Abstract
Objective
To examine the relation between hepatic reticuloendothelial system (RES) dysfunction and the development of acute biliary pancreatitis. In an opossum model, the authors tested the hypothesis that RES blockade can turn the mild pancreatitis seen after pancreatic duct obstruction (PDO) into the severe form.
Summary Background Data
Biliary obstruction is considered the decisive event in gallstone pancreatitis. Suppression of the RES occurs during biliary obstruction.
Methods
Eighteen opossums were placed into three groups of six animals each: group A, RES blockade with λ-carrageenan; group B, PDO; and group C, PDO and RES blockade with carrageenan. The severity of pancreatitis was evaluated by enzyme serum levels and percentage of pancreatic tissue necrosis. RES capacity was measured by dynamic liver scintigraphy, and hepatic blood flow was documented using the hydrogen clearance technique.
Results
No changes in hepatic blood flow occurred in groups A to C. RES capacity was suppressed in groups A and C; in group B, RES function remained unchanged. In group A, amylase and lipase levels remained normal, 3 ± 1.9% of pancreatic tissue were necrotic. The animals in group B developed mild edematous pancreatitis with an increase in amylase and lipase levels and 15 ± 10% of pancreatic necrosis. In group C, amylase and lipase increased significantly and histology revealed severe necrotizing pancreatitis, with 72 ± 11% of necrotic areas.
Conclusions
Artificial RES blockade can promote the progression from mild pancreatitis as observed after PDO to the severe necrotizing form of the disease. Thus, RES dysfunction resulting from biliary obstruction might be an important cofactor in the pathogenesis of bile-induced pancreatitis.
Acute biliary pancreatitis is an inflammatory disease that varies in severity and outcome. Mild edematous pancreatitis usually results in morphologic and functional recovery, whereas severe necrotizing pancreatitis is often associated with multisystemic complications and a death rate of 10% to 50%. 1 The reason for this variety in manifestation is not known. The identification of factors determining the severity of acute pancreatitis is essential to improve the understanding of the pathophysiology of bile-induced pancreatitis and its complications.
Obstruction of the terminal biliopancreatic ductal system is considered to be the triggering event in human gallstone pancreatitis. Opie, 2 in 1901, postulated that reflux of bile, caused by a gallstone impacted in a common terminal biliopancreatic duct, initiates the process of pancreatic inflammation. Today, most authors disagree with this so-called common channel theory, and not reflux of bile but simultaneous obstruction of the biliary and the pancreatic ducts and their pathophysiologic consequences are considered to be the determining factors in the development of severe pancreatitis. 3–5
Suppression of the hepatic part of the reticuloendothelial system (RES) is one of the multiple changes occurring during extrahepatic cholestasis. 6–9 The most important function of the RES is to clear the portal blood of foreign particles and endotoxins before they can enter the peripheral circulation, and failure of RES function is associated with many diseases. 10,11 The results of clinical and experimental studies have shown that RES dysfunction also plays an important role in the pathogenesis of acute biliary-type pancreatitis. 12–15 Recent studies using the opossum model showed that biliary obstruction causes significant suppression of hepatic RES function. Additional obstruction of the pancreatic duct results in severe hemorrhagic pancreatitis and an even greater decrease in RES capacity, whereas pancreatic duct obstruction (PDO) alone leads to mild pancreatitis without alteration of hepatic macrophage function. 16 These results suggest that RES dysfunction, coinciding with a predamaged pancreatic gland, might trigger the progression from mild to severe acute pancreatitis. Nevertheless, it cannot be excluded that impairment of the RES is simply a consequence of the inflammatory disease.
We designed the present study to examine the relation between hepatic RES dysfunction and the development of experimental acute pancreatitis and to test the hypothesis that artificial RES blockade can turn a mild edematous pancreatitis into the severe necrotizing form of the disease.
METHODS
Animals
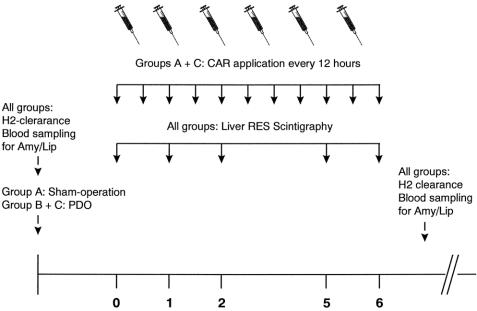
Eighteen female adult North American opossums (Didelphis virginiana) were obtained from Ray Singleton (Riverview, FL). The opossums weighed 3,250 to 4,850 g; all were healthy, dewormed, and free of Salmonella. Animals were housed in individual steel cages, maintained on an artificial 12-hour day/night cycle, and fed standard laboratory chow and water ad libitum. A schematic overview of the experimental setup is given in figure 1.
Figure 1. Experimental time table of surgical procedures, injections of carrageenan (CAR), and measurements of reticuloendothelial (RES) capacity, pancreatic enzyme activity, and liver perfusion performed in groups A to C.
Surgical Procedures
After a 12-hour overnight fast, anesthesia was induced by an intraperitoneal injection of 25 mg/kg pentobarbital (Nembutal, 6 g/100 mL; Sanofi Ceva, Düsseldorf, Germany), a dose that allowed spontaneous breathing. The animals then underwent surgery under sterile conditions. A central venous line was placed in the internal jugular vein and the catheter was externalized to the back. After the abdomen was entered using a midline laparotomy, hepatic microperfusion was measured using the hydrogen clearance technique. The opossums were then randomly placed into three experimental groups of six animals each. The animals in group A underwent mobilization of the pancreatic duct and served as sham-operated controls with solitary artificial RES blockade. This group was designed to exclude possible toxic effects of the substance used for RES blockade on the pancreas itself. In groups B and C, the pancreatic duct was ligated using prolene 4-0. Care was taken to exclude and, if present, close accessory pancreatic ducts. In group B, solitary PDO was performed. The animals in group C underwent additional RES blockade as described below. All experiments were performed in accordance with German legislation on the protection of animals and the “Guide for the Care and Use of Laboratory Animals.”
Dynamic Liver Scintigraphy
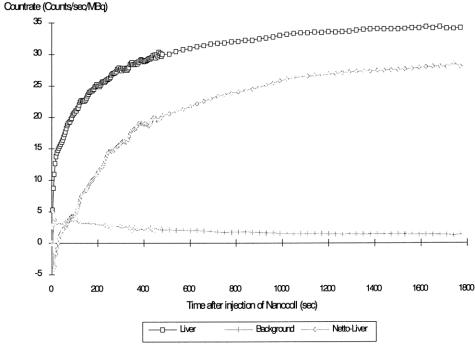
The animals were allowed to recover from anesthesia and surgery and were given free access to food and water. Two days after the surgical procedures and before induction of artificial blockade, RES capacity was determined by dynamic liver scintigraphy. The opossums were anesthetized with an intravenous injection of 0.5 mL Nembutal and placed under a computerized gamma camera. Nanocoll (Sorin Biomedica GmbH, Düsseldorf, Germany), an albumen colloid with a diameter of 25 to 50 nm, was marked with 99mTc and used as a test substance. An injection of 20 MBq in a volume of 2 mL Nanocoll colloid solution was given rapidly through the central venous line. The system was then flushed with 0.9% sodium chloride to avoid accumulation of radioactive particles in the catheter. Gamma camera pictures were taken every 4 seconds for the first 8 minutes and at intervals of 30 seconds for the next 12 minutes. To calculate the net liver uptake of the test substance, one region of interest was placed above the liver and another was used to mark an area in the lower abdomen to measure background radiation. Time-activity curves were generated for both regions of interest, and the resulting net uptake by the hepatic RES was calculated using a computer (Fig. 1).
Fig. 2). To obtain a parameter that quantitatively describes RES capacity, natural logarithmic regression of the data obtained from the gamma camera was performed using the formula: count rate (MBq/sec) = R × ln(time) + S, where S reflects the curve position in the coordinate system and R describes the shape of the uptake curve and therefore is well suited to quantify RES uptake capacity. It is referred to as “regression parameter R.”
Figure 2. Count rates of the regions of interest placed above the liver and the abdomen (background radiation) and calculation of the resulting net liver uptake curve after injection of 20 MBq 99mTc–Nanocoll, according to Baas et al. 16
Scintigraphic measurements were repeated in an identical manner on days 1, 2, 5, and 6 after RES blockade.
RES Blockade
After the initial RES capacity was determined, artificial blockade of the RES was induced in the sham-operated animals of group A and in group C, where PDO had been performed. λ-Carrageenan, a high-molecular-weight sulfated polygalactan, was used to block macrophage function. To provide a sufficient depression of RES function for the entire duration of the experiment, 25 mg/kg was injected twice a day at 12-hour intervals. The animals in group B did not undergo RES blockade and served as controls with solitary PDO.
To detect carrageenan in liver tissue, 5-mm slices were fixed in 9% buffered formalin and embedded in paraffin. Sections were stained with alcian blue at pH 2.5 and examined by light microscopy.
Hydrogen Clearance Technique
To exclude changes in hepatic blood flow as a possible cause of RES suppression, we measured the microperfusion of the liver before surgery and application of the test substances and at the end of the experiment in all animals using the hydrogen clearance technique described by Auckland et al. 17 During the surgical procedure, the liver was exposed and two platinum electrodes (18G) were inserted in the parenchyma of each lobe. The terminals of the electrodes were connected to an amplifier-recorder device (Ameflow, Ameda AG Medical Equipment, Hüneberg, Switzerland). After a short stabilization period of 5 minutes, technically pure hydrogen was added to the air and supplied over a tube that was placed in the mouth of the spontaneously breathing animals for 1 minute. The flow rate of hydrogen gas was 1 L/min. Measurements were taken at 0 and 15 minutes after insufflation and the mean value was calculated. Hepatic blood flow was expressed in mL/min/100 g.
Amylase and Lipase
Blood samples for analysis of serum amylase and lipase activity were obtained from the central venous line before surgery (day 0) and on the last day (day 6) of the experiment. The samples were analyzed using an Hitachi Analyzer (Roche Diagnostics, Mannheim, Germany).
Morphology
On day 6 the animals were killed by an overdose of Nembutal. The pancreas was harvested and fixed in 9% buffered formalin. Five random cross-sections from the head, body, and tail of each pancreas were embedded in paraffin, sectioned (1.5 μm), and stained with hematoxylin and eosin for light microscopy. The extent of pancreatic tissue necrosis was quantitated by morphometric analysis. Extrapancreatic lesions such as fatty tissue necrosis were not considered. For morphometric analysis, a representative area of each cross-section was photographed at ×65 magnification; photomicrographs were printed on glossy paper and coded. Thirty photomicrographs per group were analyzed by an experienced morphologist unaware of the experimental group under investigation. The extent of marked necrotic pancreatic tissue was determined using a computer program (Paint Shop Pro 6.0, Jasc Software Inc. Micro Basic, Munich, Germany) and expressed as a percentage of the total microscopic field.
Statistical Analysis
All data are expressed as means ± standard deviation. Results were analyzed using the Wilcoxon test for paired and unpaired samples. Differences were considered significant at P < .05 and highly significant at P < 0.005.
RESULTS
Surgery
The average operating time for central venous catheter placement, laparotomy, hydrogen clearance measurement, and PDO was 100 minutes. All surgical manipulations were completed without any intra- or perioperative complications. One opossum in group C died on day 5 after RES blockade because of severe necrotizing pancreatitis. This diagnosis was confirmed at autopsy. All other animals survived for the duration of the experiment.
Hepatic Tissue Perfusion
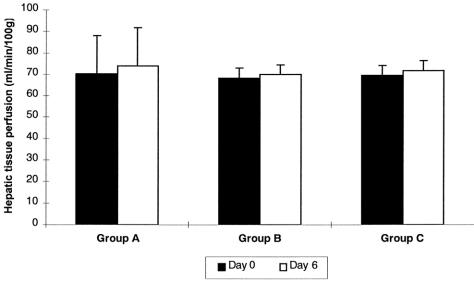
Neither surgical manipulations nor application of carrageenan and induction of RES blockade influenced hepatic blood flow. In groups A to C, values of hepatic blood flow measured before the killing of the animals on day 6 did not differ from values obtained immediately after laparotomy (day 0) (Fig. 3).
Figure 3. Hepatic blood flow in groups A to C. Values on day 0 were obtained during the surgical procedure, values on day 6 before the animals were killed. Data are presented as means ± standard deviation.
RES Phagocytic Capacity
When RES phagocytic capacity was initially determined in groups A to C after the animals had recovered from surgery (day 0), no significant differences in RES function were noted between the three groups. The mean values of regression parameter R were 4.0 ± 0.38, 4.28 ± 0.22, and 4.28 ± 0.36, respectively (Table 1).
Table 1. DEVELOPMENT OF REGRESSION PARAMETER R
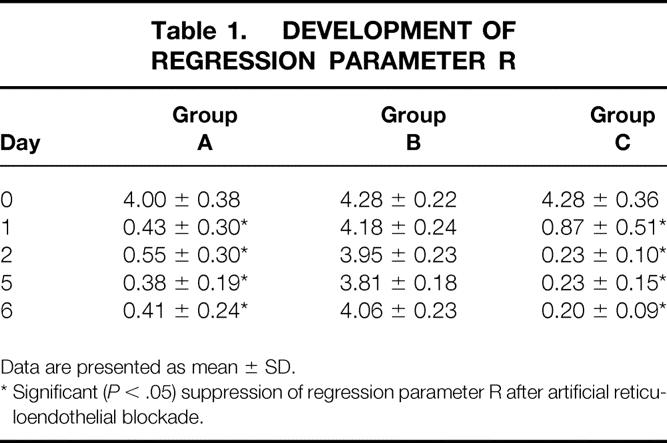
Data are presented as mean ± SD.
* Significant ( P < .05) suppression of regression parameter R after artificial reticuloendothelial blockade.
In group A (solitary RES blockade), regression parameter R dropped significantly on the first day after application of carrageenan. With daily injections of carrageenan as described above, significant RES suppression in this group continued until day 6. In group B (solitary PDO), there was no remarkable change in RES function. Regression parameter R dropped discretely over the course of the experiment, a change that could eventually be explained by the surgical manipulation. The animals in group C (PDO and RES blockade) showed a significant decrease in hepatic RES function that, as in group A, occurred on day 1 after carrageenan injection and continued until day 6.
The scintigraphic results were confirmed by the histologic liver sections stained with alcian blue that showed carrageenan in Kupffer cells (Fig. 8); no alcian blue stain was observed in group B.
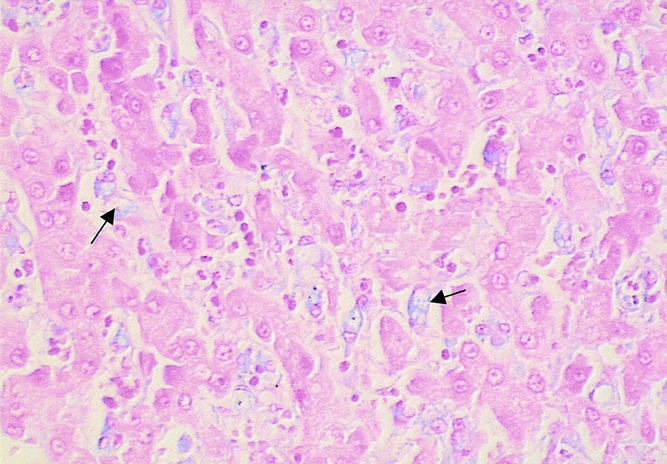
Figure 8. Liver tissue of an animal from group C showing carrageenan within Kupffer cells (arrows; alcian blue stain, ×400).
Serum Amylase and Lipase Activity
Amylase (202 ± 19 to 247 ± 36 U/L) level did not increase significantly after artificial RES blockade in group A. In group B, PDO resulted in significantly elevated amylase levels toward the end of the experiment (249 ± 42 to 745 ± 68 U/L). The highest amylase levels were observed after the combination of PDO and RES blockade in group C. The increase in amylase from day 0 to day 6 (206 ± 16 to 3,470 ± 1,418 U/L) in the latter group and the difference between amylase levels on day 6 in groups B and C (745 ± 68 U/L and 3,470 ± 1,418 U/L) were significant (P < .05;Fig. 4). Lipase activity remained nearly unchanged (60.1 ± 13 to 80 ± 21 U/L) in group A. In groups B (56 ± 9 to 100 ± 14 U/L) and C (55 ± 8 to 950 ± 404 U/L), lipase serum levels were elevated toward the end of the experiment. However, this increase was significant only in group C (P < .05).
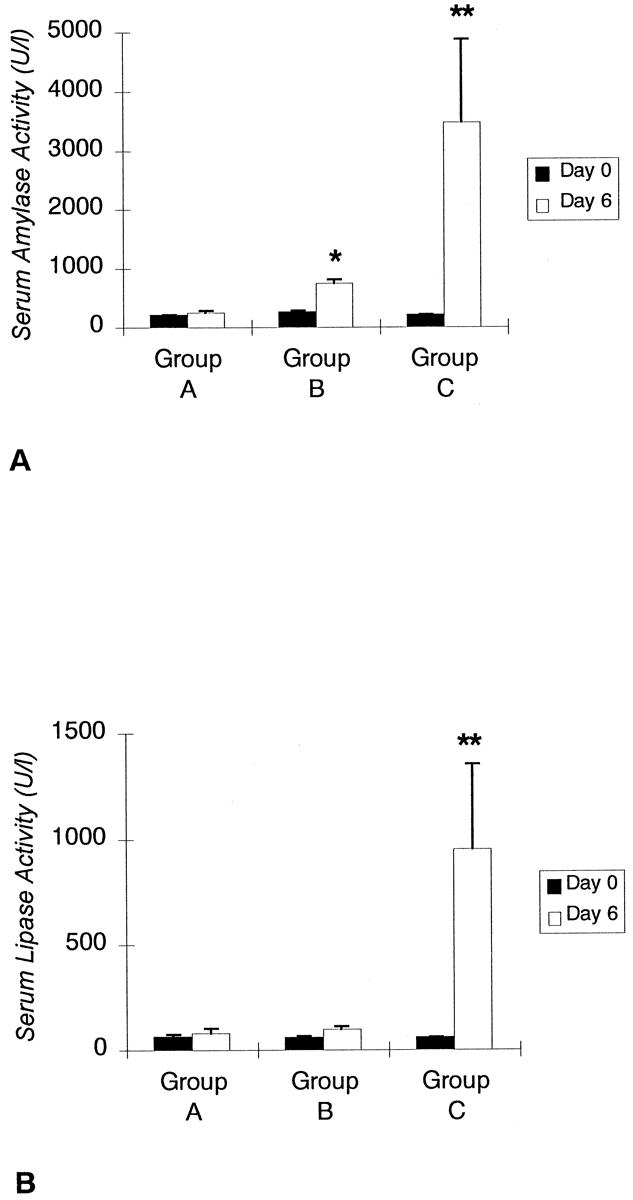
Figure 4. Comparison between preoperative (day 0) amylase and lipase levels and pancreatic enzyme activity at the end of the experiment (day 6) in groups A to C. *, significantly elevated enzyme levels. **, significant differences between groups B and C. Data are expressed as means ± standard deviation.
Morphologic Findings
The pancreas of the animals of group A (RES blockade) appeared normal. Only 3 ± 1.9% (0–8%) of pancreatic cells were necrotic (Fig. 5) (Table 2). The animals in group B, who underwent PDO without RES blockade, developed mild edematous pancreatitis typically characterized by intraacinar distention, intralobular edema, and periductal and interstitial inflammatory infiltrations. The percentage of necrotic pancreatic tissue in this group was 15 ± 10% (7–46%;Fig. 6, Table 2). PDO combined with artificial RES suppression as performed in group C resulted in the histologic reflection of severe necrotizing acute pancreatitis. The extent of pancreatic tissue necrosis, accompanied by extraparenchymal damage such as hemorrhage and fatty tissue necrosis, reached 72 ± 11% (42–88%). This was significantly higher than in group B (P < .05;Fig. 7, Table 2).
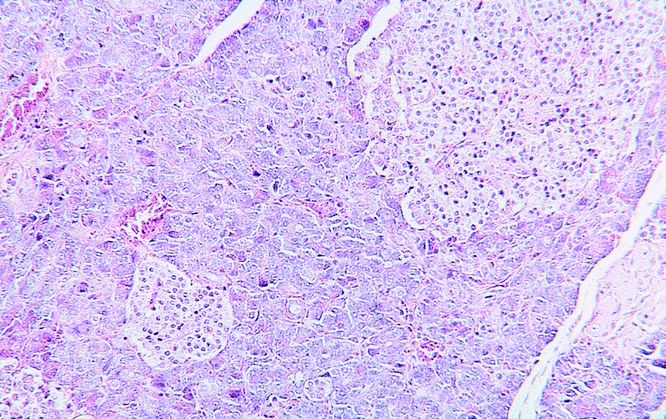
Figure 5. Normal glandular architecture of the pancreas in an animal from group A after artificial reticuloendothelial blockade without pancreatic duct obstruction (hematoxylin and eosin stain, ×125).
Table 2. PERCENTAGE OF PANCREATIC TISSUE NECROSIS
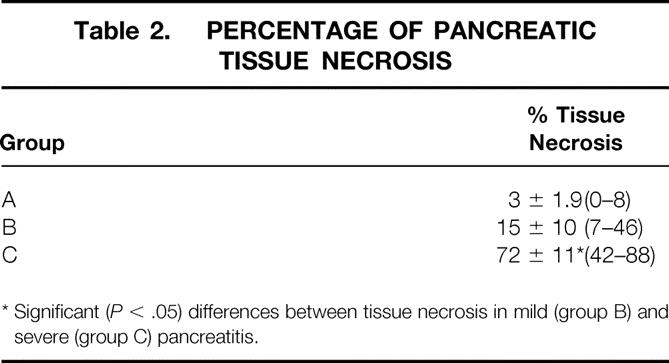
* Significant ( P < .05) differences between tissue necrosis in mild (group B) and severe (group C) pancreatitis.
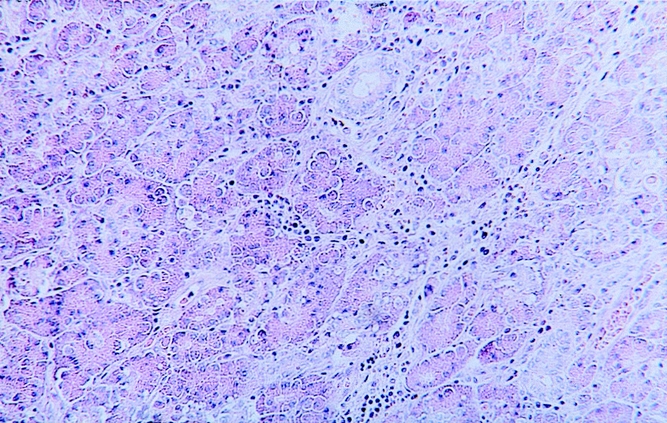
Figure 6. Mild edematous pancreatitis resulting from solitary pancreatic duct obstruction in an animal from group B. Interstitial edema and moderate leukocyte infiltration are typical (hematoxylin and eosin stain, ×125).
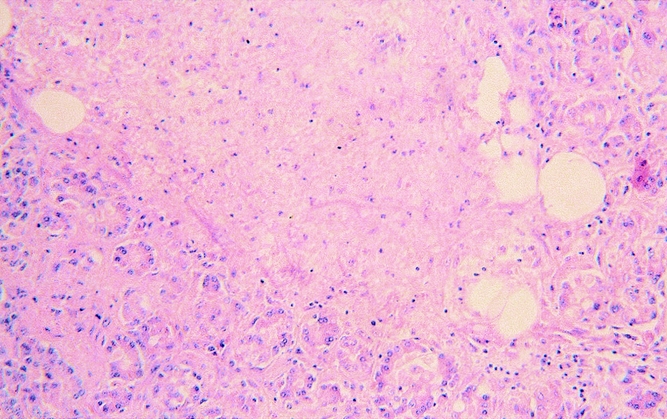
Figure 7. Severe necrotizing pancreatitis resulting from combined pancreatic duct obstruction and artificial reticuloendothelial blockade in an animal from group C. Remnants of pancreatic tissue are surrounded by extensive tissue necrosis and leukocyte infiltration (hematoxylin and eosin stain, ×125).
DISCUSSION
Most cases of acute pancreatitis manifest as mild edematous inflammation of the gland, but at least 10% of patients with acute pancreatitis develop a severe form of the disease associated with high rates of death and complications. Despite intensive research, the pathomechanisms that determine the severity of acute pancreatitis remain unclear. Because of this lack of understanding of pancreatitis pathophysiology, nospecific causal therapy is known, and clinical management still concentrates on symptomatic treatment and prevention of complications.
Biliary obstruction is the major causal factor of acute pancreatitis. The results of studies by Acosta and Ledesma 18 have shown that bile-induced pancreatitis is precipitated by the passage of a gallstone into or through the terminal biliary system. The mechanism by which stone passage triggers acute pancreatitis is still a subject of discussion. The Opie theory has been challenged by reports that bile can be infused through the pancreas at physiologic pressures without causing pancreatitis 19,20 and that a common channel is present in only a small percentage of the population. 21 Further, it has been observed that pancreatic ductal pressure exceeds biliary ductal pressure, 22 suggesting that even if a common channel existed, reflux of pancreatic juice into the bile duct rather than flow of bile into the pancreas would be expected.
Senninger et al 4 demonstrated in the American opossum that necrotizing pancreatitis can be produced by separate ligation of the bile and pancreatic ducts, a situation when reflux of bile is excluded, as well as by obstruction of the combined biliopancreatic duct. Others even described severe acute pancreatitis in the opossum after solitary PDO. 5 Therefore, we must conclude that the most important factor in the development of severe necrotizing pancreatitis is not reflux of bile but biliary and pancreatic duct obstruction and their pathophysiologic consequences.
Increased pancreatic exocrine secretion, 23 increased bile acid concentrations in pancreatic juice, 24 decreased myoelectric activity of the duodenum and the sphincter of Oddi, 25 and increased bacterial translocation from the gut and endotoxemia 26,27 are among the changes that follow biliary obstruction. Interestingly, none of these isolated factors caused severe necrotizing pancreatitis when it was experimentally combined with PDO.
Another well-known alteration occurring during extrahepatic obstructive jaundice is RES dysfunction. 6–8 The RES consists of wandering and sessile mononuclear macrophages that are derived from a common stem cell in the bone marrow. These macrophages are responsible for the identification and clearance of foreign particulate matter from the bloodstream. The Kupffer cells of the liver form the major functional mass of total body RES and account for 90% of total clearance capacity. They are located at a juxtaposition between the portal and systemic circulation and therefore are of highest importance for the phagocytosis of gut-derived bacteria and endotoxins before they can enter the peripheral bloodstream. 9,10 In former studies using the opossum model, we have shown that ligation of the choledochal duct before its junction with the pancreatic duct results in a significantly reduced clearance capacity of the Kupffer cells, but causes only hyperemia in the pancreas. Additional obstruction of the pancreatic duct, however, resulted in an even higher decrease in RES function and caused severe necrotizing pancreatitis. 16
Whether RES suppression is a pathogenic cofactor that triggers the severity of pancreatitis in this model or just a systemic reaction to the inflammatory disease had not been answered. The results presented here show that artificial blockade of the hepatic RES directly influences the development of acute pancreatitis by promoting the progression from the mild to the severe form of the disease.
In studies by other groups, the influence of the RES functional status on the development of pancreatitis was examined using an experimental design where necrotizing pancreatitis was induced first and then the effect of blockade or stimulation of the RES on the outcome of pancreatitis was evaluated. 15 To demonstrate a direct relation between hepatic RES dysfunction and the development of severe acute pancreatitis, we needed an experimental setup that allowed us to examine selectively whether RES suppression induced in animals with mild pancreatitis can turn it into severe necrotizing pancreatitis.
The duct obstruction model used in the North American opossum is a suitable model to examine mechanisms of gallstone pancreatitis. The opossum has a long and easily identified extrahepatic bile duct that is joined by the pancreatic duct 2 to 3 cm before it drains into the duodenum. Thus, ligation of the bile and pancreatic ducts is easily accomplished and surgery can mimic the kind of obstruction that would occur during the passage of a gallstone or its impaction into the papilla of Vater in the clinical situation with little manipulation of the choledochopancreatoduodenal junction. Depending on the site of ligation, standardized circumstances of varying severity of pancreatitis may be examined: pancreatic hyperemia and hypersecretion by solitary ligation of the bile duct, mild edematous pancreatitis by obstruction of the pancreatic duct alone, and necrotizing pancreatitis by ligation of the common biliopancreatic duct or bile and pancreatic ducts. 4 In contrast to the opossum, obstruction of the common channel in the rat results in mild edematous pancreatitis followed by pancreatic atrophy without the development of necrosis. That is why the rat obstruction model was not appropriate to show the histologic appearance of severe necrotizing pancreatitis after RES blockade. The other frequently used model of acute pancreatitis in the rat, cerulein-induced edematous pancreatitis, differs from the pathogenesis of gallstone pancreatitis and therefore is hampered by its lack of clinical relevance. As for the opossum model, its clinical relevance, combined with the morphologic similarities between human and opossum pancreatitis of varying severity, suggests that the opossum is an ideal experimental animal with which to address questions of pancreatitis pathophysiology.
However, opossums cannot be bred in captivity, so animals captured from the wild must be used. Large parasite and Salmonella burden and high interanimal variability are the major problems associated with the use of wild opossums in the laboratory. 28,29 Our animals were pretreated with antibiotics and anthelmintics by the vendor. Because the animals of the three groups did not differ in initial RES capacity, an influence of variable bacterial burden on the results of RES measurement and the severity of pancreatitis is not very likely.
We used carrageenan to induce depression of RES function. Carrageenan is a high-molecular-weight sulfated polygalactan extracted from marine algae. After intravenous injection, it is taken up by the macrophages and produces osmotic swelling and cell death. The use of this substance to depress Kupffer cell function in rats is frequently described in the literature, 30,31 whereas in the opossum artificial RES blockade had not been performed yet. An important factor that had to be considered was the susceptibility of RES phagocytic capacity to changes in hepatic blood flow. To achieve comparable results of macrophage function in the different groups, the effects of carrageenan and also pancreatic obstruction on the perfusion of the liver had to be documented. We measured hepatic microperfusion by the hydrogen clearance technique before surgery and application of carrageenan and toward the end of the experiment in all groups. The results of these measurements showed that neither carrageenan nor pancreatic obstruction altered liver perfusion, and hepatic blood flow remained unchanged in all three groups.
In group A (sham-operated animals with carrageenan application), the regression parameter R representing RES function significantly dropped after the injection of carrageenan. This low level of phagocytic activity was preserved by daily repetition of the carrageenan dose. Because carrageenan is also known to induce acute inflammatory edema of the rat hind paw after subcutaneous injection, 32 a toxic effect of this substance on the pancreas itself could not be excluded from the start. Nevertheless, neither a change in the serum activity of amylase and lipase nor any pathomorphologic changes occurred in the animals with solitary carrageenan-induced RES blockade.
As performed in group B, PDO caused interstitial edema of the pancreas with mild inflammatory cell infiltration and only small areas of necrosis. Serum levels of amylase and lipase were increased. These results confirm earlier observations reported by our group 4,16 using the opossum model and observations made by others who used different animal models. 33,34 Hepatic RES capacity was not significantly decreased in this mild form of pancreatitis. The results from groups A and B clearly indicate that neither RES dysfunction alone nor solitary pancreatic obstruction can cause necrotizing pancreatitis in this experimental setup. Additional carrageenan-induced blockade of the RES, as performed in group C, resulted in an increase of amylase and lipase activity that was significantly greater than in group B. Morphometric analysis in this group revealed severe acute pancreatitis with a significantly higher percentage of necrotic areas, hemorrhage, and extensive fatty tissue necrosis. Because any other alterations observed after mechanical biliary obstruction were excluded in our model by artificial suppression of the RES, we conclude from these results that RES dysfunction can turn the mild form of pancreatitis into the severe necrotizing form. It does not seem to be important whether suppression of the RES is a result of biliary obstruction or is induced pharmacologically.
The mechanism by which diminished RES phagocytic capacity triggers severe pancreatitis cannot be explained by our results. Our results show that mild inflammatory damage of the pancreas, as induced by duct obstruction, seems to be a precondition for the development of severe pancreatitis after macrophage blockade. PDO is known to result in increased pancreatic duct pressure and ductal permeability, thus enabling pancreatic enzymes such as trypsinogen to enter the blood vessels and the lymphatic system. When entering the circulation, these proteases are complexed by α2-macroglobulin. The complexes remain enzymatically active and can still activate various enzymes such as trypsin, chymotrypsin, kallikrein, and elastase. 35 Normally, the proteinase–inhibitor complexes are rapidly cleared from the bloodstream by receptor-mediated phagocytosis by the RES. 36 If this clearance is not adequately performed, these complexes could further damage the pancreas and other tissues, thereby inducing a vicious circle culminating in necrotizing pancreatitis and multisystemic complications.
Another possible explanation would be that the deficit of pancreatic enzymes in the duodenum during duct obstruction results in maldigestion and consecutive overgrowth of endotoxin-producing bacteria in the gut. 6 Bacterial endotoxins activate macrophages and mediate the production of different cytokines. These cytokines are known to activate neutrophil granulocytes that develop metabolic hyperactivity in severe acute pancreatitis. 37 Elevated cytokine levels and granulocyte hyperactivation are associated with impaired function of the phagocytic cells, 38,39 again resulting in an inadequate clearance of endotoxin and harmful protease–antiprotease complexes.
Further research will be necessary to prove or disprove these arguments and to clarify the significance of the complex mechanisms of local and systemic immune function in the progression from mild to severe acute pancreatitis. Whether support or substitution of impaired macrophages can influence the outcome of acute biliary pancreatitis must be investigated in further studies with our model. The efficiency of a so-called artificial RES for eliminating protease-complexed α2-macroglobulin has been proven in vitro. 40 However, as for the clinical situation, our results show that dysfunction of the hepatic RES like that found in obstructive jaundice promotes the progression from mild to severe acute pancreatitis, and early biliary decompression is necessary in acute pancreatitis to avoid further damage to the RES.
Footnotes
Correspondence: Dr. Christina Schleicher, Klinik und Poliklinik für Allgemeine Chirurgie, Westfalian Wilhelms-University, Waldeyerstraβe 1, 48149 Muenster, Germany.
E-mail: cschlei@medsnt01.uni-muenster.de
Accepted for publication November 13, 2000.
References
- 1.Goebell H. Klinik und Prognose der akuten Pankreatitis. In: Moessner J, Adler G, Fölsch UR, Singer MV, eds. Erkrankungen des exkretorischen Pankreas. Jena: Gustav Fischer Verlag; 1995: 256–261.
- 2.Opie EL. The etiology of hemorrhagic pancreatitis. Bull Johns Hopkins Hosp 1901; 12: 182–188. [Google Scholar]
- 3.Luethen R, Niederau C. Pathophysiology of acute pancreatitis. Z Gastroenterol 1990; 28: 211–221. [PubMed] [Google Scholar]
- 4.Senninger N, Moody FG, Coelho JCU, et al. The role of biliary obstruction in the pathogenesis of acute pancreatitis in the opossum. Surgery 1986; 99: 688–693. [PubMed] [Google Scholar]
- 5.Lerch MM, Saluja AK, Rünzi M, et al. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology 1993; 104: 853–861. [DOI] [PubMed] [Google Scholar]
- 6.Scott-Conner CE, Grogan JB. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J Surg Res 1994; 57: 316–336. [DOI] [PubMed] [Google Scholar]
- 7.Vane DW, Redlich P, Weber T, et al. Impaired immune function in obstructive jaundice. J Surg Res 1988; 45: 287–293. [DOI] [PubMed] [Google Scholar]
- 8.Clements WDB, Halliday MI, McCaigue MD, et al. Effects of extrahepatic obstructive jaundice on Kupffer cell clearance capacity. Arch Surg 1993; 128: 200–205. [DOI] [PubMed] [Google Scholar]
- 9.Saba TM. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med 1970; 126: 1031–1052. [PubMed] [Google Scholar]
- 10.Baas J, Senninger N, Elser H. The reticuloendothelial system. An overview of function, pathology and newer methods of measurement. Z Gastroenterol 1994; 32: 117–123. [PubMed] [Google Scholar]
- 11.Athlin L, Holmberg SB, Hafstrom L. Macrophage function and surgery. A clinical review with special reference to phagocytosis. Eur J Surg 1991; 157: 163–170. [PubMed] [Google Scholar]
- 12.Larvin M, Alexander DJ, Switala SF, et al. Impaired mononuclear phagocyte function in patients with severe pancreatitis: evidence from studies of plasma clearance of trypsin and monocyte phagocytosis. Dig Dis Sci 1993; 38: 18–27. [DOI] [PubMed] [Google Scholar]
- 13.Alexander D, Madan M, Hodgson J, et al. Complicated pancreatitis results in disease specific impairment of clearance of protease-antiprotease complexes. Gut 1991; 32: A342. [Google Scholar]
- 14.Banks RE, Evans SW, Alexander D, et al. Alpha2 macroglobulin state in acute pancreatitis. Raised values of α2 macroglobulin-protease complexes in severe and mild attacks. Gut 1991; 32: 430–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adham NF, Song MK, Haberfelde GC. Relationship between the functional status of the reticuloendothelial system and the outcome of experimentally induced pancreatitis in young mice. Gastroenterology 1983; 84: 461–469. [PubMed] [Google Scholar]
- 16.Baas J, Senninger N, Elser H, et al. Dynamic liver scintigraphy–a new way of measuring the function of the reticuloendothelial system of the liver. Eur Surg Res 1995; 27: 137–144. [DOI] [PubMed] [Google Scholar]
- 17.Auckland K, Bower BF, Berliner RW. Measurement of local blood flow with hydrogen gas. Circ Res 1964; 14: 164–187. [DOI] [PubMed] [Google Scholar]
- 18.Acosta JL, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med 1974; 290: 484–487. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TM, Dunphy JE. Continuous perfusion of bile protease through the pancreas. JAMA 1963; 183: 530–533. [DOI] [PubMed] [Google Scholar]
- 20.White TT, Magee DF. Perfusion of the dog pancreas with bile without producing pancreatitis. Ann Surg 1960; 131: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones BA, Salsberg BB, Bohnen JMA, et al. Common pancreatobiliary channels and their relationship to gallstone size in gallstone pancreatitis. Ann Surg 1987; 205: 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carr-Locke D, Cregg JA. Endoscopic manometry of pancreatic and biliary sphincter zone in man. Dig Dis Sci 1981; 26: 7–15. [DOI] [PubMed] [Google Scholar]
- 23.Senninger N, Moody FG, Van Buren DH, et al. Effect of biliary obstruction on pancreatic exocrine secretion in conscious opossums. Surg Forum 1984; 35: 226–228. [Google Scholar]
- 24.Senninger N, Runkel N, Zosel S, et al. Sphincter of Oddi motility and bile acid concentration in pancreatic juice following biliary obstruction in opossums. Pancreas 1992; 7: 758. [Google Scholar]
- 25.Senninger N, Zou SQ, Machens HG, et al. Myoelectric activity of the sphincter of Oddi and duodenum following temporary obstruction of the common channel. Langenbeck Arch Chir Suppl Chir Forum 1990; 107: 437–440. [Google Scholar]
- 26.Runkel NSF, Rodriguez LF, Moody FG. Mechanisms of sepsis in acute pancreatitis in opossums. Am J Surg 1995; 169: 227–232. [DOI] [PubMed] [Google Scholar]
- 27.Pain JA, Bailey ME. Measurement of operative plasma endotoxin levels in jaundiced and non-jaundiced patients. Eur Surg Res 1987; 19: 207–216. [DOI] [PubMed] [Google Scholar]
- 28.Runkel NS, Rodriguez LF, Moody FG, et al. Salmonella infection of the biliary and intestinal tract of wild opossums. Lab Anim Sci 1991; 41: 54–56. [PubMed] [Google Scholar]
- 29.Senninger N, Runkel N. Applied anatomy: opossums. In: Jensen JL, Gregersen H, Moody FG, Shokouh-Amiri M, eds. Essentials of experimental surgery: gastroenterology. Newark, NJ: The Gordon and Breach Publishing Group; 1995:1–11.
- 30.Marshall JC, Lee C, Meakins JL, et al. Kupffer cell modulation of the systemic immune response. Arch Surg 1987; 122: 191–196. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Arii S, Sasaoki T, et al. The role of the Kupffer cells in the surveillance of tumor growth in the liver. J Surg Res 1993; 55: 140–146. [DOI] [PubMed] [Google Scholar]
- 32.Winter CA, Risley EA, Nuss GW. Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 1962; 111: 544–547. [DOI] [PubMed] [Google Scholar]
- 33.Churg A, Richter WR. Early changes in the exocrine pancreas of the dog and rat after ligation of the pancreatic duct: a light and electron microscopy study. Am J Pathol 1971; 63: 521–546. [PMC free article] [PubMed] [Google Scholar]
- 34.Ohshio G, Saluja A, Steer ML. Effects of short-term pancreatic duct obstruction in rats. Gastroenterology 1991; 100: 196–202. [DOI] [PubMed] [Google Scholar]
- 35.Travis J, Savelsen GS. Human plasma proteinase inhibitors. Annu Rev Biochem 1983; 52: 655–709. [DOI] [PubMed] [Google Scholar]
- 36.Ohlsson K, Laurell CB. The disappearance of enzyme-inhibitor complexes from the circulation of man. Clin Sci Mol Med 1976; 51: 87–92. [DOI] [PubMed] [Google Scholar]
- 37.Widdison AL, Cunningham S. Immune function early in acute pancreatitis. Br J Surg 1996; 83: 633–636. [DOI] [PubMed] [Google Scholar]
- 38.Lanser ME, Mao P, Brown G, et al. Serum mediated depression of neutrophil chemilumiscence following blunt trauma. Ann Surg 1985; 202: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liras G, Carballo F. An impaired phagocytic function is associated with leucocyte activation in the early stages of severe acute pancreatitis. Gut 1996; 39: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama S, Hermann RE, Malchesky PS, et al. Removal of trypsin complexed alpha-2 macroglobulin by plasma fractionation. ASAIO J 1993; 39: M297–300. [PubMed] [Google Scholar]