Abstract
Objective
To report the authors’ experience with adult living donor liver transplantation (ALDLT) without venovenous bypass and to describe modifications that will allow for a direct duct-to-duct biliary reconstruction.
Summary Background Data
Adult living donor liver transplantation is being evaluated as a method to alleviate the organ shortage. Descriptions of the procedure have emphasized the use of venovenous bypass, portocaval decompression, and the mandatory use of a Roux-en-Y biliary enteric anastomosis. The authors describe a technique for ALDLT without venovenous bypass, portocaval decompression, or caval clamping in 11 recipients and describe the modifications to the procedure that may allow a duct-to-duct biliary reconstruction in certain cases.
Methods
Between March 1999 and March 2000, 11 ALDLTs were performed at the authors’ institution. All procedures were performed without venovenous bypass, portocaval decompression, or caval clamping. After a modification to the procedure, five of the last six recipients underwent biliary reconstruction with a direct duct-to-duct anastomosis. Data regarding donor, recipient, and graft survival, complications, and graft function were collected.
Results
Recipients comprised five women and six men, mean age 48 years. Donors comprised five women and six men, mean age 36.5 years. Donor to recipient relationships included sibling, spouse, son, and daughter. Indications for transplantation were hepatitis C, hepatitis C with hepatocellular carcinoma, primary biliary cirrhosis, primary sclerosing cholangitis, ethanol, and cryptogenic. No case required venovenous bypass or portocaval shunting. The right hepatic vein of the donor graft was anastomosed to the confluence of the left and middle hepatic veins in all cases. All donors are alive and well, with no adverse complications reported. Recipient and graft survival rates were 91% and 82%, respectively, for ALDLT versus 92% and 92% for recipients of cadaveric organs during the same time period. One recipient died of multiple organ failure and sepsis. Biliary reconstruction was performed by Roux-en-Y hepaticojejunostomy in the six cases. In five of the last six recipients, direct duct-to-duct biliary reconstruction with a T tube was used. No anastomotic leaks or strictures occurred in the patients undergoing duct-to-duct reconstruction.
Conclusions
Adult living donor liver transplantation can be performed safely and may help alleviate the organ shortage. Neither venovenous bypass nor portocaval shunting is necessary to perform the procedure, and modifications to both the donor and recipient hepatectomy procedures may allow biliary reconstruction to be performed by a direct duct-to-duct anastomosis in selected cases.
The concept of living donor liver transplantation was initially reported in the pediatric population and has achieved remarkable success. 1–3 The development of living donor liver transplantation, in the pediatric population, arose from the scarcity of appropriate-size organs for small children, many of whom died while awaiting transplantation. During the past decade, living donor liver transplantation has become an established therapeutic option for small children requiring transplantation for end-stage liver disease. 4,5 The excellent results achieved by living donor liver transplantation in children are due to several factors. First, patients receive their transplant on an elective basis when they are not receiving inpatient treatment for hepatic decompensation. Second, the cold ischemic time imparted to the graft is minimal. Third, the liver is procured from a healthy, hemodynamically stable donor. The reduced cold ischemic time and the quality of the donor contribute to the almost complete absence of primary nonfunction in the transplanted organ. Finally, there is a theoretical immunologic advantage to receiving a living related organ from a haploidentical sibling or parent. 6 Countries in which the absence of a brain-death law precludes the option of cadaveric organ donation have, in recent years, explored the feasibility of adult living donor liver transplantation (ALDLT). Several reports from these countries have confirmed the safety and effectiveness of this procedure. 7–10
With more than 14,000 adults currently awaiting liver transplantation in the United States, numerous strategies to increase organ availability are being studied. These include the use of marginal donors, split-liver transplantation, and non–heart-beating donors. Although these efforts increase the efficiency of organ utilization and should be encouraged, they do not significantly affect the overall waiting list death rate, which currently exceeds 20%. 11
With the recent introduction of ALDLT in the United States, there is significant interest in the technical aspects of the procedure. Previous reports describing the technique in adults have emphasized the need for venovenous bypass, portocaval decompression, and Roux-en-Y biliary enteric anastomosis. 12 Based on an extensive experience with the procedure in experimental animals, we introduced several technical modifications that have been used in our clinical program. We describe our initial experience with procuring and implanting a right lobe from a living adult donor to an adult recipient with neither venovenous bypass nor portocaval decompression. In addition, we report our experience of five cases of direct duct-to-duct anastomosis for biliary drainage.
METHODS
Donor Evaluation
All procedures, including informed consent, were conducted in accordance with the ethical standards of the Committee on Human Experimentation and the Institutional Review Board at the University of Tennessee, Memphis. All potential donors are evaluated in three phases. Phase 1 comprises a comprehensive history, physical examination, and laboratory profile, which includes viral serology and blood type. In phase 2, potential donors are counseled by a nontransplant physician, acting as a donor advocate, who ensures donor commitment, motivation, and understanding of the risks involved. In addition, the donor advocate attempts to detect the presence of coercion. A social worker and financial advisor also attempt to help the donor with social or financial issues that may arise during the process of donation. Finally, in phase 3 the donor undergoes volumetric magnetic resonance imaging, magnetic resonance angiography, magnetic resonance cholangiography (Figs. 1 and 2), and arteriography to determine whether there are any anatomical restrictions for donation.
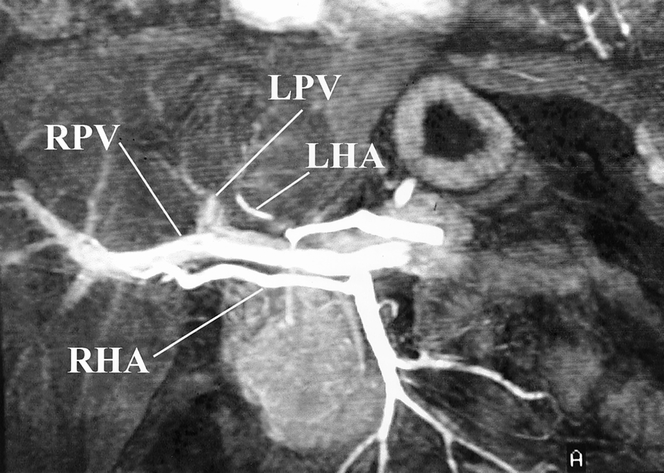
Figure 1. Magnetic resonance angiogram showing the relevant hepatic vasculature in a potential right lobe donor. This patient represents an ideal situation for right lobe donation. A replaced right hepatic artery (RHA) is seen to arise from the superior mesenteric artery, and a single right portal vein (RPV) is present. LPV, left portal vein; LHA, left hepatic artery.
Figure 2. Magnetic resonance cholangiogram showing normal biliary anatomy with a single right hepatic duct draining the right lobe.
Surgical Technique
Donor Right Lobe Hepatectomy
The donor’s abdomen is opened through a bilateral subcostal incision with a vertical extension to the xiphoid. Once unsuspected disease that would preclude donation is excluded by laparotomy, the falciform ligament is divided, its attachment to the anterior abdominal wall is separated in a cephalad direction to a point approximately halfway to the level of the hepatic veins. At this point the sulcus between the right and middle hepatic veins is clearly defined by clearing the surrounding connective tissue. No attempt is made to divide either the left triangular ligament or the gastrohepatic ligament, because this may lead to excessive mobility of the remaining left lobe and result in torsion and outflow occlusion.
Cholangiography
After a cholecystectomy, we routinely perform an intraoperative cholangiogram to provide anatomical information regarding the level of confluence of the common hepatic duct and to detect the presence, number, and size of any aberrant segmental ducts from the right lobe that may drain into the left hepatic duct. These ducts occur in about 8% of the population 13 and may necessitate the construction of additional biliary anastomoses, depending on their size and number. The cholangiogram is performed while occluding the distal common bile duct with a bulldog clamp to prevent contrast from passing into the duodenum and thus allowing better opacification of the intrahepatic ducts. In addition, a metallic marker is placed near the right hepatic duct to serve as an anatomical guide and prevent unnecessary dissection in this area.
Hilar Dissection and Mobilization of the Right Lobe
After the cholangiogram, the right hepatic artery is exposed to the right of the common hepatic duct and subsequently mobilized proximally to the right border of the common hepatic duct and distally to the hepatic parenchyma (Fig. 3). Dissection in the region where the right hepatic artery comes into contact with the bile duct is avoided because this may result in devascularization of the donor bile duct and subsequent ischemic stricture in the donor. After mobilization of the right hepatic artery, the portal vein is identified and encircled with a vessel loop. Complete mobilization of the right portal vein is undertaken to allow the maximum length of the portal vein to be obtained, while at the same time ensuring the accurate placement of vascular clamps at the time of graft excision.
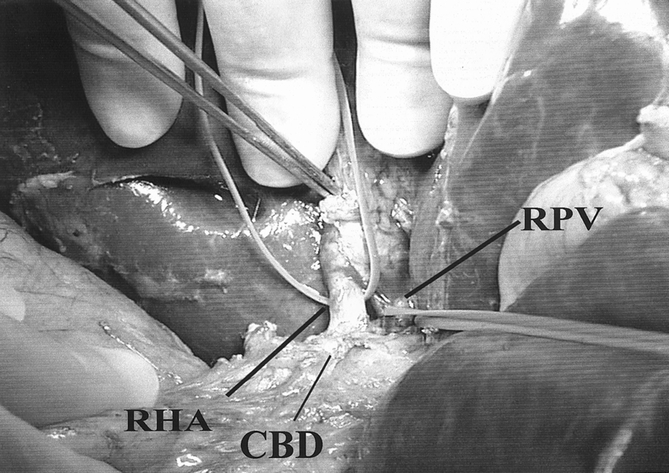
Figure 3. Dissection of the right hepatic artery (RHA) in the donor is performed to the right side of the common bile duct (CBD). Dissection is avoided between the right hepatic artery and bile duct to prevent devascularization. PV, portal vein.
The right lobe of the liver is then mobilized medially by separating its diaphragmatic attachments and taking down the right triangular and coronary ligaments, thus exposing the retrohepatic vena cava. The short hepatic veins draining the posterior aspect of the right lobe are then ligated and divided in continuity up to the level of the right hepatic vein and medially past the line of proposed transection of the right lobe (Fig. 4). The right hepatic vein is then encircled with a vessel loop. Occasionally, an accessory right hepatic vein draining the posterior aspect of the right lobe into the retrohepatic vena cava is encountered. This vessel may be contributing significantly to the venous drainage of the right lobe, and division may result in venous congestion after implantation. If such a vessel is encountered, temporary clamping followed by visual assessment of the right lobe should be performed to determine whether this vessel could be divided safely. Should venous congestion occur, two separate hepatic vein anastomoses would need to be performed to ensure adequate venous outflow of the graft.
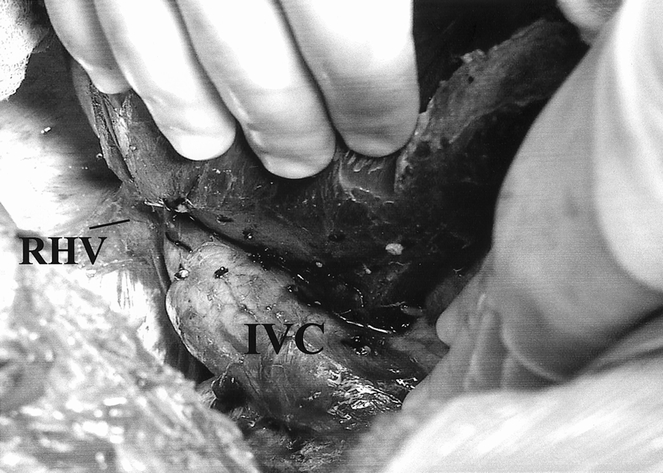
Figure 4. The donor right lobe is mobilized from the inferior vena cava (IVC). The right hepatic vein (RHV) can be seen clearly.
Parenchymal Transection
The line of parenchymal transection is determined by first occluding both the right hepatic artery and right portal vein with an atraumatic bulldog clamp for approximately 1 minute. This allows an area of demarcation to become apparent between the right and left lobes, which is marked with electrocautery. Locating the exact position of the middle hepatic vein using intraoperative ultrasound further defines this line of demarcation. Once the path of the middle hepatic vein has been traced, the line of transection is then adjusted so that it lies 1 to 2 cm to the right of the middle hepatic vein. The middle hepatic vein must remain with the left lobe, where it provides venous drainage for Couinaud segment 4 (medial segment of the left lobe). Failure to preserve the middle hepatic vein may result in venous congestion of Couinaud segment 4 and subsequent liver dysfunction in the donor.
There are many methods for performing the parenchymal transection. Important features of this part of the procedure include the ability to transect the parenchyma without vascular occlusion to either lobe, which may result in ischemia and organ dysfunction for both the donor and recipient. However, it is important to minimize blood loss when transection is performed without vascular occlusion. We prefer to use the Cavitron Ultrasonic Aspirator (CUSA; Valley Lab, Boulder, CO) to expose the blood vessels within the hepatic parenchyma. Smaller vessels are then coagulated using the Harmonic scalpel (Ethicon, Cincinnati, OH), and vessels larger than 4 mm are ligated and divided in continuity. An argon beam coagulator (Conmed, Utica, NY) provides additional hemostasis to the surface of the cut edge. Using this combination of instruments, the parenchymal transection is expedited and is usually performed in 1 to 2 hours with minimal blood loss. Before completion of the parenchymal transection, the right hepatic duct is again identified and sharply divided with scissors flush with the liver parenchyma to avoid encroachment of the confluence and left hepatic duct. The graft side of the right hepatic duct is marked with a stitch to allow easier identification of this structure should it retract into the liver substance. The stump remaining with the donor is closed with a 5-0 prolene suture.
Removal of the Graft and Back Table Preparation
After the parenchyma and the bile duct are divided, the right lobe of the donor is attached by only the right hepatic vein, portal vein, and hepatic artery. At this point the right hepatic artery, right portal vein, and right hepatic vein are clamped and divided, allowing removal of the right lobe graft from the donor. The partially divided falciform ligament is then reattached to the anterior abdominal wall to minimize the possibility of rotation of the donor’s left lobe.
The donor right lobe graft is then taken to the back table, where both the hepatic artery and portal vein are each flushed with 500 mL heparinized University of Wisconsin (UW) solution. The right hepatic duct is flushed with a syringe containing UW solution, and the graft is weighed and its volume measured by water displacement. Finally, the graft is packed in sterilely chilled UW solution until implantation.
Recipient Hepatectomy and Implantation of Right Lobe
The recipient hepatectomy, performed by a separate surgical team, is begun shortly after the start of the donor procedure. The recipient hepatectomy is performed using the piggyback technique, in which the liver is removed while preserving the recipient’s retrohepatic vena cava. 14,15 Neither venovenous bypass nor portocaval decompression is used in performing the recipient procedure.
In view of the relatively short hepatic artery and portal vein provided by the donor organ, it is important that both the right and left hepatic arteries and portal veins of the recipient are dissected into the hepatic parenchyma to preserve an adequate length for subsequent vascular reconstruction. Recently, modifications aimed at preserving the blood supply to the bile duct from the right hepatic artery have allowed the creation of a direct duct-to-duct biliary anastomosis in five patients. To maintain the blood supply from the right hepatic artery to the bile duct, dissection between the right hepatic artery and the bile duct is avoided. This can be achieved by dividing the right hepatic artery and bile duct close to the hepatic parenchyma and mobilizing both of these structures proximally as a single unit. In addition, the gastroduodenal artery is left intact to maintain the proximal blood supply to the bile duct.
Once the recipient’s liver is fully mobilized, the right hepatic vein is encircled with a vessel loop. Vascular clamps are then placed on the main portal vein, the right hepatic vein, and the confluence of the left and middle hepatic veins. The inferior vena cava is not clamped, and thus venovenous bypass is not required. The liver is then removed, preserving as much length of the hepatic veins as possible. The donor right lobe is then removed from the preservation fluid, flushed with 1 L chilled lactated Ringer’s solution, and placed in the recipient’s body to determine the most appropriate site of anastomosis for the venous outflow of the graft. The donor right hepatic vein can be anastomosed end to end either to the right hepatic vein or to the confluence of the left and middle hepatic veins of the recipient. The judgment as to which orifice to use is vital to the success of the procedure because failure to provide adequate unobstructed venous outflow from the graft leads to graft congestion and poor function. Because of its larger size and anatomical lie, we have routinely used the confluence of the left and middle hepatic veins for venous outflow. The anastomosis is performed using an intimal eversion technique, with an emphasis on shortening any redundancies between the donor and recipient hepatic veins. This is important because an excessively long anastomosis may allow rotation of the graft and subsequent kinking of the venous outflow.
After completion of the outflow anastomosis, the sutures are left untied to allow the graft to be flushed free of any remaining UW solution using the patient’s own blood. We prefer to use the patient’s own blood to flush the graft because this obviates the need for cannulation of the donor right portal vein, a procedure that invariably results in shortening of this vein. The donor right portal vein is then anastomosed end to end to the recipient’s portal vein. Because we do not perform a temporary portocaval shunt or use venovenous bypass, we prefer to reperfuse the graft as soon as the portal vein anastomosis is complete. Reperfusion with portal blood is performed by removing the portal clamp while the caval clamp is maintained. After approximately 200 mL blood is flushed through the suprahepatic caval anastomosis, the sutures of the outflow anastomosis are tied and the outflow clamp is removed.
Arterial reconstruction is performed end to end between the graft’s right hepatic artery and either the recipient’s common or right hepatic artery using either running or interrupted 7-0 prolene with loupe magnification. Biliary reconstruction mandates the use of a Roux-en-Y hepaticojejunostomy if more than one biliary anastomosis is required, or if the blood supply to the bile duct cannot be maintained, as in patients with a replaced right hepatic artery. If a Roux limb has been used, the anastomoses are performed using interrupted 6-0 PDS sutures. These anastomoses are performed over an internal/external stent that consists of a pediatric feeding tube brought out through the Roux limb in a Witzel fashion and exiting out the right side of the recipient’s abdominal wall. If the blood supply to the bile duct can be maintained, a direct duct-to-duct anastomosis is performed with interrupted 6-0 PDS sutures. All cases of duct-to-duct anastomosis are performed with placement of a T tube. The use of a T tube in this situation is important because it allows subsequent evaluation of both the anastomosis and the cut edge of the liver for biliary leaks. After completion of the biliary anastomosis, the T tube is injected with 20 mL saline in an attempt to identify biliary leaks within the cut surface of the liver.
Postoperative Imaging
Both donors and recipients undergo magnetic resonance imaging volumetric analysis to assess baseline liver volume before discharge. In addition, hepatobiliary iminodiacetic acid (HIDA) scanning is performed routinely in all donors and recipients before discharge (Fig. 5). In recipients in whom a duct-to-duct anastomosis has been used, the HIDA scan is replaced with a T-tube cholangiogram (Fig. 6).
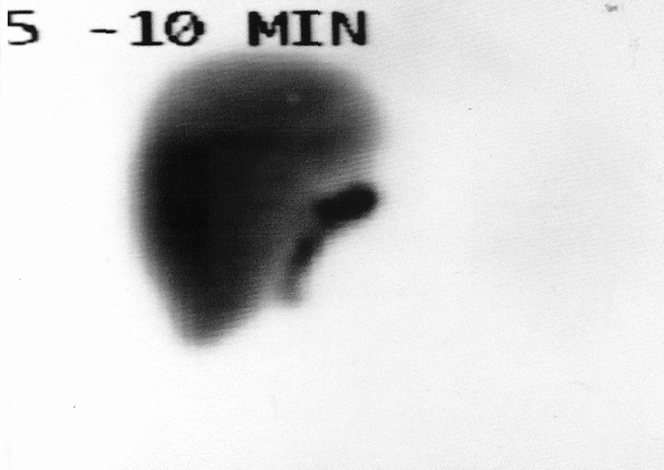
Figure 5. Postoperative T-tube cholangiogram in a recipient who received a direct duct-to-duct anastomosis for biliary reconstruction. This is made possible by preserving the blood supply to the bile duct from the right hepatic artery and gastroduodenal artery of the recipient.
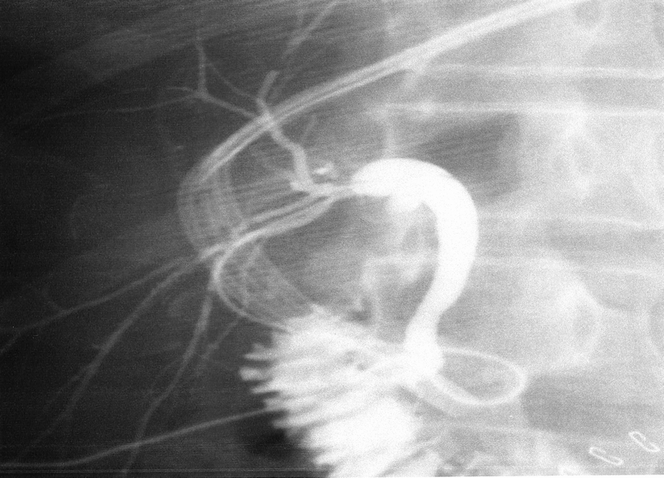
Figure 6. HIDA scan performed on postoperative day 7 in a recipient of a right lobe with a biliary enteric anastomosis. Excellent uptake and excretion are noted from the right lobe graft.
RESULTS
Between March 1999 and March 2000, 11 ALDLTs were performed at our center with a mean follow-up of 290 ± 122 days (range 90–485). During the same period, 38 cadaveric liver transplants were performed. Adult living donor recipients included six men and five women with a mean age of 48 years (range 21–61). United Network for Organ Sharing (UNOS) status at the time of transplant was IIb in five patients and III in six patients. Mean recipient weight was 71.5 ± 9.6 kg (range 56–86), and the graft to recipient body weight ratio was 1.16 ± 0.57% (range 0.64–1.8). Mean time from placement on the waiting list to the time of transplantation was 166 ± 104 days (median 88, range 24–540) in ALDLT versus 248 days (median 203, range 8–604) for patients receiving a cadaveric organ.
Mean surgical time for recipients of ALDLT was 8.8 ± 2.6 hours, and cold ischemic time averaged 89 ± 32 minutes (range 61–158). No case required venovenous bypass or portocaval shunting. Portal vein clamping averaged 50 ± 12 minutes (range 42–70). The right hepatic vein of the donor graft was anastomosed to the confluence of the left and middle hepatic veins in all cases. In no instances was it necessary to reanastomose any accessory hepatic veins, and all grafts remained soft and showed excellent venous drainage of all segments after reperfusion. Recipient and graft survival rates were 91% and 82%, respectively, for ALDLT versus 92% and 92% for recipients of cadaveric organs during the same period (P = NS). Poor initial graft function was seen in 2 of 11 grafts. Reasons for poor initial function included one episode of portal vein thrombosis that required retransplantation and a single case of primary graft nonfunction for no apparent reason. This latter case resulted in the recipient’s death from multiple organ failure and sepsis secondary to complication from a liver biopsy 1 month after transplant.
Biliary reconstruction was performed by Roux-en-Y hepaticojejunostomy in six patients. In five of the last six recipients, a direct duct-to-duct biliary reconstruction with a T tube was used. For patients undergoing a Roux-en-Y biliary reconstruction, a single biliary enteric anastomosis was required in two patients. The remaining four patients required two biliary enteric anastomoses. Biliary complications in patients undergoing a Roux-en-Y biliary reconstruction included two anastomotic leaks that required reoperation and revision of the anastomosis, two cut edge biliary leaks that required reoperation, and two patients who had reflux cholangitis that required hospital admission and antibiotic therapy. Long-term complications in this group of patients included one recipient in whom a biliary enteric stricture developed; it required percutaneous dilatation and stenting.
For patients undergoing choledochocholedochostomy, biliary reconstruction in all patients except one was performed to a single donor duct. In the remaining patient, two donor bile ducts were joined by their medial walls and anastomosed to the recipient duct as a single anastomosis. No anastomotic leaks occurred in the five patients undergoing duct-to duct reconstruction; however, two of these patients incurred minor biliary leaks from the T-tube insertion sites. Other vascular complications included one hepatic artery thrombosis secondary to an intimal dissection. This complication was recognized early, and both the patient and graft were salvaged by placement of a saphenous vein interposition graft.
All donors are alive and well, with a mean total serum bilirubin level of 0.57 mg/dL (median 0.5, range 0.3–1.0). Mean donor surgical time was 7.3 ± 0.74 hours. Mean hospital stay for donors was 8.8 days (median 9, range 7–10). The median blood loss during the donor procedure was 900 mL. All donors received cell-saver blood, and three donors required transfusion with packed red blood cells. One donor required laparoscopic removal of a drain. No adverse events were reported in any of the donors, and no donors required readmission to the hospital.
DISCUSSION
Adult living donor liver transplantation has been shown to be an effective technique for selected patients awaiting liver transplantation and has the potential of reducing the waiting list death rate by providing an organ for transplantation in a more timely fashion. In our analysis, the waiting time from listing to transplantation was 148 days for ALDLT versus 248 days for cadaveric recipients. This difference in waiting time was even more pronounced when we considered only patients who were listed after the inception of the living donor program: in this group of patients, the mean waiting time from listing to transplantation was 43 days (range 24–216).
The technique described here differs from conventional methods for graft placement that have been described for ALDLT in the literature. 8,10,12 ALDLT necessitates caval preservation in the recipient because the donor organ lacks a vena cava. However, the use of venovenous bypass would appear to be unnecessary, providing that caval clamping can be avoided, as described in this article. The avoidance of both venovenous bypass and caval clamping maintains the hemodynamic stability of the donor during the anhepatic phase and reduces the cost and complications associated with venovenous bypass. These complications include unique problems such as thromboembolic complications and mechanical injury associated with global capillary leak, leading to third-spacing of fluid after surgery. 16–18 A recent study of cadaveric transplants from our institution compared the efficacy, cost, and outcome of the piggyback technique (as described here) against those in which venovenous bypass was used. The results indicated that although there was no significant difference in outcome between the two techniques, the patients in the piggyback group had significantly shorter intensive care unit and hospital stays and incurred significantly lower hospital charges. 19
With regard to the technique used for biliary reconstruction in ALDLT, there is no doubt that the current standard remains a Roux-en-Y hepaticojejunostomy. It would appear, however, that in selected patients the application of a direct duct-to-duct anastomosis can be performed safely in recipients who would otherwise require a single biliary enteric anastomosis. This option is possible provided that the blood supply to both the recipient’s bile duct and the right hepatic duct of the donor right lobe can be maintained. The blood supply to the supraduodenal segment of the bile duct originates from the gastroduodenal artery and its retroduodenal branch, the right hepatic artery, and the cystic artery. Approximately eight small arteries supply this supraduodenal area, the most important of which run along the lateral borders of the duct at 3 and 9 o’clock. Sixty percent of these vessels course upward from the major inferior vessels and 40% run downward, originating from the right hepatic artery. The hilar ducts receive their blood supply from a rich network of blood vessels, predominantly from the right hepatic artery. 20
The ability to preserve the blood supply to the donor right hepatic duct requires sharp dissection, avoidance of electrocautery around the bile duct, and meticulous surgical technique in exposing this area during the hepatic transection. The vascular supply to the recipient’s common bile duct and hepatic duct requires preservation of the blood vessels between the right hepatic artery and the bile duct, and the gastroduodenal artery. The ability to preserve the blood supply to the recipient’s bile duct from the right hepatic artery is facilitated by dividing both structures close to the parenchyma of the liver. Both structures are then mobilized caudad as a single unit, avoiding dissection between the right hepatic artery and the bile duct in the recipient. We believe that the use of a direct duct-to-duct biliary reconstruction is beneficial because it allows both transhepatic and endoscopic access to the biliary tree for diagnostic and therapeutic instrumentation. In addition, it eliminates the possibility of ascending cholangitis, which not infrequently complicates the use of a Roux-en-Y hepaticojejunostomy.
CONCLUSIONS
Whichever technique is used for ALDLT, donor safety is of paramount importance. We believe that the experience derived from the pediatric population, technologic advancements, and improvements in surgical techniques will enable surgeons to perform this procedure safely in the adult population. Our technique demonstrates that neither venovenous bypass nor portocaval shunting is necessary to perform the procedure, and that biliary reconstruction can be performed, in selected cases, by a direct duct-to-duct anastomosis.
Footnotes
Correspondence: Dr. Hosein Shokouh-Amiri, Department of Surgery, 956 Court Ave., Suite A202, Memphis, TN 38163.
E-mail: hamiri@utmem.edu
Accepted for publication November 22, 2000.
References
- 1.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg 1998; 228: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otte JB, de Ville de Goyet J, Reding R, et al. Pediatric liver transplantation: from the full-size liver graft to reduced, split, and living related liver transplantation. Pediatr Surg Int 1998; 13: 308–318. [DOI] [PubMed] [Google Scholar]
- 3.Emond JC, Heffron TG, Kortz EO, et al. Improved results of living-related liver transplantation with routine application in a pediatric program. Transplantation 1993; 55: 835–840. [DOI] [PubMed] [Google Scholar]
- 4.Chen YS, Chen CL, Liu PP, et al. Pediatric liver transplantation from living-related donors. Transplant Proc 1998; 30: 3252–3253. [DOI] [PubMed] [Google Scholar]
- 5.de Ville de Goyet J, Reding R, Sokal E, et al. Related living donor for liver transplantation in children: results and impact. Chirurgie 1997; 122: 83–87. [PubMed] [Google Scholar]
- 6.Alonso EM, Piper JB, Echols G, et al. Allograft rejection in pediatric recipients of living related liver transplants. Hepatology 1996; 23: 40–43. [DOI] [PubMed] [Google Scholar]
- 7.Ichida T, Matsunami H, Kawasaki S, et al. Living related-donor liver transplantation from adult to adult for primary biliary cirrhosis. Ann Intern Med 1995; 122: 275–276. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki S, Makuuchi M, Matsunami H, et al. Living related liver transplantation in adults. Ann Surg 1998; 227: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg 1997; 226: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wachs ME, Bak TE, Karrer FM, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation 1998; 66: 1313–1316. [DOI] [PubMed] [Google Scholar]
- 11.Harper AM, Rosendale JD. The UNOS OPTN waiting list and donor registry. Clin Transplant 1997:61–80. [PubMed]
- 12.Marcos A, Fisher RA, Ham JM, et al. Right lobe living donor liver transplantation. Transplantation 1999; 68: 798–803. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida J, Chijiiwa K, Yamaguchi K, et al. Practical classification of the branching types of the biliary tree: an analysis of 1,094 consecutive direct cholangiograms. J Am Coll Surg 1996; 182: 37–40. [PubMed] [Google Scholar]
- 14.Lerut J, Gertsch P, Blumgart LH. “Piggy back” adult orthotopic liver transplantation. Helv Chir Acta 1989; 56: 527–530. [PubMed] [Google Scholar]
- 15.Jovine E, Mazziotti A, Grazi GL, et al. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transplant Int 1997; 10: 109–112. [DOI] [PubMed] [Google Scholar]
- 16.Ellis JE, Lichtor JL, Feinstein SB, et al. Right heart dysfunction, pulmonary embolism, and paradoxical embolization during liver transplantation. A transesophageal two- dimensional echocardiographic study. Anesth Analg 1989; 68: 777–782. [PubMed] [Google Scholar]
- 17.Khoury GF, Mann ME, Porot MJ, et al. Air embolism associated with veno-venous bypass during orthotopic liver transplantation. Anesthesiology 1987; 67: 848–851. [DOI] [PubMed] [Google Scholar]
- 18.Navalgund AA, Kang Y, Sarner JB, et al. Massive pulmonary thromboembolism during liver transplantation. Anesth Analg 1988; 67: 400–402. [PubMed] [Google Scholar]
- 19.Shokouh-Amiri MH, Gaber AO, Bagous WA, et al. Choice of surgical technique influences perioperative outcomes in liver transplantation. Ann Surg 2000; 231: 814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg 1979; 66: 379–384. [DOI] [PubMed] [Google Scholar]


