Abstract
Objective
To determine outcome in diabetic pancreas transplant recipients according to risk factors and the surgical techniques and immunosuppressive protocols that evolved during a 33-year period at a single institution.
Summary Background Data
Insulin-dependent diabetes mellitus is associated with a high incidence of management problems and secondary complications. Clinical pancreas transplantation began at the University of Minnesota in 1966, initially with a high failure rate, but outcome improved in parallel with other organ transplants. The authors retrospectively analyzed the factors associated with the increased success rate of pancreas transplants.
Methods
From December 16, 1966, to March 31, 2000, the authors performed 1,194 pancreas transplants (111 from living donors; 191 retransplants): 498 simultaneous pancreas–kidney (SPK) and 1 simultaneous pancreas–liver transplant; 404 pancreas after kidney (PAK) transplants; and 291 pancreas transplants alone (PTA). The analyses were divided into five eras: era 0, 1966 to 1973 (n = 14), historical; era 1, 1978 to 1986 (n = 148), transition to cyclosporine for immunosuppression, multiple duct management techniques, and only solitary (PAK and PTA) transplants; era 2, 1986 to 1994 (n = 461), all categories (SPK, PAK, and PTA), predominately bladder drainage for graft duct management, and primarily triple therapy (cyclosporine, azathioprine, and prednisone) for maintenance immunosuppression; era 3, 1994 to 1998 (n = 286), tacrolimus and mycophenolate mofetil used; and era 4, 1998 to 2000 (n = 275), use of daclizumab for induction immunosuppression, primarily enteric drainage for SPK transplants, pretransplant immunosuppression in candidates awaiting PTA.
Results
Patient and primary cadaver pancreas graft functional (insulin-independence) survival rates at 1 year by category and era were as follows: SPK, era 2 (n = 214) versus eras 3 and 4 combined (n = 212), 85% and 64% versus 92% and 79%, respectively; PAK, era 1 (n = 36) versus 2 (n = 61) versus 3 (n = 84) versus 4 (n = 92), 86% and 17%, 98% and 59%, 98% and 76%, and 98% and 81%, respectively; in PTA, era 1 (n = 36) versus 2 (n = 72) versus 3 (n = 30) versus 4 (n = 40), 77% and 31%, 99% and 50%, 90% and 67%, and 100% and 88%, respectively. In eras 3 and 4 combined for primary cadaver SPK transplants, pancreas graft survival rates were significantly higher with bladder drainage (n = 136) than enteric drainage (n = 70), 82% versus 74% at 1 year (P = .03). Increasing recipient age had an adverse effect on outcome only in SPK recipients. Vascular disease was common (in eras 3 and 4, 27% of SPK recipients had a pretransplant myocardial infarction and 40% had a coronary artery bypass); those with no vascular disease had significantly higher patient and graft survival rates in the SPK and PAK categories. Living donor segmental pancreas transplants were associated with higher technically successful graft survival rates in each era, predominately solitary (PAK and PTA) in eras 1 and 2 and SPK in eras 3 and 4. Diabetic secondary complications were ameliorated in some recipients, and quality of life studies showed significant gains after the transplant in all recipient categories.
Conclusions
Patient and graft survival rates have significantly improved over time as surgical techniques and immunosuppressive protocols have evolved. Eventually, islet transplants will replace pancreas transplants for suitable candidates, but currently pancreas transplants can be applied and should be an option at all stages of diabetes. Early transplants are preferable for labile diabetes, but even patients with advanced complications can benefit.
The world’s first clinical pancreas transplant was performed, simultaneously with a kidney graft, on December 16, 1966, to treat a uremic diabetic patient at the University of Minnesota Hospital. 1 Since then, more than 12,000 pancreases have been transplanted worldwide;2 of these, nearly 10% have been done at the University of Minnesota, as reported here (Fig. 1).

Figure 1. Number of pancreas transplants according to recipient category (SPK, simultaneous pancreas–kidney; PTA, pancreas transplant alone; PAK, pancreas after kidney; SPL, simultaneous pancreas–liver) from living related donors (LRD) or living unrelated donors (LURD). Inclusive dates are December 16, 1966, to March 31, 2000.
The cumulative experience with pancreas transplantation at the University of Minnesota has been periodically presented at the American Surgical Association, 3–6 and the historical evolution of transplantation for diabetes (kidney, pancreas, islets) at this institution has been described. 7
For diabetic patients dependent on exogenous insulin for survival, the objectives of pancreas transplantation are to make them insulin-independent and normoglycemic, improve day-to-day quality of life, and ameliorate secondary complications. That the first objective could be achieved was obvious from the first case;1 the others had to be proven, and that is part of the multidecade story told here.
The evolution of pancreas transplantation at the University of Minnesota is closely intertwined with the advances in surgical techniques, 8–13 organ preservation technology, 14 and immunosuppressive modalities 15–19 that have occurred in other pioneering programs, but some aspects of the Minnesota program have been decidedly different. 7 An analysis of outcome over such a long time span in a constantly changing field can be done only by eras. Although each era has distinctive features, there is much overlap between eras as well as heterogeneity within eras. The similarities and differences with other programs are indicated in the following description of the Minnesota pancreas transplant program by eras.
DESCRIPTION OF ERAS
Era 0
From 1966 to 1973, 14 pancreas transplants were done, the first a duct-ligated segmental graft in which William Kelly and Richard Lillehei collaborated. 1 The next 13 were whole pancreas grafts (the first 5 with a cutaneous graft duodenostomy, the next 7 with internal exocrine drainage using a Roux-en-Y duodenojejunostomy, and the last with only the graft papilla of Vater retained for anastomosis to recipient bowel) in a personal series by Lillehei et al. 20 Era 0 will not be included in the detailed analyses to come; this era and its influence will simply be summarized in this introduction.
In brief, the first 11 cases were performed in uremic diabetic patients, 10 as simultaneous pancreas–kidney (SPK) transplants and 1 as a pancreas transplant alone (PTA) in a patient who remained on dialysis. At the outset it was thought that to do kidney transplants successfully in uremic patients would require correction of the diabetic state. 3 However, because of the initially high complication rate with pancreaticoduodenal transplants, this concept was challenged internally. 21 A series of kidney transplants alone (KTA) from living or cadaver donors was initiated in uremic diabetic patients, as reported to the American Surgical Association, 22,23 ultimately producing a large number of candidates 24 for pancreas after kidney (PAK) transplants in era 1. 4 Lillehei switched to doing PTA in nonuremic diabetic patients but had only three cases, and all grafts were rejected within 3 months. 20,25 Interestingly, none of the pancreas allografts were rejected in the uremic diabetic patients (all SPK transplants except one), all losses resulting from technical complications (thrombosis, infection, anastomotic leak) or death with a functioning graft (DWFG). 20 One SPK recipient (case number 6, or number 5 as tabulated by Lillehei;20 he called the first case done with Kelly number 0), however, was insulin-independent for more than 1 year 3 until dying with a functioning pancreas graft, after losing the kidney to renal artery stenosis and returning to dialysis. 20 This was the longest functioning pancreas graft in the world until a series of SPK segmental transplants drained into the ureter by Gliedman et al, 26 beginning in the early 1970s, produced a recipient whose new pancreas functioned (insulin-independent) for 5 years.
The Lillehei series ended in 1973 with the hope that islet transplantation would quickly be developed for clinical application and would succeed pancreas transplantation as total endocrine replacement therapy for diabetes. 27 Islet transplantation research had begun at Minnesota in the late 1960s 28 and has continued to this day, 7,29 but it was apparent by the late 1970s that clinical application, 30 at least for islet allografts (islet autografts were successful, 31,32 even the first case 30), would require many more years of research 33,34 —research that is just coming to fruition. 29,35 Thus, after further laboratory experiments designed to refine surgical techniques, 7,36 a new series of clinical pancreas transplants was begun in 1978 (era 1). 4,37
We took with us several lessons from the Lillehei series. 25 In era 0, only azathioprine and prednisone were used for immunosuppression; although adequate to prevent at least early rejection of SPK grafts, such a regimen was inadequate for PTA cases. The Lillihei series was the first to make a distinction in immunologic risk for PTA versus SPK transplants 20,25 a distinction that persisted until only recently. 38 Conversely, in the Lillehei series, PTA recipients had many fewer complications than SPK recipients. 20 Thus, in era 1 we were swayed to reduce the magnitude of the surgical procedures for uremic diabetic patients by doing a KTA with a PAK after an interval of recovery. 4 We also were impressed by the high complication rate in the Lillehei series, seemingly associated with the duodenal portion of the whole pancreas graft. 20 So, like others, 8–10 we initially believed that it was better to do a segmental graft and avoid the duodenum. 37,39,40 These concepts changed as era 1 progressed, 4,5 but early on much of what we did was related to what we perceived as good or bad from era 0. We continue to expound Lillehei’s belief that PTA should be the norm and not simply the future of pancreas transplantation. 7, 38 Lillehei deserves the credit for being the first proponent. 20
Era 1
Era 1 began July 25, 1978, with a cadaver segmental pancreas transplant. 37 The graft duct was left open, allowing the exocrine secretions to drain freely into the peritoneal cavity of a diabetic woman who had received a successful kidney transplant from her mother 6 years earlier. The recipient was insulin-independent for 17.5 years, when she died with a functioning graft after being thrown off a horse. At the time she died, she had the world’s longest functioning graft, a record not exceeded until 2 years later when another PAK segmental graft, this one duct injected and from a living donor, reached the same duration of function; the latter still functions, now 20 years after the transplant. Thus, era 1 initiated the first series of pancreas transplants where truly long-term graft function was destined to occur, 41 even though our initial success rate was low and we had not yet developed a good method for monitoring solitary pancreas transplants for rejection.
Era 1 ended in July 1986 when we resumed SPK transplants, giving us the maximal flexibility to manage diabetic patients with uremia. In the 8-year period of era 1 (July 1978 to June 1986), we performed only solitary (65 PAK and 83 PTA) pancreas transplants (n = 148). In contrast, every other program in the world performed only or predominately SPK transplants during this period. 42
Initially, we did not have a good marker to monitor for solitary pancreas graft rejection episodes, because an elevation in the plasma glucose level is a late manifestation (as opposed to elevation of the serum creatinine level in kidney graft recipients, which is a relatively early marker of renal allograft rejection, and one that gives an immunologic advantage to SPK recipients because serum creatinine can be used as a surrogate marker to detect rejection that usually affects both organs from the same donor). Duct management techniques later solved the problem, but initially we used other tactics. One was to administer Minnesota antilymphocyte globulin, which we had already shown reduced the rejection response in renal allograft recipients. 43 The other was to use living donors for segmental grafts 44 because we knew that the rejection episode rate was much less for kidney grafts from living than from cadaver donors. 45,46 Although the technical failure rate of living donor segmental pancreas transplants was initially high, the tactic was an immunologic success; particularly when the living donor had previously given a kidney to the recipient, the rejection rate was low. 47
For cadaver solitary pancreas transplants, the rejection rate remained high with the immunosuppression available at the time (Minnesota antilymphocyte globulin, azathioprine, and prednisone). Although International Pancreas Transplant Registry data 42 showed that SPK transplants gave an advantage in terms of pancreas graft survival, we believed that the immunologic problems of solitary pancreas transplants could be overcome. We persisted with the philosophy that PTA was the most logical application: why wait for secondary complications? 48
In era 1, the emphasis was on developing the best surgical technique, particularly for duct management. We sequentially used every duct management technique devised (open duct, 1978; duct injection, 1980; enteric drainage, 1981; urinary drainage via the bladder, 1983), with overlap in application. 49 The open duct technique was used in our first few cases. 39 We then compared 4 open duct to the polymer duct injection technique developed by Dubernard et al 9 in France and to a variant of the enteric drainage technique for segmental grafts popularized by Groth et al 10 in Sweden. By mid-1983 we stopped doing cadaver segmental grafts as a routine 4 and returned to the whole pancreas technique (with papilla of Vater) used by Lillihei in his last case. 20 We performed only a few cases by this technique before following the lead of Starzl et al 12 at Pittsburgh to include the entire duodenum, 50 as originally described by Lillihei. 3 Urinary drainage, initially introduced by Gliedman et al 8 in the early 1970s by anastomosing the pancreatic duct of a segmental graft to the recipient ureter, was modified by Sollinger et al 11 in the early 1980s by directly anastomosing a whole pancreatic graft to the bladder. Once we learned how useful a decline in urine amylase activity was as a marker for rejection episodes, 51,52 we used bladder drainage almost exclusively for solitary pancreas transplants. 50
In the beginning, however, we used the open duct technique clinically because of its uniformly successful application in dogs that were controls for an experiment testing the duct injection technique. 53 Half of the human recipients of open duct segmental grafts did well surgically, presumably because the enzymes remained inactive and the peritoneal cavity absorbed the pancreatic secretions, but in the other half, chemical peritonitis developed, and the grafts had to be removed. 39 For this reason, we switched to the duct injection and enteric drainage techniques until these were superseded by bladder drainage near the end of era 1. 50,52
Other technical aspects of era 1 included portal venous drainage of segmental pancreas grafts using the interior mesenteric vessels in a few cases, 54 routine procurement of whole pancreas grafts from liver donors 55 with reconstruction of the graft arterial system using a Y graft of donor iliac artery, 56 and development of a reliable method of cold storage of pancreas grafts for more than 24 hours in silica gel-filtered plasma. 57,58 The latter was superseded in era 2 by the nonbiologic (eliminating the risk of disease transmission) University of Wisconsin (UW) solution developed by Belzer. 14 We also began to do pancreas graft biopsies 59 to help diagnose the cause of graft dysfunction. 60
During era 1, immunosuppression evolved 61 from azathioprine and prednisone to cyclosporine and prednisone to cyclosporine, azathioprine, and prednisone (triple therapy), using Minnesota antilymphocyte globulin for induction in nearly all cases. 62 Uniquely, we performed segmental pancreas transplants from nondiabetic identical twin living donors to their diabetic twin counterparts under the mistaken impression that we could do so without immunosuppression 63; we observed recurrence of autoimmune isletitis 64 and diabetes, 63 a confirmation of the autoimmune etiology of type 1 diabetes, 65 and a hard lesson. 66
In era 1, nearly all pancreas candidates and recipients participated in baseline and serial follow-up studies of metabolism 67 and secondary diabetic complications of the eyes, 68 nerves, 69 and kidneys 70 to determine whether their lesions progressed, stabilized, or regressed. 71 The studies have been continued longitudinally across all subsequent eras.
Era 1 was an exciting period of development, with numerous international conferences bringing together clinicians from the few institutions applying pancreas transplantation as a treatment for diabetes. 72–74 The lessons from our experience and that of others during era 1 were undoubtedly responsible for the improved results in era 2.
Era 2
Era 2 began in July 1986, when we resumed SPK transplants. 75 From then on, we offered pancreas transplants liberally in all three recipient categories. 76,77 Our basic immunosuppressive regimen (quadruple therapy) at that time consisted of Minnesota antilymphocyte globulin for induction and the combination of cyclosporine, azathioprine, and prednisone for maintenance. 62 We conducted a randomized study of Minnesota antilymphocyte globulin versus OKT3 for induction therapy in KTA and SPK recipients, 78 we substituted antithymocyte globulin (ATGAM) for Minnesota antilymphocyte globulin when the latter became unavailable at the end of 1993, 79 and we instituted anti-T-cell agents as the first line of therapy for all first rejection episodes in pancreas recipients. 80 We found that aggressive treatment of rejection episodes could preserve long-term endocrine function even when exocrine function was lost. 81,82 However, the pancreas graft failure rate was high enough 83 for us to accumulate a large series of pancreas retransplants. 84 Era 2 ended in June 1994, when new agents for maintenance immunosuppression became available. 16,85
During the 8-year interval of era 2, we performed 461 pancreas transplants, of which 51% were SPK, the others being nearly equally divided between the solitary PAK (23%) and PTA (26%) categories (with 1 simultaneous pancreas–liver [SPL] transplant). Living donors were used for 6% of the transplants in era 2, predominately in recipients of solitary pancreas transplants (12% of cases equally distributed in the PAK and PTA categories). 86 The incentive for solitary pancreas transplants with living donors lessened as the results of cadaver pancreas transplants improved and waiting times became short by the use of outside donors. 87 Thus, near the end of era 2 (1994), we expanded our living donor program to include SPK transplants for diabetic patients with uremia who wanted one operation to become insulin-independent as well as dialysis-free without a long wait. 88 The goal of one operation to receive both a kidney and a pancreas was also achieved in a few era 2 patients who received a cadaver pancreas simultaneously with a living donor kidney. 7
Bladder drainage predominated as the duct management technique, with enteric drainage done in only 5% of our cases, most early in era 2 to complete a study, initiated with the 101st case in era 1, of enteric versus bladder drainage for solitary pancreas transplants. 50 This study showed that the solitary pancreas rejection loss rate was significantly lower in bladder drainage recipients treated for rejection based on a decline in urine amylase activity (units per hour). 50 Thus, we began to use bladder drainage for almost all solitary pancreas transplants. 76 A decrease in exocrine function, as detected by urine amylase monitoring, always preceded hyperglycemia as a manifestation of rejection in pancreas grafts, 52 and the incidence of rejection episodes was high with the immunosuppressants available during this era. 89 In special cases, such as with patients who had both pancreatic exocrine and endocrine deficiency as a result of pancreatectomy-induced diabetes, we would use enteric drainage to correct both. 90
For SPK bladder drainage transplants, monitoring of urine amylase was less important to detect rejection because a serum creatinine elevation usually preceded a urine amylase decline when the rejection episode affected both organs. 91 However, experimental studies showed that the incidence of discordant rejection (one organ involved when the other was not) was on the order of 10%, 92 and clinical observations 93 corroborated the experimental ones. 94 Thus, we continued to do bladder drainage with urine amylase monitoring even for SPK transplants in era 2, because it allowed the salvage of the occasional pancreas affected by a discordant rejection, as documented by transcystoscopic biopsies. 95–97 We managed the chronic bladder-related complications (primarily recurrent urinary tract infections and metabolic acidosis from bicarbonate loss) by converting to enteric drainage 98,99; during era 2, our conversion rate was 9% to 19% by 2 years, depending on the recipient category. 100
We made modifications in surgical technique during era 2, such as using a stapler for the duodenocystostomy of bladder drainage grafts, 101 using every donor for both liver and pancreas procurement, 102,103 and splitting the cadaver pancreases into two segments for transplantation to two recipients 104 (especially useful when two patients with a high panel-reactive antibody level to human leukocyte antigens [HLAs] have a negative crossmatch to the same donor). We began using pancreas grafts procured by surgeons outside our region, 87 leading to an increase in our pancreas transplant volume with no detrimental affect on outcome. We converted from using silica gel-filtered plasma to UW for pancreas organ preservation, with no difference in results. 105 Long-term endocrine function was similar for grafts preserved with either solution for up 30 hours. 106
Donor 107 and recipient risk factors were redefined in this era. 108 We accepted all referrals 7,108 but initiated measures to treat risk factors such as coronary artery disease before the transplant. 109
The observations and studies we initiated in era 1 on the pathogenesis of diabetes and the course of its complications continued in era 2. We added to our series of identical twin donor segmental pancreas transplants, but with prophylactic immunosuppression. 110 Of the three twin transplants done in era 2, only one recipient has manifested disease recurrence; with immunosuppression, it progressed slowly and graft function was maintained for 8 years. The other two recipients had no evidence of disease recurrence in follow-up graft biopsies 7 and are currently insulin-independent at 10 and 13 years, respectively.
Studies of metabolism 81,111–115 and secondary diabetic complications 7 in pancreas recipients continued in era 2. Pancreas transplantation did not alter the immediate course of advanced eye disease, but by 3 years stabilization occurred. 68 Neuropathy improved 116 and neuropathic patients with successful pancreas transplants had a survival advantage. 117 Recurrence of diabetic nephropathy in renal allografts was also ameliorated by a successful pancreas transplant. 118
Although the results of solitary pancreas transplants improved in era 2, we were not satisfied. 7 The success rate was still higher in SPK recipients, particularly in the subgroup of young patients without vasculopathy, but we wanted to continue to offer pancreas transplants for all categories and stages of diabetic complications. Although rejection episodes were usually readily reversed in SPK recipients, the incidence of rejection episodes was high in all categories. Rejection episodes were harder to reverse or had a higher recurrence rate for solitary pancreas transplant recipients. We knew that new immunosuppressive approaches were needed.
Era 3
Era 3 began in January 1994, when tacrolimus was approved for clinical use by the U.S. Food & Drug Administration (FDA). We immediately used it for clinical pancreas transplants. 119 A year later, mycophenolate mofetil (MMF) was also FDA-approved, and again, we immediately used it for pancreas transplants, 120 including in combination with tacrolimus. 121 We continued to use an anti-T-cell agent (ATGAM) for induction immunosuppression and Orthoclone (OKT3) for treatment of rejection episodes. Bladder drainage predominated for pancreas graft duct management in all recipient categories in era 3.
In the 3.75 years of era 3, with tacrolimus initially and then tacrolimus and MMF as the principal maintenance immunosuppressants, we did 286 pancreas transplants. As in era 2, about half of the era 3 transplants were in the SPK category (51%) and half in the solitary pancreas transplant categories. However, the proportion of PAK transplants was higher (36%) in era 3 than in era 2.
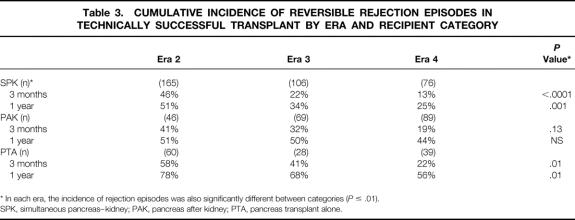
The surgical complication rate declined in all categories in era 3. 122 The incidence of rejection episodes declined more in SPK and PAK than in PTA, 123 and percutaneous pancreas graft biopsies were used routinely to confirm the diagnosis. 124
The metabolic studies initiated in the previous eras continued in era 3. 125 The durability of pancreas graft insulin secretory reserve for more than one or nearly two decades was documented. 126 Likewise, the studies on diabetic secondary complications in pancreas graft recipients continued. 127,128 Nerve regeneration after successful pancreas transplantation in patients with diabetic neuropathy was clearly shown, 129 and the survival advantage of a successful pancreas transplant in neuropathic patients was confirmed. 130 In regard to diabetic nephropathy in native kidneys, surprisingly over a 10-year period, even structural glomerular lesions could regress. 131
Era 3 ended on these optimistic notes. The main remaining challenges were to reduce the surgical complication rate further and to refine immunosuppression to improve PTA results, both early and late. 132
Era 4
Era 4 began in March 1998, when we added daclizumab (Zenapax), alone or in combination with polyclonal anti-T-cell antibody (ATGAM, initially; thymoglobulin when it was FDA-approved in 1999), to our induction immunosuppressive regimen. We also began to give anti-T-cell agents before graft revascularization, following the lead of others. 133 In the PTA category, we also began to give tacrolimus and MMF to the candidates while waiting. Concomitantly, we began to use enteric drainage as our principle exocrine drainage technique in SPK recipients of cadaver grafts (exceptions were some high-risk elderly or obese patients or patients with chronic peritonitis from peritoneal dialysis). Bladder drainage remained the preferred drainage technique for cadaver solitary and all living donor pancreas transplants.
Through March 31, 2000, we did 285 pancreas transplants in era 4, and those transplanted as of March 5, 2000, are included in these analyses (n = 276). Even though the absolute annual number was greater, the proportion of SPK transplants in era 4 (38%) was less than in eras 2 and 3, whereas the proportion of PAKs increased (44%). Enteric drainage was used in 75% of primary SPK transplant recipients but in only 12% of PAK and in no PTA. The outcomes for era 4 are presented here for the first time.
SUMMARY OF PROCEDURES
Between December 16, 1966, and March 31, 2000, we performed 1,194 pancreas transplants at the University of Minnesota (498 SPK, 404 PAK, 291 PTA, 1 SPL). Of these, 1,003 were primary and 191 were retransplants (157 second, 30 third, and 4 fourth); 18 retransplants (16 second, 2 third) were in 17 patients who had had their first transplant elsewhere, so the total number of patients enrolled in our program as of March 31, 2000 was 1,022.
We did 1,083 cadaver (188 retransplants) and 111 living donor (3 retransplants) pancreas transplants. Of the SPK transplants, the pancreas graft came from a living donor in 29 (kidney from same donor) and a cadaver in 468 cases (in 9 the kidney was from a living donor); of the PAK, from a living donor in 32, a cadaver in 373; of the PTA, from a living donor in 50, a cadaver in 241. Of the retransplants, 36 were SPK (25 second, 10 third, 1 fourth), 96 PAK (77 second, 13 third, 3 fourth), and 59 PTA (52 second, 7 third).
Pancreas graft duct management or the exocrine drainage technique involved a cutaneous graft duodenostomy in 5 (all era 0); open duct free intraperitoneal drainage in 15 (all era 1); duct occlusion (4 simply ligated, 44 polymer-injected) in 48 (eras 1, 2, 3); enteric drainage in 203 (all eras); and urinary drainage (4 ureter, 919 bladder) in 923 cases (all eras). The recipient portal venous system was used for the pancreas graft venous effluent in seven cases (eras 1, 2 and 4).
STATISTICAL ANALYSES
Analyses were done on all pancreas transplants (n = 1,171) performed at the University of Minnesota from July 25, 1978, through March 5, 2000 (the 14 from era 0 [10 SPK, 4 PTA] and the last 9 from era 4 [1 SPK, 5 PAK, 3 PTA] were not included), and all primary KTA (n = 515) in uremic diabetic patients from July 1, 1986 (the beginning of era 2), to the present. Patient, pancreas, and kidney graft functional survival rates were calculated by the Kaplan-Meier method using SAS 6.12 software.
Pancreas grafts were considered functioning as long as the recipients were insulin-independent. Death with a functioning graft (DWFG) was considered a graft failure in our analyses of all cases. In our analyses of immunologic events (rejection), we excluded technical failures and pancreas graft primary nonfunction cases. Technical failures included primary graft thrombosis or removal of functioning grafts for complications such as anastomotic leak, perigraft infection, or bleeding. The remaining cases were considered technically successful. In all analyses of technically successful cases, those with DWFG were censored at the time of death.
Kidney grafts were considered functioning as long as the graft was still in place and the patients who were receiving dialysis before the transplant were dialysis-free after the transplant or the posttransplant serum creatinine level was below the pretransplant level in recipients who were never dialyzed.
In univariate actuarial analyses, probability values were calculated by the Wilcoxon and log-rank tests and refer to the significance of the differences between the overall survival curves. The Wilcoxon test primarily reflects the probability that early differences are significant; the log-rank test is weighted to detect late differences. Probability values of less than 0.2 are indicated numerically; all others are designated nonsignificant. We also performed logistic and Cox multivariate regression analyses using multiple variables in the models to ascertain the relative risks for patient death, graft failure in general, technical failure, and rejection loss. The significance of differences in proportions of events comparing categorical variables of one group with another was determined by the chi-square or Fisher exact test. Continuous variables were analyzed parametrically using the t test. Means are given with one standard deviation.
For SPK and PAK transplants, we compared outcomes with the outcome in uremic diabetic KTA recipients. In era 2, 40% of uremic diabetic patients received a primary cadaver SPK transplant; 60% received either a living donor (34%) or a cadaver (26%) KTA. In eras 3 and 4 combined, the distribution was as follows: 53% SPK, 47% KTA (27% living donor, 20% cadaver), reflecting our emphasis on living donor KTA followed by a PAK.
RECIPIENT CATEGORIES AND DUCT MANAGEMENT
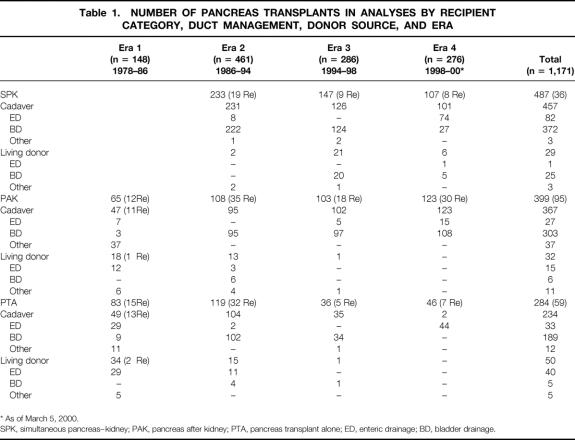
In era 1 (M = 148), we did only PAK (44%) and PTA (56%) procedures (15 open duct, 41 duct injection, 3 ligation, 77 enteric drainage, and 12 bladder drainage) (Table 1). In era 2 (n = 461), we did all categories: SPK (51%), PAK (23%), PTA (26%), and 1 SPL (430 bladder drainage, 4 ureter, 24 enteric drainage, and 3 duct injection). In era 3 (n = 286), 51% were SPK, 36% PAK, and 13% PTA (276 bladder drainage, 5 enteric drainage, and 5 duct injection).
Table 1. NUMBER OF PANCREAS TRANSPLANTS IN ANALYSES BY RECIPIENT CATEGORY, DUCT MANAGEMENT, DONOR SOURCE, AND ERA
* As of March 5, 2000.
SPK, simultaneous pancreas–kidney; PAK, pancreas after kidney; PTA, pancreas transplant alone; ED, enteric drainage; BD, bladder drainage.
In era 4, as of March 5, 2000 (n = 276), 39% were SPK, 45% PAK, and 17% PTA (184 bladder drainage, 92 enteric drainage). (One PTA retransplant was with enteric drainage to provide intestinal exocrine function in a native pancreatectomized recipient with a functioning primary bladder drainage pancreas transplant.)
Our past and current surgical techniques are detailed elsewhere. 134
RECIPIENT DEMOGRAPHICS
Age of Onset and Type of Diabetes
The age of onset of diabetes in our recipients ranged from younger than 1 to 51 (SPK), 39 (PAK), and 43 (PTA) years, with 25% younger than 8 and 25% older than 16 (5% of SPK recipients had onset of diabetes later than age 30). The mean age of onset tended to increase in all categories for each era: in SPK, from 13 ± 7 years (n = 228) in era 2 to 16 ± 1 (n = 89) in era 4; in PAK, from 9 ± 4 (n = 64) in era 1 to 13 ± 7 (n = 103) in era 4; and in PTA from 10 ± 6 (n = 81) in era 1 to 15 ± 9 (n = 89) in era 4.
Almost all recipients (99%) had type 1 diabetes, based on the history of abrupt clinical presentation and the need for exogenous insulin from the time of diagnosis. Nine patients, although receiving insulin before the transplant, were classified as having type 2 diabetes (8 SPK, 1 PAK), based on clinical features of age of onset later than 35 years, a long duration (more than 10 years) before requiring insulin, and detectable serum C-peptide levels at the time of evaluation for a pancreas transplant.
Seven recipients had diabetes mellitus secondary to total pancreatectomy for benign disease (chronic pancreatitis in four, trauma in two, tumor in one). A retransplant was done in six recipients, five after primary graft failure; in the other, an enteric drainage pancreas graft was added to provide exocrine function into the recipient’s intestine, after a failed attempt to convert the still-functioning primary bladder drainage graft to enteric drainage.
Duration of Diabetes
The duration of diabetes before the transplant ranged from less than 1 to 52 years (in 5% it was 40 years or more) and did not differ by era. The range was extreme in all categories, but PTA recipients tended to have a shorter duration of diabetes (mean 21 ± 8 years) than SPK (26 ± 8 years) or PAK (26 ± 7 years) recipients. The duration of diabetes was less than 10 years in 10% of PTA recipients but only 1% of PAK and SPK recipients.
Age
The age range of the recipients was also extreme (11–64 years) in all categories and all eras. Only seven, however, were children (younger than 18): four PTA (15–17 years), one SPL (16 years), and 2 SPK (11 and 12 years). 135 The mean age tended to increase in each category. In SPK recipients, it went from 38 ± 7 years in era 2 to 44 ± 8 in era 4; in PAK recipients, from 33 ± 6 to 41 ± 8; and in PTA recipients, from 31 ± 7 to 38 ± 11.
Analyses of outcome were done according to whether the recipients were younger than 45 or 45 years or older. The proportion of younger patients significantly (P = .001) increased in each era. In eras 2, 3, and 4, in SPK, 17%, 30%, and 45% were 45 or older, respectively; in PAK, 16%, 27%, and 30%; and in PTA, 9%, 21%, and 27%.
Gender
Overall (1978–2000), the proportion of female pancreas recipients was slightly less than male in the SPK (44%) and PAK (48%) categories, but substantially greater than male in the PTA category (73%). The female-to-male ratio in each category was constant across eras. The slightly higher proportion of males in the SPK and PAK categories can be explained by the fact that diabetic male patients are more likely to be afflicted by end-stage renal disease than diabetic female patients. 136 However, this fact does not explain why female patients represent such a high proportion of PTA recipients, because the incidence of type 1 diabetes is equal in male and female patients. 136 It appears that nonuremic diabetic female patients are more likely to seek pancreas transplantation as an alternative to treatment with exogenous insulin.
General Vascular Disease
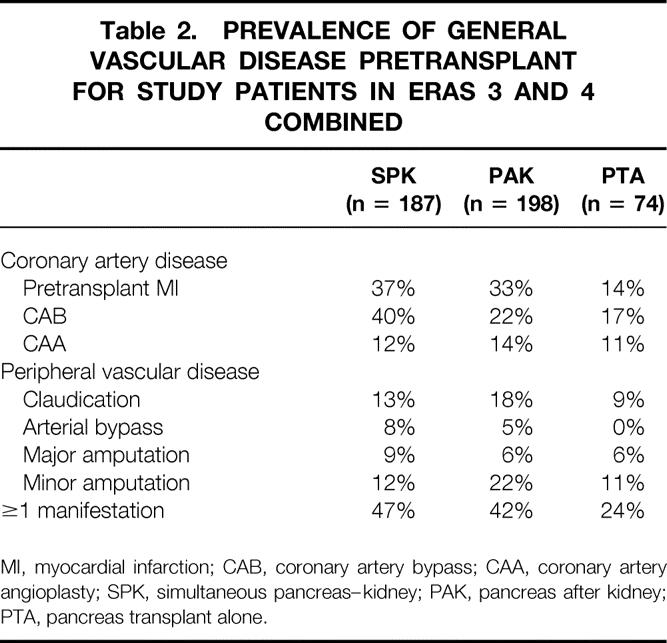
The proportion of recipients with preexisting cardiac, cerebral, or peripheral vascular disease, as indicated by events or the need for therapeutic intervention before the transplant, was determined by a formal study in eras 3 and 4. Cardiac vascular disease was defined as a documented myocardial infarction or the need for pretransplant coronary artery bypass or angioplasty. Cerebral vascular disease was defined as a documented stroke or transient ischemic attack. Peripheral vascular disease was defined as a history of claudication with documented arterial lesions, previous arterial bypass, or a major (extremity) or minor (digit) amputation.
The overall prevalence of one or more of these manifestations of general vascular disease was 47% in SPK, 42% in PAK, and 24% in PTA recipients in eras 3 and 4 combined (Table 2). The incidence of general vascular disease was significantly less (P < .003) in PTA recipients, but even in this category of nonuremic diabetic patients, nearly a quarter were affected. The age range of the patients with any vascular disease was 25 to 61 years. Overall, 50% of the recipients with general vascular disease were younger than 44. The mean age was significantly (P < .001) higher in recipients with versus without vascular disease (overall, 44 ± 7 vs. 38 ± 8 years): SPK, 44 ± 7 versus 40 ± 8; PAK, 45 ± 8 versus 37 ± 7; and PTA, 40 ± 8 versus 36 ± 10. In terms of gender, 38% of female patients and 46% of male patients had general vascular disease (P = .08).
Table 2. PREVALENCE OF GENERAL VASCULAR DISEASE PRETRANSPLANT FOR STUDY PATIENTS IN ERAS 3 AND 4 COMBINED
MI, myocardial infarction; CAB, coronary artery bypass; CAA, coronary artery angioplasty; SPK, simultaneous pancreas–kidney; PAK, pancreas after kidney; PTA, pancreas transplant alone.
A documented myocardial infarction occurred before the transplant in twice as many SPK and PAK than PTA recipients. The age range of those with a pretransplant myocardial infarction was 26 to 64 years (mean 43 ± 8, vs. 44 ± 7 for those without). The overall incidence of pretransplant coronary artery bypass was higher in SPK than PAK or PTA recipients. We noted no difference by gender.
Strokes and transient ischemic attacks occurred before the transplant in 7% and 5%, respectively, of era 3 and 4 pancreas recipients (all categories). We found no significant difference in the incidence of cerebral vascular disease between categories, but the prevalence of transient ischemic attacks was significantly (P = .03) higher in female patients (9%) than male patients (2%).
Peripheral vascular disease was common in the pancreas transplant population of era 3 and 4 combined. By gender, the overall incidence of claudication, arterial bypass, and major and minor amputations was 19%, 2%, 4%, and 6%, respectively, in female patients and 15%, 8%, 10%, and 16%, in male patients.
Waiting Time
We attempted to do preemptive (predialysis) kidney or kidney–pancreas transplants whenever possible for uremic diabetic patients. 137 In eras 3 and 4 combined, we achieved the goal of no dialysis before the transplant in only 33% of primary cadaver SPK recipients (n = 172) because of the long waiting time for cadaver kidneys done with or without a pancreas. The other recipients either underwent pretransplant hemodialysis (47%) or peritoneal dialysis (21%). For our living donor SPK recipients, 138 the proportion of preemptive transplants doubled: 19 of 29 (66%) were not receiving dialysis at the time of transplant.
From 1996 to 1999, the median waiting time for cadaver SPK grafts was nearly 1 year (354 days) for blood group A recipients and almost 2 years for blood group B (716 days) and O (690 days) recipients. For KTA recipients, the waiting time was about 1.5 times longer in each blood group. For cadaver SPK candidates, the percentage transplanted by 1 year was 45% for blood group A, 40% for B, and 22% for O. By contrast, for solitary pancreas transplants the median waiting times for blood group A, B, and O recipients were about 3 months (80 days), 6 months (172 days), and 6 months (186 days), respectively, with 80%, 92%, and 69% transplanted by 1 year.
DONOR DEMOGRAPHICS
Cadaver Donor Age
The cadaver donor age was available only for eras 2, 3, and 4. The age range was 1 to 68 years. In each era, donors tended to be older for SPK recipients than for PAK and PTA. In era 4, the mean donor age for SPK recipients was 32 ± 14 years (n = 83); for PAK, 31 ± 12 (n = 103); and for PTA, 32 ± 13 (n = 42). Combining all eras, half of the donors were 17 to 46 years; 25% each were older or younger. In eras 3 and 4 combined, 19% of SPK, 11% of PAK, and 14% of PTA donors were older than 45 years.
Cadaver Donor Cause of Death
The proportion of cadaver donors who died of trauma decreased in successive eras. For cadaver donors of primary pancreas transplants in eras 3 and 4 combined (n = 397), 69% of those younger than 45 and 20% of those 45 or older died of trauma. The incidence of cardiocerebral vascular disease was 18% in those younger than 45 and 63% in those 45 or older. Donor demographics were similar in all recipient categories.
Surgical Team
Our local organ procurement agency, LifeSource, encompasses Minnesota, North Dakota, and South Dakota, with six transplant centers; two, including ours, perform pancreas transplants. The annual number of cadaver donors for LifeSource ranged from 109 to 127 in era 2 and from 146 to 174 in eras 3 and 4 combined, of which about two thirds were judged to be suitable for pancreas donation. The proportion of pancreas grafts procured by a LifeSource local team was much higher for SPK than for solitary (PAK and PTA) transplants, primarily because sharing of SPK transplants between agencies is largely limited to zero-HLA-mismatched donor–recipient pairs. For solitary pancreas transplants, sharing is common to place a cadaver pancreas that otherwise would not be used locally.
For primary cadaver pancreas transplants, in era 2, the proportion of grafts procured by nonlocal surgical teams was 25% in SPK (n = 200), 61% in PAK (n = 56), and 71% in PTA recipients. For eras 3 and 4 combined, the proportion of primary cadaver pancreas grafts procured by nonlocal teams was 15% in SPK (n = 185), 61% in PAK (n = 142), and 67% in PTA (n = 60) recipients.
Our dependence on outside teams for nonlocal donors to sustain the number of solitary pancreas transplants we do is apparent from the 1999 statistics. For all cadaver pancreas transplants done, 29% of SPK (n = 51) and 80% of solitary (n = 96) grafts were from nonlocal (other agency) donors.
Cadaver Donor Organ Preservation Time
During era 1, we made a transition from Collins solution to silica gel-filtered plasma for cold storage preservation, and during era 2, we moved from silica gel-filtered plasma to UW solution. The preservation times were shorter during era 1 than during eras 2, 3, or 4. Preservation times varied little between recipient categories or between eras after era 1.
In era 1 (SPK, PAK, and PTA), the range of preservation time was 5 to 24 hours, and the mean time was 11 ± 4 hours. Preservation time was 1 to 11, 12 to 23, and more than 24 hours for 56%, 42%, and 2% of the cases, respectively.
In era 2, preservation times for primary cadaver grafts ranged from 2 to 38 hours, with a mean time of 18 ± 5 hours. Preservation times were 1 to 11, 12 to 23, and 24 or more hours in 10%, 77%, and 13% of the cases, respectively.
In eras 3 and 4 combined, preservation time for primary cadaver grafts ranged from 6 to 30 hours, with a mean time of 17 ± 5 hours. Preservation times were 1 to 11, 12 to 23, and more than 24 hours in 12%, 79%, and 9% of the cases, respectively.
Cadaver Donor HLA Mismatches
Beginning in the middle of era 2 to the present, we have deliberately attempted to minimize pancreas donor HLA mismatches with solitary (PAK and PTA), but not SPK, recipients. However, because of the United Network for Organ Sharing (UNOS) policy of mandatory sharing of donor organs for zero-mismatch SPK transplants, the proportion of zero-mismatch recipients at all three HLA loci (A, B, and DR) with the donor was higher in the technically successful SPK (n = 295) than in the PAK (n = 161) or PTA (n = 114) categories: 7%, 3%, and 2%, respectively.
Nevertheless, the percentage of recipients with a low number of donor HLA mismatches (one to three antigens) was significantly higher in the PAK (68%) and PTA (71%) than in the SPK (50%) categories. Conversely, the percentage of grafts from donors with a high number of HLA mismatches (four to six antigens) was significantly higher (P < .05) in the technically successful SPK (42%) than in the either the PAK (29%) or PTA (25%) categories.
Regarding individual HLA loci, in all three categories (SPK, PAK and PTA), about half the recipients had a mismatch for one antigen at a given locus (A, B, or DR); one quarter had a mismatch for zero antigens and the other quarter a mismatch for two antigens. We compared functional survival rates for technically successful grafts according to the number of HLA mismatches at either the class I (A, B) or class II (DR) HLA loci.
Living Donors
Of 111 living segmental pancreas donors (all eras), 37 were HLA-identical siblings (22 sisters, 15 brothers), 10 were identical twins (9 female, 1 male), 61 were HLA-mismatched relatives, including 31 siblings (20 sisters, 11 brothers), and 28 parents (18 mothers, 10 fathers); 1 was a male cousin, 1 a wife, and 1 a female friend. Of the 32 PAK and 50 PTA donors, all but 2 (1 in each category) were done in era 1 and 2. The 29 SPK donors were all in eras 3 and 4. The age range of the donors was 20 to 59 years.
The first donor (1979) remains normoglycemic, as do nearly all the others. However, in three donors, diabetes developed that was treated with insulin; all had a body mass index of more than 28 kg/m2. The donors have been studied extensively by metabolic testing. 139,140 Donors who had a 300% increase in plasma insulin levels from baseline during the first 1 to 3 minutes after a predonation intravenous glucose or arginine challenge have remained with normal glucose tolerance. Our current criteria to be a pancreas living donor include a body mass index of less than 28 kg/m2, normal results on a glucose tolerance test, and a threefold increase in plasma insulin concentration after both glucose and arginine stimulation.
OUTCOME ANALYSIS
Patient and graft (DWFG counted as a failure) survival rates, technical failure rates, and immunologic loss rates (technically successful grafts analyzed with DWFG censored) for our pancreas and diabetic kidney recipients are given according to era, recipient category, pancreas graft duct management, primary or retransplant status, donor source, and recipient and donor demographic features.
Era Analysis of Outcome for Primary Cadaver Transplants by Recipient Category
SPK Transplants
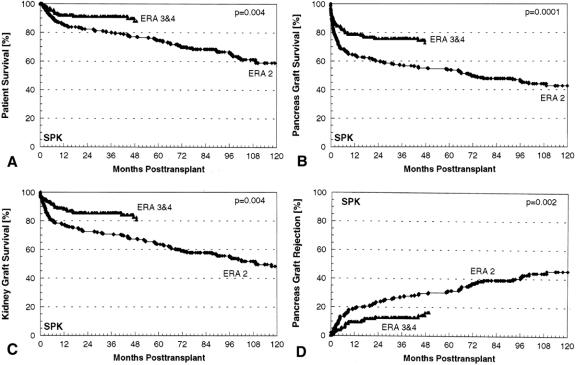
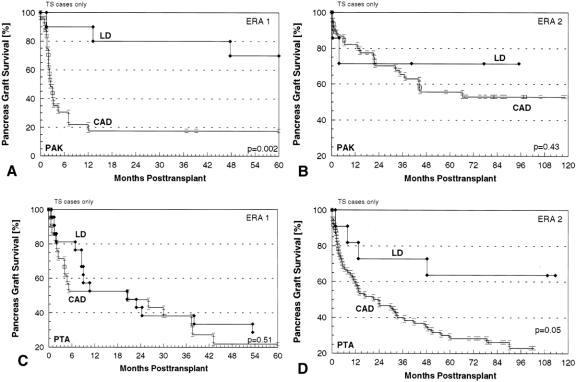
We did no SPK transplants in era 1. The primary cadaver SPK patient, pancreas graft, and kidney graft survival rates were significantly higher in eras 3 and 4 combined (n = 212) than in era 2 (n = 214) (Fig. 2). The principal difference between era 2 versus eras 3 and 4 was the use of cyclosporine and azathioprine in the former and tacrolimus and azathioprine or tacrolimus and MMF in the latter for maintenance immunosuppression. Bladder drainage predominated for duct management of SPK transplants in eras 2 and 3 and enteric drainage predominated in era 4, but for this analysis we combined eras 3 and 4 because of the common rejection prophylaxis protocol and the fact that rejection monitoring in the SPK category is primarily by serum creatinine, regardless of duct management technique. In eras 3 and 4 combined, 1-year patient, pancreas, and kidney survival rates were 92%, 79%, and 88%, respectively; at 5 years, the corresponding figures were 88%, 73%, and 81%.
Figure 2. Primary cadaver simultaneous pancreas–kidney transplant (A) patient, (B) pancreas graft, and (C) kidney graft functional survival rates and (D) pancreas graft rejection failure rates for era 2 versus eras 3 and 4 combined.
The principal cause of early pancreas graft loss was technical failure, but it was significantly lower (P = .01) in eras 3 and 4 combined (13%) than in era 2 (22%). For technically successful cases (DWFG censored), the rejection loss rate was significantly lower in eras 3 and 4 combined than in era 2: at 1 year, 9% versus 19%.
PAK Transplants
Primary cadaver PAK patient and pancreas graft survival rates (Fig. 3) improved significantly from era 1 (n = 36) to era 2 (n = 61), and pancreas graft survival rates further improved in eras 3 and 4 combined (n = 176). The principal differences between era 1 versus 2 were the consistent use of cyclosporine and azathioprine for immunosuppression and bladder drainage for pancreas graft duct management in era 2, allowing early diagnosis and treatment of rejection episodes. The principal difference between era 2 versus eras 3 and 4 was the use of tacrolimus and azathioprine or tacrolimus and MMF for maintenance immunosuppression.
Figure 3. Primary cadaver pancreas after kidney transplant (A) patient and (B) pancreas graft functional survival rates and (C) pancreas graft rejection failure rates for era 1 versus era 2 versus eras 3 and 4 combined.
In eras 3 and 4 combined, patient and pancreas graft survival rates at 1 year were 97% and 78%; at 5 years, they were 89% and 62%. Both the technical failure rate and the rejection loss rate declined significantly in successive eras. The technical failure rates were 25%, 23%, 18%, and 8% for the four eras, respectively (P = .001). The rejection loss rates at 1 year in the respective eras were 81%, 24%, 16%, and 10% (P = .001). In era 4, PAK patient and graft survival rates at both 1 and 2 years were 98% and 81%.
PTA Transplants
Primary cadaver PTA patient and pancreas graft survival rates (Fig. 4) also improved from era 1 (n = 36) to era 2 (n = 72), and pancreas graft survival rates further improved in eras 3 and 4 combined (n = 70). The principal differences between the eras were similar to those for PAK transplants. In eras 3 and 4 combined, patient and pancreas graft survival rates at 1 year were 95% and 76%; at 5 years, they were 78% and 57%. As in the other categories, the technical failure rate and the rejection loss rate declined significantly in successive eras, particularly in era 4. The technical failure rates were 33%, 15%, 7%, and 2% for the four eras, respectively (P = .002). The rejection loss rates at 1 year in the respective eras were 54%, 40%, 35%, and 9% (P = .0004). In era 4, PTA patient and graft survival rates at 1 year were 100% and 88%; at 2 years, they were 100% and 83%.
Figure 4. Primary cadaver pancreas transplant alone (A) patient and (B) pancreas graft functional survival rates and (C) pancreas graft rejection failure rates for era 1 versus era 2 versus eras 3 and 4 combined.
Influence of Duct Management on Outcome
Era 1
All duct management techniques were used in era 1. The technical failure rate was high except with duct injection (7%), but each technique was compatible with long-term success. The first open duct graft (segmental cadaver PTA) functioned for 17 years, until DWFG occurred. A duct injection graft (segmental living donor PAK) is still functioning at 20 years, and the longest functioning cadaver donor duct injection graft (PTA) is at 17 years. The longest functioning enteric drainage graft (living donor PTA) is at 18 years; the longest functioning cadaver enteric drainage graft (PAK) is at 16 years. The longest functioning bladder drainage graft is also from era 1 (cadaver PTA), now at 14 years.
In era 1, we did only solitary pancreas transplants. The open duct technique was used in 12 primary PAK cases (technical failures in 3/9 cadaver, 2/3 living donor) and in 3 primary PTA cases (all technical failure; 1 cadaver, 2 living donor). The duct injection technique was used in 28 PAK cases (technical failure in 1/25 cadaver, 1/3 living donor) consisting of 23 primary cases (technical failure in 1/20 cadaver, 1/3 living donor) and 5 retransplants (all cadaver, no technical failures), and in 12 PTA cases (technical failure in 1/9 cadaver, 0/3 living donor), consisting of 10 primary cases (technical failure in 1/7 cadaver, 0/3 living donor) and 2 retransplants (both technical successes, cadaver). Overall, the technical failure rate was 53% with open duct (n = 15) and 7% with duct injection (n = 40) in era 1.
Enteric drainage was also used in era 1: in 19 PAK cases (technical failure in 3/7 cadaver, 5/12 living donor), consisting of 15 primary cases (technical failure in 3/4 cadaver, 4/11 living donor) and 4 retransplants (technical failure in 0/3 cadaver, 1/1 living donor), and in 58 PTA cases (technical failure in 10/29 cadaver, 9/29 living donor), consisting of 50 primary cases (technical failure in 9/23 cadaver, 8/27 living donor) and 9 retransplants (technical failure in 1/7 cadaver, 2/2 living donor).
We began to use bladder drainage near the end of era 1: in three cadaver PAK cases (all technical failures), consisting of two primary cases and one retransplant, and in nine cadaver PTA cases (two technical failures), consisting of five primary cases (one technical failure) and four retransplants (one technical failure). The overall technical failure rate for bladder drainage in era 1 (n = 12) was 42%. However, the rejection loss rate in the technically successful bladder drainage cases in era 1 was low; of the seven technically successful PTA transplants, only one was rejected at less than 1 year, with a 2-year graft survival rate of 86%. After era 1, we rarely used any technique other than bladder or enteric drainage, and predominantly bladder drainage until era 4.
Era 2
Of the 24 enteric drainage transplants done in era 2, 8 were in cadaver SPK recipients (technical failure in 3/7 primary, 0/1 retransplant), 3 in primary living donor PAK (2 technical failures), and 13 in PTA recipients, consisting of 2 cadaver retransplants (no technical failure) and 11 primary living donor (3 technical failures). The one technically successful enteric drainage PAK retransplant in era 2 was rejected at less than 1 year.
In era 2, the 1-year graft survival rates were 50% for all enteric drainage cadaver SPK procedures (n = 8) and 64% for all enteric drainage living donor PTA procedures (n = 11). The 1- and 5-year graft survival rates for the corresponding technically successful transplants were 80% and 40% in the enteric drainage cadaver SPK recipients (n = 5) and 88% and 75% in the enteric drainage living donor PTA recipients (n = 8).
Of the primary cadaver bladder drainage grafts in era 2, the technical failure rate was 23% in PAK (n = 61) and 15% in PTA (n = 72) recipients. For cadaver bladder drainage retransplants in era 2, the technical failure rate was 29% in PAK (n = 35) and 10% in PTA (n = 30) recipients.
The 1-year graft survival rates in all bladder drainage cadaver PAK (n = 95) and PTA (n = 102) recipients in era 2 were 47% and 51%, respectively; for the corresponding technically successful cases, the figures were 72% (n = 69) and 64% (n = 85).
For primary bladder drainage cadaver PAK (n = 46) and PTA (n = 40) transplants that were technically successful, the 1-year graft survival rates were 82% and 60%, respectively. Bladder drainage was used in four living donor PTA recipients in era 2 (one technical failure; two of the three technically successful grafts functioned for more than 1 year [67%] and one is still functioning at more than 10 years). Urinary drainage was used in 12 living donor PAK recipients in era 2: via the bladder in 8 (2 technical failures; 5/6 technically successful grafts [83%] are still functioning at 6–15 years) and via the ureter in 4 (2 technical failures; the 2 technically successful grafts are still functioning at more than 6 years).
Eras 3 and 4
With rare exceptions, we continued to use bladder drainage in cadaver PTA recipients in era 3 (n = 35) and era 4 (n = 44), with technical failure rates of 3% and 2%, respectively. For all cadaver bladder drainage PTA cases (n = 76) in eras 3 and 4 combined, the 1-year graft survival rate was 77%; for the corresponding technically successful cases (n = 74), it was 82%. For primary cadaver PTA cases that were technically successful, the 1-year graft survival rates were 80% in era 3 (n = 28) and 91% in era 4 (n = 38) (P = .002), the improvement perhaps reflecting our change to pretransplant immunosuppression.
Most cadaver PAK transplants were also done with bladder drainage in era 3 (n = 96) and era 4 (n = 107), with technical failure rates of 16% and 9%, respectively. However, more exceptions were made in the PAK category: 6 (4 primary, 2 retransplants) in era 3 and 15 (11 primary, 4 retransplants) in era 4 were done with enteric drainage (technical failure rates of 25% and 9%, respectively, for primary grafts, and 0% and 25%, respectively, for retransplants).
In eras 3 and 4 combined, the 1-year primary cadaver graft survival rate was 67% for enteric drainage PAK transplants (n = 15) versus 79% for bladder drainage PAK transplants (n = 162) (P = .04). For technically successful primary cadaver enteric drainage PAK transplants (n = 13), the 1-year graft survival rate was 77%. For technically successful primary cadaver bladder drainage PAK transplants, the 1-year graft survival rate was 88% in era 3 (n = 66) versus 94% in era 4 (n = 74) (P = .01).
We did not do an enteric drainage SPK transplant in era 3, but did 123 bladder drainage cadaver SPK transplants with an overall technical failure rate of 10%: 9% for primary grafts (n = 115) and 22% for retransplants (n = 9). We resumed enteric drainage cadaver SPK transplants in era 4 (n = 72), with an overall technical failure rate of 19%: 20% for primary grafts (n = 69) and 0% for retransplants (n = 3). For 28 bladder drainage cadaver SPK transplants in era 4, the overall technical failure rate was 11%: 9% for primary grafts (n = 23) and 20% for retransplants (n = 5).
Regarding the outcome for primary cadaver SPK transplants done with bladder drainage (n = 136) versus enteric drainage (n = 70) in eras 3 and 4 combined, patient survival rates were not significantly different: 92% at 1 year in both groups. However, the pancreas graft survival rate was significantly higher in the bladder drainage group than the enteric drainage group: 82% versus 74% at 1 year. There was no difference in the kidney graft survival rates for bladder drainage versus enteric drainage SPK transplants: 89% versus 88% at 1 year. We also found no significant difference in graft loss from rejection for technically successful bladder drainage (n = 24) and enteric drainage (n = 60) SPK transplants; no pancreas grafts had failed by 1 year in the enteric drainage group and only one graft (4%) had failed in the bladder drainage group.
In our hands, there was a technical penalty for using enteric drainage for SPK transplants. However, enteric drainage avoids the chronic complications of bladder drainage (urinary infections, hematuria, metabolic acidosis, dysuria) that may lead to the need to convert to enteric drainage. 141 In eras 3 and 4 combined, the actuarial incidence of conversion of a technically successful SPK bladder drainage graft (n = 126) to enteric drainage was 3% at 6 months, 8% at 1 year, and 14% at 2 years; for technically successful bladder drainage PAK grafts (n = 140), the conversion rate was 6% by 1 year and 16% by 2 years; for technically successful bladder drainage PTA grafts (n = 66), the rate at 1 and 2 years was 6% and 19%. Thus, if the technical failure rate for SPK enteric drainage transplants is reduced, there is an advantage in terms of the chronic complication rate. Certainly the immunologic risk for graft failure is minimal with modern antirejection prophylaxis.
Outcome by Recipient Risk Factors
Recipient Age
The age range of the pancreas recipient population expanded in each successive era, mainly in the direction of older. Across all eras, only seven recipients were younger than 18 years (four PTA, two SPK, one SPL). One pediatric living donor PTA graft functioned for 4 years; the other PTA grafts failed between 2 and 6 months. The two pediatric SPK recipients 135 currently have functioning grafts at 5 years. At the other extreme, for primary cadaver pancreas transplants in eras 3 and 4 combined, 39% of SPK, 31% of PAK, and 24% of PTA recipients were 45 years of age or older.
In the SPK category, patient survival rates were significantly higher (P ≤ .07) in those younger than 45 (n = 128) compared with those 45 or older (n = 81): 95% and 88% at 1 year. However, the pancreas graft survival rates were nearly identical in the two age groups: 80% and 77%, respectively, at 1 year. This finding reflects a lower rejection rate in older recipients, because DWFG was counted as a graft failure. Likewise, kidney graft survival rates were not significantly different for the SPK recipients younger than 45 versus 45 or older: 90% and 85%, respectively, at 1 year.
In the PAK category, patient survival rates were virtually identical for those younger than 45 (n = 122) and 45 or older (n = 54): 97% and 98% at 1 year. The same was true for pancreas graft survival rates: 76% and 83% at 1 year.
In the PTA category, the older recipients did extremely well. Patient survival rates for those younger than and older than 45 were 94% and 100% at 2 years; graft survival rates were 72% and 91%, significantly higher in the older age group (P = .05). Older recipients seem less likely to reject a PTA graft than younger recipients.
The pancreas transplant technical failure rate was not significantly different for recipients younger than 45 versus 45 or older in any category in any era, except for SPK in era 2 (20% [n = 191] vs. 33% [n = 39], P = .07). In eras 3 and 4 combined the technical failure rates for recipients younger than 45 versus 45 or older were 13% and 10% for SPK, 12% and 13% for PAK, and 3% and 5% for PTA. In all eras, technical failure rates were lowest in the PTA category.
Thus, in eras 3 and 4 combined, pancreas graft survival rates were at least equivalent in older and younger recipients in all categories, if not better in older PTA recipients. In the SPK category, patient survival rates were lower in older recipients. In the PAK and PTA categories, no effect of age was seen, at least during the first 4 years of follow-up.
Duration of Diabetes
The duration of diabetes had only a modest impact on pancreas transplant outcome. The results for eras 3 and 4 combined are given for recipients who were diabetic for less than 25 years versus 25 years or more.
In the SPK category, for those with diabetes less than 25 years (n = 73) versus 25 years of more (n = 101), the 1-year patient survival rates were 93% and 90%; pancreas survival rates were 78% and 80%; kidney survival rates were 87% and 90% (P = NS for all comparisons).
In the PAK category, for those with diabetes less than 25 years (n = 66) versus 25 years or more (n = 92), the 1-year patient survival rates were 98% and 97%; pancreas survival rates were 78% and 78% (P = NS). At 5 years, the patient survival rate for recipients with diabetes of 25 years’ duration or more had declined to 79%, significantly different from the 98% for those with diabetes of less than 25 years’ duration (P = .03).
In the PTA category, for those with diabetes less than 25 years (n = 41) versus 25 years or more (n = 24), the 1-year patient survival rates were 92% and 100%; graft survival rates were 65% and 100% (P ≤ .02 for graft survival). At 5 years, the patient survival rate was still 100% for recipients with diabetes for more than 25 years, so long duration of diabetes had no negative impact in the PTA (as opposed to the PAK) category. Indeed, a long duration of diabetes before PTA was associated with a high graft survival rate, paralleling the effect of recipient age in this category.
Vascular Disease
A comparison of outcome was done for the study cohort recipients with one or more vascular risk factors versus those without a vascular risk factor in combined eras 3 and 4.
For SPK recipients with vascular disease (n = 91), the 1-year patient, pancreas, and kidney graft survival rates were 85%, 76%, and 83%, respectively; at 4 years, the corresponding figures were 79%, 68%, and 70%. In contrast, for SPK recipients (n = 96) with no vascular disease, the 1-year patient, pancreas, and kidney survival rates were 97%, 81%, and 94%, respectively; at 4 years, the figures were 95%, 75%, and 89% (P ≤ .03 for patient and kidney survival;P = NS for pancreas graft survival). The pancreas graft technical failure rates were not significantly different for those with (11%) versus those without (16%) vascular disease.
For PAK recipients with vascular disease (n = 83), the 1-year patient and pancreas graft survival rates were 98% and 68%, respectively; at 4 years, they were 86% and 49%. In contrast, for PAK recipients (n = 115) without vascular disease, the 1-year patient and pancreas graft survival rates at 1 year were 98% and 82%; at 4 years, they were 89% and 68% (P ≤ .04 for both comparisons between the two groups). In the PAK category, the technical failure rate was higher for those with (19%) versus without (10%) vascular disease (P = .04).
For PTA recipients with vascular disease risk (n = 18), the 1-year patient and graft survival rates were 94% and 68%, respectively; at 4 years, they were 94% and 58%. In contrast, for PTA recipients (n = 56) without vascular disease, the 1-year patient and graft survival rates were 96% and 75%; at 4 years, they were 83% and 52%. In the PTA category, none of the differences between the two groups were statistically significant. Likewise, the technical failure rate was not statistically different for those with (8%) versus without (4%) vascular disease.
In the study cohort, we also analyzed outcome by one specific vascular risk factor: presence or absence of a pretransplant myocardial infarction. An infarction had occurred in 27% of SPK, 33% of PAK, and 14% of PTA recipients.
In the SPK category, at 1 year after the transplant, 79% of recipient with (n = 25) versus 87% of those without (n = 66) a pretransplant infarction were alive. The corresponding 1-year pancreas graft survival rates were 68% versus 79%; kidney graft survival rates were 74% versus 86% (P = .1 for all comparisons).
In the PAK category, the 1-year patient survival rates for those with (n = 27) versus without (n = 56) a pretransplant infarction were 96% versus 92%. The corresponding pancreas graft survival rates were 70% versus 67% (P = NS for both comparisons).
In the PTA category, the 1-year patient survival rate was 100% for those with (n = 3) versus 93% for those without (n = 18) a pretransplant infarction. The corresponding graft survival rates were 67% versus 66% (P = NS for both comparisons).
The pancreas transplant outcomes for diabetic recipients without vascular disease were good in all categories, but satisfactory outcomes are also achieved in most recipients with vascular disease.
SPK Outcome by Pretransplant Dialysis Modality
We compared patient and pancreas and kidney graft survival rates in eras 3 and 4 combined for primary cadaver SPK recipients (n = 172) according to whether they had no dialysis before the transplant (preemptive kidney graft) or were receiving hemodialysis or peritoneal dialysis at the time of transplantation.
The 1-year patient survival rates for the no dialysis (n = 56), hemodialysis (n = 80), and peritoneal dialysis (n = 56) groups were 96%, 91%, and 88%; 1-year pancreas graft survival rates were 82%, 80%, and 75%; and 1-year kidney graft survival rates were 93%, 88%, and 89% (P = NS).
Bladder drainage (n = 107) and enteric drainage (n = 64) cases were analyzed separately; we found no substantial differences. With bladder drainage, the 1-year patient, pancreas, and kidney graft survival rates were 100%, 89%, and 94% for recipients not receiving dialysis (n = 37); 88%, 79%, and 86% for those receiving hemodialysis (n = 43); and 88%, 85%, and 88% for those receiving peritoneal dialysis (n = 27). With enteric drainage, 1-year patient, pancreas, and kidney survival rates were 87%, 68%, and 88% in the group without dialysis (n = 19); 94%, 80%, and 88% for those receiving hemodialysis (n = 37); and 88%, 50%, and 88% for those receiving peritoneal dialysis (n = 8).
Technical failure rates were not significantly different for the no dialysis, hemodialysis, and peritoneal dialysis recipients: 14%, 11%, and 14%, respectively. With bladder drainage, technical failure rates were 8%, 10%, and 4%; with enteric drainage, they were 26%, 13%, and 38%. The technical failure rate may be higher with peritoneal dialysis for enteric drainage recipients, so our current general policy is to do bladder drainage SPK transplants for peritoneal dialysis recipients and enteric drainage for hemodialysis or no dialysis recipients.
Outcome by Interval Between Grafts for PAK Recipients
In our series of PAK transplants, 72% were a primary pancreas after a primary kidney transplant; 22% were a pancreas retransplant after a primary kidney transplant; 4% were a primary pancreas after a kidney retransplant; and 2% were a pancreas retransplant after a kidney retransplant. The interval between the primary kidney and primary pancreas transplant for PAK recipients varied widely in all eras, ranging from 2 days (pancreas retransplant) to 25 years. The median intervals for all PAK transplants in eras1, 2, 3, and 4 were 39, 19, 17, and 13 months, respectively: 25% received the pancreas within 20 months of the kidney in era 1, 8 months in era 2, 7 months in era 3, and 3 months in era 4.
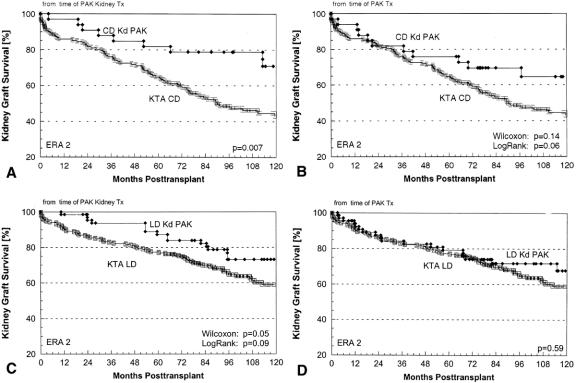
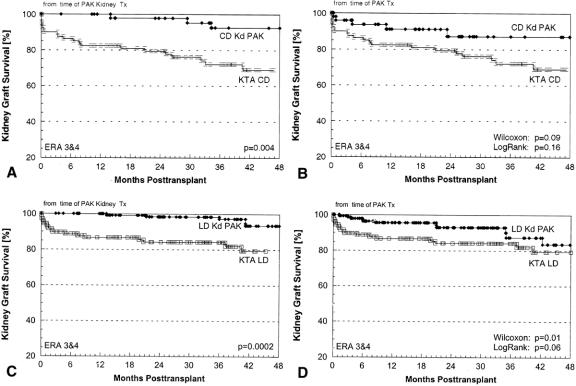
PAK transplants were much more common after a living donor (vs. cadaver) kidney transplant: 70% (vs. 30%) for the entire population across all eras (the proportions were similar in all eras). A higher proportion of diabetic recipients of living donor KTAs (vs. cadaver KTAs) went on to have a PAK transplant (what prevents a uremic diabetic patient without a living donor from getting a cadaver SPK may also be an impediment to getting a PAK transplant; candidates who could get a cadaver SPK but opt for a living donor KTA are more likely to have the resources for a PAK transplant). Nevertheless, the proportion of diabetic cadaver KTA recipients who went on to have a PAK transplant increased in successive eras: from 33/175 in era 2 (19%), to 50/130 in eras 3 and 4 combined (38%).
The proportions of living donor KTA recipients who opted for a PAK transplant also increased in successive eras. Of 253 living donor KTA recipients in era 2, 66 went on to have a PAK transplant (26%); of 258 living donor recipients in eras 3 and 4, 148 (58%) did so. The median interval to a PAK transplant was significantly shorter after a living donor (vs. cadaver) KTA: 22 (vs. 37) months in era 1, 18 (vs. 21) months in era 2, 14 (vs. 39) months in era 3, and 10 (vs. 20) months in era 4.
During eras 3 and 4 combined, 54% of PAK transplants were done more than 1 year after the KTA and 12% were done less than 2 months after. For primary PAK recipients, the 1-year graft survival rates were 80%, 67%, 81%, and 79%, respectively (P = NS) at these intervals after the primary KTA: 0 to 1 (n = 5), 1 to 2 (n = 17), 2 to 12 (n = 67), and more than 12 (n = 74) months (P = NS).
In a separate analysis for PAK recipients in eras 3 and 4 combined, the 1-year kidney survival rate from the time of the pancreas transplant was 95%: 96% for cadaver kidneys (n = 59) and 95% for living donor kidneys (n = 150). Kidney graft survival rates were also not affected by the interval between the pancreas and the kidney transplants. The 1-year kidney graft survival rates were 100%, 100%, 98%, and 92%, respectively (P = NS), at these intervals after the KTA: 0 to 1, 1 to 2, 2 to 12, and more than 12 months.
The outcome for PAK transplants done very early (less than 2 months) after a kidney is possibly less good, but statistically not different from those done later. Certainly after 2 months the outcome is not influenced by the interval.
Outcome by Donor Risk Factors
Donor Age
Cadaver donor age per se was significantly associated with pancreas graft survival rates only in the SPK category. In eras 3 and 4 combined for primary SPK recipients with cadaver donors younger than 45 (n = 141) versus 45 years or older (n = 33), the 1-year pancreas graft survival rate was 81% versus 69% (P ≤ .08). The 1-year SPK kidney graft survival rate was not significantly different with younger (vs. older) donors: 89% (vs. 87%). In eras 3 and 4 combined, for primary PAK recipients with cadaver donors younger than 45 (n = 141) versus 45 or older (n = 17), the 1-year pancreas graft survival rate was 77% versus 81% (P = NS). For primary PTA recipients with cadaver donors younger than 45 (n = 56) versus 45 or older (n = 9), the 1-year graft survival rate was 78% versus 73% (P = NS).
Technical failure rates in eras 3 and 4 combined were also not significantly different for primary cadaver pancreas recipients with donors younger than 45 versus 45 or older: for SPK, 11% versus 18%; for PAK, 13% versus 12%; and for PTA, 4% versus 0%. The minimal differences seen in outcome for older versus younger donors may reflect selective use of older cadaver donors; they represented only 19%, 11%, and 14% of donors, respectively, in the SPK, PAK, and PTA categories.
Cause of Cadaver Donor Death
In eras 3 and 4 combined, 60% of SPK (n = 174), 64% of PAK (n = 158), and 62% of PTA (n = 65) cadaver donors for primary pancreas transplants died of trauma. In the SPK category, the 1-year functional survival rates for pancreas grafts from trauma (n = 103) versus nontrauma (n = 71) donors were 81% versus 76%; for PAK grafts from trauma (n = 101) versus nontrauma (n = 57) donors, 77% versus 80%; and for PTA grafts from trauma (n = 40) versus nontrauma (n = 25) donors, 73% versus 86% (P = NS for all comparisons).
We also analyzed for the presence of vascular disease in donors (24%). Donor vascular disease slightly influenced outcome in the SPK category: for transplants from donors with (n = 47) versus without (n = 127) vascular disease, the 1-year pancreas graft survival rate was 71% versus 82% (P ≤ .2). In the PAK category, for transplants from donors with (n = 35) versus without (n = 123) vascular disease, the 1-year pancreas graft survival rate was 76% versus 78% (P = NS). In the PTA category, for transplants from donors with (n = 15) versus without (n = 50) vascular disease, the 1-year rate was 83% versus 76% (P = NS).
In analyses of the combination of cadaver donor age (15% were 45 years or older) with a risk factor (vascular disease vs. no vascular disease; or trauma vs. nontrauma as the cause of death), a marginally significant effect was seen only in the SPK category: for recipients with older donors (n = 18) with versus without (n = 15) vascular disease, the 1-year graft survival rate 78% versus 58% (P ≤ .1).
Local or Nonlocal Source
Most cadaver organs for SPK transplants were procured from local donors, whereas most pancreases for solitary (PAK and PTA) transplants were procured outside our area by other surgeons. Pancreas graft survival rates were not significantly different for local versus nonlocal organs within each category, except possibly for PTA in era 2. In era 2, the 1-year function rates for local versus nonlocal pancreas grafts for SPK were 61% (n = 149) versus 69% (n = 51); for PAK, 50% (n = 22) versus 65% (n = 34); and for PTA (P = .13), 65% (n = 17) versus 52% (n = 42). In eras 3 and 4 combined, the 1-year function rates for local versus nonlocal primary pancreas grafts for SPK were 81% (n = 157) versus 75% (n = 28); for PAK, 82% (n = 56) versus 78% (n = 86); and for PTA, 77% (n = 20) versus 74% (n = 40).
These relatively good results may reflect our selectivity in acceptance of nonlocal pancreas grafts. For example, in eras 3 and 4, the proportion of outside offers accepted was 9%, 13%, 17%, and 21% in 1996 (n = 203), 1997 (n = 270), 1998 (n = 283), and 1999 (n = 514). About half of the offers were turned down because of an unacceptable HLA mismatch; most of the others were refused because of perceived donor unsuitability (age, history, anatomy, infections, preservation time, and so forth).
Duration of Graft Preservation
The graft survival rates for primary cadaver pancreas transplants were not statistically different by preservation time in any recipient category or in any era. For eras 3 and 4 combined, the 1-year functional survival rates for pancreas grafts stored 1 to 11, 12 to 23, or more than 24 hours were as follows: for SPK, 86% (n = 21), 79% (n = 141), and 67% (n = 12); for PAK, 77% (n = 18), 77% (n = 123), and 88% (n = 17); and for PTA, 75% (n = 8), 75% (n = 51), and 100% (n = 6). In the SPK category, the functional survival rates for the kidney (nearly always implanted before the pancreas) for the corresponding preservation times were 95%, 84%, and 78% (P = .13). In eras 3 and 4 combined, there were no preservation times of more than 30 hours. However, cold storage for at least this time is feasible, because the 1-year functional survival rate for all pancreas grafts stored 24 to 30 hours (n = 35, all categories) was 83%.
HLA Matching
In our analyses of technically successful primary cadaver grafts by number of donor HLA class I (A and B loci) mismatches with the recipients, we found no significant differences in the SPK category in any era. In era 2, the 1-year technically successful SPK pancreas graft survival rates for 0 versus 1, versus 2, versus 3, versus 4 HLA-A, B mismatches were 92% (n = 12), 95% (n = 23), 93% (n = 48), 89% (n = 40), and 100% (n = 17); in eras 3 and 4 combined, the rates were 100% (n = 5), 92% (n = 13), 100% (n = 46), 97% (n = 33), and 100% (n = 15). In era 4, for technically successful SPK recipients enteric drainage, the 1-year graft survival rate was 100% for each number of mismatches: 0 (n = 10), 1 (n = 8), 2 (n = 12), 3 (n = 10), and 4 (n = 2).
Each HLA locus (A, B, or DR) was also analyzed separately. For SPK transplants, a modest effect on outcome was seen at the DR locus. For bladder drainage SPK transplants in era 2, the 1-year functional survival rates with 0 (n = 30), 1 (n = 79%) or 2 (n = 31) DR mismatches were 100%, 91%, and 93% (P = .18). For technically successful bladder drainage SPK pancreas transplants in eras 3 and 4 combined, the 1-year functional survival rates for 0 (n = 8), 1 (n = 55), and 2 (n = 29) DR mismatches were 100%, 98%, and 96% (P = .08).
For solitary technically successful bladder drainage pancreas transplants, the effect of HLA mismatching on outcome was stronger in the PTA than the PAK category. For PAK recipients in era 2 with 0, versus 1, versus 2, versus 3, versus 4 mismatches, the 1-year pancreas graft functional survival rates were 83% (n = 7), 90% (n = 12), 88% (n = 16), 60% (n = 10), and 67% (n = 3); in eras 3 and 4 combined, the rates were 83% (n = 6), 92% (n = 29), 95% (n = 42), 90% (n = 21), and 80% (n = 5) (P = .14).
For technically successful bladder drainage PTA recipients in eras 3 and 4 combined, the 1-year graft functional survival rates for 0 (n = 7), 1 (n = 25), 2 (n = 18), and 3 (n = 3) HLA-A,B mismatches were 100%, 87%, 85%, and 0% (P = .004).
In our analyses of separate loci, only at the B locus did we find a significant effect on solitary pancreas transplant outcome. For technically successful bladder drainage PAK recipients in era 2 (n = 46), the 1-year graft survival rates for 0 (n = 12), 1 (n = 22), and 2 (n = 12) HLA-B mismatches were 100%, 82%, and 67% (P = .06); at 5 years, the rates were 89%, 48%, and 40% (P = .05). In eras 3 and 4 combined, the effect of mismatching at the B locus for technically successful bladder drainage PAK recipients disappeared: the 1-year functional survival rates for 0 (n = 22), 1 (n = 59), and 2 (n = 22) mismatches were 91%, 95%, and 86% (P = NS).
However, for technically successful bladder drainage PTA recipients, we saw a beneficial effect of minimizing mismatching at the B locus in both era 2 and in eras 3 and 4 combined. For 0 versus 1, versus 2 HLA-B mismatches in era 2, the 1-year graft survival rates were 50% (n = 10), 73% (n = 26), and 50% (n = 24) (P = .03); in eras 3 and 4 combined, the rates were 92% (n = 18), 86% (n = 31), and 25% (n = 4) (P = .005).
Subsequent Cox regression analyses confirmed that minimizing mismatching at the HLA-B locus had a significant impact on pancreas graft survival (see below).
Kidney Transplant Outcome for Uremic Diabetic Pancreas Versus Nonpancreas Recipients
Virtually all uremic diabetic patients referred to our program since era 1 were offered the option of a living donor or cadaver KTA (with the possibility of a subsequent PAK transplant) or an SPK transplant (in era 2 from a cadaver donor, in eras 3 and 4 from either a living donor or a cadaver donor). We strongly encourage living donor kidney transplants, 142 and a high proportion of our uremic diabetic pancreas transplant candidates underwent a living donor KTA. For those without a living donor, a cadaver SPK transplant was possible only in those with adequate financial coverage: this proportion increased from era 2 to eras 3 and 4. The proportion of living donor and cadaver KTA recipients who went on to get a PAK transplant also depended on their financial coverage; again, this proportion increased from era to era. However, many living donor and cadaver KTA recipients were not able to, or chose not to, have a subsequent pancreas transplant. Thus, we can compare both patient and kidney graft survival rates both in era 2 and eras 3 and 4 combined for cadaver SPK versus living donor or cadaver KTA recipients as well as for cadaver PAK (with the previous kidney from either a living donor or a cadaver donor) versus living donor or cadaver KTA recipients.
Our PTA recipients were nonuremic at the time of transplant, but some with moderate nephropathy before the transplant developed progressive renal insufficiency after the pancreas transplant and went on to have a kidney after pancreas (KAP) transplant. 143 The outcome in this group is also described.
Cadaver SPK Versus Living Donor or Cadaver KTA Patient and Kidney Graft Survival Rates
In era 2, uremic diabetic recipients of a cadaver SPK early on had lower patient and kidney graft survival rates (Fig. 5) than their KTA counterparts. In the long term (more than 5 years), however, patient and kidney graft survival rates for SPK recipients were actually slightly higher than for cadaver KTA recipients. In era 2, patient survival rates for primary cadaver SPK (n = 212) versus cadaver KTA (n = 142) transplants were 85% versus 92% at 1 year and 59% versus 56% at 10 years (P = NS). The corresponding kidney graft survival rates were 77% versus 86% at 1 year and 48% versus 45% at 10 years (P = NS). However, in era 2, for living donor KTA recipients (n = 186), patient and kidney graft survival rates were significantly higher than for either SPK or KTA cadaver recipients: 94% and 90% at 1 year and 65% and 59% at 10 years (P ≤ .03 for both comparisons, patient and kidney survival).
Figure 5. (A) Patient and (B) kidney graft survival rates in era 2 for primary cadaver simultaneous pancreas–kidney transplant versus cadaver or living donor kidney transplant alone; (C) patient and (D) kidney graft survival rates in eras 3 and 4 combined for the same categories.
In eras 3 and 4 combined, there was no penalty, early or late, for adding a pancreas to a kidney for cadaver uremic diabetic recipients. Patient survival rates at 1 year for primary cadaver SPK (n = 209) versus cadaver KTA (n = 80) were 92% versus 91% at 1 year and 88% versus 80% at 4 years (P = NS). The corresponding kidney graft survival rates were 88% versus 82% at 1 year (P = .06) and 81% versus 69% at 4 years (P = .04). Thus, in this era, kidney graft functional survival rates were even better for cadaver SPK than for cadaver KTA recipients. Further, cadaver SPK outcomes did not differ significantly from those of living donor KTA (n = 107) recipients, in terms of either patient survival (91% at 1 and 84% at 4 years) or kidney graft functional survival (87% at 1 and 79% at 4 years).
Cadaver Pancreas PAK (Living Donor or Cadaver Kidney) Versus Living Donor or Cadaver KTA Kidney Graft Survival Rates
In era 1, we did not compare kidney graft survival rates for PAK and KTA recipients, but we did calculate long-term kidney function rates for PAK recipients. In era 1, for PAK cadaver kidney recipients (n = 19), the kidney graft functional survival rates from the time of the kidney transplant were 100% and 57% at 1 and 10 years; from the time of the pancreas transplant, the rates were 79% and 20% at 1 and 10 years. For PAK living donor kidney recipients (n = 46), kidney survival rates from the time of the kidney transplant were 100% and 59% at 1 and 10 years; from the time of the pancreas transplant, they were 91% and 41%.
In era 2 (Fig. 6) and in eras 3 and 4 combined (Fig. 7), we compared kidney graft functional survival rates for primary cadaver PAK recipients with either a cadaver or living donor kidney versus primary cadaver or living donor KTA recipients. In both eras, we found no penalty for adding a pancreas after a kidney; indeed, the addition of a pancreas was associated with superior long-term kidney graft functional survival rates.
Figure 6. Kidney graft survival rates in era 2 for cadaver pancreas after kidney transplant (PAK) recipients with cadaver kidneys versus cadaver kidney transplant alone (KTA) from the time of the (A) kidney transplant or (B) pancreas transplant (in PAK); and for primary cadaver PAK recipients with living donor kidneys versus living donor KTA from the time of the (C) kidney transplant or (D) pancreas transplant (PAK).
Figure 7. Kidney graft survival rates in eras 3 and 4 combined for primary cadaver pancreas after kidney transplant (PAK) recipients with cadaver kidneys versus cadaver kidney transplant alone (KTA) from the time of the (A) kidney or (B) pancreas transplant (PAK); and for primary cadaver PAK recipients with living donor kidneys versus living donor KTA from the time of the (C) kidney transplant or (D) pancreas transplant (PAK).
In era 2, the cadaver kidney graft functional survival rates from the time of the kidney transplant for recipients who went on to get a PAK (n = 142) transplant were 97% and 71% at 1 and 10 years. For cadaver KTA diabetic recipients (n = 33), the corresponding rates were lower: 86% (P = .007) and 43% (P = .003). However, to get a PAK transplant, a kidney recipient had to survive an interval to the pancreas transplant with a functioning kidney. Therefore, we did a separate analysis of kidney graft survival rates in PAK recipients from the time of the pancreas (PAK) transplant. In this analysis, cadaver kidney graft functional survival rates at 1 and 10 years after the pancreas transplant in PAK recipients were 94% and 64%, slightly better than the kidney graft survival rates at 1 and 10 years from the time of the kidney transplant in the cadaver KTA recipients (P = .14 at 1 and .06 at 10 years). Correcting diabetes seems to promote long-term cadaver kidney graft function.
The same trend was seen in our analysis of living donor kidney graft functional survival rates in PAK and KTA recipients in era 2. In era 2, living donor kidney graft functional survival rates from the time of the kidney transplant in recipients who went on to get a PAK (n = 66) were 98% and 73% at 1 and 10 years versus 90% (P = .05) and 59% (P = .09) in living donor KTA recipients (n = 185).
Again, PAK living donor recipients had to survive the interval to the pancreas transplant with a functioning kidney, so we also did a separate analysis of living donor kidney graft survival rates in PAK recipients from the time of the pancreas (PAK) transplant. In this analysis, living donor kidney graft functional survival rates at 1 and 10 years after the pancreas transplant in PAK recipients were 92% and 68%, at least as good as the kidney graft survival rates from the time of the kidney transplant in living donor KTA recipients (P = NS).
In eras 3 and 4 combined, it was even more apparent that long-term kidney functional survival rates were better in PAK than KTA recipients. The cadaver kidney graft functional survival rates from the time of the kidney transplant in recipients who went on to get a PAK (n = 50) were 100% and 93% at 1 and 4 years versus 82% (P = .002) and 69% (P = .004) in diabetic cadaver KTA recipients (n = 80).
In eras 3 and 4 combined, we analyzed cadaver kidney graft survival rates in PAK recipients from the time of the pancreas (PAK) transplant. The rates at 1 and 4 years were 91% and 87%, again slightly better than the cadaver living donor kidney graft survival rates in KTA recipients from the time of the kidney transplant (P = .09 at 1 year and .16 at 4 years).
For living donor kidney recipients in eras 3 and 4 combined, the 1- and 4-year kidney graft functional survival rates from the time of the living donor kidney transplant in recipients who went on to get a PAK transplant (n = 148) were 100% and 94% at 1 and 4 years versus 87% (P = .0001) and 79% (P = .0002) in diabetic living donor KTA recipients. In our separate analysis of living donor kidney graft survival in PAK recipients from the time of the pancreas transplant, the function rates were 96% and 84% at 1 and 4 years, again significantly higher than the kidney survival rates from the time of the kidney transplant in living donor KTA recipients (P = .01 at 1 and .06 at 4 years).
In each analysis, the addition of a pancreas (PAK) was associated with kidney graft survival rates that were higher than in KTA recipients, whether the kidney was from a cadaver or a living donor.
Kidney After Pancreas (KAP) Transplants in PTA Recipients
In the entire cohort of primary PTA recipients in all eras (n = 223), 36 went on to have a kidney transplant, 25 after the primary pancreas transplant (12 with and 13 without the original pancreas graft functioning at the time of the KAP transplant), 10 after a second PTA (2 functioning), and 1 after a third PTA (functioning). Only 2 of the 36 KAP recipients were receiving dialysis at the time of the primary kidney transplant (6%), because early transplants are indicated for those already receiving immunosuppression.
Our cumulative incidence of KAP after primary PTAs was 4% by 1 year. The incidence with (n = 90) versus without (n = 133) sustained pancreas graft function after PTAs (all cases) was nearly identical: 10% versus 10% at 5 years and 16% versus 12% at 10 years (P = NS). The incidence of KAP transplants diminished slightly in successive eras: at 5 years after the pancreas transplant, it was 15% in era 1 (n = 83), 12% in era 2 (n = 119), and 8% in eras 3 and 4 combined (n = 80) (P = .2).
Our total number of KAPs was 40 (one recipient had 4; one had 2). Of these 40, 13 were cadaver SPK (primary kidney) transplants and 27 (23 primary, 4 secondary) were a kidney alone (11 cadaver, 10 primary; 16 living donor, 13 primary). In the recipients who had four KAP transplants, an SPK kidney functioned for 1 year (the pancreas for 11 years) and the next three (all kidneys alone) for 3, 6, and more than 2 (current) years (a pancreas retransplant done after the fourth kidney is also currently functioning). In the recipient with two KAP transplants, the first kidney functioned for only 4 months, while the second (an SPK) is currently functioning (as is the pancreas) after more than 1 year.
For the 36 primary KAP transplants (including the SPK transplants), the 1-year kidney graft survival rate was 80%: 45% for SPK (n = 13) versus 96% for kidney alone (n = 23) (P = .0004). With these results, our approach for PTA recipients with declining renal function is to add a kidney early rather than risk pancreas graft rejection by lowering the dose of calcineurin inhibitors to suboptimal immunosuppression levels in an attempt to lessen nephrotoxicity.
Rejection Episode Incidence by Recipient Category
Immunosuppressive protocols for pancreas recipients changed in each era and were associated with a decrease in pancreas graft rejection loss rates in each recipient category. From era 2 on, rejection episodes were nearly always treated with anti-T-cell agents in addition to steroids.
To determine whether the protocol changes in prophylactic immunosuppression altered the probability of a rejection episode, we calculated the cumulative incidence to the first treated episode in primary cadaver technically successful pancreas recipients (DWFG censored) in eras 2, 3, and 4. In the SPK category, the episode was included in the tabulation if the kidney, the pancreas, or both were involved. The diagnosis of a rejection episode in SPK recipients was based on an elevation of serum creatinine (usually with renal biopsy confirmation). Diagnosis of a rejection episode in bladder drainage pancreas recipients was based on a 50% decline in urine amylase (units per hour). For nonbladder drainage pancreas recipients, an elevation in serum pancreatic enzyme levels was used to diagnose a rejection episode (usually with graft biopsy confirmation). For the purposes of analysis, however, rejection episode identification was based on the need for treatment.
The cumulative incidence of rejection episodes for technically successful grafts in each recipient category, regardless of duct management, is given in Table 3 (a separate analysis showed no difference with the overall analysis, even though 75% of primary SPK grafts in era 4 were enteric drainage). In each category, the incidence of rejection episodes declined in successive eras. In each era, the incidence of rejection episodes was highest in the PTA and lowest in the SPK recipients.
Table 3. CUMULATIVE INCIDENCE OF REVERSIBLE REJECTION EPISODES IN TECHNICALLY SUCCESSFUL TRANSPLANT BY ERA AND RECIPIENT CATEGORY
* In each era, the incidence of rejection episodes was also significantly different between categories ( P ≤ .01).
SPK, simultaneous pancreas–kidney; PAK, pancreas after kidney; PTA, pancreas transplant alone.
In the SPK and PAK categories, after 1 year the probability of having a rejection episode, if one had not previously occurred, was almost nonexistent. In the PTA category, recipients who escaped a rejection episode in the first year were still at relatively high risk for one to occur. In a separate analysis of eras 3 and 4 combined, the incidence of rejection episodes at 1 year in the SPK (n = 182) versus PAK (n = 153) versus PTA (n = 67) recipients was 31% versus 47% versus 61% (P = .001). By 4 years, the cumulative incidences of rejection episodes in the SPK and PAK categories had risen to only 36% and 54%; in the PTA category, the rate was 77%. Thus, for PTA recipients, laboratory parameters must be monitored frequently to detect late rejection episodes, but in the SPK and PAK categories, monitoring can be less intense.
Pancreas Retransplants
In our entire series (all eras) of pancreas transplants since 1978 (n = 1,180), 191 (16%) were retransplants (13% second transplants, 2.5% third, 0.3% fourth); all but three were from cadaver donors. Of the 154 pancreas recipients who went on to have one or more retransplants, the primary was an SPK in 47 (the second was a SPK in 12 [26%] and a PAK in 35 [74%]); a PAK in 47 (the second was an SPK in 5 [11%] and a PAK in 42 [89%]); and a PTA in 60 (the second was an SPK in 8 [13%] and a PTA in 52 [87%]).
Analyses were done by era, recipient category, interval between the primary and retransplant, and cause of first graft failure.
SPK Retransplants
In eras 2, 3, and 4 combined, we did 36 SPK retransplants. Of the 25 second SPK transplants, 12 (48%) were after an SPK, 5 (20%) after a PAK, and 8 (32%) after a PTA (in which case the kidney transplant was a primary graft). In the SPK category for era 2, the 1-year patient, pancreas, and kidney graft survival rates were 79%, 58%, and 53% for pancreas retransplants (n = 19) versus 85% (P = .05), 64% (P = .17), and 77% (P = .004) for primary transplants (n = 214). The outcome was better for SPK retransplants in eras 3 and 4 combined. The 1-year patient, pancreas, and kidney graft survival rates were 87%, 70%, and 87% for retransplants (n = 17) versus 93% (P = .02), 80% (P = .04), and 90% (P = .03) for primary transplants (n = 234).
PAK Retransplants
In all eras combined, we did 93 PAK retransplants. Of the 77 second PAK transplants, 35 (45%) were after an SPK and 42 (45%) after a PAK. In the PAK category for era 1, the 1-year patient and pancreas graft survival rates for retransplants (n = 12) were 92% and 25% versus 89% and 26% for primary transplants (n = 53) (P = NS). In era 2 we saw little improvement for PAK retransplants (n = 35): 1-year patient and graft survival rates were 80% and 29% versus 99% (P = .006) and 55% (P = .005) for primary transplants (n = 73). In eras 3 and 4 combined, however, the PAK retransplant (n = 46) outcome improved, with 1-year patient and graft survival rates of 93% and 75% versus 97% (P = .06) and 79% (P = NS) for primary transplants (n = 171).
PTA Retransplants
In all eras combined, we did 60 PTA retransplants (52 second transplants). In the PTA category for era 1, the 1-year patient and pancreas graft survival rates for retransplants (n = 15) were 100% and 33% versus 85% and 34% for primary transplants (n = 68) (P = NS). PTA outcome improved in era 2 for both primary transplants and retransplants. The 1-year patient and graft survival rates for retransplants (n = 32) were 84% and 53%; for primary transplants (n = 87), the figures were 94% (P = .16) and 52% (P = NS). In eras 3 and 4 combined, the improvement continued for primary but not retransplant PTA recipients. The 1-year patient and graft survival rates were 100% and 44% for retransplants (n = 12) versus 95% (P = NS) and 82% (P = .08) for primary transplants (n = 68).
Retransplant Outcome by Cause of First Graft Failure
We compared outcome of second pancreas transplants in each category in all eras by cause of primary graft loss: rejection versus no rejection. For SPK second transplants, the 1-year pancreas graft functional survival rate was 74% for rejection (n = 20) versus 60% for no rejection (n = 5) recipients (P = NS). For PAK second transplants, the 1-year function rate was 50% for rejection (n = 32) versus 51% for no rejection (n = 44) recipients (P = NS). For PTA second transplants, the 1-year function rate was 44% for rejection (n = 32) versus 42% for no rejection (n = 19) recipients (P = NS). It appears that the probability of a successful retransplant is not influenced by the cause of primary graft failure.
Influence of Interval From Primary Transplant to Retransplant on Outcome
At the time of a pancreas graft technical failure, it is tempting to do an immediate retransplant if a donor is available. Doing so achieves the objective of insulin independence at once and avoids a late reoperation in a scarred surgical field. In all eras combined, we compared the graft survival rates of pancreas retransplants done early (less than 2 weeks after the original transplant) versus late (more than 2 weeks).
Of the 137 Minnesota-done primary pancreas transplant losses (all categories) in which the recipient had a second transplant, the second was done early in 29 (21%). Of the early second transplants, 17 (1 SPK, 16 PAK) (49%) were after a primary SPK (n = 35); 7 (1 SPK, 6 PAK) (15%) were after a primary PAK (n = 46); and 5 (all PTA) (9%) were after a primary PTA (n = 58). Of the 18 late second transplants after a Minnesota primary pancreas graft failure in the SPK category, the retransplant was in the SPK category in 4 (22%) and in the PAK category in 14 (78%). Of the 39 late second transplants after a PAK pancreas graft failure, the retransplant was in the SPK category in 2 (5%) and in the PAK category in 37 (95%). Of the 53 late second transplants after a primary PTA failure, the retransplant was in the SPK category in 8 (15%) and in the PTA category in 45 (85%). (One enteric drainage PTA second transplant was done to correct pancreas exocrine deficiency in a pancreatectomized [native] patient with a functioning bladder drainage PTA.)
Of our 17 second transplants (7 SPK, 8 PAK, 2 PTA) in which the primary transplant was done elsewhere (11% of our second transplants), all were late (5 SPK after an SPK, 7 SPK after a PAK, 2 PAK after an SPK, 1 PAK after a PAK, and 2 PTA after a PTA). These cases are included in our comparison of outcome for early versus late second pancreas transplants.
Of all 154 second transplants, 3 were from living donors, all late (1 sequential PAK and 2 sequential PTA cases). Most of the second transplants were after a cadaver primary, but 31 were after a primary living donor transplant (1 SPK after a PTA; 5 PAK after an SPK [2 early]; 11 PAK after a PAK; and 14 PTA after a PTA [1 early]).
For our analysis, we divided retransplants into those done at less than 2 weeks versus more than 2 weeks after the original transplant. Nearly all of the latter were months or years after the original transplant, although the interval from the primary graft failure to the retransplant might be short.
For SPK retransplants, the mean interval from the primary (Minnesota) to the second pancreas transplant was 4.5 years (in half, the interval was between 16 and 76 months). For PAK and PTA retransplants, the mean interval from the first was about 2 years in both (the interval was between 7 and 34 months for half of the PAK and between 11 and 44 months for half of the PTA).
For SPK second transplants (10 after an SPK, 1 early; 5 after a PAK, 1 early; 8 after a PTA, none early), the 1-year function rate was 50% for those done early (n = 2) versus 73% for those done late (n = 21) (P = .07). For PAK second transplants (30 after an SPK, 16 early; 43 after a PAK, 6 early), the 1-year function rate was 60% for early (n = 22) versus 48% for late (n = 51) (P = NS). For PTA second transplants, the 1-year function rate was 40% for early (n = 5) versus 44% for late (n = 47) (P = NS).
Few (9%) of the second pancreas transplants in the SPK category were in the early subgroup because of limitations in kidney allocation. Of the second pancreas transplants in the PAK category, 28% were in the early subgroup. The outcome for solitary pancreas retransplants was similar for those done early or late.
We did 30 third pancreas transplants (all but one recipient had had the second pancreas at Minnesota, 29 from cadaver donors). Of the 30 third transplants (all cadaver), 1 SPK and 1 PAK were after a second SPK; 3 SPK and 12 PAK were after a second PAK; and 6 SPK and 7 PTA were after a second PTA. Only one (3%), an SPK after a PAK, was early. The 1-year third graft survival rate was 50% for SPK (n = 10), 46% for PAK (n = 13) (the early retransplant is functioning), and 71% for PTA (n = 7) recipients.
We did four fourth pancreas transplants (all cadaver), with a 50% 1-year graft survival rate. One was an SPK after PAK (it functioned for more than 3 years until the patient died), and three were after a PAK (one is functioning at more than 1 year).
Living Donor Pancreas Transplants
Nearly all of the solitary (PAK and PTA) pancreas transplants were done in eras 1 and 2 (Fig. 8). All but two of the living donor SPK transplants (n = 29) were done in eras 3 and 4 combined (Fig. 9).
Figure 8. Functional survival rates of technically successful pancreas grafts from living donor versus cadaver donors for pancreas after kidney transplant recipients in (A) era 1 and (B) era 2 and for pancreas transplant alone recipients in (C) era 1 and (D) era 2.
Figure 9. Patient, pancreas, and kidney graft survival rates in all simultaneous pancreas–kidney transplant recipients of both grafts from a living donor.
In era 1, we primarily used living donors because the rejection rate for solitary cadaver pancreas transplants was high with the immunosuppressive regimens then used. The technical failure rate was high for both cadaver and living donor PAK transplants in this era (23% and 33%, respectively), so the main gain of using living donors was immunologic, as reflected by the graft survival rates for technically successful PAK transplants. For technically successful PAK transplants from living donors (n = 10) versus cadaver (n = 37) donors, the pancreas graft survival rates were 90% versus 22% at 1 year (P = .004) and 70% versus 9% at 5 years (P = .002).
Also in era 1, the improvement in pancreas graft survival rates with living donors was much higher for PAK than for PTA transplants. It may be that the chronic immunosuppression PAK recipients were already receiving contributed to this difference, but also most (84% of technically successful cases) of the living donor PAK recipients had previously received a kidney from the same donor and thus had been selected as having an immunologically favorable state with their particular donor. That situation was not the case with cadaver PAK transplants, where the pancreas always came from a different donor than the kidney, or with PTA transplants, where donor selection based on a previously favorable outcome was not possible.
Living Donor PAK
Of 10 technically successful living donor PAK recipients in era 1, 9 had the same donor for the kidney and pancreas (sequential operations). The only early failure (less than 1 year) was part of the identical twin series (the only one with a kidney transplant 63); the recipient was not immunosuppressed for either organ (inconsequential for the kidney), and the pancreas graft developed recurrent autoimmune isletitis. 64 The immunologic privilege in this case did not extend to the beta cells. The other nine technically successful living donor PAK grafts in era 1 (including the one from a different donor, who was HLA identical with both the kidney donor and the recipient) all functioned for more than 1 year, the longest now for nearly 20 years (a duct injection segmental graft done in 1980, a year after a kidney transplant from the same donor).
In era 2, the rejection rate of technically successful cadaver PAK transplants (n = 46) was much lower than in era 1; thus, the advantage of a technically successful living donor PAK (n = 7) was less in era 2, at least early on (functional survival rates for living donor vs. cadaver grafts were 71% vs. 82% at 1 year and 71% vs. 53% at 10 years [P = NS]). Of the seven technically successful living donor PAK transplants in era 2, the only two graft losses occurred when the pancreas came from a different donor than the kidney. In the other five, the kidney and pancreas grafts came from the same donor in sequential operations, and all are functioning between 6 and 13 years.
The living donor PAK transplant outcomes in era 1 and 2 demonstrate the potential for lifelong function of a pancreas graft. However, because of the immunologically privileged nature of a living donor PAK transplant from the same donor as the kidney, the immunologic advantage, or lack thereof, of a living donor pancreas transplant over a cadaver transplant is more easily discerned in the PTA category.
Living Donor PTA
In era 1, the technical failure rates for primary living donor PTA (n = 33) versus cadaver PTA (n = 36) were 33% and 33%. Our hope that the rejection rate would be less for technically successful living donor PTA (n = 22) than for technically successful cadaver PTA (n = 24) grafts turned out not to be the case for era 1: primary graft survival rates at 1 year were 53% versus 52%; at 10 years, they were 24% versus 22% (P = NS).
Also in era 1, we were making the transition from azathioprine and prednisone to cyclosporine and prednisone for maintenance immunosuppression. It was not until era 2, when we went to triple therapy for maintenance, that we saw an improvement in technically successful PTA graft survival rates, but with living donor only. In era 2, graft survival rates for technically successful living donor PTA (n = 11) versus technically successful cadaver PTA (n = 60) procedures were 82% versus 60% at 1 year (P = .05) and 64% versus 23% at 10 years (P = .04).
With triple therapy in era 2, living donors definitely gave an advantage for PTA. (The introduction of triple therapy had much more of an impact on cadaver PAK than on cadaver PTA results.) It was not until era 3 (tacrolimus and MMF) that cadaver PTA 1-year graft survival rates 38 equaled those of living donor PTA in era 2.
Thus, in era 3 we placed less emphasis on living donors for PTA transplants because cadaver donor pancreas grafts were readily available in the country for the small number of PTA candidates listed, 87 and the success rate with cadaver grafts had improved.
The potential for PTA grafts to function for a lifetime was shown in the era 1 series. Eight of those grafts (five from living donors and three from cadaver donors) are currently functioning 14 to 18 years after the transplant.
Living Donor SPK
We initiated living donor SPK transplants in March 1994, just before era 3 began. 88 Of the 29 donors, 7 were HLA-identical siblings, 21 were HLA-mismatched relatives, and 1 was an ABO-incompatible (AB-to-B) friend donating to a recipient with a low titer of anti-A antibodies made even lower by plasmapheresis (both grafts are currently functioning at more than 1 year).
We used duct injection in the first 2 SPK segmental pancreas grafts (both still functioning after more than 6 years), as well as in a later duct injection case (the pancreas failed at 4 months; the kidney is still functioning at more than 3 years). We used enteric drainage in 1 case (both organs are functioning at more than 1 year) and bladder drainage in 25 (3 were technical failures, all thromboses, 2 at less than 1 week and 1 at 5 months from a proximal iliac artery occlusion; 2 pancreas grafts were rejected at 15 and 25 months, but both the kidney grafts are still functioning at 3 and 4 years after the transplant).
For all living donor SPK transplants (n = 29), the 1-year patient, pancreas, and kidney graft survival rates were 100%, 86%, and 100% (vs. 92%, 79%, and 88% for primary cadaver SPK transplants in eras 3 and 4 combined [n = 212]). The corresponding 5-year rates were 100%, 77%, and 94% (vs. 88%, 73%, and 81%).
One living donor kidney graft failed at more than 3 years as a result of chronic sequelae of an early posttransplant hemolytic-uremic syndrome. The pancreas graft in this patient continues to function at more than 4 years, and a second kidney graft is also functioning.
Of the six living donor SPK recipients whose primary pancreas grafts failed, five have been retransplanted (one twice) with cadaver pancreas grafts. The two with early thrombosis of the primary graft were retransplanted during the same hospital stay. Four of these recipients currently have a functioning pancreas at 4, 30, 30, and 44 months after the retransplant. Thus, of the 29 living donor SPK recipients, all are currently alive with functioning kidneys (1 retransplanted), and 27 have functioning pancreas grafts (4 retransplanted).
Cox and Logistic Multivariate Analyses of Pancreas Transplant Outcome
The univariate analyses in the preceding sections were supplemented by Cox multivariate and logistic regression analyses for eras 3 and 4 combined, done separately in each recipient category (SPK, PAK, and PTA). The Cox method was used to determine the effect of the chosen variables (retransplant vs. primary transplant; bladder drainage vs. enteric drainage; recipient or donor age younger than or older than 45 years; preservation for more than or less than 20 hours; recipient body mass index [BMI] less than or more than 25 kg/m2; presence or absence of vascular disease; 0 vs. 1 vs. 2 HLA antigen mismatches at a given locus; and era 3 vs. era 4) on the relative risk (RR) for patient death, graft failure from all causes, and technically successful graft failure from rejection. The logistic regression method was used to determine the effect of the chosen variables (as in the Cox) with two more added: trauma versus no trauma as the cause of donor death, and right iliac versus left iliac vessel placement of the graft on RR for graft loss from a technical failure.
In the SPK category, the only variable associated with an increased risk for patient death was vascular disease (RR 4.9, P = .03). The only variable associated with an increased risk for graft failure in SPK recipients was a retransplant (RR 2.6, P = .07), which was also the only variable associated with an increased risk for rejection loss in SPK recipients (RR 3.7, P = .05). In the logistic regression analysis, no variables sorted out as having a significant impact on RR for technical failure in the SPK category.
In the PAK category, vascular disease was the only variable associated with an increased risk for patient death (RR 4.5, P = .03). Regarding graft failure from any cause, again, a retransplant was a risk in PAK recipients (RR 2.0, P = .04), but so was vascular disease (RR 2.3, P = .008), as was a BMI of 25 or more (RR 1.6, P = .011). The risk for PAK graft loss also increased as the number of HLA-B locus mismatches increased (RR 1.5, P = .08). The risk for graft loss was decreased in older PAK recipients (RR 0.6, P = .15). Overall, the RR for graft loss in PAK recipients was lower in era 4 (RR 0.6, P = .12).
In the technically successful PAK cases, again, a retransplant increased the risk for rejection loss (RR 3.2, P = .001). Surprisingly, vascular disease was also associated with an increased risk for rejection loss in PAK recipients (RR 2.7, P = .02), possibly because we were less likely to treat rejection episodes vigorously.
The logistic regression analysis showed that the RR for technical failure in PAK recipients was increased with preservation for 20 hours or more (RR 3.7, P = .02) and vascular disease (RR 2.3, P = .13) or a BMI of 25 or more (RR 2.0, P = .17) in the recipient. Surprisingly, the RR for technical failure in PAK recipients also increased as the number of HLA-B locus mismatches increased (RR 2.2, P = .06), suggesting that some rejection losses are misinterpreted as technical failure (e.g., an early acute rejection episode leading to secondary thrombosis). Older PAK recipients were not at increased risk for technical failure (RR 0.4, P = .17). The risk for technical failure in PAK recipients decreased in era 4 (RR 0.4, P = .08).
In the PTA category, no variable was associated with an increased risk for patient death except possibly a recipient BMI of 25 or more (RR 15.9, P = .13). Regarding graft failure, as in the other categories, a retransplant was associated with an increased risk (RR 3.0, P = .06). Vascular disease also increased the risk for graft loss in PTA recipients (RR 5.6, P = .03). The risk of graft loss also increased as the number of HLA-B locus mismatches increased (RR 3.1, P = .009). Interestingly, older PTA recipients were at decreased risk for graft failure (RR 0.2, P = .05), consistent with our univariate analyses. Again, the risk of graft loss in PTA recipients decreased in era 4 (RR 0.3, P = .07). Regarding technically successful PTA rejection loss, the risk was slightly higher with a retransplant (RR 2.9, P = .1). The risk for PTA rejection loss significantly increased as the number of HLA-B locus mismatches increased (RR 3.4, P = .008). However, older PTA recipients were much less likely to reject an organ (RR 0.1, P = .04), and the rejection loss risk in general decreased in era 4 (RR 0.3, P = .07). In logistic regression analysis, no variables sorted out as having a significant impact on RR for technical failure in the PTA category.
Although we found slight differences in each category, the multivariate analyses support the impression that recipient vascular disease increases the risk for transplant failure. Retransplanted grafts are also generally at higher risk to fail than primary grafts. HLA matching at the B locus is important in solitary pancreas (PAK and PTA) but not SPK recipients. The multivariate analyses also indicate that outcome has improved in era 4 for solitary pancreas transplants.
Quality of Life Study
In eras 2, 3, and 4 combined, 316 SPK, 204 PAK, and 98 PTA recipients enrolled in a prospective study of quality of life (QOL) changes after pancreas transplantation. For QOL assessment, we used four dimensions of the Karnofsky Index: status of health, management of life, life satisfaction, and health satisfaction. Each recipient’s response was recorded on a 1 (low) to 5 (high) scale for each parameter. A total score was calculated from the sum of the four parameters (maximum score possible, 20). The impact of a successful or failed transplant was assessed by the changes in scores from baseline in annual follow-up evaluations.
The baseline (prepancreas transplant) median total scores (all eras) were significantly higher (P < .0001) in the PAK (13.3) than in the SPK (11.3) and PTA (10.9) candidates. The ranges of baseline scores for the two midquarters were 8.4 to 14.6 for SPK, 11.7 to 15.9 for PAK, and 8.1 to 13.4 for PTA recipients.
Interestingly, pretransplant (baseline) QOL scores became successively higher in era 2 versus 3 versus 4. The mean baseline scores in these eras were 9.5 ± 2.6 (n = 109), 12.3 ± 3.9 (n = 131), and 13.0 ± 3.7 (n = 62) for SPK (P = .0001); 10.9 ± 2.6 (n = 32), 13.9 ± 3.3 (n = 82), and 15.2 ± 2.8 (n = 46) for PAK (P = .0001); and 9.9 ± 2.9 (n = 26), 10.3 ± 3.6 (n = 30), and 12.7 ± 3.3 (n = 24) for PTA (P = .009) candidates. Possibly diabetic patients come to pancreas transplantation in better health than in the past.
It is not the absolute QOL score that is telling, but rather the change in score from the pretransplant baseline to the posttransplant evaluation that is important. The total score changes for each recipient category are shown in Figure 10 (all eras).
Figure 10. Change in total quality of life (QOL) scores by quartile from before the transplant to 1 year after the transplant for (A) simultaneous pancreas–kidney, (B) pancreas after kidney, and (C) pancreas transplant alone recipients with functioning versus failed pancreas grafts.
SPK
SPK recipients were divided into four groups by graft status: 1) both grafts had sustained function; 2) the pancreas had sustained function, but the kidney graft failed; 3) the kidney graft had sustained function, but the pancreas graft failed; or 4) both grafts failed. At 1 year after the transplant, the mean increase in total QOL scores was 5.2 ± 4.0 points in the SPK recipients with both grafts functioning (n = 130) (P = .0001); 2.4 ± 1.5 points in those with a functioning pancreas but a failed kidney (n = 5) (P = .12); and virtually nil (0.2 ± 3.7) in those with a functioning kidney but a failed pancreas (n = 24). Only two recipients in whom both grafts failed completed the follow-up evaluation at 1 year: the total score did not change in one and was lower compared with baseline in the other. The results in the recipients in whom only one graft failed suggest that achieving insulin independence improves QOL more than becoming dialysis-free.
PAK
At 1 year, the mean total score increased 3.7 ± 4.1 points from baseline in PAK recipients with sustained graft function (n = 55) (P = .0001) versus 0.9 ± 2.5 points in those with failed grafts (n = 16) (P = .09).
PTA
At 1 year, the mean total score increased 5.9 ± 4.2 points from baseline in PTA recipients with sustained graft function (n = 25) (P = .0001) versus 2.8 ± 4.8 points in those with a failed graft (n = 12) (P = .07).
Long-Term QOL
The increase in mean total points from pretransplant baseline was sustained in succeeding years in patients with functioning grafts. At 2 years, the mean increases were 4.3 ± 0.8 points for SPK (n = 100), 3.7 ± 5.6 for PAK (n = 32), and 6.4 ± 4.3 for PTA (n = 8) (P = .0001). For 50 SPK study patients who completed the evaluation at 4 years, the mean increase in total points from baseline was 6.2 ± 4.6 (n = 50) (P = .0001).
Overall, our results show that diabetic patients who become insulin-independent perceive their QOL as having improved despite immunosuppression. The data presented here is original and complements past QOL studies, done by independent investigators, 144–150 of the Minnesota pancreas recipients.
Metabolic Studies
Formal metabolic studies of the Minnesota pancreas recipients have been concluded since the inception of our program 27 and are still ongoing. 126 No original data are presented in this section; instead, the results of past studies are summarized.
In era 1, the studies were initiated by coauthor F.C.G. and were basic: 24 metabolic profiles of glucose and insulin values before and after meals, and standard oral or intravenous glucose tolerance tests in pancreas recipients who were insulin-independent as a result of a functioning graft. 67 The profiles usually resembled those of nondiabetic persons, or at least those of nondiabetic kidney allograft recipients, with or without portal drainage of the graft venous effluent. 54
The metabolic profile and glucose tolerance test studies were used in era 2 to compare posttransplant endocrine function by duration of pancreas graft preservation 106 and to compare function in recipients who did or did not have reversible rejection episodes. 81 The results were similar regardless of preservation time or occurrence of rejection episodes in recipients with sustained insulin independence; glycosylated hemoglobulin levels, 112 both in the short term 151 and the long term, 114 were normal.
Era 2 saw the introduction of more sophisticated metabolic studies using new methods initiated by coauthor R.P.R. 152 and carried out by a series of fellows and associate faculty members in the Division of Endocrinology. 111,113,139,140,153–158 Parallel studies of our islet auto- and allograft recipients were also done. 159–163 These studies examined not only pancreatic graft beta-cell function but also alpha-cell function, glucose counterregulatory mechanisms, and the impact of the site of venous drainage (systemic or portal) of a pancreas graft.
Diem et al 111 were the first to establish systemic venous drainage as the principal cause of systemic venous hyperinsulinemia after pancreas transplantation. A smaller portion of the hyperinsulinemia could be attributed to recipients’ glucocorticoid use. Despite this metabolic abnormality, virtually all measures of carbohydrate metabolism in the fasting state and after a mixed meal remained normal. 153
Possible adverse effects of immunosuppressive drugs on beta-cell function and glucose tolerance were also studied. Many of the drugs are known to interfere with insulin synthesis or secretion, or action. Teuscher et al 158 assessed insulin secretory reserve in pancreas transplant recipients by measuring glucose potentiation of arginine-induced insulin secretion and observed abnormally low insulin responses. Because diminished insulin secretory reserve was also observed in nondiabetic kidney recipients, the immunosuppressive drugs were the likely causes of this metabolic abnormality. A similar defect was observed in psoriasis patients treated with cyclosporine, but not in arthritis patients treated with glucocorticoids; thus, cyclosporine was the likely cause of diminished insulin secretory reserve. 158 However, despite the hyperinsulinemia consequent to systemic drainage and glucocorticoids, and despite the diminished insulin secretory reserve attributable to cyclosporine, we (R.P.R., D.E.R.S.) have recently reported normal levels of fasting plasma glucose and hemoglobin A1C in a group of pancreas recipients followed up for 10 to 18 years. 126
Defective glucagon and epinephrine counterregulatory responses to hypoglycemia are serious consequences of type 1 diabetes. These abnormalities can lead to dangerous levels of hypoglycemia that incapacitate patients and seriously compromise QOL. This scenario is made all the worse because such patients lose normal symptom recognition of hypoglycemia, which prevents them from taking early corrective measures. The results of studies by Diem et al 113 showed that a successful pancreas transplant restores normal glucagon responses. Later studies by Kendall et al 161 concluded that the transplanted pancreas, rather than the alpha cells in the native pancreas, provided the restored glucagon response. Barrou et al 156 used isotopic infusions and hypoglycemic clamp methodology to show that the restored glucagon response normalized hepatic glucose production during hypoglycemia. Kendall et al 157 showed that a successful pancreas transplant partially restored epinephrine response during hypoglycemia. More important, these studies also documented that recipients of a successful pancreas transplant reestablish normal symptom recognition.
Although most of our pancreas transplants were from cadaver donors, nearly 10% were segmental grafts from living donors. The metabolic responsivity of the transplanted hemipancreas is generally indistinguishable that of whole pancreas grafts. Donors of the pancreatic segments generally maintain normal glucose levels, but follow-up studies of the donors of era 1 and the early part of era 2 (before we established our current criteria to be a living donor) showed that about 25% had metabolic evidence of acquired glucose intolerance several years after donation. 139 Studies by Seaquist and Robertson 154 established that both beta-cell and alpha-cell responses were compromised in hemipancreatectomized donors during measurements of insulin secretory reserve. Later studies by Seaquist et al 140 showed that hemipancreatectomy was also associated with elevated circulating levels of proinsulin, presumably as a result of release of immature insulin granules in which cleavage of C-peptide from proinsulin was not yet complete.
The results of these studies prompted us to modify our criteria to be a living donor. Now, all living donors must have a BMI of less than 28, in addition to having normal glucose tolerance test results, and plasma insulin levels must increase by 300% within 1 to 2 minutes after intravenous stimulation with glucose or arginine. Living donors who meet these criteria have so far remained euglycemic and insulin-independent, but they must be carefully studied in the years to come.
Studies of Diabetic Secondary Complications.
Formal studies on the course of preexisting diabetic secondary complications after pancreas transplantation were initiated at the beginning of era 1. 71,164 The multicenter Diabetes Control and Complications Trial (DCCT) (of which the University of Minnesota was a part) was just beginning. 165 Until it was completed in 1993 (near the end of era 2), the best evidence that a constant euglycemic state mitigated the progression of secondary complications was from studies by us 68,69,116–118 and others 166,167 of pancreas recipients. These studies were carried out by members of our faculty from ophthalmology, 68 pediatric nephrology, 118,131 and neurology. 116,117,129,130 As in the preceding section, no original data on secondary complications are presented here, but the studies completed to date are briefly summarized.
The failure rate of pancreas transplants was relatively high in eras 1 and 2, generating a control group for these studies. Recipients were studied at baseline and subsequently divided into two groups: 1) those with early pancreas graft failure (less than 3 months) and 2) those with sustained graft function for more than 1 year.
Retinopathy
Ramsay et al 68 studied solitary pancreas recipients in eras 1 and 2. Retinopathy and visual acuity were quantitated before and serially after transplantation. Most candidates had advanced, proliferative retinopathy. At 2 years after the transplant, the incidence of progression to a higher grade of retinopathy was the same (~30%) in the eyes of recipients with versus without graft function. After 3 years, however, no further progression occurred in the recipients with functioning grafts. However, 70% with failed transplants advanced to a higher grade by 5 years. Only a few recipients had no retinopathy at the pretransplant baseline examination, but disease has not emerged in the subgroup with continuously functioning pancreas grafts.
Nephropathy
Studies of diabetic nephropathy focused on disease recurrence or prevention in the kidney grafts of diabetic KTA, SPK, or PAK recipients, 118,168,169 as well as on disease progression, stabilization, or regression of disease in the native kidneys of PTA recipients. 131
Mauer et al documented the recurrence of diabetic nephropathy (vascular lesions 170 and an increase in glomerular and tubular basement membrane and mesangial matrix 168) in nearly half of kidneys transplanted without a pancreas in uremic diabetic recipients. 171
Initial evidence that a successful pancreas transplant can influence the course of diabetic nephropathy came from kidney allograft biopsy studies in PAK recipients by Bilous et al. 118 At the time of the pancreas transplant 1 to 7 years (mean 4) after the kidney transplant, the graft glomerular mesangial volume was moderately increased and glomerular basement membrane was moderately thickened. There was no progression; indeed, there was regression of glomerular lesions in follow-up biopsies taken 2 to 10 years later (mean 4.5). These findings are in contrast to those in the KTA recipients, where progressive diabetic glomerulopathy occurred, 171 leading to kidney graft failure and the need for a kidney retransplant in some recipients. 172
The most dramatic and surprising findings came from studies by Fioretto et al 131 of native kidneys in our PTA recipients. We obtained baseline biopsies of native kidneys in most of the PTA recipients. 173 Follow-up biopsies in some have shown cyclosporine-induced lesions that were associated with a progressive decline in kidney function, independent of the diabetic lesions already present. 128,169,174 The diabetic kidney lesions were distinct. In eight PTA recipients who were nonuremic at the time of the pancreas transplant but who had mild to moderately advanced lesions of diabetic nephropathy at baseline, 10-year follow-up biopsies showed that glomerular and tubular basement membrane thickness and mesangial fractional volume of the glomerulus had decreased and, indeed, returned to normal. 131
Thus, although it takes at least 5 years of normoglycemia, a pancreas transplant can reverse the lesions of diabetic nephropathy. Such reversal does not guarantee normal function because independent damage to the kidney may occur from the calcineurin inhibitors needed to prevent pancreas rejection. 128 If an effective nonnephrotoxic immunosuppressive regimen is developed, nearly all patients with early diabetic nephropathy would benefit from a pancreas transplant.
Neuropathy
As with the eye and kidney, our pancreas recipients had baseline neurologic studies with serial follow-up. 69,116,129 More than 80 of our recipients had symptomatic neuropathy, and more than 90% had abnormal results on the baseline neurologic examination. 127 Kennedy et al 116 showed significant improvement in motor and sensory indices as well as autonomic function between 1 and 4 years after the transplant; we concluded that progression of diabetic neuropathy is halted and that improvement is possible with sustained normoglycemia.
Navarro et al 117 found higher death rates in patients with autonomic dysfunction or abnormal nerve conduction studies, compared with those with minimal disease. The death rate was also high in nontransplanted diabetic patients with neuropathy. However, in neuropathic patients with a successful pancreas transplant, the death rate was significantly lower, even if neuropathy improved only minimally. 130 The combination of diabetes and severe neuropathy is lethal; correction of diabetes improves survival even if neuropathy persists.
Navarro et al 129 did follow-up studies at 10 years of diabetic pancreas recipients. In control patients (those with a failed transplant), neuropathy progressively worsened, whereas in recipients with sustained graft function, the improvement in neuropathy seen early on was sustained.
General
The most remarkable feature of the studies of diabetic secondary complications in our pancreas recipients was the positive impact on even advanced disease. In the DCCT, 165 at entry, diabetic patients had either no or minimal manifestations of secondary complications. Even with intensive insulin treatment, new lesions emerged, and lesions already present progressed in some patients. 175 The secondary diabetic lesions at baseline were much more advanced in our patients than in the DCCT. The regression of neuropathic and nephropathic lesions seen after a successful pancreas transplant did not occur with intensified insulin treatment. 176 Other groups have now also shown that a successful pancreas transplant can ameliorate microvascular complications, 177,178 including retinopathy, 179 nephropathy, 167 and neuropathy. 166,180
Although a main objective of pancreas transplantation is to improve day-to-day QOL by omitting the need for insulin injections and glucose monitoring, the fact that secondary complications are also favorably influenced gives even greater impetus to apply this treatment modality.
GENERAL DISCUSSION
Clinical pancreas transplantation, begun at Minnesota in 1966, now encompasses a third of a century. Transplantation of endocrine tissue (pancreatic islet beta cells) is the only treatment that can induce insulin independence for type 1 diabetic patients. To date, transplantation of islets within an immediately vascularized graft (pancreas) has been much more successful 2 than as a free cellular graft. 181
The promise that islet transplantation can replace pancreas transplantation has been propagated over nearly three decades. Recent successes with islet transplantation 182 suggest that the dream of eliminating the major surgery of pancreas transplantation may soon be achieved. Meanwhile, pancreas transplantation continues to be done, and the lessons learned over many years at our own and other centers (only a few examples are referenced) 183–189 have contributed to the high success rate now achieved in both uremic and nonuremic diabetic recipients. Indeed, there is good evidence that a pancreas transplant prolongs survival of both nephropathic 185,190–192 and neuropathic 130 diabetic patients.
Our program differs from most in that we emphasized solitary pancreas transplants from the beginning, 20,39,76 and continue to do so. A few other programs have increased the proportion of solitary pancreas transplants in recent years, 188,193 with results equivalent to our own. 38
Although a few aspects of our program are original, much of what we have done has been adapted from the pioneering efforts of others. We were the first group to use a living donor for a pancreas transplant, in 1979, 44 with extension to identical twin donors in 1980. 39,63 The living donor option has been exercised by only a few other centers, but includes the use of an identical twin. 194
Our practice of splitting a cadaver pancreas to give a segment to each of two recipients 104 appears to be unique. However, our use of living donor kidney transplants simultaneous with a cadaver pancreas 7 has been followed by many cases elsewhere. 195 The introduction of an immediate retransplant 196 for a primary technical failure (e.g., thrombosis) has been adopted by other groups. 197,198 Even our use of enteric drainage pancreas grafts to correct exocrine deficiency 90 has been duplicated. 199
Regarding surgical techniques, segmental pancreas transplantation has largely disappeared except with living donors, as has the use of duct management techniques other than bladder or enteric drainage. In the past decade, many groups have compared outcome with enteric versus bladder drainage and have concluded that the results are equivalent, at least for SPK transplants. 184,200–203 Although portal drainage of pancreas graft venous effluent was done in a few cases at several centers in the 1980s, 204–206 including our own, 54 Rosenlof et al 207 reported its routine use for SPK transplants in 1992, stimulating others to adopt the technique. 184,188,208 Portal drainage must be more physiologic than systemic drainage, and the metabolic perturbations of systemic drainage include pseudohyperinsulinemia, 111 but the relevance is unknown.
Surgical complications of pancreas transplantation decreased at our center from era 83 to era. 122 This decrease was paralleled at other centers. 201,209,210 Chronic complications of bladder drainage, however, have persisted, 211–213 and our rate of conversion to enteric drainage exceeded 10% throughout the 1990s. 141
The most immediate and frequent posttransplant complication is pancreas graft thrombosis. 214 Some groups have not found that heparinization reduces the risk, 215 but in our experience it seems to have helped. 122
Infections after pancreas transplantation can be local or systemic. Our incidence of local infections has been reduced, 122 but local infection can necessitate graft removal. 216,217 The most common systemic infection is due to cytomegalovirus or Epstein-Barr virus. We showed that drug prophylaxis is effective in reducing the incidence of cytomegaloviral infections in pancreas recipients, 218 as have others. 219 The risk of posttransplant lymphoproliferative disorder (PTLD) after Epstein-Barr virus infection is a risk in all organ allograft recipients. Our incidence of PTLD after pancreas transplantation has been less than 2%, including only 0.6% in PTA recipients. 220 Other groups report a similarly low rate of PTLD after pancreas transplantation. 221–225
Regarding immunosuppression, the practice in our center, 121 along with many others, has evolved to include the use of tacrolimus and/or MMF in all recipient categories. Sirolimus is just beginning to be used for pancreas transplantation, 226 and our experience is limited. We also use anti-T-cell therapy routinely for induction; its use is variable at other centers. 183,184,227–234 We have used adjunctive measures such as blood transfusion, as have others, but we have not adopted donor bone marrow administration. 183 The immunosuppressive protocol changes initiated by us and others have been associated with a reduction in the previously high rejection episode rates seen even in SPK recipients. 235 In PTA recipients, however, the rejection episode rate is still high, but reversal is more readily accomplished than in previous eras.
Early treatment of rejection episodes is important. SPK recipients of grafts from the same donor can be monitored by serum creatinine. For solitary pancreas transplant recipients, serum creatinine cannot be used as a surrogate marker for rejection. Thus, we still favor bladder drainage and use a decline in the urine amylase level as a marker for rejection. 97 A decline in the urine amylase level is sometimes preceded or accompanied by a rise in serum pancreatic enzyme levels, 236 but we have seen several rejection episodes where only the urine amylase level declined; they would have been missed by relying on serum enzyme levels alone. Thus, we believe that urine amylase is still superior for immunologic monitoring of solitary bladder drainage pancreas transplants and should be used, at least for PTA, until protocols that further lower the rejection episode incidence have been developed.
Pancreas allograft biopsies and pathologic assessment are less important in SPK recipients than in solitary pancreas recipients for the reasons mentioned above. However, for solitary pancreas recipients, a pancreas graft biopsy has the same utility as a kidney graft biopsy in KTA recipients. We have used pancreas allograft biopsies for solitary pancreas recipients by one technique or another since the early 1980s. 59,60,97 The introduction of the percutaneous needle technique by Allen et al 237 and Gaber et al 238 in the early 1990s made routine biopsies practical. Pathologic features and histologic grading of pancreatic allograft biopsies have been well described by many groups, 221,239–244 including our own. 97,245 Our use of pancreas graft biopsies in the 1980s was critical in identifying disease recurrence (autoimmune isletitis). 64 Although recurrence (selective beta-cell loss) is occasionally seen in human allografts, 110,246 it is rare. One center that is liberal in performing pancreatic allograft biopsies has never seen a case of recurrent disease. 247
In SPK recipients, documented discordant rejection episodes (i.e., involving only one organ) are rare, but they do occur. 248,249 In our experience they can lead to discordant graft loss as well. 93 HLA matching reduced rejection failures in solitary pancreas transplants at a time when the overall results were not as good as now. 250 Although not all agree, 251 our own data reported here, as well as that of the Registry, 2 indicate that HLA matching, particularly at the B locus, is still beneficial in the tacrolimus and MMF era.
If graft loss does occur for any reason, a pancreas retransplant is feasible. Although some have considered retransplantation a high-risk procedure, 252 we have not been deterred. We have had a large number of candidates for retransplants because of the low success rate with primary transplants in the early eras. Pancreas graft survival rates were significantly lower for retransplants than for primary transplants in each era, but the current retransplant success rate is much higher than the primary pancreas transplant success rate in earlier eras. Thus, we have no hesitation in routinely offering a retransplant to recipients whose primary grafts fail.
Even apart from retransplantation, many risk factors influence outcome. Multivariate analyses have been done by others, looking at both recipient 253 and donor 254 risk factors, but with many fewer patients than in our analyses. Some groups have assessed individual risk factors, such as obesity 255 or recipient race;256 the former has a moderate impact, but the latter does not seem to influence outcome.
The question as to whether early SPK transplants to preempt dialysis give an advantage in nephropathic diabetic recipients 257 is answered by our good results in patients who did not receive dialysis. Other questions, such as the impact of vascular disease, have not been well studied because most groups have excluded vasculopathic patients from pancreas transplantation in the first place. 258 In our program, nearly all uremic diabetic patients undergo pretransplant coronary angiography followed by intervention (bypass or angioplasty), if indicated, before a pancreas transplant. 109 Although patient survival rates are lower for those with versus without preexisting vascular disease, correcting diabetes is beneficial for both groups. A few other centers also do pancreas transplants in diabetic patients with coronary artery disease 259,260 and believe it safe. Even uremic patients with type 2 diabetes have routinely received SPK transplants in some programs. 261 We, too, have found no difference in insulin independence rates in the few type 2 diabetic patients we have transplanted.
As important as recipient risk are donor risk factors. 254 Although pancreas grafts from both pediatric 262 and older donors 263 have been successfully transplanted, 107 we 107 and others 264,265 are selective. Whatever the age range, one group has shown that the outcomes for paired grafts from the same donor are similar in KTA and SPK recipients. 266
Restoration of normal metabolism is the immediate goal of pancreas transplantation. Although we have described delayed endocrine function, 267 most recipients become insulin independent immediately after the transplant. Nearly all are euglycemic and have normal glycosylated hemoglobin levels as long as the graft functions. Several other groups have also performed sophisticated metabolic studies after pancreas transplantation. 268–273 Even though metabolic perturbations are described, it is interesting that recipient lipid profiles usually improve after a successful pancreas transplant. 274–276 Whether the improvement in lipid profiles translates into a lower risk for vascular disease in pancreas recipients has not been determined. It is clear from the results of our studies and others that microangiopathy can improve after a successful pancreas transplant, 177,178 including retinopathy if the intervention is early enough. 68,179 Of course, advanced retinopathy is difficult to influence, as we 68 and others 277 have found.
Every group that has reported on neuropathic studies has shown improvement after pancreas transplantation. 166,180,278 Even autonomic dysfunction, including cardiopathy, 279 vesicopathy, 280 and gastropathy, 281 can improve.
As expected, diabetic nephropathy does not recur in kidney grafts of recipients with sustained insulin independence after an SPK transplant. 167 The only real surprise in the area of kidney disease is our finding that advanced lesions of diabetic nephropathy in native kidneys can resolve over time after a successful PTA. 131
The improved metabolism after a pancreas transplant not only ameliorates secondary complications; concomitantly there is an improvement in QOL. The independent studies done in our patients 144–150 show the same findings as those of other groups in their patients:282–287 recipients are more satisfied before than after the pancreas transplant.
Pancreas transplantation is a highly effective therapy for diabetes mellitus. There are surgical complications, and immunosuppression is required, but QOL improves. At least in the short run, a pancreas transplant is more expensive than exogenous insulin treatment, 288 but better treatment is worth the higher cost. The cost of pancreas transplantation in the short term has been studied by ourselves 289 and others, 290–293 but the long-term overall economic impact of preventing or ameliorating secondary complications, thereby recouping initial start-up costs, has only been projected. 288 Nevertheless, pancreas transplantation is so effective that a new American Diabetes Association position statement 294 says that SPK and PAK should be routine in diabetic kidney transplant recipients and that PTA is appropriate for nonuremic labile diabetic patients.
Our own program is more liberal than the American Diabetes Association. We have done pancreas transplants as prophylaxis for secondary complications or for adult patients who would rather manage immunosuppression and its risks than diabetes and its risks. We take it as a matter of informed consent as to which route patients want to take: the diabetes or immunosuppression. There are risks with each, but the benefits are greater with a transplant.
Of course, if immunosuppression is required for other reasons in a diabetic patient, a pancreas transplant might as well be done, even in children. 135 Certainly the current American Diabetes Association guidelines are appropriate for children. Whatever hesitation there may be to recommend endocrine transplantation as a treatment for diabetes, it will be less with free grafts of islets, which eliminate surgical complications. 181 If tolerance-inducing protocols become successful clinically, 29 either pancreas or islet transplantation could be performed without the fear of immunosuppressive complications. If islets are as successful as the pancreas transplant technically, in the absence of immunosuppression (tolerance), virtually every diabetic patient would want to be treated. Because of the limited supply of human cadaver donors, treating all diabetic patients would require the development of propagated beta-cell lines that are suitable for transplantation, 295 or the application of xenografts. 181 Ultimately, neither strategy may be needed if beta-cell regeneration can be induced in the native pancreas 296 and the autoimmune threat thwarted. 297,298 When the last scenario will materialize is uncertain.
Certainly, pancreas or islet transplantation will continue to be in the therapeutic armamentarium for diabetes in the immediate future. Our experience shows that large-scale application is possible.
Acknowledgments
The authors thank Barbara Bland, M.S., M.L.I.S., B.A.N., RN, and Mary Beth Drangstveit, RN, C.C.T.C., for assistance with the database, and Linda Ridgway for preparation of the manuscript. The authors also greatly appreciate the roles of Barbara Elick, RN, and the nurse transplant coordinators in facilitating our pancreas transplant program over the years.
Footnotes
Presented at the American Surgical Association, Philadelphia, Pennsylvania, April 6, 2000.
Correspondence: Dr. David E. R. Sutherland, Department of Surgery, University of Minnesota, Box 280 Mayo, 420 Delaware St. SE, Minneapolis, MN 55455.
E-mail: dsuther@tc.umn.edu
Accepted for publication December 18, 2000.
References
- 1.Kelly WD, Lillehei RC, Merkel FK. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61: 827–835. [PubMed] [Google Scholar]
- 2.Gruessner A, Sutherland DER. Analyses of pancreas transplant outcomes for United States cases reported to the United Network for Organ Sharing (UNOS) and non-US cases reported to the International Pancreas Transplant Registry. In: Cecka JM, Terasaki PI, eds. Clinical Transplant—1999. Los Angeles: UCLA Immunogenetics Center; 2000: 51–69. [PubMed]
- 3.Lillehei RC, Simmons RL, Najarian JS, et al. Pancreatico-duodenal allotransplantation: experimental and clinical experience. Ann Surg 1970; 172: 405–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland DER, Goetz FC, Najarian JS. One hundred pancreas transplants at a single institution. Ann Surg 1984; 200: 414–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland DER, Dunn DL, Goetz FC, et al. A 10-year experience with 290 pancreas transplants at a single institution. Ann Surg 1989; 210: 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruessner RWG, Kendall DM, Drangstveit MB, et al. Simultaneous pancreas-kidney transplantation from live donors. Ann Surg 1997; 226: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland DER, Gores PF, Farney AC, et al. Evolution of kidney, pancreas, and islet transplantation for patients with diabetes at the University of Minnesota. Am J Surg 1993; 166: 456–491. [DOI] [PubMed] [Google Scholar]
- 8.Gliedman ML, Gold M, Whittaker J, et al. Clinical segmental pancreatic transplantation with ureter-pancreatic duct anastomosis for exocrine drainage. Surgery 1973; 74: 171–180. [PubMed] [Google Scholar]
- 9.Dubernard JM, Traeger J, Neyra P, et al. New method of preparation of segmental pancreatic grafts for transplantation: trials in dogs and in man. Surgery 1978; 84: 633–640. [PubMed] [Google Scholar]
- 10.Groth CG, Collste H, Lundgren G. Successful outcome of segmental human pancreatic transplantation with enteric exocrine diversion after modifications in technique. Lancet 1982; 2: 522–524. [DOI] [PubMed] [Google Scholar]
- 11.Sollinger HW, Cook K, Kamps D. Clinical and experimental experience with pancreaticocystostomy for exocrine pancreatic drainage in pancreas transplantation. Transplant Proc 1984; 16: 749–751. [PubMed] [Google Scholar]
- 12.Starzl TE, Iwatsuki S, Shaw BW Jr, et al. Pancreaticoduodenal transplantation in humans. Surg Gynecol Obstet 1984; 159: 265–272. [PMC free article] [PubMed] [Google Scholar]
- 13.Nghiem DD, Corry RJ. Technique of simultaneous pancreaticoduodenal transplantation with urinary drainage of pancreatic secretion. Am J Surg 1987; 153: 405–406. [DOI] [PubMed] [Google Scholar]
- 14.Belzer FO. Clinical organ preservation with UW solution. Transplantation 1989; 47: 1097–1098. [DOI] [PubMed] [Google Scholar]
- 15.Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979; 2: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE, Todo S, Fung J, et al. FK 506 for liver, kidney, and pancreas transplantation. Lancet 1989; 2: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayhill SC, Kirk AD, Odorico JS, et al. Simultaneous pancreas-kidney transplantation at the University of Wisconsin. Clin Transplant 1995; 261–269. [PubMed] [Google Scholar]
- 18.Vincent F, Kirkman R, Light S, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med 1998; 338: 161–165. [DOI] [PubMed] [Google Scholar]
- 19.Sollinger HW, Bruce DS, Humar A, et al. Multicenter retrospective analysis of simultaneous kidney–pancreas transplant recipients receiving daclizumad induction. Transplantation 1999; 67: S543. [DOI] [PubMed] [Google Scholar]
- 20.Lillehei RC, Ruiz JO, Aquino C, et al. Transplantation of the pancreas. Acta Endocrinol 1976; 83 (suppl 205): 303–320. [PubMed] [Google Scholar]
- 21.Kjellstrand CM, Simmons RL, Goetz FC, et al. Renal transplantation in patients with insulin-dependent diabetes. Lancet 1973; 2: 4–8. [DOI] [PubMed] [Google Scholar]
- 22.Najarian JS, Kjellstrand CM, Simmons RL, et al. Renal transplantation for diabetic glomerulosclerosis. Ann Surg 1973; 4: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najarian JS, Sutherland DER, Simmons RL, et al. Ten-year experience with renal transplantation in juvenile-onset diabetics. Ann Surg 1979; 190: 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland DER, Morrow CE, Fryd DS, et al. Improved patient and primary renal allograft survival in uremic diabetic recipients. Transplantation 1982; 34: 319–325. [DOI] [PubMed] [Google Scholar]
- 25.Sutherland DER, Goetz FC, Carpenter AM, et al. Pancreaticoduodenal grafts: clinical and pathological observations in uremic versus nonuremic recipients. Amsterdam-Oxford: Excerpta Medica; 1979: 190–195.
- 26.Gliedman ML, Tellis VA, Soberman R. Long-term effects of pancreatic transplant function in patients with advanced juvenile-onset diabetes. Diabetes Care 1978; 1: 1–9. [DOI] [PubMed] [Google Scholar]
- 27.Matas AJ, Sutherland DE, Najarian JS. Current status of islet and pancreas transplantation in diabetes. Diabetes 1976; 25: 785–795. [DOI] [PubMed] [Google Scholar]
- 28.Younoszai R, Sorenson RL, Lindall AW. Homotransplantation of isolated pancreatic islets. Diabetes 1970; 19 (suppl): 406. [Google Scholar]
- 29.Hering BJ, Ricordi C, Sutherland DE, et al. Islet transplantation: at the forefront of clinical research on immune tolerance. In: Norman D, Suki WN, eds. Primer on transplantation. Thorofare, NJ: American Society of Transplant Physicians; 2000.
- 30.Sutherland DER, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am 1978; 58: 365–382. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland DER, Matas AJ, Goetz FC, et al. Transplantation of dispersed pancreatic islet tissue in humans: autografts and allografts. Diabetes 1980; 29: 3l–44. [DOI] [PubMed] [Google Scholar]
- 32.Najarian JS, Sutherland DER, Baumgartner D, et al. Total or near-total pancreatectomy and islet autotransplantation for treatment of chronic pancreatitis. Ann Surg 1980; 192: 526–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland DER. Pancreas and islet transplantation. I. Experimental studies. Diabetologia 1981; 20: 161–185. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland DER. Pancreas and islet transplantation. II. Clinical trials. Diabetologia 1981; 20: 435–450. [DOI] [PubMed] [Google Scholar]
- 35.Gores PF, Najarian JS, Stephanian E, et al. Insulin independence in type I diabetes after transplantation of unpurified islets from a single donor using 15-deoxyspergualin. Lancet 1993; 341: 19–21. [DOI] [PubMed] [Google Scholar]
- 36.Kyriakides GK, Sutherland DER, Miller JB, et al. Segmental pancreatic transplantation in dogs after suppression of exocrine function by intraductal injection of neoprene. Transplant Proc 1979; 11: 530–532. [PubMed] [Google Scholar]
- 37.Sutherland DER, Goetz FC, Najarian JS. Intraperitoneal transplantation of immediately vascularized segmental pancreatic grafts without duct ligation: a clinical trial. Transplantation 1979; 28: 485–491. [PubMed] [Google Scholar]
- 38.Gruessner RWG, Sutherland DER, Najarian JS, et al. Solitary pancreas transplantation for nonuremic patients with labile insulin-dependent diabetes mellitus. Transplantation 1997; 64: 1572–1577. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland DER, Goetz FC, Rynasiewicz JJ, et al. Segmental pancreas transplantation from living related and cadaver donors: a clinical experience. Surgery 1981; 90: 159–169. [PubMed] [Google Scholar]
- 40.Sutherland DER, Goetz FC, Elick BA, et al. Experience with 49 segmental pancreas transplants in 45 diabetic patients. Transplantation 1982; 34: 330–338. [DOI] [PubMed] [Google Scholar]
- 41.Najarian JS, Gruessner A, Drangstveit MB, et al. Insulin independence for more than 10 years in 32 pancreas transplant recipients from an historical era. Transplant Proc 1998; 30: 279–279. [DOI] [PubMed] [Google Scholar]
- 42.Sutherland DER, Moudry KC. Report of the pancreas transplant registry. In: Terasaki PI, ed. Clinincal Transplants, 1986. Los Angeles: UCLA Tissue Typing Laboratory; 1986: 7–15. [PubMed]
- 43.Najarian JS, Simmons RL, Condie RM, et al. Seven years’ experience with antilymphoblast globulin for renal transplantation from cadaver donors. Ann Surg 1976; 184: 352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland DER, Goetz FC, Najarian JS. Living-related donor segmental pancreatectomy for transplantation. Transplant Proc 1980; 12: 19–25. [PubMed] [Google Scholar]
- 45.Simmons RL, Kjellstrand CM, Condie RM, et al. Parent-to-child and child-to-parent kidney transplants: experience with 101 transplants at one center. Lancet 1976; 1: 321–324. [DOI] [PubMed] [Google Scholar]
- 46.Simmons RL, Van Hook EJ, Yunis EJ, et al. 100 sibling kidney transplants followed 2 to 7-1/2 years: a multifactorial analysis. Ann Surg 1977; 185: 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutherland DER, Goetz FC, Najarian JS. Pancreas transplants from related donors. Transplantation 1984; 38: 625–633. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland DER. Pancreas transplantation in non-uremic diabetic patients. Transplant Proc 1986; 18: 1747–1749. [Google Scholar]
- 49.Sutherland DER, Kendall DM, Goetz FC, Najarian JS. Pancreas transplantation. Surg Clin North Am 1986; 66: 557–582. [DOI] [PubMed] [Google Scholar]
- 50.Prieto M, Sutherland DER, Goetz FC, et al. Pancreas transplant results according to the technique of duct management: bladder versus enteric drainage. Surgery 1987; 102: 680–691. [PubMed] [Google Scholar]
- 51.Prieto M, Sutherland DER, Fernandez-Cruz L, et al. Urinary amylase monitoring for early diagnosis of pancreas allograft rejection in dogs. J Surg Res 1986; 40: 597–604. [DOI] [PubMed] [Google Scholar]
- 52.Prieto M, Sutherland DER, Fernandez-Cruz L, et al. Experimental and clinical experience with urine amylase monitoring for early diagnosis of rejection in pancreas transplantation. Transplantation 1987; 43: 71–79. [DOI] [PubMed] [Google Scholar]
- 53.Sutherland DER, Baumgartner D, Najarian JS. Free intraperitoneal drainage of segmental pancreas grafts: clinical and experimental observations on technical aspects. Transplant Proc 1980; 12: 26–32. [PubMed] [Google Scholar]
- 54.Sutherland DER, Goetz FC, Moudry KC, et al. Use of recipient mesenteric vessels for revascularization of segmental pancreas grafts: technical and metabolic considerations. Transplant Proc 1987; 19: 2300–2304. [PubMed] [Google Scholar]
- 55.Sutherland DER, Ascher NL. Whole pancreas donation from a cadaver. In: Simmons RL, Finch ME, Ascher NL, Najarian JS, eds. Manual of vascular access, organ donation, and transplantation. New York: Springer-Verlag; 1984: 144–152.
- 56.Sutherland DER, Moudry KC, Najarian JS. Pancreas transplantation. In: Cerilli J, ed. Organ transplantation and replacement. Philadelphia: Lippincott; 1987: 535–574.
- 57.Florack G, Sutherland DER, Heise JW, et al. Successful preservation of human pancreas grafts for 28 hours. Transplant Proc 1987; 19: 3882–3885. [PubMed] [Google Scholar]
- 58.Abouna GM, Sutherland DER, Florack G, et al. Function of transplanted human pancreatic allografts after preservation in cold storage for 6 to 26 hours. Transplantation 1987; 43: 630–636. [DOI] [PubMed] [Google Scholar]
- 59.Sutherland DER, Casanova D, Sibley RK. Role of pancreas graft biopsies in the diagnosis and treatment of rejection after pancreas transplantation. Transplant Proc 1987; 19: 2329–2331. [PubMed] [Google Scholar]
- 60.Sibley RK, Sutherland DER. Pancreas transplantation: an immunohistologic and histopathologic examination of 100 grafts. Am J Pathol 1987; 128: 151–170. [PMC free article] [PubMed] [Google Scholar]
- 61.Sutherland DER. Cyclosporine vs. azathioprine in pancreas transplantation: a comparison. Transplant Immunol 1985; 2: 1–3. [Google Scholar]
- 62.Sutherland DER, Goetz FC, Najarian JS. Improved pancreas graft survival by use of multiple drug combination immunotherapy. Transplant Proc 1986; 18: 1770–1773. [Google Scholar]
- 63.Sutherland DER, Sibley RK, Xu XZ, et al. Twin-to-twin pancreas transplantation: reversal and reenactment of the pathogenesis of type I diabetes. Trans Assoc Am Physicians 1984; 97: 80–87. [PubMed] [Google Scholar]
- 64.Sibley RK, Sutherland DER, Goetz FC, et al. Recurrent diabetes mellitus in the pancreas iso- and allograft: a light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest 1985; 53: 132–144. [PubMed] [Google Scholar]
- 65.Eisenbarth GS. Type I diabetes mellitus: a chronic autoimmune disease. N Engl J Med 1986; 314: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 66.Sutherland DER, Sibley RK. Recurrence of disease in pancreas transplants. In: van Schilfgaarde R, Hardy MA, eds. Transplantation of the endocrine pancreas in diabetes mellitus. Amsterdam: Elsevier Science Publishers; 1988: 60–66.
- 67.Sutherland DER, Najarian JS, Greenberg BZ, et al. Hormonal and metabolic effects of a pancreatic endocrine graft: vascularized segmental transplantation in insulin-dependent diabetic patients. Ann Intern Med 1981; 95: 537–541. [DOI] [PubMed] [Google Scholar]
- 68.Ramsay RC, Goetz FC, Sutherland DER, et al. Progression of diabetic retinopathy after pancreas transplantation for insulin-dependent diabetes mellitus. N Engl J Med 1988; 318: 208–214. [DOI] [PubMed] [Google Scholar]
- 69.Van der Vliet JA, Navarro X, Kennedy WR, et al. The effect of pancreas transplantation on diabetic polyneuropathy. Transplantation 1988; 45: 368–370. [DOI] [PubMed] [Google Scholar]
- 70.Mauer SM, Steffes MW, Ellis EN, et al. Structural-functional relationships in diabetic nephropathy. J Clin Invest 1984; 74: 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutherland DER. Effect of pancreas transplants on secondary complications of diabetes: review of observations of a single institution. Transplant Proc 1992; 24: 859–860. [PubMed] [Google Scholar]
- 72.Dubernard JM, Traeger J. Pancreas and islet transpantation workshop. Transplant Proc 1980; 12: 1–2. [Google Scholar]
- 73.Land W, Landgraf R. Segmental pancreatic transplantation international workshop. Horm Metab Res 1983; 13: 1–104. [PubMed] [Google Scholar]
- 74.Land W, Landgraf R. The world experience in clinical pancreas transplantation. Transplant Proc 1987; 19 (suppl): 1–2. [PubMed] [Google Scholar]
- 75.Sutherland DER, Goetz FC, Najarian JS. Pancreas transplantation at the University of Minnesota: donor and recipient selection, operative and postoperative management, and outcome. Transplant Proc 1987; 19 (suppl 4): 63–74. [PubMed] [Google Scholar]
- 76.Sutherland DER, Kendall DM, Moudry KC, et al. Pancreas transplantation in nonuremic, type I diabetic recipients. Surgery 1988; 104: 453–464. [PubMed] [Google Scholar]
- 77.Sutherland DER, Moudry KC, Dunn DL, et al. Pancreas-transplant outcome in relation to presence or absence of end-stage renal disease, timing of transplant, surgical technique, and donor source. Diabetes 1989; 38 (suppl 1): 10–12. [DOI] [PubMed] [Google Scholar]
- 78.Frey DJ, Matas AJ, Gillingham KJ, et al. Sequential therapy: a prospective randomized trial of Minnesota antilymphocyte globulin versus OKT3 for prophylactic immunosuppression in cadaver renal allograft recipients. Transplantation 1992; 54: 50–56. [DOI] [PubMed] [Google Scholar]
- 79.Wilson LG. The crime of saving lives. The FDA, John Najarian, and Minnesota ALG. Arch Surg 1995; 130: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 80.Sutherland DER. Immunosuppression for clinical pancreas transplantation. Clin Transplant 1991; 5 (special issue): 549–553. [Google Scholar]
- 81.Morel P, Brayman KL, Goetz FC, et al. Long-term metabolic function of pancreas transplants and influence of rejection episodes. Transplantation 1991; 51: 990–1000. [DOI] [PubMed] [Google Scholar]
- 82.Barrou B, Barrou Z, Gruessner A, et al. Probability of retaining endocrine function (insulin-independence) after definitive loss of exocrine function in bladder-drained pancreas transplants. Transplant Proc 1994; 26: 473–474. [PubMed] [Google Scholar]
- 83.Gruessner RWG, Sutherland DER, Troppmann C, et al. The surgical risk of pancreas transplantation in the cyclosporine era: an overview. J Am Coll Surg 1997; 185: 128–144. [DOI] [PubMed] [Google Scholar]
- 84.Morel P, Schlumpf RB, Dunn DL, et al. Pancreas retransplants compared with primary transplants. Transplantation 1991; 51: 825–833. [DOI] [PubMed] [Google Scholar]
- 85.Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1995; 60: 225–232. [DOI] [PubMed] [Google Scholar]
- 86.Sutherland DER, Gruessner RWG, Moudry-Munns KC, et al. Pancreas transplants from living-related donors. Transplant Proc 1994; 26: 443–445. [PubMed] [Google Scholar]
- 87.Morel P, Gillingham KJ, Schlumpf RB, et al. Effect of simultaneous liver retrieval, retrieval team, and preservation time on cadaver whole-organ, bladder-drained pancreatic allograft survival rates. Transplant Proc 1991; 23: 1640–1642. [PubMed] [Google Scholar]
- 88.Gruessner RWG, Sutherland DER. Simultaneous kidney and segmental pancreas transplants from living related donors: the first two successful cases. Transplantation 1996; 61: 1265–1268. [DOI] [PubMed] [Google Scholar]
- 89.Gruessner RWG, Dunn DL, Tzardis PJ, et al. An immunological comparison of pancreas transplants alone in nonuremic patients versus simultaneous pancreas/kidney transplants in uremic diabetic patients. Transplant Proc 1990; 22: 1581–1581. [PubMed] [Google Scholar]
- 90.Gruessner RWG, Manivel DC, Dunn DL, et al. Pancreaticoduodenal transplantation with enteric drainage following native total pancreatectomy for chronic pancreatitis: a case report. Pancreas 1991; 6: 479–488. [DOI] [PubMed] [Google Scholar]
- 91.Gruessner RWG, Dunn DL, Tzardis PJ, et al. Simultaneous pancreas and kidney transplants versus single kidney transplants and previous kidney transplants in uremic patients and single pancreas transplants in nonuremic diabetic patients: comparison of rejection, morbidity, and long-term outcome. Transplant Proc 1990; 22: 622–623. [PubMed] [Google Scholar]
- 92.Gruessner RWG, Nakhleh RE, Tzardis P, et al. Rejection patterns after simultaneous pancreaticoduodenal-kidney transplants in pigs. Transplantation 1994; 57: 756–760. [PubMed] [Google Scholar]
- 93.Sutherland DER, Gruessner RWG, Moudry-Munns KC, et al. Discordant graft loss from rejection of organs from the same donor in simultaneous pancreas-kidney recipients. Transplant Proc 1995; 27: 907–908. [PubMed] [Google Scholar]
- 94.Gruessner RWG, Nakhleh RE, Tzardis P, et al. Differences in rejection grading after simultaneous pancreas and kidney transplantation in pigs. Transplantation 1993; 56: 1357–1364. [DOI] [PubMed] [Google Scholar]
- 95.Jones JW, Nakhleh RE, Casanova D, et al. Cystoscopic transduodenal pancreas transplant biopsy: a new needle. Transplant Proc 1994; 26: 527–528. [PubMed] [Google Scholar]
- 96.Nakhleh RE, Sutherland DER, Benedetti E, et al. Diagnostic utility and correlation of duodenal and pancreas biopsy tissue in pancreaticoduodenal transplants with emphasis on therapeutic use. Transplant Proc 1995; 27: 1327–1328. [PubMed] [Google Scholar]
- 97.Benedetti E, Najarian JS, Gruessner A, et al. Correlation between cystoscopic biopsy results and hypoamylasuria in bladder-drained pancreas transplants. Surgery 1995; 118: 864–872. [DOI] [PubMed] [Google Scholar]
- 98.Burke GW, Gruessner RWG, Dunn DL, et al. Conversion of whole pancreaticoduodenal transplants from bladder to enteric drainage for metabolic acidosis or dysuria. Transplant Proc 1990; 22: 651–652. [PubMed] [Google Scholar]
- 99.Stephanian E, Gruessner RWG, Brayman KL, et al. Conversion of exocrine secretions from bladder to enteric drainage in recipients of whole pancreaticoduodenal transplants. Ann Surg 1992; 216: 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gruessner RWG, Stephanian E, Dunn DL, et al. Cystoenteric conversion after whole pancreaticoduodenal transplantation: indications, risk factors, and outcome. Transplant Proc 1993; 25: 1179–1181. [PubMed] [Google Scholar]
- 101.Pescovitz MD, Dunn DL, Sutherland DER. Use of the circular stapler in construction of the duodenoneocystostomy for drainage into the bladder in transplants involving the whole pancreas. Surg Gynecol Obstet 1989; 169: 169–171. [PubMed] [Google Scholar]
- 102.Marsh CL, Perkins JD, Sutherland DER, et al. Combined hepatic and pancreaticoduodenal procurement for transplantation. Surg Gynecol Obstet 1989; 168: 254–258. [PubMed] [Google Scholar]
- 103.Dunn DL, Schlumpf RB, Gruessner RWG, et al. Maximal use of liver and pancreas from cadaveric organ donors. Transplant Proc 1990; 22: 423–424. [PubMed] [Google Scholar]
- 104.Sutherland DER, Morel P, Gruessner RWG. Transplantation of two diabetic patients with one divided cadaver donor pancreas. Transplant Proc 1990; 22: 585–585. [PubMed] [Google Scholar]
- 105.Morel P, Moudry-Munns KC, Balakumar M, et al. Influence of preservation time on the early function of pancreas transplants. Transplant Proc 1990; 22: 527–528. [PubMed] [Google Scholar]
- 106.Morel P, Moudry-Munns KC, Najarian JS, et al. Influence of preservation time on outcome and metabolic function of bladder-drained pancreas transplants. Transplantation 1990; 49: 294–303. [DOI] [PubMed] [Google Scholar]
- 107.Gores PF, Gillingham KJ, Dunn DL, et al. Donor hyperglycemia as a minor risk factor and immunologic variables as major risk factors for pancreas allograft loss in multivariate analysis of a single institution’s experience. Ann Surg 1992; 215: 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gruessner RWG, Dunn DL, Gruessner A, et al. Recipient risk factors have an impact on technical failure and patient and graft survival rates in bladder-drained pancreas transplants. Transplantation 1994; 57: 1598–1606. [PubMed] [Google Scholar]
- 109.Manske CL, Wang Y, Rector T. Coronary revascularization in insulin-dependent diabetic patients with chronic renal failure. Lancet 1992; 340: 998. [DOI] [PubMed] [Google Scholar]
- 110.Sutherland DER, Goetz FC, Sibley RK. Recurrence of disease in pancreas transplants. Diabetes 1989; 38: 85–87. [DOI] [PubMed] [Google Scholar]
- 111.Diem P, Abid M, Redmon JB, et al. Systemic venous drainage of pancreas allografts as independent cause of hyperinsulinemia in type I diabetic recipients. Diabetes 1990; 39: 534–540. [DOI] [PubMed] [Google Scholar]
- 112.Morel P, Goetz FC, Moudry-Munns KC, et al. Serial glycosylated hemoglobin levels in diabetic recipients of pancreatic transplants. Transplant Proc 1990; 22: 649–650. [PubMed] [Google Scholar]
- 113.Diem P, Redmon JB, Abid M, et al. Glucagon, catecholamine, pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J Clin Invest 1990; 86: 2008–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morel P, Goetz FC, Moudry-Munns KC, et al. Long-term glucose control in patients with pancreatic transplants. Ann Intern Med 1991; 115: 694–699. [DOI] [PubMed] [Google Scholar]
- 115.Robertson RP, Kendall DM, Sutherland DER. Long-term metabolic control with pancreatic transplantation. Transplant Proc 1994; 26: 386–387. [PubMed] [Google Scholar]
- 116.Kennedy WR, Navarro X, Goetz FC, et al. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 117.Navarro X, Kennedy WR, Loewensen RB, et al. Influence of pancreas transplantation on cardiorespiratory reflexes, nerve conduction, and mortality in diabetes Mellitus. Diabetes 1990; 39: 802–806. [DOI] [PubMed] [Google Scholar]
- 118.Bilous RW, Mauer SM, Sutherland DER, et al. The effects of pancreas transplantation on the glomerular structure of renal allografts in patients with insulin-dependent diabetes. N Engl J Med 1989; 321: 80–85. [DOI] [PubMed] [Google Scholar]
- 119.Gruessner RWG, Sutherland DER, Drangstveit MB, et al. Use of FK506 in pancreas transplantation. Transplant Intl 1996; 9: 251–258. [Google Scholar]
- 120.Gruessner RWG, Sutherland DER, Drangstveit MB, et al. Mycophenolate mofetil in pancreas transplantation. Transplantation 1998; 66: 318–323. [DOI] [PubMed] [Google Scholar]
- 121.Gruessner RWG, Sutherland DER, Drangstveit MB, et al. Mycophenolate mofetil and tacrolimus for induction and maintenance therapy after pancreas transplantation. Transplant Proc 1998; 30: 518–520. [DOI] [PubMed] [Google Scholar]
- 122.Humar A, Kandaswamy R, Granger DK, et al. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 2000; 231: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laftavi MR, Gruessner A, Bland BJ, et al. Diagnosis of pancreas rejection. Transplantation 1998; 65: 528–532. [DOI] [PubMed] [Google Scholar]
- 124.Laftavi MR, Gruessner A, Bland BJ, et al. Significance of pancreas graft biopsy in detection of rejection. Transplant Proc 1998; 30: 642–644. [DOI] [PubMed] [Google Scholar]
- 125.Robertson RP, Sutherland DER, Kendall DM, et al. Characterization of long-term successful pancreas transplants in type I diabetes. J Invest Med 1996; 44: 549–555. [PubMed] [Google Scholar]
- 126.Robertson RP, Sutherland DER, Lanz KJ. Normoglycemia and preserved insulin secretory reserve in diabetic patients 10–18 years after pancreas transplantation. Diabetes 1999; 48: 1737–1740. [DOI] [PubMed] [Google Scholar]
- 127.Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45: 773–780. [DOI] [PubMed] [Google Scholar]
- 128.Fioretto P, Steffes MW, Mihatsch MJ, et al. Cyclosporine-associated lesions in native kidneys of diabetic pancreas transplant recipients. Kidney Int 1995; 48: 489–495. [DOI] [PubMed] [Google Scholar]
- 129.Navarro X, Sutherland DER, Kennedy WR. Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol 1997; 42: 727–736. [DOI] [PubMed] [Google Scholar]
- 130.Navarro X, Kennedy WR, Aeppli D, et al. Neuropathy and mortality in diabetes: influence of pancreas transplantation. Muscle Nerve 1996; 19: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 131.Fioretto P, Steffes MW, Sutherland DER, et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339: 69–75. [DOI] [PubMed] [Google Scholar]
- 132.Sutherland DER, Gruessner RWG, Najarian JS, et al. Solitary pancreas transplants: a new era. Transplant Proc 1998; 30: 280–281. [DOI] [PubMed] [Google Scholar]
- 133.Schulz T, Martin D, Heimes M, et al. Tacrolimus/mycophenolate mofetil/steroid-based immunosuppression after pancreas-kidney transplantation with single-shot antithymocyte globulin. Transplant Proc 1998; 30: 1533–1535. [DOI] [PubMed] [Google Scholar]
- 134.Brayman KL, Sutherland DER, Najarian JS. Pancreas transplantation. In: Trede M, Carter DC, eds. Pancreatic surgery. London: Churchill Livingstone; 1993: 593–619.
- 135.Bendel-Stenzel MR, Kashtan CE, Sutherland DER, et al. Simultaneous pancreas-kidney transplant in two children with hemolytic-uremic symptom. Pediatr Nephrol 1997; 11: 485–487. [DOI] [PubMed] [Google Scholar]
- 136.LaPorte RE, Matsushima M, Chang YF. Prevalence and incidence of insulin-dependent diabetes. In: Harris MI, ed. Diabetes in America. Washington DC: NIH; 1995: 37–46.
- 137.Papalois BE, Moss A, Gillingham KJ, et al. Preemptive transplants for patients in renal failure. Transplantation 2000; 70: 625–631. [DOI] [PubMed] [Google Scholar]
- 138.Sutherland DER, Najarian JS, Gruessner RWG. Living versus cadaver donor pancreas transplants. Transplant Proc 1998; 30: 2264–2266. [DOI] [PubMed] [Google Scholar]
- 139.Kendall DM, Sutherland DER, Najarian JS, et al. Effects of hemipancreatectomy on insulin secretion and glucose tolerance in healthy humans. N Engl J Med 1990; 322: 898–903. [DOI] [PubMed] [Google Scholar]
- 140.Seaquist ER, Kahn SE, Clark PM, et al. Hyperproinsulinemia is associated with increased beta cell demand after hemipancreatectomy in humans. J Clin Invest 1996; 97: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.West M, Gruessner A, Metrakos P, et al. Conversion from bladder to enteric drainage after pancreaticoduodenal transplantations. Surgery 1998; 124: 883–893. [PubMed] [Google Scholar]
- 142.Matas AJ, Payne WD, Sutherland DE, et al. 2,500 living donor kidney transplants: a single-center experience. Ann Surg (in press). [DOI] [PMC free article] [PubMed]
- 143.Wang YL, Stevens RB, Fioretto P, et al. Correlation of preoperative renal function and identification of risk factors for eventual native renal failure in cyclosporine-treated nonuremic diabetic recipient of pancreas transplants alone. Transplant Proc 1993; 25: 1291–1292. [PubMed] [Google Scholar]
- 144.Zehrer CL, Gross CR. Quality of life of pancreas transplant recipients. Diabetologia 1991; 34 (suppl 1): S145–149. [DOI] [PubMed] [Google Scholar]
- 145.Gross CR, Zehrer CL. Health-related quality of life outcomes of pancreas transplant recipients. Clin Transplant 1992; 6: 165–171. [PubMed] [Google Scholar]
- 146.Gross CR, Zehrer CL. Impact of the addition of a pancreas to quality of life in uremic diabetic recipients of kidney transplants. Transplant Proc 1993; 25: 1293–1295. [PubMed] [Google Scholar]
- 147.Zehrer CL, Gross CR. Pevalence of “low blood glucose” symptoms and quality of life in pancreas transplant recipients. Clin Transplant 1993; 7: 312–319. [Google Scholar]
- 148.Zehrer CL, Gross CR. Patient perceptions of benefits and concerns following pancreas transplantation. Diabetes Educator 1994; 20: 216–220. [DOI] [PubMed] [Google Scholar]
- 149.Gross CR, Kangas JR, Lemieux AM, et al. One-year change in quality-of-life profiles in patients receiving pancreas and kidney transplants. Transplant Proc 1995; 27: 3067–3068. [PubMed] [Google Scholar]
- 150.Gross CR, Limwattananon C, Matthees BJ. Quality of life after pancreas transplantation: a review. Clin Transplant 1998; 12: 351–361. [PubMed] [Google Scholar]
- 151.Morel P, Chau C, Brayman KL, et al. Quality of metabolic control 2 to 12 years after a pancreas transplant. Transplant Proc 1992; 24: 835–838. [PubMed] [Google Scholar]
- 152.Robertson P. Pancreatic and islet transplantation for diabetes: cures or curiosities? N Engl J Med 1992; 327: 1861–1868. [DOI] [PubMed] [Google Scholar]
- 153.Katz H, Homan M, Velosa J, et al. Effects of pancreas transplantation on postprandial glucose metabolism. N Engl J Med 1991; 325: 1278–1283. [DOI] [PubMed] [Google Scholar]
- 154.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pancreatic alpha and beta cell function in healthy human donors. J Clin Invest 1992; 89: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Robertson RP. Pancreatic and islet transplantation for diabetes–cures or curiosities? N Engl J Med 1992; 327: 1861–1868. [DOI] [PubMed] [Google Scholar]
- 156.Barrou B, Seaquist ER, Robertson P. Pancreas transplantation in diabetic humans normalizes hepatic glucose production during hypoglycemia. Diabetes 1994; 43: 661–666. [DOI] [PubMed] [Google Scholar]
- 157.Kendall DM, Rooney DP, Smets YF, et al. Pancreas transplantation restores epinephrine response and symptom recognition during hypoglycemia in patients with long-standing type I diabetes and autonomic neuropathy. Diabetes 2000; 46: 249–257. [DOI] [PubMed] [Google Scholar]
- 158.Teuscher AU, Seaquist ER, Robertson P. Diminished insulin secretory reserve in diabetic pancreas transplant and nondiabetic kidney transplant recipients. Diabetes 1994; 43: 593–598. [DOI] [PubMed] [Google Scholar]
- 159.Pyzdrowski KL, Kendall DM, Halter JB, et al. Preserved insulin secretion and insulin independence in recipients of islet autografts. N Engl J Med 1992; 327: 220–226. [DOI] [PubMed] [Google Scholar]
- 160.Wahoff DC, Papalois BE, Najarian JS, et al. Autologous islet transplantation to prevent diabetes after pancreatic resection. Ann Surg 1995; 22: 562–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kendall DM, Teuscher AU, Robertson P. Defective glucagon secretion during sustained hypoglycemia following successful islet allo- and autotransplantation in humans. Diabetes 1997; 46: 23–27. [DOI] [PubMed] [Google Scholar]
- 162.Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46: 28–33. [DOI] [PubMed] [Google Scholar]
- 163.Teuscher AU, Kendall DM, Smets YF, et al. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes 1998; 47: 324–330. [DOI] [PubMed] [Google Scholar]
- 164.Sutherland DER. Effect of pancreas transplantation on secondary complications of diabetes. In: Dubernard JM, Sutherland DER. eds. International handbook of pancreas transplantation. Boston: Kluwer Academic Publishers; 1989: 257–289.
- 165.DCCT Research Group. Diabetes Control and Complications Trial (DCCT): the effect of intensive diabetes treatment in long-term complications in IDDM. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 166.Solders G, Tydén G, Persson A, et al. Improvement of nerve conduction in diabetic neuropathy: a follow-up study 4 years after combined pancreatic and renal transplantation. Diabetes 1992; 41: 946–951. [DOI] [PubMed] [Google Scholar]
- 167.Wilczek H, Jaremko G, Tydén G, et al. A pancreatic graft protects a simultaneously transplanted kidney from developing diabetic nephropathy: a 1–6-year follow-up study. Transplant Proc 1993; 25: 1314–1315. [PubMed] [Google Scholar]
- 168.Mauer SM, Steffes MW, Connett J, et al. The development of lesions in the glomerular basement membrane and mesangium after transplantation of normal kidneys to diabetic patients. Diabetes 1983; 32: 948–952. [DOI] [PubMed] [Google Scholar]
- 169.Morel P, Sutherland DER, Almond PS, et al. Assessment of renal function in type I diabetic patients after kidney, pancreas or combined kidney-pancreas transplantation. Transplantation 1991; 51: 1184–1189. [DOI] [PubMed] [Google Scholar]
- 170.Mauer SM, Barbosa J, Vernier RL, et al. Development of diabetic vascular lesions in normal kidneys transplanted into patients with diabetes mellitus. N Engl J Med 1976; 295: 916–920. [DOI] [PubMed] [Google Scholar]
- 171.Mauer SM, Goetz FC, McHugh LE, et al. Long-term study of normal kidneys transplanted into patients with type I diabetes. Diabetes 1989; 38: 516–523. [DOI] [PubMed] [Google Scholar]
- 172.Najarian JS, Kaufman DB, Fryd DS, et al. Long-term survival following kidney transplantation in 100 type I diabetic patients. Transplantation 1989; 47: 106–113. [DOI] [PubMed] [Google Scholar]
- 173.Fioretto P, Mauer SM, Bilous RW, et al. Effects of pancreas transplantation on glomerular structure in insulin-dependent diabetic patients with their own kidneys. Lancet 1993; 342: 1193–1196. [DOI] [PubMed] [Google Scholar]
- 174.Troppmann C, Gruessner RWG, Matas AJ, et al. Results with renal transplants performed after previous solitary pancreas transplants. Transplant Proc 1994; 26: 448–449. [PubMed] [Google Scholar]
- 175.DCCT Research Group. Diabetes Control and Complications Trial (DCCT): update. Diabetes Care 1990; 13: 427–433. [DOI] [PubMed] [Google Scholar]
- 176.DCCT Research Group. Lifetime benefits and costs of intensive therapy as practiced in the Diabetes Control and Complications Trial. JAMA 1997; 277: 372. [PubMed] [Google Scholar]
- 177.Cheung AT, Perez RV, Chen PC. Improvements in diabetic microangiopathy after successful simultaneous pancreas-kidney transplantation: a computer-assisted intravital microscopy study on the conjunctival microcirculation. Transplantation 1999; 68: 927–932. [DOI] [PubMed] [Google Scholar]
- 178.Abendroth D, Landgraf R, Pfeiffer M, et al. Diabetic microangiopathy and blood viscosity changes after pancreas and kidney transplantation. Transplant Proc 1994; 26: 491–492. [PubMed] [Google Scholar]
- 179.Chow VC, Pai RP, Chapman JR, et al. Diabetic retinopathy after combined kidney-pancreas transplantation. Clin Transplant 1999; 13: 356–362. [DOI] [PubMed] [Google Scholar]
- 180.Allen RD, Al Harbi IS, Morris JG, et al. Diabetic neuropathy after pancreas transplantation: determinants of recovery. Transplantation 1997; 63: 830–838. [DOI] [PubMed] [Google Scholar]
- 181.Hering BJ, Ricordi C. Islet transplantation for patients with type I diabetes. Graft 1999; 2: 12–27. [Google Scholar]
- 182.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343: 230–238. [DOI] [PubMed] [Google Scholar]
- 183.Corry RJ, Chakrabarti PK, Shapiro R, et al. Simultaneous administration of adjuvant donor bone marrow in pancreas transplant recipients. Ann Surg 1999; 230: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stratta RJ, Gaber AO, Shokouh-Amiri MH, et al. Evolution in pancreas transplantation techniques: simultaneous kidney-pancreas translantation using portal-enteric drainage without antilymphocyte induction. Ann Surg 2000; 229: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tyden G, Bolinder J, Solders G, et al. Improved survival in patients with insulin-dependent diabetes mellitus and end-stage diabetic nephropathy 10 years after combined pancreas and kidney transplantation. Transplantation 1999; 67: 645–648. [DOI] [PubMed] [Google Scholar]
- 186.Sollinger HW, Odorico JS, Knechtle SJ, et al. Experience with 500 simultaneous pancreas-kidney transplants. Ann Surg 1998; 228: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Henry ML, Elkhammas EA, Bumgardner GL, et al. Outcome of 300 consecutive pancreas-kidney transplants. Transplant Proc 1998; 30: 291. [DOI] [PubMed] [Google Scholar]
- 188.Bartlett ST, Schweitzer EJ, Johnson LB, et al. Equivalent success of simultaneous pancreas kidney and solitary pancreas transplantation. A prospective trial of tacrolimus immunosuppression with percutaneous biopsy. Ann Surg 1996; 224: 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Di Landro D, Koenigsrainer A, Oefner D, et al. Experience with 100 combined pancreatic renal transplantations in a single center. Nephron 1996; 72: 547–551. [DOI] [PubMed] [Google Scholar]
- 190.Rayhill SC, D’Alessandro AM, Odorico JS, et al. Simultaneous pancreas-kidney transplantation and living related donor renal transplantation in patients with diabetes: is there a difference in survival? Ann Surg 2000; 231: 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Becker BN, Pintar TJ, Becker Y. Simultaneous pancreas kidney transplantation reduces excess mortality in type I diabetes patients with ESRD. Kidney Int 2000; 57: 2129–2135. [DOI] [PubMed] [Google Scholar]
- 192.Smets YF, Westendorp RG, van der Pijl JW, et al. Effect of simultaneous pancreas-kidney transplantation on mortality of patients with type 1 diabetes mellitus and end-stage renal failure. Lancet 1999; 353: 1915–1919. [DOI] [PubMed] [Google Scholar]
- 193.Stratta RJ, Weide LG, Sindhi R, et al. Solitary pancreas transplantation. Experience with 62 consecutive cases. Diabetes Care 1997; 20: 362–368. [DOI] [PubMed] [Google Scholar]
- 194.Benedetti E, Dunn T, Massad MG, et al. Successful living related simultaneous pancreas-kidney transplant between identical twins. Transplantation 1999; 67: 915–918. [DOI] [PubMed] [Google Scholar]
- 195.Farney AC, Cho E, Schweitzer E, et al. Simultaneous cadaver pancreas living donor kidney transplantation (SPLK): a new approach for the type 1 diabetic uremic patient. Ann Surg 2000; 232: 646–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boudreaux JP, Corry RJ, Dickerman R, et al. Combined experience with immediate pancreas retransplantation. Transplant Proc 1991; 23: 1628–1629. [PubMed] [Google Scholar]
- 197.Fernandez-Cruz L, Gilabert R, Sabater L, et al. Pancreas graft thrombosis: prompt diagnosis and immediate thrombectomy or retransplantation. Clin Transplant 1993; 7: 230. [Google Scholar]
- 198.Sansalone CV, Aseni P, Follini ML, et al. Early pancreas retransplantation for vascular thrombosis in simultaneous pancreas-kidney transplants. Transplant Proc 1998; 30: 253–254. [DOI] [PubMed] [Google Scholar]
- 199.Stern RC, Mayes JT, Weber FL Jr, et al. Restoration of exocrine pancreatic function following pancreas-liver-kidney transplantation in a cystic fibrosis patient. Clin Transplant 1994; 8: 1–4. [PubMed] [Google Scholar]
- 200.Kuo PC, Johnson LB, Schweitzer EJ, et al. Simultaneous pancreas/kidney transplantation–a comparison of enteric and bladder drainage of exocrine pancreatic secretions. Transplantation 1997; 63: 238–243. [DOI] [PubMed] [Google Scholar]
- 201.Pirsch JD, Odorico JS, D’Alessandro AM, et al. Posttransplant infection in enteric versus bladder-drained simultaneous pancreas-kidney transplant recipients. Transplantation 1998; 66: 1746–1750. [DOI] [PubMed] [Google Scholar]
- 202.Feitosa Tajra LC, Dawhara M, Benchaib M, et al. Effect of the surgical technique on long-term outcome of pancreas transplantation. Transplant Int 1998; 11: 295–300. [DOI] [PubMed] [Google Scholar]
- 203.Sugitani A, Gritsch HA, Shapiro R, et al. Surgical complications in 123 consecutive pancreas transplant recipients: comparison of bladder and enteric drainage. Transplant Proc 1998; 30: 293–294. [DOI] [PubMed] [Google Scholar]
- 204.Calne RY. Paratopic segmental pancreas grafting: a technique with portal venous drainage. Lancet 1984; 1: 595–597. [DOI] [PubMed] [Google Scholar]
- 205.Gil-Vernet JM, Fernandez-Cruz L, Caralps A, et al. Whole organ and pancreaticoureterostomy in clinical pancreas transplantation. Transplant Proc 1985; 17: 2019–2022. [PubMed] [Google Scholar]
- 206.Tyden G, Lundgren G, Ostman J, et al. Grafted pancreas with portal venous drainage. Lancet 1984; 1: 964. [PubMed] [Google Scholar]
- 207.Rosenlof LK, Earnhardt RC, Pruett TL, et al. Pancreas transplantation: an initial experience with systemic and portal drainage of pancreatic allografts. Ann Surg 1992; 215: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bruce DS, Newell KA, Woodle ES, et al. Synchronous pancreas-kidney transplantation with portal venous and enteric exocrine drainage: outcome in 70 consecutive cases. Transplant Proc 1998; 30: 270–271. [DOI] [PubMed] [Google Scholar]
- 209.Reddy KS, Stratta RJ, Shokouh-Amiri MH, et al. Surgical complications after pancreas transplantation with portal-enteric drainage. J Am Coll Surg 1999; 189: 305–313. [DOI] [PubMed] [Google Scholar]
- 210.Tyden G, Tibell A, Sandberg J, et al. Improved results with a simplified technique for pancreaticoduodenal transplantation with enteric exocrine drainage. Clin Transplant 1996; 10: 306–309. [PubMed] [Google Scholar]
- 211.Kaplan B, Wang Z, Abecassis MM, et al. Frequency of hyperkalemia in recipients of simultaneous pancreas and kidney transplants with bladder drainage. Transplantation 1996; 62: 1174–1175. [DOI] [PubMed] [Google Scholar]
- 212.Sindhi R, Stratta RJ, Lowell JA, et al. Experience with enteric conversion after pancreatic transplantation with bladder drainage. J Am Coll Surg 1997; 184: 281–289. [PubMed] [Google Scholar]
- 213.Ploeg RJ, Eckhoff DE, D’Alessandro AM, et al. Urological complications and enteric conversion after pancreas transplantation with bladder drainage. Transplant Proc 1994; 26: 458–459. [PubMed] [Google Scholar]
- 214.Troppmann C, Gruessner A, Benedetti E, et al. Vascular graft thrombosis after pancreatic transplantation: univariate and multivariate operative and nonoperative risk factor analysis. J Am Coll Surg 1996; 182: 285–316. [PubMed] [Google Scholar]
- 215.Sollinger H. Pancreatic transplantation and vascular graft thrombosis [editorial]. J Am Coll Surg 1996; 182: 362. [PubMed] [Google Scholar]
- 216.Benedetti E, Gruessner AC, Troppmann C, et al. Intra-abdominal fungal infections after pancreatic transplantation: incidence, treatment, and outcome. J Am Coll Surg 1996; 183: 307–316. [PubMed] [Google Scholar]
- 217.Benedetti E, Troppmann C, Gruessner AC, et al. Pancreas graft loss caused by intra-abdominal infection. A risk factor for a subsequent pancreas retransplantation. Arch Surg 1996; 131: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 218.Dunn DL, Gillingham KJ, Kramer MA, et al. A prospective randomized study of acyclovir versus ganciclovir plus human immune globulin prophylaxis of cytomegalovirus infection after solid organ transplantation. Transplantation 1994; 57: 876–884. [DOI] [PubMed] [Google Scholar]
- 219.Somerville T, Hurst G, Alloway R, et al. Superior efficacy of oral ganciclovir over oral acyclovir for cytomegalovirus prophylaxis in kidney-pancreas and pancreas alone recipients. Transplant Proc 1998; 30: 1546–1548. [DOI] [PubMed] [Google Scholar]
- 220.Hassoun A, Humar A, Kandaswamy R, et al. The low incidence of PTLD after pancreas and simultaneous kidney-pancreas transplant. Transplantation 1999; 67: S208. [Google Scholar]
- 221.Drachenberg CB, Abruzzo LV, Klassen DK, et al. Epstein-Barr virus-related posttransplantation lymphoproliferative disorder involving pancreas allografts: histological differential diagnosis from acute allograft rejection. Hum Pathol 1998; 29: 569–577. [DOI] [PubMed] [Google Scholar]
- 222.Keay S, Oldach D, Wiland A, et al. Posttransplantation lymphoproliferative disorder associated with OKT3 and decreased antiviral prophylaxis in pancreas transplant recipients. Clin Infect Dis 1998; 26: 596–600. [DOI] [PubMed] [Google Scholar]
- 223.Darenkov IA, Marcarelli MA, Basadonna GP, et al. Reduced incidence of Epstein-Barr virus-associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation 1997; 64: 848–852. [DOI] [PubMed] [Google Scholar]
- 224.Martinenghi S, Dell’Antonio G, Secchi A, et al. Cancer arising after pancreas and/or kidney transplantation in a series of 99 diabetic patients. Diabetes Care 1997; 20: 272–275. [DOI] [PubMed] [Google Scholar]
- 225.Sasaki TM, Pirsch JD, D’Alessandro AM, et al. Increased beta 2-microglobulin (B2M) is useful in the detection of post-transplant lymphoproliferative disease. Clin Transplant 1997; 11: 29–33. [PubMed] [Google Scholar]
- 226.McAlister VC, Gao Z, Peltekian K, et al. Sirolimus–tacrolimus combination immunosuppression [letter]. Lancet 2000; 355: 376–377. [DOI] [PubMed] [Google Scholar]
- 227.Kaufman DB, Leventhal JR, Stuart J, et al. Mycophenolate mofetil and tacrolimus as primary maintenance immunosuppression in simultaneous pancreas-kidney transplantation: initial experience in 50 consecutive cases. Transplantation 1999; 67: 586–593. [DOI] [PubMed] [Google Scholar]
- 228.Jordan ML, Shapiro R, Gritsch HA, et al. Long-term results of pancreas transplantation under tacrolimus immunosuppression. Transplantation 1999; 67: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Odorico JS, Pirsch JD, Knechtle SJ, et al. A study comparing mycophenolate mofetil to azathioprine in simultaneous pancreas-kidney transplantation. Transplantation 1998; 66: 1751–1759. [DOI] [PubMed] [Google Scholar]
- 230.Peddi VR, Kamath S, Munda R, et al. Use of tacrolimus eliminates acute rejection as a major complication following simultaneous kidney and pancreas transplantation. Clin Transplant 1998; 12: 401–405. [PubMed] [Google Scholar]
- 231.Stratta RJ. Sequential pancreas after kidney transplantation: is anti-lymphocyte induction therapy needed? Transplant Proc 1998; 30: 1549–1551. [DOI] [PubMed] [Google Scholar]
- 232.Burke GW, Ciancio G, Alejandro R, et al. Use of tacrolimus and mycophenolate mofetil for pancreas-kidney transplantation with or without OKT3 induction. Transplant Proc 1998; 30: 1544–1545. [DOI] [PubMed] [Google Scholar]
- 233.Elkhammas EA, Yilmaz S, Henry ML, et al. Simultaneous pancreas/kidney transplantation: comparison of mycophenolate mofetil versus azathioprine. Transplant Proc 1998; 30: 512. [DOI] [PubMed] [Google Scholar]
- 234.Peddi VR, Kamath S, Schroeder TJ, et al. Efficacy of OKT3 as primary therapy for histologically confirmed acute renal allograft rejection in simultaneous kidney and pancreas transplant recipients. Transplant Proc 1998; 30: 285–287. [DOI] [PubMed] [Google Scholar]
- 235.Tesi RJ, Henry ML, Elkhammas EA, et al. The frequency of rejection episodes after combined kidney-pancreas transplant–the impact on graft survival. Transplantation 1994; 58: 424–430. [DOI] [PubMed] [Google Scholar]
- 236.Sugitani A, Egidi MF, Gritsch HA, et al. Serum lipase as a marker for pancreatic allograft rejection. Clin Transplant 1998; 12: 224–227. [PubMed] [Google Scholar]
- 237.Allen RD, Wilson TG, Grierson JM, et al. Percutaneous biopsy of bladder-drained pancreas transplants. Transplantation 1991; 51: 1213–1216. [DOI] [PubMed] [Google Scholar]
- 238.Gaber AO, Gaber L, Shokouh-Amiri MH, et al. Percutaneous biopsy of pancreas transplants. Transplantation 1992; 54: 548–550. [DOI] [PubMed] [Google Scholar]
- 239.Drachenberg CB, Klassen DK, Weir MR, et al. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation 1999; 68: 396–402. [DOI] [PubMed] [Google Scholar]
- 240.Papadimitriou JC, Drachenberg CB, Wiland A, et al. Histologic grading of acute allograft rejection in pancreas needle biopsy: correlation to serum enzymes, glycemia, and response to immunosuppressive treatment. Transplantation 1998; 66: 1741–1745. [DOI] [PubMed] [Google Scholar]
- 241.Drachenberg CB, Papadimitriou JC, Klassen DK, et al. Evaluation of pancreas transplant needle biopsy: reproducibility and revision of histologic grading system. Transplantation 1997; 63: 1579–1586. [DOI] [PubMed] [Google Scholar]
- 242.Egidi MF, Corry RJ, Sugitani A, et al. Enteric-drained pancreas transplants monitored by fine-needle aspiration biopsy. Transplant Proc 1997; 29: 674–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gill IS, Stratta RJ, Taylor RJ, et al. Correlation of serologic and urinary tests with allograft biopsy in the diagnosis of pancreas rejection. Transplant Proc 1997; 29: 673. [DOI] [PubMed] [Google Scholar]
- 244.Klassen DK, Hoen-Saric EW, Weir MR, et al. Isolated pancreas rejection in combined kidney–pancreas tranplantation. Transplantation 1996; 61: 974–977. [DOI] [PubMed] [Google Scholar]
- 245.Benedetti E, Gruessner A, et al. Nakhleh rejection episode: Cystoscopic biopsies in pancreaticoduodenal transplantation. Are duodenal biopsies indicative of pancreas dysfunction? Transplantation 1995; 60: 541–546. [DOI] [PubMed] [Google Scholar]
- 246.Tyden G, Reinholt FP, Sundkvist G, et al. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts [published erratum appears in N Engl J Med 1996 Dec 5;335(23): 1778]. N Engl J Med 1996; 335: 860–863. [DOI] [PubMed] [Google Scholar]
- 247.Drachenberg CB, Papadimitriou JC, Weir MR, et al. Histologic findings in islets of whole pancreas allografts: lack of evidence for recurrent cell-mediated diabetes mellitus. Transplantation 1996; 62: 1770–1772. [DOI] [PubMed] [Google Scholar]
- 248.Stratta RJ. Patterns of graft loss following simultaneous kidney-pancreas transplantation. Transplant Proc 1998; 30: 288. [DOI] [PubMed] [Google Scholar]
- 249.Hawthorne WJ, Allen RD, Greenberg ML, et al. Simultaneous pancreas and kidney transplant rejection: separate or synchronous events? Transplantation 1997; 63: 352–358. [DOI] [PubMed] [Google Scholar]
- 250.So SKS, Moudry-Munns KC, Gillingham KJ, et al. Short-term and long-term effects of HLA matching in cadaveric pancreas transplantation. Transplant Proc 1991; 23: 1634–1636. [PubMed] [Google Scholar]
- 251.Basadonna GP, Auersvald LA, Oliveira SC, et al. Pancreas after kidney transplantation: HLA mismatch does not preclude success. Transplant Proc 1997; 29: 667. [DOI] [PubMed] [Google Scholar]
- 252.Stratta RJ, Lowell JA, Sudan D, et al. Retransplantation in the diabetic patient with a pancreas allograft. Am J Surg 1997; 174: 759–762. [DOI] [PubMed] [Google Scholar]
- 253.Douzdjian V, Rice JC, Carson RW, et al. Renal allograft failure after simultaneous pancreas-kidney transplantation: univariate and multivariate analyses of donor and recipient risk factors. Clin Transplant 1996; 10: 271–277. [PubMed] [Google Scholar]
- 254.Douzdjian V, Gugliuzza KG, Fish JC. Multivariate analysis of donor risk factors for pancreas allograft failure after simultaneous pancreas-kidney transplantation. Surgery 1995; 118: 73–81. [DOI] [PubMed] [Google Scholar]
- 255.Bumgardner GL, Henry ML, Elkhammas E, et al. Obesity as a risk factor after combined pancreas/kidney transplantation. Transplantation 1995; 60: 1426–1430. [DOI] [PubMed] [Google Scholar]
- 256.Douzdjian V, Thacker LR, Blanton JW. Effect of race on outcome following kidney and kidney-pancreas transplantation in type I diabetics: the Southeastern Organ Procurement Foundation experience. Clin Transplant 1997; 11: 470–475. [PubMed] [Google Scholar]
- 257.Stratta RJ, Taylor RJ, Lowell JA, et al. Preemptive combined pancreas-kidney transplantation: is earlier better? Transplant Proc 1994; 26: 422–424. [PubMed] [Google Scholar]
- 258.Mellinghoff AC, Reininger AJ, Land W, et al. Cardiovascular risk profile after simultaneous pancreas and kidney transplantation. Exp Clin Endocrinol Diabetes 1998; 106: 460–464. [DOI] [PubMed] [Google Scholar]
- 259.Schweitzer EJ, Anderson L, Kuo PC, et al. Safe pancreas transplantation in patients with coronary artery disease. Transplantation 1997; 63: 1294–1299. [DOI] [PubMed] [Google Scholar]
- 260.Kim SI, Elkhammas EA, Henry ML, et al. Outcome of combined kidney/pancreas transplantation in recipients with coronary artery disease. Transplant Proc 1995; 27: 3071. [PubMed] [Google Scholar]
- 261.Sasaki TM, Gray RS, Ratner RE, et al. Successful long-term kidney-pancreas transplants in diabetic patients with high C-peptide levels. Transplantation 1999; 67: 586A. [DOI] [PubMed] [Google Scholar]
- 262.Van der Werf WJ, Odorico J, D’Alessandro AM, et al. Utilization of pediatric donors for pancreas transplantation. Transplant Proc 1999; 31: 610–611. [DOI] [PubMed] [Google Scholar]
- 263.Kapur S, Bonham CA, Dodson SF, et al. Strategies to expand the donor pool for pancreas transplantation. Transplantation 1999; 67: 284–290. [DOI] [PubMed] [Google Scholar]
- 264.Odorico JS, Heisey DM, Voss BJ, et al. Donor factors affecting outcome after pancreas transplantation. Transplant Proc 1998; 30: 276–277. [DOI] [PubMed] [Google Scholar]
- 265.Stratta RJ. Donor age, organ import, and cold ischemia: effect on early outcomes after simultaneous kidney-pancreas transplantation. Transplant Proc 1997; 29: 3291–3292. [DOI] [PubMed] [Google Scholar]
- 266.Cosio FG, Elkhammas EA, Henry ML, et al. Function and survival of renal allografts from the same donor transplanted into kidney-only or kidney-pancreas recipients. Transplantation 1998; 65: 93–99. [DOI] [PubMed] [Google Scholar]
- 267.Troppmann C, Gruessner AC, Papalois BE, et al. Delayed endocrine pancreas graft function after simultaneous pancreas-kidney transplantation. Incidence, risk factors, and impact on long- term outcome. Transplantation 1996; 61: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 268.Secchi A, Taglietti MV, Socci C, et al. Insulin secretory patterns and blood glucose homeostasis after islet allotransplantation in IDDM patients: comparison with segmental- or whole-pancreas transplanted patients through a long-term longitudinal study. J Mol Med 1999; 77: 133–139. [DOI] [PubMed] [Google Scholar]
- 269.Nankivell BJ, Chapman JR, Bovington KJ, et al. Glucose homeostasis standards for pancreas transplantation. Clin Transplant 1998; 12: 434–438. [PubMed] [Google Scholar]
- 270.La Rocca E, Gobbi C, Ciurlino D, et al. Improvement of glucose/insulin metabolism reduces hypertension in insulin-dependent diabetes mellitus recipients of kidney-pancreas transplantation. Transplantation 1998; 65: 390–393. [DOI] [PubMed] [Google Scholar]
- 271.Tibell A, Tyden G, Bolinder J, et al. Metabolic control in recipients of segmental pancreatic grafts functioning beyond 8 years. Transplant Proc 1995; 27: 3029–3030. [PubMed] [Google Scholar]
- 272.Christiansen E, Tibell A, Volund A, et al. Insulin secretion, insulin action and non-insulin-dependent glucose uptake in pancreas transplant recipients. J Clin Endocrinol Metab 1994; 79: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 273.Osei K, Henry M, Ferguson R. Differential effects of systemic insulin delivery and prednisone on tissue glucose and insulin sensitivity in insulin-dependent diabetes mellitus pancreas recipients. Transplant Proc 1994; 26: 480–482. [PubMed] [Google Scholar]
- 274.Konigsrainer A, Foger B, Steurer W, et al. Influence of hyperinsulinemia on lipoproteins after pancreas transplantation with systemic insulin drainage. Transplant Proc 1998; 30: 637–638. [DOI] [PubMed] [Google Scholar]
- 275.Foger B, Konigsrainer A, Palos G, et al. Effects of pancreas transplantation on distribution and composition of plasma lipoproteins. Metabolism 1996; 45: 856–861. [DOI] [PubMed] [Google Scholar]
- 276.Foger B, Konigsrainer A, Palos G, et al. Effect of pancreas transplantation on lipoprotein lipase, postprandial lipemia, and HDL cholesterol. Transplantation 1994; 58: 899–904. [DOI] [PubMed] [Google Scholar]
- 277.Wang Q, Klein R, Moss SE, et al. The influence of combined kidney-pancreas transplantation on the progression of diabetic retinopathy. A case series. Ophthalmology 1994; 101: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 278.Laftavi MR, Chapuis F, Vial C, et al. Diabetic polyneuropathy outcome after successful pancreas transplantation: 1- to 9-year follow-up. Transplant Proc 1995; 27: 1406–1409. [PubMed] [Google Scholar]
- 279.Gaber AO, el Gebely S, Sugathan P, et al. Changes in cardiac function of type I diabetics following pancreas-kidney and kidney-alone transplantation. Transplant Proc 1995; 27: 1322–1323. [PubMed] [Google Scholar]
- 280.Martin X. Improvement of diabetic vesicopathy after pancreatic transplantation. Transplant Proc 1995; 27: 2441–2443. [PubMed] [Google Scholar]
- 281.Gaber AO, Hathaway DK, Abell T, et al. Improved autonomic and gastric function in pancreas-kidney vs. kidney-alone transplantation contributes to quality of life. Transplant Proc 1994; 26: 515–516. [PubMed] [Google Scholar]
- 282.Secchi A, Martinenghi S, Castoldi R, et al. Effects of pancreas transplantation on quality of life in type I diabetic patients undergoing kidney transplantation. Transplant Proc 1998; 30: 339–342. [DOI] [PubMed] [Google Scholar]
- 283.Piehlmeier W, Bullinger M, Kirchberger I, et al. Evaluation of the quality of life of patients with insulin-dependent diabetes mellitus before and after organ transplantation with the SF 36 health survey. Eur J Surg 1996; 162: 933–940. [PubMed] [Google Scholar]
- 284.Nakache R, Tyden G, Groth CG. Long-term quality of life in diabetic patients after combined pancreas-kidney transplantation or kidney transplantation. Transplant Proc 1994; 26: 510–511. [PubMed] [Google Scholar]
- 285.Adang EM, Engel GL, van Hooff JP, et al. Comparison before and after transplantation of pancreas-kidney and pancreas-kidney with loss of pancreas–a prospective controlled quality of life study. Transplantation 1996; 62: 754–758. [DOI] [PubMed] [Google Scholar]
- 286.Esmatjes E, Ricart MJ, Fernandez-Cruz L, et al. Quality of life after successful pancreas-kidney transplantation. Clin Transplant 1994; 8: 75–78. [PubMed] [Google Scholar]
- 287.Nathan DM, Fogel H, Norman D, et al. Long-term metabolic and quality of life results with pancreatic/renal transplantation in insulin-dependent diabetes mellitus. Transplantation 1991; 52: 85–91. [DOI] [PubMed] [Google Scholar]
- 288.Stratta RJ. The economics of pancreas transplantation. Graft 2000; 3: 19. [Google Scholar]
- 289.Gruessner A, Troppmann C, Sutherland DER, et al. Donor and recipient risk factors significantly affect cost of pancreas transplants. Transplant Proc 1997; 29: 656–657. [DOI] [PubMed] [Google Scholar]
- 290.Douzdjian V, Escobar F, Kupin WL, et al. Cost-utility analysis of living-donor kidney transplantation followed by pancreas transplantation versus simultaneous pancreas-kidney transplantation. Clin Transplant 1999; 13: 51–58. [DOI] [PubMed] [Google Scholar]
- 291.Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31: 794–802. [DOI] [PubMed] [Google Scholar]
- 292.Stratta R, Bennett L. Pancreas underutilization according to United Network for Organ Sharing. Transplant Proc 1998; 30: 264. [DOI] [PubMed] [Google Scholar]
- 293.Stratta RJ, Cushing KA, Frisbie K, et al. Analysis of hospital charges after simultaneous pancreas-kidney transplantation in the era of managed care. Transplantation 1997; 64: 287–292. [DOI] [PubMed] [Google Scholar]
- 294.American Diabetes Association. Pancreas transplantation for patients with type 1 diabetes. Diabetes Care 2000; 23: S85. [DOI] [PubMed] [Google Scholar]
- 295.Ramiya VK, Maraist M, Arfors KE, et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 2000; 6: 278–282. [DOI] [PubMed] [Google Scholar]
- 296.Vinik A, Rafaeloff R, Pittenger G, et al. Induction of pancreatic islet neogenesis. Horm Metab Res 1997; 29: 278–293. [DOI] [PubMed] [Google Scholar]
- 297.Smith JA, Bluestone JA. T cell inactivation and cytokine deviation promoted by anti-CD3 mAbs. Curr Opin Immunol 1997; 9: 648–654. [DOI] [PubMed] [Google Scholar]
- 298.Giannoukakis N, Rudert WA, Robbins PD, et al. Targeting autoimmune diabetes with gene therapy. Diabetes 1999; 48: 2107–2121. [DOI] [PubMed] [Google Scholar]