetic deficiencies of inherited disorders of metabolism, current interest is mainly focused on its potential therapeutic roles in cardiovascular disease and cancer. Approaches to cancer gene therapy include genetic marking, cancer vaccination, inhibition of oncogene expression, restoration of tumor suppressor genes, and the use of suicide genes, which may be one of the most successful therapeutic strategies to date. The suicide gene strategy aims at the induction of drug sensitivity by introducing genes such as the herpes simplex thymidine kinase gene into the tumor cells, whose expression initiates the formation of prodrug metabolizing enzymes. The herpes simplex thymidine kinase gene converts ganciclovir into phosphorylated metabolites that act as chain terminators during DNA synthesis, in this way causing selective cell death. 3,4
A major concern in the enzyme/prodrug approach is the transfer of suicide genes to organs other than the tumor, especially organs with a rapid cell turnover. The risk of infecting cell types other than target cells is negligible in strategies involving the ex vivo suicide gene transfer. 5,6 However, apart from a possible role in cancer vaccination, ex vivo gene transfer is clearly not applicable in anticancer gene therapy. In vivo gene delivery, in contrast, should be targeted to tumor cells to avoid complications resulting from leakage of genes to other cells in the body. One way to achieve tumor-specific in vivo gene delivery is by tissue-specific administration of viral vectors to tumor cells. 2
In surgical oncology trials, isolated limb perfusion (ILP) is successfully used for administration of chemotherapeutic agents and cytokines to locally advanced soft tissue extremity sarcomas and in-transit melanoma metastases. 7–10 ILP involves the recirculation of high drug concentrations in a vascularly isolated extremity, resulting in minimal drug exposure to organs outside the closed circuit. In the present study, we evaluated the efficiency and tumor specificity of adenovirus-mediated gene transfer using ILP in an established sarcoma-bearing rat model. 11–13 We quantified the activity of a marker gene in limb sarcomas after ILP with adenoviral vectors carrying the luciferase marker gene. The intratumoral luciferase gene expression was compared with the luciferase activity in other organs either inside or outside the isolated vascular circuit. The efficiency and tumor specificity of ILP-mediated gene transfer was compared with other delivery routes, including systemic administration, regional infusion, and intratumoral injection. Moreover, adenoviral vectors carrying the lacZ marker gene were used to determine the intratumoral localization of transfected cells after both ILP and intratumoral administration.
METHODS
Adenoviral Vectors
All adenoviral vectors used in this study were derived from human adenovirus type 5 and were deleted for the El region in which the transgenes were cloned. The E3 region was retained in all vectors. The cytomegalovirus (CMV) promoter and adenoviral major late promoter (MLP) were used to drive the lacZ and luciferase marker genes. The construction and production of IG.Ad.MLP.Luc, IG.Ad.CMV.Luc, and IG.Ad.CMV.LacZ recombinant adenoviruses is described in detail elsewhere. 14 Briefly, recombinant adenoviral vectors were plaque-purified twice, propagated on 293 or PER.C6 cells, purified by CsCl density centrifugation, dialyzed, and stored at −80°C in buffer containing (in mmol/L) 13 Na2HPO4 (pH 7.4), 140 NaCl, 0.9 CaCl2, and 0.5 MgCl2 and 5% (m/v) sucrose. The virus titers (infectious units [iu]/mL) were determined by end-point cytopathogenic effect titrations using 911 cells. 4 All recombinant adenoviral vectors were produced at IntroGene, Leiden, The Netherlands.
Animals
Inbred male Brown Norway rats and WagRij rats, weighing 200 to 300 g, were obtained from Harlan (Zeist, The Netherlands). Animals were kept in standard laboratory conditions and were fed a standard laboratory diet (Hope Farms, Woerden, The Netherlands). The experimental protocols adhered to the rules described in the Dutch Animal Experimentation Act and the Guidelines on the Protection of Experimental Animals by the Council of the European Community. Before the experiments, the protocols were approved by the Animal Research Committee of Erasmus University in Rotterdam and of the University of Leiden, The Netherlands.
Tumor Model
The spontaneous BN-175 sarcoma and ROS-1 osteosarcomas were implanted in the flank of donors and passaged serially. Both tumors are nonimmunogenic and rapidly growing and metastasizing, with a doubling time of approximately 5 to 7 days. 15 Small tumor fragments were subcutaneously implanted into the right hind limb just above the ankle. All surgical interventions were performed at a tumor diameter of 5 to 10 mm, at least 7 days after implantation.
Administration Techniques
All surgical procedures were performed under Hypnorm anesthesia (Janssen Pharmaceutica, Tilburg, The Netherlands). For ILP, we used the technique described by Manusama et al 11 (Fig. 1). Briefly, the femoral vessels were approached through an incision parallel to the inguinal ligament after systemic heparin administration (50 IU). Subsequently, the femoral artery and vein were cannulated with Silastic tubing (0.30 mm inner diameter, 0.64 mm outer diameter; 0.64 mm inner diameter, 1.19 mm outer diameter, respectively; Dow Corning, Ann Arbor, MI). Collaterals were temporarily occluded by applying a tourniquet around the groin. Perfusion was performed with recombinant adenoviral vectors (1 × 109 iu IG.Ad.MLP.Luc or 1 × 109 iu IG.Ad.CMV.LacZ) added as a bolus in 5 mL Haemaccel (Behring Pharma, Amsterdam, The Netherlands). An oxygenation reservoir and a roller pump were included in the isolated circuit. The perfusate was circulated at a flow speed of 2 mL per minute for 5 to 30 minutes. After ILP, the isolated circuit was perfused with Haemaccel for another 5 minutes to wash out the nonbound viruses. During ILP and washout, the hind leg was kept at a constant temperature of 38° to 39°C by a warm-water mattress applied around the leg. After washout, the isolated circuit was discontinued, and after tube removal the femoral vessels were ligated. Previous experiments have shown that the collateral circulation to the leg is so extensive that ligation of the femoral vessels can be performed without detrimental effects. 11
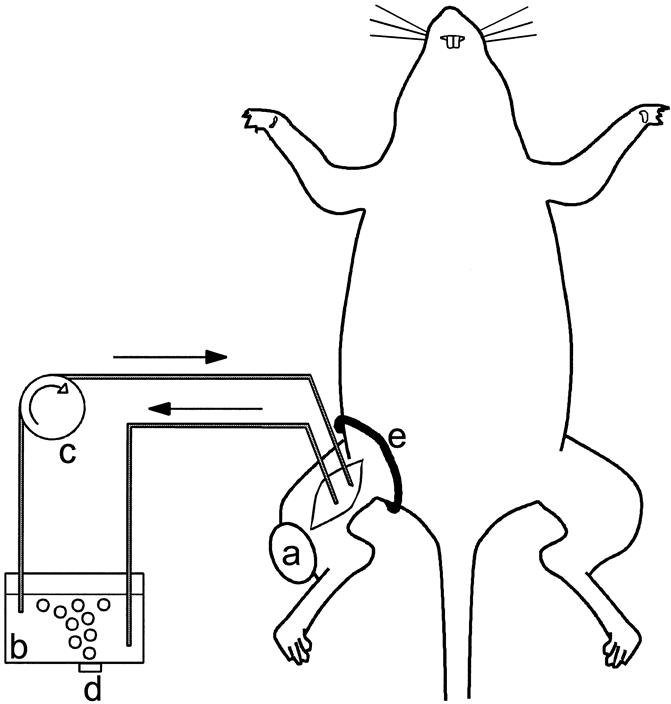
Figure 1. Isolated limb perfusion setting in the rat: a, BN-175 soft tissue sarcoma; b, perfusion reservoir; c, roller pump; d, oxygenation of the perfusate; e, tourniquet. (Adapted from ten Hagen, Eggermont. Adv Drug Deliv Rev 1997; 24:245–256.)
For intratumoral injection, the same amount of recombinant adenoviral vectors (1 × 109 iu IG.Ad.MLP.Luc or 1 × 109 iu IG.Ad.CMV.LacZ) was injected into the center of the BN-175 tumor using a 25-gauge needle. Leakage of virus was minimized by tamponade of the injection site with a cotton tip. For systemic administration, adenoviral vectors (1 × 109 iu IG.Ad.MLP.Luc or 1 × 109 iu IG.Ad.CMV.Luc) were injected into the penile vein using a 25-gauge needle, followed by tamponade to prevent virus and blood leakage. For regional administration, a Silastic tube (0.30 mm inner diameter, 0.64 mm outer diameter) was implanted into the femoral artery. Recombinant adenoviruses (1 × 109 iu IG.Ad.MLP.Luc), dissolved in 1 mL Haemaccel, were infused through the implanted tube, followed by 1 mL Haemaccel to wash out the adenoviral vectors from the Silastic tube. Also in these animals, the femoral artery was ligated after tube removal.
Luciferase Assay
Two days after administration of luciferase, the experimental animals were killed. Tumor and quadriceps muscle in the isolated circuit were removed. In addition, liver, spleen, heart, lung, kidney, intestine, gonads, and aorta, all outside the isolated limb, were harvested for measurement of luciferase activity. Cross-contamination of the tissue samples was avoided by cleaning the equipment thoroughly with 1% SDS, H2O, and ethanol after dissection of each sample. Removed tissues were weighed, frozen in liquid N2, and stored at −20°C. Later, samples were thawed in 2 mL ice-cold lysis buffer (8 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L DTT, 1% Triton X-100, and 15% glycerol in phosphate-buffered saline [PBS]) then homogenized. Lysed cells were collected and centrifuged (14,000 rpm for 7 minutes) at 2° to 6°C to remove cell debris. Luciferase activity present in 20 μL lysate was determined by adding 100 μL of luciferase assay reagent (Promega, Madison, WI). After 10 seconds of preincubation, the produced light was measured for 30 seconds in a Lumat LB9501 luminometer (Berthold, Wildbad, Germany). Protein concentrations of different tissues were determined using the Biorad Protein Assay kit (Biorad, München, Germany), and luciferase activity was calculated as relative light units per milligram protein.
β-Galactosidase Histochemistry
Two days after ILP or intratumoral injection, the experimental animals exposed to Ad.CMV.LacZ were killed and the tumor was dissected. Tumor tissues were fixed for 1 hour in ice-cold 2% paraformaldehyde/0.25% glutaraldehyde solution. After fixation, the tissues were washed with PBS and incubated for 1 day in freshly prepared X-gal staining solution (Boehringer, Mannheim, Germany). The tissue was washed again with PBS and fixed in 10% buffered formalin solution (40 g NaH2PO4, 81.5 g NaH2PO4, and 1 L 100% formalin in 10 L demineralized H20). Subsequently, histologic slices were prepared for qualitative analysis.
Statistical Analysis
The Mann-Whitney test was used to compare luciferase activity in the various organs after different routes of administration.
RESULTS
Optimal Duration of ILP Using Recombinant Adenoviral Vectors
To determine the optimal duration of ILP for maximal gene transfer into tumor tissue, luciferase activity was determined and compared after 5, 15, or 30 minutes of perfusion with 1 × 109 iu IG.Ad.MLP.Luc. As shown in Figure 2, luciferase activity in tumor tissue increased with longer perfusion times. However, increments in luciferase gene expression tended to decrease with longer perfusion times. There was no increase in luciferase activity in tumor tissue after 30 minutes of ILP versus 15 minutes (P = 1.0); therefore, 15 minutes was used for further experiments. Systemic leakage of adenoviral vectors to other organs did not increase with longer perfusions (data not shown).
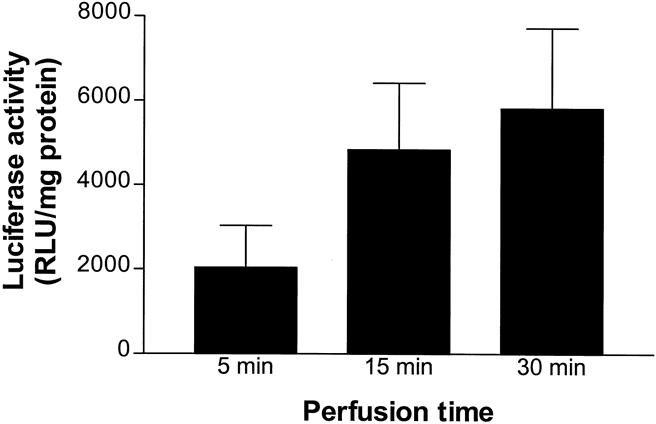
Figure 2. Mean luciferase activity (±SEM) in BN-175 tumors 48 hours after adenovirus-mediated gene transfer using isolated limb perfusion. Limbs were perfused with 1 × 109 infectious units IG.Ad.MLP.Luc for 5 minutes (n = 6), 15 minutes (n = 6), or 30 minutes (n = 6).
Efficiency of Gene Transfer in Tumor Tissue Using Different Administration Methods
The efficiency of luciferase gene transfer in tumors after 15 minutes of perfusion with 1 × 109 iu IG.Ad.MLP.Luc in an isolated limb was compared with systemic, regional, and intratumoral administration with the same amount of recombinant adenovirus (Fig. 3). Both ILP and intratumoral injections resulted in a significantly greater mean intratumoral luciferase activity compared with systemic administration (both P < .005) and regional infusion (both P < .005). Intratumoral injection resulted in greater gene expression in tumor tissue compared with ILP, but the difference was not significant (P = .7). In the intratumoral group, a larger standard error of the mean (SEM) was observed than in the other routes of administration, indicating a large variance in gene transfer between the different injections of adenoviral vectors in the tumor.
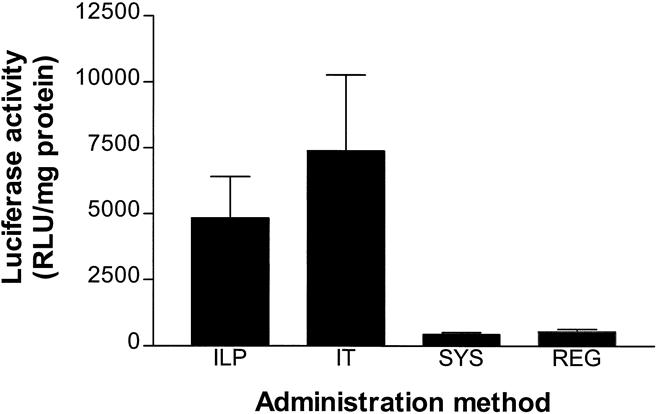
Figure 3. Efficacy of gene transfer in BN-175 tumors after administration of 1 × 109 infectious units IG.Ad.MLP.Luc using isolated limb perfusion (ILP, n = 6), intratumoral injection (IT, n = 6), regional administration (REG, n = 6), or systemic administration (SYS, n = 6). Mean luciferase activity (±SEM) 48 hours after ILP and IT administration was significantly different from SYS (both P < .005) and REG administration (both P < .005).
Systemic Leakage of Adenoviral Vectors
Luciferase activity after ILP was measured in various organs outside and inside the isolated circuit during perfusion with 1 × 109 iu IG.Ad.MLP.Luc. Figure 4 shows negligible luciferase activity in organs outside the isolated circuit. Luciferase gene expression fluctuated around the detection level (100 relative light units per milligram protein), indicating that the isolated limb perfusion was leakage-free. Mean luciferase activity in the tumor was significantly greater (P < .005) than its activity in quadriceps muscles, suggesting a preference for tumor cell transfection in the isolated limb.
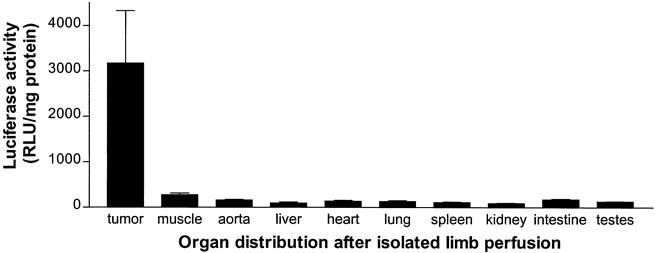
Figure 4. Organ specificity of gene delivery 48 hours after isolated limb perfusion using 1 × 109 infectious units IG.Ad.MLP.Luc (n = 6). Mean luciferase activity (±SEM) was significantly greater in tumor tissue than in all other organs inside or outside the isolated circuit (P < .005).
Distribution of Adenoviral Vectors After Systemic Administration
Luciferase activity was measured in various organs after systemic administration of 1 × 109 iu IG.Ad.CMV.Luc in WagRij rats bearing a ROS-1 osteosarcoma (Fig. 5). The CMV promoter was stronger than the MLP promoter, and thus absolute luciferase gene expression was greater. Much greater luciferase activity was demonstrated in spleen and liver, with some activity in tumor tissue comparable to heart and lung. The muscle of the hind limb and kidney showed negligible luciferase activity.
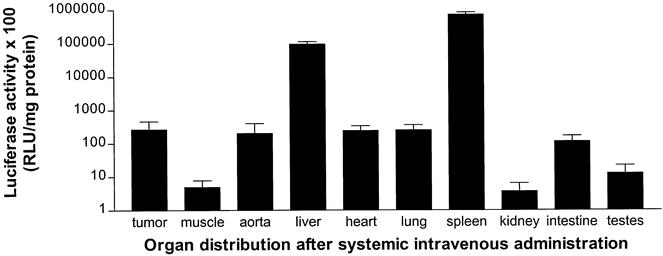
Figure 5. Organ specificity of gene delivery 48 hours after systemic intravenous administration using 1 × 109 infectious units IG.Ad.CMV.Luc in WagRij rats (n = 3). Mean luciferase activity (±SEM) was significantly greater in spleen and liver tissue compared with all other organs (P < .005).
Intratumoral Location of Transfected Marker Genes
Animals underwent ILP (n = 6) or intratumoral (n = 6) administration of 1 × 109 iu IG.Ad.CMV.LacZ and were killed after 48 hours. Tumors were harvested and histologic slides were prepared with staining for 13-galactosidase. In these slides (three per tumor), lacZ-positive cells were identified and analyzed for cell type and location in the tumor. After intratumoral administration, lacZ-positive cells were found along the needle track, without staining tumor cells in other parts of the tumor (Fig. 6). After ILP, lacZ expression was more homogenous and was observed around tumor-associated vessels. Moreover, lacZ-positive cells were found in several areas of the tumor, with a preferential location in the viable rim. Routine examination of the numerous histologic slides of tumors after both ILP and intratumoral injection did not show large numbers of inflammatory cells surrounding the transfected cells.
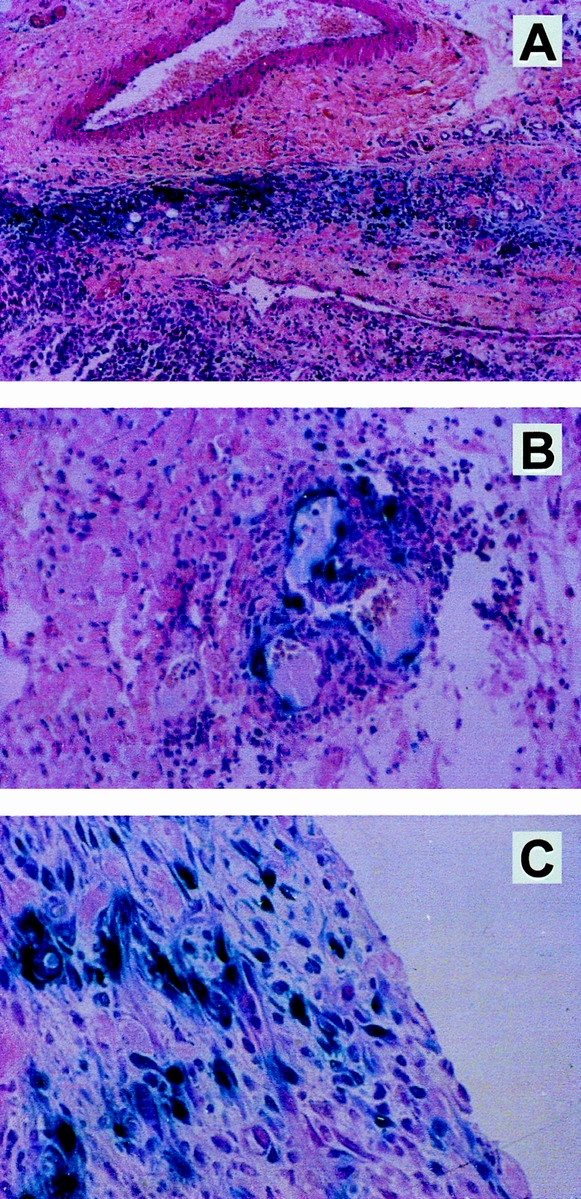
Figure 6. Adenovirus-mediated lacZ gene transfer to BN-175 soft tissue sarcoma in rats. Blue staining represents cells actively expressing β-gal 48 hours after treatment with 1 × 109 infectious units IG.Ad.CMV.LacZ. After intratumoral injection, blue staining was found only around the needle track and not in other parts of the tumor (A). After isolated limb perfusion, lacZ gene transfer was demonstrated in various parts of the tumor, including tumor-associated vessels (B) and the viable rim of the tumor (C).
DISCUSSION
To compete with conventional modalities, anticancer gene therapy should be both effective and safe. 2 The first requirement implies the use of viral or nonviral vector systems that guarantee efficient gene transfer, in addition to the application of promoters that offer appropriate expression of the desired genes. For safety, tissue specificity of gene expression is essential, because expression of transfected genes in organs other than tumor tissue may cause potentially dangerous complications. Tissue specificity may be accomplished at the level of gene transfer by vector targeting, which necessitates the use of ligands or antibodies that can be conjugated to both viral and nonviral vector systems. 2 Ligands can target a vector system to specific tumor cell types by interacting with receptors that are exclusively present on the surface of these target cells. Various ligands have been used for vector targeting to tumor cells, including folate, asialo-orosomucoid, and epidermal growth factor, allowing for tumor-specific gene delivery in ovarian cancer, hepatocellular carcinoma, and lung cancer, respectively. 16,17 Apart from targeted vector delivery, tumor-specific gene expression can be achieved with the use of tumor-specific promoters such as those for carcinoembryonic antigen and human surfactant protein A, which are activated only in the nuclei of tumor cells containing these substances. 18,19
The most widely applied gene transfer vectors, derived from retroviruses or adenoviruses as well as standard promoters, do not generate tumor-specific gene expression. In the present study, systemic administration of adenoviral vectors carrying the luciferase marker gene did not result in significant tumor tissue expression, as was expected. The predominant expression of luciferase was demonstrated in spleen and liver tissue. Gene uptake in tumor tissue was low and comparable to that of other organs, such as heart and lung. These observations confirm recommendations by other authors who advise against using systemically administered anticancer gene therapy for clinical trials unless tissue-specific vector systems are included. 2
Apart from systemic delivery, a catheter into the tumor vasculature can be used to administer recombinant viruses. Regional infusion of a target organ has been explored for lung, 20 liver, 21 and brain, 22 and effective viral-mediated gene transfer has been demonstrated. In the present study, gene transfer of adenoviral vectors after intraarterial infusion was remarkably ineffective and was not superior to systemic intravenous administration. The observed low gene expression might be explained by the fact that there is only a single passage of a small fraction of the administered adenoviral vectors through the tumor vasculature. The vast majority of adenoviral vectors bypass the tumor and disappear in the normal vessels of the hind limb to end up in the systemic circulation.
In principle, the simplest route of tumor-specific gene delivery is local administration of vectors into tumors by direct injection; this has been successfully performed in subcutaneous malignancies 23 and brain gliomas, 24 the latter with stereotactic guidance. The present experiments demonstrated that intratumoral injection of adenoviral vectors resulted in an efficient transfer of luciferase genes to tumor cells. However, localization studies demonstrated that the lacZ-positive tumor cells were confined to the injection site in the tumor (needle track staining). With the ILP technique, an extremity can be exposed to high drug concentrations for various periods of time. Higher tissue uptake may result, as has been demonstrated for melphalan. 25,26 Moreover, the ILP technique allows a washout procedure to remove nonbound drug, thereby minimizing systemic contamination after recirculation. In previous studies using tumor necrosis factor and melphalan, we demonstrated an almost leakage-free isolated system in the hind limb of the rat. 13 This sarcoma-bearing rat model closely mimics the clinical situation and can be used as a preclinical model for pharmacokinetic studies and antitumor responses. In the present study, ILP of adenoviral vectors carrying the luciferase marker gene resulted in a significantly higher luciferase activity in the tumor than after systemic or regional administration, indicating an efficient gene transfer using this technique. Intratumoral luciferase activity increased with longer durations of perfusion, indicating the importance of repeated passages of the adenoviral vectors for tumor cell infection. Moreover, gene delivery using ILP showed a homogeneous lacZ gene transfer around tumor-associated vessels and in the viable rim of the tumor, in contrast to the expression limited only around the needle track, as seen after intratumoral administration.
The efficacy of adenoviral-mediated gene delivery has previously been demonstrated in other isolated perfusion settings, including isolated liver perfusion and isolated lung perfusion. 27,28 In these studies, no tumor was included in the isolated circuit, so it is unknown whether there was preferential transfection of tumor cells in relation to other cell types in the perfused circuit. The current quantitative analysis of luciferase activity in the perfused limb clearly demonstrates a higher uptake of luciferase genes by tumor cells compared with muscle tissue. This might be explained by the fact that normal muscle tissue is inert, and the muscle vasculature has a normal endothelial lining, in contrast to the rapidly dividing cells and leaky vasculature in tumor tissue. Moreover, luciferase activity in systemic organs was low because a nearly leakage-free circuit is obtained during ILP, and a washout procedure is performed after ILP to remove unbound viruses before the limb is enclosed to the circulation.
The concept of using ILP for tumor-specific gene transfer was recently explored by Milas et al, 29 who demonstrated efficient gene delivery in tumor tissue by means of an isolated limb perfusion model in the rat using an adenovirus Ad.LacZ. Systemic leakage was, however, not directly quantified by measuring marker gene activity, as in the present study, but with the help of radioactively labeled red blood cells. Moreover, the efficiency and tumor specificity of adenoviral-mediated gene delivery by ILP were not quantitatively compared with other methods of administration.
In conclusion, our results indicate that in sarcoma-bearing rats, delivery of adenoviral vectors by ILP is effective and reproducible. Consequently, ILP might be useful for safe, efficient, transvascular, and tumor-specific delivery of recombinant adenoviruses carrying various therapeutic gene constructs, including genes encoding for cytokines, angiogenesis inhibitors, and suicide genes, to enhance tumor control. We recently demonstrated that interleukin (IL)-3β gene therapy by ILP was effective in the treatment of ROS-1 and BN-175 sarcoma, whereas intratumoral injections appeared not to be effective. 30 Replacement of genes to increase sensitivity for tumor necrosis factor (e.g., EMAP-II) is another promising therapeutic option to increase responses to tumor necrosis factor/melphalan perfusion. 31 Preclinical studies are ongoing to explore these possibilities in limb and organ perfusion settings, which may ultimately prove beneficial to cancer patients.
Discussion
Prof. B. Jeppsson (Malmö, Sweden): I guess your aim is that you want to avoid the complications that we already have seen when you transfer cell systems that we do not want to transfer. My guess is also that you fairly soon want to go on to patients. You showed us the time–response curve, which was also in the abstract. What do you think will be the optimal time, in a patient, when you want to isolate the limb? That is my first question. I also wonder if you have any idea of the amount of gene you want to transfer to have any clinical efficiency with your system. And finally, you also showed us that you did not have any systemic leak. Was that also estimated a long time after the end of the perfusion?
Dr. W. de Roos (Rotterdam, The Netherlands): To answer your last question first, we did not have much time to evaluate the long-term systemic leakage because the rats were sacrificed after 2 days, so the results on systemic leakage represent the situation at 48 hours after perfusion. In answer to the second question, we have performed this kind of research. Recently we performed studies in which we used the gene encoding for the cytokine IL-3β in this rat model. We compared the administration of adenoviral vectors by isolated limb perfusion to intratumoral injection, systemic (intravenous) administration, and regional (intraarterial) infusion. Only after isolated limb perfusion was a significant antitumor response of the IL-3β gene observed. Moreover, we demonstrated that this effect was dose-dependent. However, on this basis I cannot predict the concentration of adenoviral vectors that would have to be used in humans. Your first question is about the perfusion time. Our data suggests that the adenovirus infects the cells very quickly, so there is no need to prolong the perfusion time beyond 15 minutes because you do not get a significantly higher uptake by the tumor after 30 minutes of perfusion.
Prof. P. Kinnaert (Brussels, Belgium): My question is related to the safety of the procedure. How can you be sure that the genetic information of the transfected cancer cells will not be transmitted later on to the surrounding tissues? How do you protect the medical personnel?
Dr. de Roos: To answer your first question on the construction of the virus factor, what you do is remove the pathological genes from the virus and replace them with the therapeutic gene that you need. In this removal of the pathological genes, you remove the capacity of the virus to infect again. By using this isolated technique, you minimize the infection of other tissues. That is the whole issue of the isolated perfusion.
Prof. B. Kremer (Kiel, Germany): Can you speculate on the tumor specificity of the virus? Is it transferred to the tumor and not to the surrounding tissue because of the more activated cell division of endothelial cells in the tumor vessels?
Dr. de Roos (Closing Discussion): Tumor-associated vessels differ from normal vessels. We speculate that the endothelial lining of tumor vasculature is more penetrable for adenoviral vectors in comparison to vasculature in normal tissue—e.g., muscle tissue.
Footnotes
Correspondence: Alexander M.M. Eggermont, MD, PhD, Dept. of Surgical Oncology, University Hospital Rotterdam, Daniel den Hoed Cancer Center, Groene Hilledijk 301, 3075 EA Rotterdam, The Netherlands.
Presented at the Seventh Annual Meeting of the European Surgical Association, Amstel Intercontinental Hotel, Amsterdam, The Netherlands, April 14–15, 2000.
E-mail: eggermont@.chih.azr.n1
Accepted for publication July 2000.
References
- 1.Kong HL, Crystal RG. Gene therapy strategies for tumor antiangiogenesis. J Natl Cancer Inst 1998; 90: 273–286. [DOI] [PubMed] [Google Scholar]
- 2.Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst 1997; 89: 21–39. [DOI] [PubMed] [Google Scholar]
- 3.Esandi MC, van Someren GD, Vincent AJPE, et al. Gene therapy of experimental malignant mesothelioma using adenovirus vectors encoding the HSVtk gene. Gene Ther 1997; 4: 280–287. [DOI] [PubMed] [Google Scholar]
- 4.Vincent Al, Vogels R, Someren GV, et al. Herpes simplex virus thymidine kinase gene therapy for rat malignant brain tumors. Hum Gene Ther 1996; 7: 197–205. [DOI] [PubMed] [Google Scholar]
- 5.Tamura M, Shimizu K, Yamada M, et al. Targeted killing of migrating gliomal cells by injection of HTK-modified gliomal cells. Hum Gene Ther 1997; 8: 381–391. [DOI] [PubMed] [Google Scholar]
- 6.Namba H, Tagawa M, Iwadate Y, et al. Bystander effect-mediated therapy of experimental brain tumor by genetically engineered tumor cells. Hum Gene Ther 1998; 9: 5–11. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AMM, Schraffordt Koops H, Lienard D, et al. Isolated limb perfusion with high-dose tumor necrosis factor-α in combination with interferon-gamma and melphalan for nonresectable extremity soft tissue sarcomas: a multicenter trial. J Clin Oncol 1996; 14: 2653–2665. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont AMM, Schraffordt Koops H, Klausner J, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas: the cumulative multicenter European experience. Ann Surg 1996; 224: 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraker DL, Alexander HR, Andrich M, Rosenburg SA. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: results of a tumor necrosis factor dose-escalation study. J Clin Oncol 1996; 14: 479–489. [DOI] [PubMed] [Google Scholar]
- 10.Liénard D, Ewalenko P, Delmotte JJ, et al. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol 1992; 10: 52–60. [DOI] [PubMed] [Google Scholar]
- 11.Manusama ER, Nooijen PTGE, Stavast J, et al. Synergistic antitumour effect of recombinant human tumour necrosis factor-α with melphalan in isolated limb perfusion in the rat. Br J Surg 1996; 83: 551–555. [DOI] [PubMed] [Google Scholar]
- 12.Manusama ER, Stavast J, Durante NMC, et al. Isolated limb perfusion with TNF-α and melphalan in a rat osteosarcoma: a new anti-tumour approach. Eur J Surg Oncol 1996; 22: 152–157. [DOI] [PubMed] [Google Scholar]
- 13.de Wilt JHW, Manusama ER, van Tiel ST, et al. Prerequisites for effective isolated limb perfusion using tumour necrosis factor alpha and melphalan in rats. Br J Cancer 1999; 80: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallaux FJ, Bout A, van der Velde I, et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 1998; 9: 1909–1917. [DOI] [PubMed] [Google Scholar]
- 15.Kort WJ, Zondervan PE, Hulsman LO, et al. Incidence of spontaneous tumors in a group of retired breeder female brown Norway rats. J Natl Cancer Inst 1984; 72: 709–713. [PubMed] [Google Scholar]
- 16.Gottschalk S, Cristiano RJ, Smith L, Woo SL. Folate receptor-mediated DNA delivery and expression in vitro. Gene Ther 1994; 1: 185–191. [PubMed] [Google Scholar]
- 17.Cristiano R, Roth R. Epidermal growth factor mediated DNA delivery into lung cancer cells via the epidermal growth factor receptor. Cancer Gene Ther 1996; 3: 4–10. [PubMed] [Google Scholar]
- 18.DiMaio JM, Clary BM, Via DF, et al. Directed enzyme pro-drug gene therapy for pancreatic cancer in vivo. Surgery 1994; 116: 205–213. [PubMed] [Google Scholar]
- 19.Smith MJ, Rousculp MD, Goldsmith KT, et al. Surfactant protein A-directed toxin gene kills lung cancer cells in vitro. Hum Gene Ther 1994; 5: 29–35. [DOI] [PubMed] [Google Scholar]
- 20.Nabel EG, Yang Z, Muller D, et al. Safety and toxicity of catheter gene delivery to the pulmonary vasculature in a patient with metastatic melanoma. Hum Gene Ther 1994; 5: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 21.Kay MA, Landen CN, Rothenberg SR, et al. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci USA 1994; 91: 2353–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauvet AE, Kesava PP, Goh CS, Badie B. Selective intraarterial gene delivery into a canine meningioma. J Neurosurg 1998; 88: 870–873. [DOI] [PubMed] [Google Scholar]
- 23.Nabel GJ, Nabel EG, Yang Z-Y, et al. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proc Natl Acad Sci USA 1993; 90: 11307–11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spear MA, Herrlinger U, Rainov N, et al. Targeting gene therapy vectors to CNS malignancies. J Neurovirol 1998; 4: 133–147. [DOI] [PubMed] [Google Scholar]
- 25.Scott RN, Kerr DJ, Blackie R, et al. The pharmacokinetic advantages of isolated limb perfusion with melphalan for malignant melanoma. Br J Cancer 1992; 66: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaase JM, Kroon BB, Beijnen JH, et al. Melphalan tissue concentrations in patients treated with regional isolated perfusion for melanoma of the lower limb. Br J Cancer 1994; 70: 151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Roos WK, Fallaux FJ, Marinelli AW, et al. Isolated-organ perfusion for local gene delivery: efficient adenovirus-mediated gene transfer into the liver. Gene Ther 1997; 4: 55–62. [DOI] [PubMed] [Google Scholar]
- 28.Lee R, Boasquevisque CH, Boglione MM, et al. Isolated lung liposome-mediated gene transfer produces organ-specific transgenic expression. Ann Thor Surg 1998; 66: 903–907. [DOI] [PubMed] [Google Scholar]
- 29.Milas M, Feig B, Yu D, et al. Isolated limb perfusion in the sarcoma-bearing rat: a novel preclinical gene delivery system. Clin Cancer Res 1997; 3: 2197–2203. [PubMed] [Google Scholar]
- 30.de Wilt JHW, Bout A, Eggermont AMM, et al. Adenovirus-mediated IL-3β gene transfer using isolated limb perfusion inhibits growth of limb sarcoma in rats. Hum Gene Ther 2000 (in press). [DOI] [PubMed]
- 31.Gnant MF, Berger AC, Huang J, et al. Sensitization of tumor necrosis factor alpha-resistant human melanoma by tumor-specific in vivo transfer of the gene encoding endothelial monocyte-activating polypeptide II using recombinant vaccinia virus. Cancer Res 1999; 59: 4668–4674. [PubMed] [Google Scholar]


