Abstract
Objective
To assess the outcomes of current treatment strategies for Budd-Chiari syndrome.
Summary Background Data
Budd-Chiari syndrome, occlusion or obstruction of hepatic venous outflow, is a disease traditionally managed by portal or mesenteric-systemic shunting. The development of other treatment options, such as catheter-directed thrombolysis, transjugular portosystemic shunting (TIPS), and liver transplantation, has expanded the therapeutic algorithm.
Methods
The authors reviewed the medical records of all patients diagnosed with Budd-Chiari syndrome at the Johns Hopkins Hospital during the past 20 years.
Results
A total of 54 patients were identified: 13 (24%) male patients and 41 (76%) female patients, ranging in age from 2 to 76 years (median 33 years). Twenty-one (39%) had polycythemia vera, 3 (5.6%) used estrogens, 11 (20%) had a myeloproliferative or coagulation disorder, and in 7 (13%) the cause remained unknown. Forty-three patients were treated with surgical shunting, 24 mesocaval and 19 mesoatrial. Actuarial survival rates at 1, 3, and 5 years after shunting were 83%, 78%, and 75%, respectively. Of 33 patients surviving more than 4 years, 28 (85%) had relief of clinical symptoms. Five patients required shunt revision and eight had radiologic procedures to maintain shunt patency. Primary and secondary shunt patency rates were 46% and 69% respectively for mesoatrial shunts and 70% and 85% respectively for mesocaval shunts. Clot lysis was successful as primary treatment in seven patients. TIPS was performed in three patients, one after a failed mesocaval shunt. During an average of 4 years of follow-up, these patients required multiple procedures to maintain TIPS patency. Six patients underwent liver transplantation. Of these, three had previous shunt procedures. Five of the transplant recipients are alive with follow-up of 2 to 9 years (median 6).
Conclusions
Both shunting and transplantation can result in a 5-year survival rate of at least 75%, and other treatment modalities may be appropriate for highly selected patients. Optimal management requires that treatment be directed by the predominant clinical symptom (liver failure or portal hypertension) and anatomical considerations and be tempered by careful assessment of surgical risk.
Budd-Chiari syndrome (BCS) results from obstruction of hepatic venous outflow. Several series of 20 to 50 patients with BCS, published during the past 10 years, have established that surgical shunting provides excellent long-term survival with relief of symptoms in most instances. 1–6 The principal authors of these publications have tended to be surgeons. This raises the question of whether the patients described in these articles represent a biased sample, with patients successfully treated by other means not included. In addition, significant improvements in other therapies, such as transjugular intrahepatic portosystemic shunting (TIPS), catheter-directed thrombolysis, and liver transplantation, have been made during the past 10 years. The place of these therapies in the overall management of BCS has not been clearly established. To answer these questions, we reviewed the records of all patients diagnosed with BCS at the Johns Hopkins Hospital during the past 20 years and quantified the effectiveness of the primary therapy selected. Our study was designed to access the utility of surgical shunting, focusing on long-term survival and relief of symptoms, and to evaluate the role of TIPS and liver transplantation in the management of BCS.
METHODS
The medical records of all patients with a discharge diagnosis of BCS or hepatic vein occlusion, as well as all patients undergoing a procedure to image the hepatic veins, were reviewed. A total of 364 patient records were examined. Of these, 54 patients were accurately diagnosed as having BCS (obstruction of hepatic venous drainage). Excluded were patients with venoocclusive disease, a proliferative disorder characterized by concentric narrowing at the level of the terminal hepatic venules or sinusoids that typically occurs after combined radiation and chemotherapy for malignancy. 7
Medical records were reviewed for patient demographics, radiology, laboratory and pathology reports, and surgical reports. Follow-up data were obtained from hospital medical records and clinic notes.
All patients undergoing surgical decompression of their splanchnic circulation were treated by either mesocaval or mesoatrial shunts. The shunts performed at Johns Hopkins Hospital were done using prosthetic material, either woven Dacron or PTFE (Meadox, Oakland, NJ), as previously described. 3,8
RESULTS
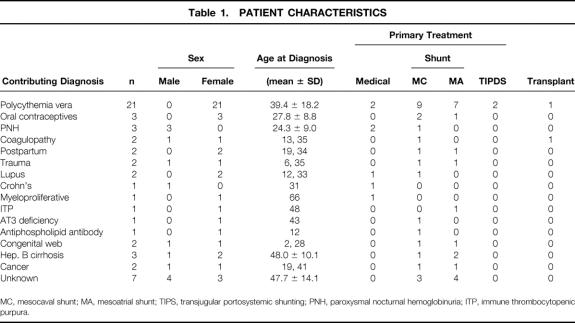
A total of 54 patients identified as having BCS were treated at the Johns Hopkins Hospital between 1976 and 1996. There were 13 (24%) male patients and 41 (76%) female patients, ranging in age from 2 to 76 years (median 33). Patient follow-up ranged from 1 to 20 years (median 8). Various causes were identified, as shown in Table 1. The most common underlying disease was polycythemia rubra vera (n = 21). Forty-six of the 54 patients (85%) had ascites as their presenting complaint. Most patients with ascites also had mild to moderate abdominal discomfort. In decreasing order of occurrence, other signs and symptoms noted on initial presentation were splenomegaly (12 patients), jaundice (10 patients), gastrointestinal bleeding (7 patients), and encephalopathy (2 patients).
Table 1. PATIENT CHARACTERISTICS
MC, mesocaval shunt; MA, mesoatrial shunt; TIPS, transjugular portosystemic shunting; PNH, paroxysmal nocturnal hemoglobinuria; ITP, immune thrombocytopenic purpura.
Primary treatment modalities were medical (no invasive therapy except for clot lysis) for 7 patients, surgical shunting for 43 patients, radiologic shunting (TIPS) for 2 patients, and orthotopic liver transplant for 2 patients. Of the seven (13%) patients treated primarily by medical therapy, one was a 66-year-old patient with a myeloproliferative disorder who developed hepatorenal syndrome, refused dialysis, and died. One patient with paroxysmal nocturnal hemoglobinuria also had progressive myotonic dystrophy and was not considered a candidate for surgical therapy. The remaining five patients, aged 12 to 29 years, were treated with an initial course of thrombolysis followed by long-term anticoagulation using warfarin. To date, these five patients have been followed up for 6 to 10 years (mean 8.2 ± 1.7), and all are alive and well, with minimal ascites well controlled with diuretics.
Surgical shunting was the most common primary treatment modality, performed in 43 patients. Nineteen patients had mesoatrial shunts and 24 had mesocaval shunts. In 40 patients, portal venous pressures were recorded before and after shunting. The mean reduction in venous pressure after shunting was 19 ± 7.7 mmHg. There was no difference in the magnitude of pressure drop with respect to shunt type (data not shown).
The overall survival rate after shunting was 77% (33/43). Of the 10 patients who died, 6 had mesoatrial and 4 had mesocaval shunts. The mean age of the patients who died was 51.6 ± 17.8 years. Six patients died within 1 month after surgery, three of liver failure and three of sepsis. Of the four patients with late deaths (1, 2, 3, and 8 years), one died of sepsis and three of progression or recurrence of the underlying disease, with varying degrees of hepatic dysfunction. Postshunt deaths are detailed in Table 2 During follow-up after surgical shunting, 21 of 33 (64%) surviving patients have remained asymptomatic. One asymptomatic patient was found to have an occluded mesoatrial shunt on routine duplex imaging, and no further therapy has been required.
Table 2. POSTSHUNT DEATHS
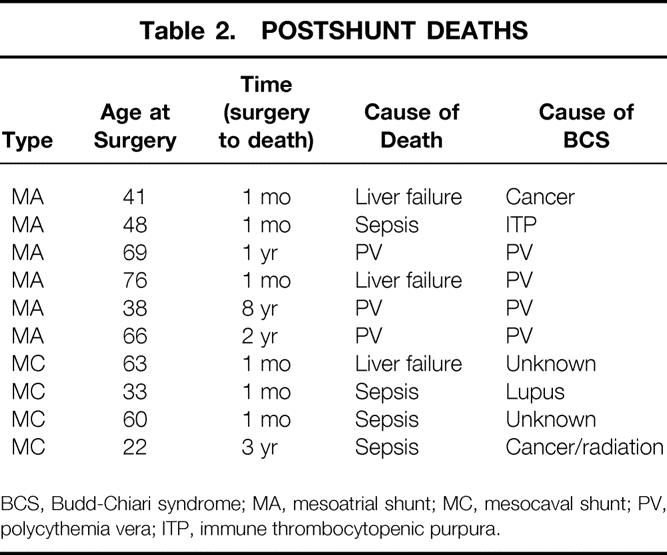
BCS, Budd-Chiari syndrome; MA, mesoatrial shunt; MC, mesocaval shunt; PV, polycythemia vera; ITP, immune thrombocytopenic purpura.
Twelve surviving patients (36%) developed symptoms suggestive of recurrent BCS. Recurrent ascites was the predominant symptom in this group, although one had upper gastrointestinal bleeding. Secondary procedures are shown in Table 3. Five patients with recurrent symptoms had surgical revision of their shunts for stenosis, three mesoatrial and two mesocaval. One mesocaval shunt was converted to a mesoatrial shunt. Three patients had failure of shunt revision and eventually required liver transplantation. Eight symptomatic patients had various interventional radiologic procedures to maintain shunt patency, including urokinase infusion, angioplasty, atherectomy, and stenting. One patient underwent TIPS. Five patients with occluded shunts that could not be revised had a LeVeen shunt placed for control of ascites. Two patients with LeVeen shunts have survived more than 4 years.
Table 3. POSTSHUNT SECONDARY PROCEDURES
TIPS, transjugular portosystemic shunting.
In two patients, TIPS was used as the primary treatment. To date, these patients have been followed up for 3 and 5 years. One, a 40-year-old woman with polycythemia, underwent liver transplantation for recurrent symptoms after TIPS, which had been revised seven times and could not be revised further. Because of the multiple secondary TIPS procedures, the metallic stent material extended proximally into the patient’s right atrium, throughout the length of the portal vein, and distally to the confluence of the superior mesenteric and splenic veins. The stent could not be removed from the atrium because scarring had narrowed the suprahepatic vena caval anastomosis, requiring stenting after the transplant. In addition, the inferior portion of the stent was incorporated within the posterior wall of the portal vein, making dissection difficult and a standard end-to-end portal vein anastomosis impossible. To revascularize the donor portal vein, a graft from the superior mesenteric vein was used. The other patient, a 75-year-old woman with polycythemia, had her initial TIPS placed in 1992. To date, she has had 18 repeat procedures to maintain stent patency.
We have performed orthotopic liver transplantation for six patients with BCS (Table 4). The indications for transplant were refractory ascites in all patients with biochemical evidence of significant hepatocellular dysfunction and histologic confirmation of severe liver injury in four of the six patients. The two patients without significant hepatocellular dysfunction had either an occluded shunt or TIPS that could not be revised, and severe, refractory ascites. One patient who underwent a transplant for a coagulopathy of undetermined cause in 1988 died after surgery of sepsis. All five liver recipients who underwent transplants since 1990 are alive and well 2, 5, 6, 8, and 9 years after the transplant. All have been maintained on warfarin therapy (INR 2.0–2.5 times control) and continued medical management of the underlying disease where indicated.
Table 4. LIVER TRANSPLANT FOR BUDD-CHIARI SYNDROME
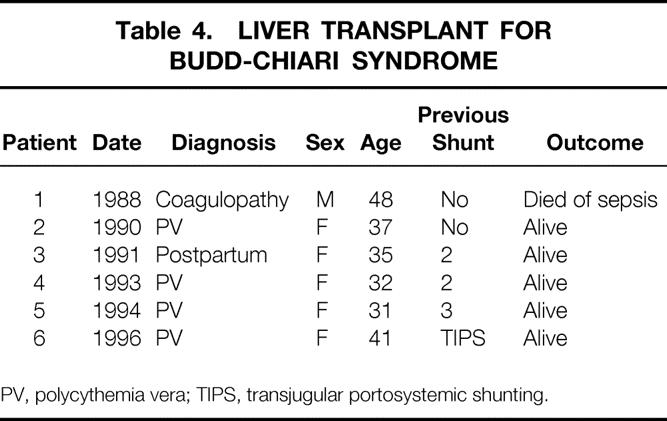
PV, polycythemia vera; TIPS, transjugular portosystemic shunting.
DISCUSSION
This series of 54 patients with BCS provides a review of the evolution of treatment strategies during the past 20 years and underlines the importance of providing appropriate secondary therapies when required for symptomatic recurrence. The primary goals of treatment are relief of symptoms and elimination of hepatic congestion and consequently of ascites. The secondary but essential goal is to prevent recurrence. The underlying diseases responsible for the development of BCS in our series are primarily hematologic, a consistent finding in patients in the Western Hemisphere. 6,9,10 Only a few cases can be cured by surgical therapy: hepatic venous obstruction caused by congenital anomalies or traumatic injury, or inborn errors of metabolism (e.g., antithrombin III deficiency) that can be reversed by liver transplantation. 11 Most patients, therefore, irrespective of the primary treatment, require life-long anticoagulation and treatment of the underlying disease.
The diagnosis of BCS should be suspected in any patient with ascites and evidence of hepatic congestion. Screening for hepatic vein occlusion can be done by duplex ultrasonography and confirmed by hepatic venography and mesenteric angiography with delayed portography, magnetic resonance angiography, or three-dimensional computed tomography may provide the same information, depending on availability and local experience. 12,13 Liver biopsy is diagnostic when characteristic centrilobular congestion is present and is useful to rule out concomitant cirrhosis. Not all three major hepatic veins are always affected equally by the thrombotic process, so a bilobar biopsy is essential. Laboratory testing to define the underlying disease, if not already known, is an important adjunct to defining the anatomical lesion responsible for hepatic venous outflow obstruction.
Once the diagnosis of BCS has been made and the cause identified, definitive treatment must be undertaken without delay. Unless the hepatic congestion is relieved, the natural history of BCS is generally progressive liver failure and death. In our series, the most common initial treatment was surgical shunting (43/54 [80%]). The few patients who had primary medical treatment fell into two categories: those who had limited life expectancies because of concomitant disease processes and declined interventional therapy, 2 and those found to have only partial hepatic vein occlusion on imaging studies. 5 In addition, these patients had an acute form of BCS, and the diagnosis was made expediently. These five patients were thus considered appropriate candidates for a trial of thrombolytic drug infusion followed by long-term anticoagulation. In this select group of patients, clot lysis was achieved, and all had relief of symptoms clinically. Although this group of patients with acute BCS was successfully managed without shunting, our experience is that this is the exception. Reports of successful nonsurgical therapy for BCS are uncommon. Case reports citing effective treatment with thrombolytic therapy are similar to our experience in that thrombolysis was instituted early in the evolution of the syndrome. 1,14–16 These patients had surgical consultation, and provisions were made for mesenteric-systemic shunting in the event that clot lysis was unsuccessful. We believe, as do most authors, that most patients with BCS require decompression of their portal venous circulation by shunting and that unnecessarily delaying such procedures increases the risk of liver failure and death.
Once the decision to shunt has been made, the appropriate procedure must be chosen. Mesocaval and mesoatrial shunts were performed in this series. Both decompress the mesenteric venous system, and the choice is based on preoperative radiographic findings. A mesoatrial shunt is used when venography shows severe narrowing or obstruction of the vena cava below the level of the hepatic veins (defined as a reduction in luminal diameter of >75%, or a pressure gradient between the right atrium and infrahepatic inferior vena cava of >15 mmHg) that is not amenable to stenting. Relief of symptoms, patient survival rates, and equivalent long-term shunt patency were demonstrated for both mesoatrial and mesocaval shunts in this series. Other authors have published similar results. 1,2,6,9,17
Reoperation can provide significant secondary patency rates, as shown in our patients. Two patients with mesocaval (10.5%) and three with mesoatrial (12.5%) shunts required surgical revision for stenosis. One mesocaval shunt was converted to a mesoatrial shunt. Eight patients had angioplasty and/or stenting of a partially occluded shunt, with restoration of flow (elimination of a pressure gradient) in all cases. The effectiveness of secondary procedures in maintaining long-term shunt patency was clear in these patients. Interventional radiology procedures are necessary for diagnosing the cause of shunt failure, and the ability to follow the diagnostic study with therapy such as thrombolysis, stenting, or angioplasty is essential. The use of atherectomy in two patients (see Table 3) allowed differentiation between intimal hyperplasia and thrombus within a stent. Although shunt patency is critical to long-term successful management of BCS, significant secondary patency can be achieved only by carefully following up these patients, clinically monitoring shunt patency by duplex scanning, measuring pressures during catheterization, and urgently performing angiography and/or stenting if occlusion or stenosis is suspected.
In this series, TIPS was used as the primary method of shunting in two patients and as a secondary shunt in one patient with a stenotic mesocaval shunt. When patent, the TIPS has provided excellent relief of symptoms and of hepatic congestion, as reported by others. 18,19 However, all three of our patients treated by TIPS placement have required secondary angioplasty and/or placement of additional stents to maintain patency. One patient, after seven revisions, developed stenosis of the TIPS that could not be corrected and required liver transplantation. As described, the ultimate configuration of the stent material significantly complicated the liver transplant. Now, 2 years after the transplant, the patient is doing well with normal liver function; however, this case illustrates the potential hazards of persisting with one therapy for too long, and the importance of having the ability to provide alternative therapy should it be required.
Overall, the survival rate after liver transplantation for BCS was 83%, with a median follow-up of 6 years (range 2–9). One patient who received a transplant in 1988 died after surgery of sepsis. The survival rate of the more recently transplanted patients reflects the state of liver transplantation overall. Liver transplantation has been reported as effective treatment of BCS by other authors, with survival rates ranging from 69% to 87%. 2,11,17,20,21
Unequivocal indications for liver transplantation as treatment of BCS include inborn errors of metabolism, such as antithrombin III deficiency, which can be cured by transplantation. 11 Selection of other patients with BCS for liver transplantation as a primary therapy is, in contrast, difficult, because characteristics predictive of a poor outcome after surgical shunting are poorly defined. In fact, hepatic function has been shown to recover after shunting even with preoperative biopsies showing fibrosis. 4 Of course, pathologic interpretation of the degree of fibrosis is subjective and not standardized, making specific recommendations problematic. However, patients whose liver biopsies show established cirrhosis or fibrosis can be expected to develop progressive hepatic decompensation (which may be rapid or gradual) after shunting and ultimately are best treated by transplantation. 9,11,21 The widening gap between the numbers of potential transplant candidates in need of liver replacement and the supply of suitable liver allografts has tempered our enthusiasm for choosing liver transplantation as the preferred therapy for patients with BCS.
The most important factor in the selection of therapy for patients with BCS is the physician’s assessment of the reversibility of hepatic damage. This is multifactorial and requires careful assessment of the duration of symptoms, hepatic synthetic function, degree of encephalopathy, and the extent of cirrhosis or fibrosis on biopsy. Liver transplantation was used as the primary treatment in only two patients in this series. Both had evidence of advanced hepatocellular dysfunction at presentation, and biopsy confirmed extensive cirrhosis and fibrosis. Even if liver transplantation is chosen as the definitive treatment for a given patient, a method of portal decompression must be chosen if local pretransplant waiting times are long. As documented by other authors and reflected in our experience, the presence of a surgical shunt (especially those in which access to the splanchnic circulation has been accomplished by means of a direct connection with the portal vein) complicates the liver transplant, potentially increasing blood loss and the death rate. 22 As a temporary method of decompression, a TIPS may be the procedure of choice if technically feasible. If it is not, and a surgical shunt is performed, a mesocaval shunt offers the advantage of avoiding dissection of the porta hepatis. In addition, should transplantation eventually be required, a mesocaval shunt allows decompression of the hypertensive splanchnic circulation during the anhepatic phase of the surgical procedure and is easily ligated at the conclusion of the procedure.
Our results illustrate the importance of understanding the potential complications and limitations of each treatment modality and of being able to provide the patient with the entire range of therapies available. It also emphasizes that secondary strategies must be considered when the primary therapy fails. Multiple attempts to revise or redo the primary procedure itself (i.e., placement of multiple stents either in the setting of a TIPS or to treat hepatic vein or caval stenosis) may jeopardize the long-term therapeutic success for these patients. In the opinion of one of us (A.C.V.), angioplasty alone to treat a venous stenosis or chronic occlusion is the initial treatment of choice. Only after angioplasty fails should stent placement be considered. Clearly, effective treatment of BCS requires a multidisciplinary approach.
Early diagnosis and decompression of the portomesenteric venous system can provide excellent survival for most patients with BCS. Accurate assessments of the duration of symptoms, the relevant venous anatomy and extent of thrombosis, and hepatocellular function and reserve are critical in determining the optimal initial course of treatment. To ensure long-term survival, the underlying cause must be treated. All patients, irrespective of treatment modality, should be maintained on oral anticoagulants.
Footnotes
Correspondence: Dr. Andrew S. Klein, Johns Hopkins Hospital, 600 N. Wolfe St., Baltimore, MD 21287-8611.
E-mail: aklein@jhmi.edu
Accepted for publication October 3, 2000.
References
- 1.Ahn S, Yellin A, Sheng F, et al. Selective surgical therapy of the Budd-Chiari syndrome provides superior survivor rates than conservative medical management. J Vasc Surg 1987; 5: 28–37. [PubMed] [Google Scholar]
- 2.Bismuth H, Sherlock D. Portasystemic shunting versus liver transplantation for the Budd-Chiari syndrome. Ann Surg 1991; 214: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron J, Herlong F, SanFey H, et al. The Budd-Chiari syndrome: treatment by mesenteric-systemic venous shunts. Ann Surg 1983; 198: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein A, Cameron J. Diagnosis and management of the Budd-Chiari syndrome. Am J Surg 1990; 160: 128–133. [DOI] [PubMed] [Google Scholar]
- 5.Orloff M, Orloff M, Daily P. Long-term results of treatment of Budd-Chiari syndrome with portal decompression. Arch Surg 1992; 127: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 6.Panis Y, Belghiti J, Valla D, et al. Portosystemic shunt in Budd-Chiari syndrome: long-term survival and factors affecting shunt patency in 25 patients in Western countries. Surgery 1994; 115: 276–281. [PubMed] [Google Scholar]
- 7.Jones R, Lee K, Beschorner W, et al. Venocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783. [DOI] [PubMed] [Google Scholar]
- 8.Klein A, Sitzmann J, Herlong F, Cameron J. Current management of the Budd-Chiari syndrome. Ann Surg 1990; 212: 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringe B, Lang H, Oldhafer K, et al. Which is the best surgery for Budd-Chiari syndrome: venous decompression or liver transplantation? A single-center experience with 50 patients. Hepatology 1995; 21: 1337–1343. [PubMed] [Google Scholar]
- 10.Valla D, Casadevall N, Lacombe C, et al. Primary myeloproliferative disorder and hepatic vein thrombosis. A prospective study of erythroid colony formation in vitro in 20 patients with Budd-Chiari syndrome. Ann Intern Med 1985; 103: 329–334. [DOI] [PubMed] [Google Scholar]
- 11.Lang H, Oldhafer K, Kupsch E, et al. Liver transplantation for Budd-Chiari syndrome: palliation or cure? Transplant Int 1994; 7: 115–119. [DOI] [PubMed] [Google Scholar]
- 12.Kane R, Eustace S. Diagnosis of Budd-Chiari syndrome: comparison between sonography and MR angiography. Radiology 1995; 195: 117–121. [DOI] [PubMed] [Google Scholar]
- 13.Stark D, Hahn P, Trey C, et al. MRI of the Budd-Chiari syndrome. AJR Am J Roentgenol 1986; 146: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 14.Cassel G, Morley J. Hepatic vein thrombosis treated with streptokinase. S Afr Med J 1974; 48: 2319–20. [PubMed] [Google Scholar]
- 15.Greenwood L, Yrizarry J, Hallett J, et al. Urokinase treatment of Budd-Chiari syndrome. AJR Am J Roentgenol 1983; 141: 1057–1059. [DOI] [PubMed] [Google Scholar]
- 16.Sholar P, Bell W. Thrombolytic therapy for inferior vena cava thrombosis in paroxysmal nocturnal hemoglobinuria. Ann Intern Med 1985; 103: 539–541. [DOI] [PubMed] [Google Scholar]
- 17.Shaked A, Goldstein R, Klintmalm G, et al. Portosystemic shunt versus orthotopic liver transplantation for the Budd-Chiari syndrome. Surg Gynecol Obstet 1992; 174: 453–459. [PubMed] [Google Scholar]
- 18.Ochs A, Sellinger M, Haag K, et al. Transjugular intrahepatic portosystemic stent-shunt (TIPS) in the treatment of Budd-Chiari syndrome. J Hepatol 1993; 18: 217–225. [DOI] [PubMed] [Google Scholar]
- 19.Rogopoulos A, Gavelli A, Sakai H, et al. Transjugular intrahepatic portosystemic shunt for Budd-Chiari syndrome after failure of surgical shunting. Arch Surg 1995; 130: 227–228. [DOI] [PubMed] [Google Scholar]
- 20.Halff G, Todo S, Tzakis A, et al. Liver transplantation for the Budd-Chiari syndrome. Ann Surg 1990; 211: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoop M, Lemmens H-P, Langrehr J, et al. Liver transplantation for Budd-Chiari syndrome. Transplant Proc 1994; 26: 3577–3578. [PubMed] [Google Scholar]
- 22.Brems J, Hiatt J, Millis J. Effect of prior portosystemic shunt on subsequent liver transplantation. Ann Surg 1989; 209: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]